Abstract
Cancer progression is driven by an accumulation of numerous genetic and epigenetic alterations in cancer cells themselves as well as constructional changes in their microenvironment. Metadherin (MTDH)/Astrocyte elevated gene-1 (AEG-1) has emerged in recent years as a key contributor to the carcinogenic process in diverse organs and tissues. As a multifunctional mediator of carcinogenesis, MTDH/AEG-1 has been found to be involved in multiple signaling pathways, such as: PI3K/Akt, NF-κB, Wnt/β-catenin and MAPK. Overexpression of MTDH/AEG-1 is observed in a variety of cancers belonging to all biological systems, and has crucial relevance with cancer progression, including initiation, proliferation, invasion, metastasis and chemoresistance. In addition, a plethora of studies have convincingly demonstrated that MTDH/AEG-1 overexpression markedly correlates with poor clinical prognosis. These findings suggest that MTDH/AEG-1 may be used as a potential biomarker for the diagnosis of cancer, monitoring of cancer progression, and target therapies which may simultaneously inhibit tumor growth, block metastasis, and intensify the efficacy of chemotherapeutic treatments.
Keywords: MTDH/AEG-1, cancer progression, carcinogenesis, signaling pathway, target therapy
Introduction
The evolution of cancer is a heterogeneous and continuous process involving multiple genetic and epigenetic alterations which promote cancer initiation, proliferation, invasion and metastasis [1]. Concerted efforts over the past two decades have made significant strides in the molecular pathogenesis of cancer resulting in the development of various treatments including small molecule inhibitors of specific target genes involved in the carcinogenic process. However, each malignant neoplasm has unique genetic makeup leading to differences in behavior and progression, which limit the effective application of a single standard modality of treatment in all cancers. These complexities increase the challenges and impediments to developing targeting novel molecules and effective cancer therapies. Theoretically, identification of molecule (s) which might play a prominent regulatory role in almost all types of cancer would be a significant advancement in the treatment of cancer.
Metadherin (MTDH), also known as Astrocyte elevated gene-1 (AEG-1) and Lysine-rich CEACAM1 co-isolated (LYRIC), fulfills many of the aforementioned characteristics to serve as an important molecule regulating multiple events in carcinogenesis. Since its initial cloning in 2002 [2], plenty of studies have demonstrated that MTDH/AEG-1 expression is elevated in a wide variety of cancers [3,4], such as hepatocellular, gallbladder and renal cell carcinomas, breast, lung, prostate, ovarian, esophageal, gastric and colorectal cancers, and glioma, neuroblastoma, melanoma, osteosarcoma and so on. With further studies, the evidence is that MTDH/AEG-1 affects most of the hallmarks in diverse cancers. MTDH/AEG-1 has emerged as a potentially crucial regulator of malignant tumors mediated by a complex network of oncogenic signaling pathways. This review provides current understanding of the role of this multifunctional gene in the progression of cancer.
Cloning and structure of MTDH/AEG-1
MTDH/AEG-1 was first identified and cloned in 2002 [2] as a neuropathology-associated gene induced at elevated levels in primary human fetal astrocytes after human immunodeficiency virus-1 (HIV-1) infection or treatment with HIV-1 envelope glycoprotein (gp120). Subsequently, four independent groups, reported the full-length cloning and functional characterization of MTDH/AEG-1, and named it depending on its discovery and postulated function [3,5-7]. In 2004, Brown and colleagues [5] used a phage expression library of breast carcinoma cDNAs to identify a lung-homing peptide in MTDH/AEG-1 which was involved in the lung metastasis of mouse 4T1 mammary tumors. Based on its overexpression in metastatic breast cancer and promoting the adhesion of 4T1 mammary tumor cells to lung, it was named metadherin (metastasis adhesion protein). In the same year, Britt et al [6] and Sutherland et al [7] also reported a novel protein, which was named LYRIC and 3D3/LYRIC separately. Data from rat and human epithelial tissues show that MTDH/AEG-1 colocalizes with tight junction protein ZO-1 in polarized epithelial cells [6], and as a transmembrane protein it is presented in cytoplasm, endoplasmic reticulum, nuclear envelope, and nucleolus [7]. The protein is highly conserved in mammals, and can be found in most vertebrates, but not in non-vertebrate species [7].
The full-length MTDH/AEG-1 cDNA includes 3611bp, excluding the poly-A tail. Genomic BLAST search demonstrated that the MTDH/AEG-1 gene consists of 12 exons and 11 introns, and is located at 8q22 where cytogenetic analysis of human gliomas manifests recurrent amplification. The open reading frame from 220 to 1968 nts encodes a single-pass transmembrane protein, including 582 amino acid, with molecular weight of 64 kDa and PI of 9.33 [3]. Multiple isoforms of the protein with different molecular weight 80 kDa, 70 kDa, 50 kDa and 37 kDa were also detected by antibodies against MTDH/AEG-1, possibly because of posttranslational processing or modification [7]. Several independent protein motif analysis methods have predicted that MTDH/AEG-1 has a single transmembrane domain. However, it is still controversial about whether MTDH/AEG-1 is a type Ib membrane protein (C-terminal inside), or a type II protein (C-terminal outside) [3,5-7].
MTDH/AEG-1 contains three putative lysine-rich nuclear localization signals (NLS), which may regulate its distribution and function in cells [7-10]. Emdad et al [8,9] revealed the presence of putative NLS between amino acids 79-91, 432-451 and 561-580. Subsequently, Thirkettle et al [10] observed that NLS-3 (amino acids 546-582) was a primary determinant of MTDH/AEG-1 nuclear localization, while extended NLS-1 (amino acids 78-130) regulated its nucleolar localization. In addition, at NLS-2 extension (amino acids 415-486), MTDH/AEG-1 could be modified by ubiquitin within the cytoplasm, and its ubiquitination was postulated to direct its cytoplasmic distribution. The examination of MTDH/AEG-1 promoter activity indicated that the negative transcriptional control elements were located in the region-2710/-459, while transcription factors binding to the region-459/-301 could enhance the activity of MTDH/AEG-1 promoter. With further analysis, it was found that the region-356/-302 were transcription factor binding sites, including two E-box elements, which were critical for basal MTDH/AEG-1 promoter activity as well as Ha-ras-mediated MTDH/AEG-1 promoter activation [11].
Crucial signaling pathways involved in MTDH/AEG-1-mediated cancer progression
MTDH/AEG-1 plays key roles in the activation of a group of signaling pathways, such as PI3K/Akt, nuclear factor-κB (NF-κB), Wnt/β-catenin, and mitogen-activated protein kinase (MAPK) pathways, which are involved in cancer proliferation, invasion, angiogenesis, metastasis, turning on epithelial-mesenchymal transition (EMT) and chemoresistance [12]. As a multifunctional protein, MTDH/AEG-1 profoundly modulates a various array of signaling networks and effector molecules to implement diverse biological processes in cancers (Figure 1).
Figure 1.
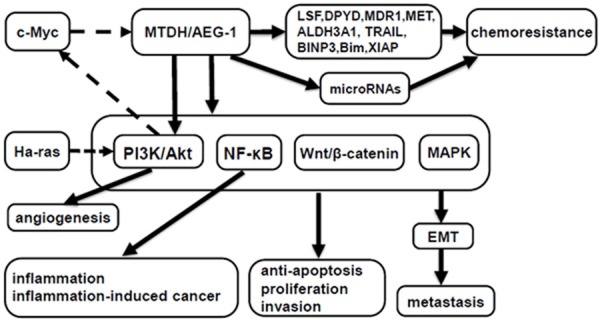
Schematic representation of the signaling networks and effector molecules modulated by MTDH/AEG-1 in carcinogenesis. Solid arrows denote signaling pathways and/or molecules regulated by MTDH/AEG-1, while dotted arrows denote signaling pathways and/or molecules that regulate MTDH/AEG-1.
PI3K/Akt pathway
PI3K/Akt pathway is a major signaling pathway regulated by MTDH/AEG-1. MTDH/AEG-1 is a downstream target gene of oncogenic Ha-ras, and is induced mainly at a transcriptional level by Ha-ras, which activates the PI3K/Akt signaling pathway leading to the binding of c-Myc to two E-box elements in the MTDH/AEG-1 promoter, thereby regulates MTDH/AEG-1 transcription [11]. Intriguingly, MTDH/AEG-1 overexpression also increases the phosphorylation of Akt and downstream substrates of Akt (including GSK3β/c-Myc, p21/mda-6, MDM2/p53 and Bad), resulting in activation of PI3K/Akt pathway [13]. These findings demonstrate that there is a feedback loop between MTDH/AEG-1 and PI3K/Akt pathway. Overexpression of MTDH/AEG-1 protects against serum starvation-induced apoptosis [13], conversely, inhibition of MTDH/AEG-1 is respectively shown to induce apoptosis by upregulating FOXO3a activity in prostate cancer cells [14] and the activity of FOXO1 in breast cancer cells [15] through PI3K/Akt signaling pathway. In non-small cell lung cancer (NSCLC), MTDH/AEG-1 significantly increases the levels of PI3Kp110 and the phosphorylation of Akt leading to the activation of PI3K/Akt pathway, and inhibits apoptosis by suppressing caspase-3 and enhancing the level of anti-apoptotic protein Bcl-2 [16]. In addition, MTDH/AEG-1 mediates aggressive ability of NSCLC through the activation of both PI3K/Akt and NF-κB signaling pathways [17]. In a recent study, we found that MTDH/AEG-1 knockdown could block the carcinogenesis and progression of human hepatocellular carcinoma (HCC) cell line MHCC97-H and highlight the therapeutic potential of Huaier polysaccharide (HP) on the migration and invasion of MHCC97-H cells, at least partly, by enhancing the NK cell-mediated immune response and inhibiting the activation of the PI3K/Akt pathway [18].
NF-κB pathway
Emdad et al [8] first reported that treatment with tumor necrosis factor-α (TNF-α) in HeLa cells resulted in MTDH/AEG-1 translocation into the nucleus where it combined with the p65 subunit of NF-κB and augmented the expression of NF-κB downstream genes such as cell adhesion molecules (ICAM-2, ICAM-3, selectin P ligand, selectin E, selectin L), toll-like receptor TLR4 and TLR5, FOS, JUN, and cytokines IL-8, which were involved in tumor progression and metastasis. Inhibition of NF-κB pathway by employing an IκB super repressor (Ad.IκBα-mt32) significantly reverted MTDH/AEG-1-induced agar cloning efficiency and invasion in HeLa cells [8]. Coimmunoprecipitation (Co-IP) assays revealed that MTDH/AEG-1 might function as a bridging molecule between p65, cyclic AMP-responsive element binding protein–binding protein (CBP) and the basal transcription machinery, and act as a coactivator for NF-κB, consequently facilitating transcriptional activation of NF-κB downstream genes which were necessary for migration and invasion [19]. Activation of NF-κB pathway by MTDH/AEG-1 has also been reported in prostate cancer and NSCLC, as well as in HCC [14,17,20,21].
Moreover, the potential role of MTDH/AEG-1 in inflammatory processes is also suggested by the observation that knockdown of MTDH/AEG-1 inhibits the ubiquitylated NF-κB signaling components from accumulating at the endoplasmic reticulum, reduces the activation of NF-κB, and decreases the production of proinflammatory cytokines [22]. In U937 human promonocytic cells, lipopolysaccharide (LPS) augmented the expression of MTDH/AEG-1 via NF-κB pathway, and MTDH/AEG-1 induced by LPS might further affect LPS-induced NF-κB activation. The prevention of MTDH/AEG-1 expression suppressed the production of LPS-induced TNF-α and prostaglandin E2 [23]. Another study showed that knockdown of MTDH/AEG-1 in human breast cancer cell line MDA-MB-231 abrogated LPS-induced cell migration and invasion, and decreased NF-kB activation by LPS and also inhibited LPS-induced production of IL-8 and MMP9 [24]. These findings suggest that MTDH/AEG-1 might be a target molecule for the treatment of LPS-related diseases such as inflammation and inflammation-induced tumor.
Wnt/β-catenin pathway
The Wnt/β-catenin pathway is another significant signaling pathway which is associated with MTDH/AEG-1 in several cancer indications. In HCC cells, MTDH/AEG-1 was found to activate Wnt/β-catenin pathway directly by upregulating lymphoid-enhancing factor 1 (LEF1) levels and indirectly by activating ERK42/44, so facilitating nuclear translocation of β-catenin. Inhibition of MTDH/AEG-1 by siRNA could significantly downregulate both MTDH/AEG-1 and LEF1 protein levels, while LEF1 siRNA-mediated inhibition only downregulated LEF1 protein levels but not the levels of MTDH/AEG-1 protein, indicating that LEF1 is a downstream gene of MTDH/AEG-1. It was also confirmed that both MTDH/AEG-1 siRNA and LEF1 siRNA could profoundly inhibit the invasion of HCC cells [20]. In gastric cancer, inhibition of MTDH/AEG-1 decreased the level of β-catenin, downregulated LEF1 and cyclin D1 proteins which were two critical downstream effectors in Wnt/β-catenin pathway, thereby inhibiting tumor cells growth and enhancing apoptosis [25]. The study of colorectal carcinoma (CRC) suggested that there was a positive correlation between MTDH/AEG-1 high expression and β-catenin nuclear expression, and overexpression of MTDH/AEG-1 remarkably increased nuclear β-catenin accumulation in CRC cell lines. It was documented that MTDH/AEG-1 promoted invasion and metastasis of CRC by activation of Wnt/β-catenin pathway [26]. Furthermore, the contribution of MTDH/AEG-1 to the pathogenesis of diffuse large-B-cell lymphoma (DLBCL) is also mediated by activation of Wnt/β-catenin pathway [27].
MAPK pathway
It is frequently observed that MTDH/AEG-1 can regulate cancer progression by activating MAPK pathway, of which extra cellular signal-regulated kinase (ERK) and p38 mitogen-activated protein kinases (p38MAPK) pathway are mainly involved. Yoo et al [20] demonstrated that overexpression of MTDH/AEG-1 could enhance the phosphorylated forms of ERK and p38 MAPK, and activation of both ERK and p38 MAPK pathways played an important role in mediating MTDH/AEG-1-inducedl invasion and anchorage-independent growth in HCC cells. Meanwhile, they found ERK pathway was associated with the Wnt/β-catenin pathway. After being activated by MTDH/AEG-1, ERK increased the level of phosphorylated GSK3β which could promote β-catenin to translocate into the nucleus, and resulted in activating the Wnt signaling pathway indirectly [20]. In the breast cancer cells, it is showed that knockdown of MTDH/AEG-1 could enhance the sensitivity of breast cancer cells to AZD6244 which is a novel ATP-noncompetitive inhibitor of MAP/ERK kinase, by regulating the expression and activity of FOXO3a [28]. Another study has suggested that MTDH/AEG-1 participates in transforming growth factor (TGF) β1-induced epithelial-mesenchymal transition through activation of p38 MAPK pathway in proximal tubular epithelial cell [29].
Multifaceted functions of MTDH/AEG-1 in cancer progression
Analysis in human Multiple Tissue Northern blots displays variable MTDH/AEG-1 expression in almost all tissues. MTDH/AEG-1 expression is high in two kinds of organs, muscle-dominating organs such as heart, skeletal muscle, small intestine and tongue, and endocrine glands including adrenal gland and thyroid. Northern blot analysis of MTDH/AEG-1 expression in human cancer cells identifies that MTDH/AEG-1 expression is upregulated in diverse cancer cells including breast cancer cells (MCF-7, MDA-MB-157, -231 and-453), melanoma cells (HO-1, C8161 and MeWo), and glioblastoma multiforme cells (T98G and G18) [3]. So far, the spectrum of cancers studied has covered all organs and tissues belonging to almost all biological systems (Table 1). Through overexpression or repression of MTDH/AEG-1 in cancer cells, these studies have demonstrated more and more clearly that MTDH/AEG-1 plays an important role in the proliferation, invasion, angiogenesis, metastasis, chemoresistance and prognosis of cancers.
Table 1.
Studies on MTDH/AEG-1 in diverse cancers
| Cancer types | First author/s, year (ref.) |
|---|---|
| Gastrointestinal system | |
| HCC | Yoo et al, 2009 [20]; Zhu et al, 2011 [55]; Gong et al, 2012 [73]; Zhou et al, 2012 [44]; Srivastava et al, 2012 [38]; Ahn et al, 2013 [74]; Zheng et al, 2014 [18]; Robertson et al, 2014 [21] |
| Gastric cancer | Jian-bo et al, 2011 [25]; Baygi&Nikpour, 2012 [75]; Zhang et al, 2013 [76]; Huang et al, 2013 [77] |
| ESCC | Yu et al, 2009 [30] |
| CRC | Song et al, 2010 [78]; Jiang et al, 2012 [45]; Gnosa et al, 2012 [79]; Wang et al, 2012 [80]; Zhang et al, 2013 [26]; Huang et al, 2014 [71]; Casimiro et al, 2014 [81] |
| GBC | Sun et al, 2011 [58]; Liu&Yang, 2013 [46] |
| TSCC | Ke et al, 2013 [59] |
| SGC | Liao et al, 2011 [82] |
| Genitourinary system | |
| RCC | Chen et al, 2010 [47]; Erdem et al, 2013 [83] |
| Bladder cancer | Zhou et al, 2012 [60]; Nikpour et al, 2014 [31] |
| PC | Kikuno et al, 2007 [14]; Thirkettle et al, 2009 [10]; Wan et al, 2014 [48] |
| Breast cancer | Brown et al, 2004 [5]; Li et al, 2008 [61]; Hu et al, 2009 [43]; Li et al, 2009 [15]; Su et al, 2010 [34]; Li et al, 2011 [39]; Li et al, 2011 [52]; Kong et al, 2012 [28]; Ward et al, 2013 [53]; Tokunaga et al, 2014 [62]; Zhang et al, 2014 [35]; Xu et al, 2014 [84]; Wan et al, 2014 [85] |
| Ovarian cancer | Meng et al, 2011 [86]; Li et al, 2011 [49]; Li et al, 2012 [87]; Yuan et al, 2012 [88]; Li et al, 2014 [63] |
| Endometrial cancer | Song et al, 2010 [64]; Meng et al, 2011 [70] |
| Nervous system | |
| Glioma | Emdad et al, 2010 [32]; Liu, et al, 2010 [89]; Xia et al, 2010 [90]; Yang et al, 2012 [72] |
| Neuroblastoma | Liu et al, 2009 [33]; Lee et al, 2009 [40]; Liu et al, 2012 [91] |
| Respiratory system | |
| NSCLC | Song et al, 2009 [17]; Sun et al, 2012 [41]; Ke et al, 2013 [16]; Liu et al, 2013 [54]; Yao et al, 2014 [92] |
| Kinetic system | |
| Osteosarcoma | Wang et al, 2011 [42]; Liu et al, 2013 [93]; Tang et al, 2014 [56] |
| Hematological system | |
| Lymphoma | Ge et al, 2012 [27]; Yan et al, 2012 [94]; Li et al, 2014 [95] |
| Others | |
| HNSCC | Nohata et al, 2011 [96]; Wang et al, 2013 [97]; Yu et al, 2014 [57] |
| Melanoma | Kang et al, 2005 [3]; Emdad et al, 2007 [9] |
HCC, hepatocellular carcinoma; ESCC, esophageal squamous cell carcinoma; CRC, colorectal cancer; GBC, gallbladder carcinoma; TSCC, squamous cell carcinoma of the tongue; SGC, Salivary gland carcinoma; RCC, renal cell carcinoma; PC, prostate cancer; NSCLC, non-small cell lung cancer; HNSCC, head and neck squamous cell carcinoma.
Proliferation
The proliferation of malignant neoplasm is a consequence of acquisition of unlimited growth potential and resistance to apoptosis. It is demonstrated that MTDH/AEG-1 potentiates serum-independent cell growth by inhibiting serum starvation-induced apoptosis [13]. In NSCLC, it is also elucidated that MTDH/AEG-1 could inhibit apoptosis via augmenting the level of anti-apoptotic protein Bcl-2 and activating PI3K/Akt pathway [16].The studies above provide the evidences that MTDH/AEG-1 has anti-apoptotic potential. MTDH/AEG-1 combined with Ha-ras can enhance soft agar colony forming ability of human astrocytes and immortalized normal human melanocyte, suggesting a potential role in oncogenesis [3].
Overexpression of MTDH/AEG-1 can significantly enhance cell proliferation and anchorage-independent growth ability in a variety of cancers, such as breast cancer [15], esophageal squamous cell carcinoma (ESCC) [30], HCC [20], bladder cancer [31], malignant gliomas [32] and so on. Liu et al [33] reported that knockdown of MTDH/AEG-1 resulted in cell arrest in the G0/G1 phase of cell cycle, and then inhibited colony forming ability and promoted apoptosis in neuroblastoma cells. In breast cancer studies, immunohistochemistry was used to analyze MTDH/AEG-1 expression in normal breast tissue, usual ductal hyperplasia (UDH), atypical ductal hyperplasia (ADH), ductal carcinoma in situ (DCIS) and invasive cancer. The results showed that MTDH/AEG-1 was nearly no expressed in normal cases, but with the progression of intraductal proliferative lesions, the level and intensity of expression were higher and stronger. The highest expression in DCIS cases may suggest that MTDH/AEG-1 plays a more significant role in initiation of ductal carcinoma. It was also found that MTDH/AEG-1 overexpression was significantly correlated with high Ki67 which is a common proliferative marker [34]. In addition, inhibition of MTDH/AEG-1 by employing microRNA-30a can suppress proliferation, colonigenic ability, migration and invasion of breast cancer lines [35].
Angiogenesis
Angiogenesis is a fundamental event in the maintenance and development of solid tumors and their metastases. The process of angiogenesis is regulated by an interaction of proangiogenic factors such as vascular endothelial growth factor (VEGF), fibroblast growth factor (FGF), angiopoietin (ANG), platelet-derived growth factor (PDGF), TGFβ, and matrix metalloproteinase (MMP) and angiogenic inhibitors including thrombospondin-1, platelet factor-4, angiostatin and endostatin, and so on [36]. In vivo, Immunohistochemistry analysis of tumors derived from nude mice which were injected with cloned rat embryo fibroblasts (CREF)-AEG-1 clones, demonstrates that the tumors have high microvessel density (MVD) and levels of molecular markers of angiogenesis, including ANG-1, MMP-2, and hypoxia-inducible factor 1-α. In the chicken chorioallantoic membrane (CAM) angiogenesis model, inhibition of MTDH/AEG-1 made glioma cells exhibit little neovascularization. In vitro, angiogenesis assays also demonstrated that MTDH/AEG-1 enhances tube formation of human umbilical vein endothelial cells in matrigel, which is an important quantified parameter of endothelial function in angiogenesis [37]. The above studies confirm that MTDH/AEG-1 has a dominant function in regulating angiogenesis. The angiogenesis-promoting function of MTDH/AEG-1 is also verified in human HCC [20,38]. Additionally, analysis of specimens from 125 patients with breast cancer showed that MTDH/AEG-1 correlated positively with VEGF levels and MCV [39].
Metastasis
Acquisition of metastatic potential by an evolving cancer is a multifactorial and multistep process involving a dynamic interplay between multiple metastasis-associated genes which elicit changes in diverse signal transduction pathways, and encode various kinds of adhesion molecules, extracellular matrix protein hydrolases, angiogenic factors, and so on. A plethora of studies demonstrate MTDH/AEG-1 is prominently relevant with the invasion and metastasis of cancers. In matrigel invasion assays, varied cancer cells for example Hela cells [8], HCC cells [20], malignant glioma cells [19,32] and neuroblastoma cells [40], showed markedly higher invasive ability via increasing the expression of MTDH/AEG-1. Upregulation of MTDH/AEG-1 was manifested to improve invasive and metastatic abilities through the upregulation of MMP-2 in osteosarcoma and MMP-9 in NSCLC, respectively [41,42]. Different groups validated successively that MTDH/AEG-1 is important in breast cancer metastasis [5,43]. MTDH/AEG-1 overexpression was found to not only accelerated lung metastasis significantly, but also led to a modest increase of bone and brain metastasis, and shortened the survival of mice that injected with breast cancer cells. On the contrary, MTDH/AEG-1 knockdown significantly reduced the adhesion of breast cancer cells to lung microvascular endothelial cells, as well as to the bone marrow and the umbilical vein endothelial cells, albeit to a lesser extent [43]. Zhou et al [44] revealed that MTDH/AEG-1 promoted the lung-specific metastasis of HCC by augmenting orientation chemotaxis and adhesion abilities of HCC cells. Moreover, MTDH/AEG-1 play a pivotal role in metastasis of cancers, which is also identified in CRC, gallbladder carcinoma (GBC), renal cell carcinoma, prostate cancer and ovarian cancer [45-49].
More recently, it has been found that epithelial-mesenchymal transition (EMT) is critically associated with the local invasion and distant metastasis of tumor. Epithelial cells transitioning to mesenchymal cells lose polarity, increase metastatic potential and upregulate nuclear expression of several transcription factors (Snail, Twis, ZEB1 et al) with the loss of epithelial phenotype markers for example E-cadherin and the acquisition of mesenchymal markers such as vimentin and β-catenin which are cytoskeletal proteins [50,51]. Numerous studies have shown that regulating the expression of MTDH/AEG-1 can mediate EMT of tumor cell. In breast cancer cells, overexpression of MTDH/AEG-1 not only led to the conversion from tightly packed cobblestone-like appearance which is typical of the epithelial phenotype, to loosely packed spindle-like morphology without tight cell-cell junction which is a typical characteristic of mesenchymal cells, but also increased invasion and migration of cells. Moreover, upregulation of MTDH/AEG-1 manifested that epithelial markers E-cadherin and CK-18 were decreased, while the mesenchymal markers vimentin and fibronectin, and transcription factors Snail and Slug were increased, accompanied with nuclear accumulation of β-catenin [52]. Afterwards, Ward et al [53] established a tamoxifen-resistant (TamR) breast cancer model which displayed mesenchymal properties and had increased invasiveness with the reduction of E-cadherin expression and the upregulation of fibronectin and ZEB1. MiRNA microarray analysis revealed that miRNA-375 of which MTDH/AEG-1 is a direct target, was among the top downregulated miRNAs in TamR cells. Knockdown of MTDH/AEG-1 sensitized TamR cells to tamoxifen and partly reversed EMT. The studies above suggest that MTDH/AEG-1 enhances the EMT of breast cancer and plays an important role in chemoresistance. Another study revealed that MTDH/AEG-1 was involved in ursolic acid (UA)-mediated EMT inhibition of lung cancer [54]. Furthermore, it is also confirmed in HCC, osteosarcoma and head and neck squamous cell carcinoma (HNSCC), that MTDH/AEG-1 is able to promote the metastasis of tumor by inducing EMT of tumor cells [55-57].
Prognosis and treatment
A large number of clinical analyses demonstrate that there is a significant correlation between the level of MTDH/AEG-1 expression and a series of clinicopathological parameters including differentiation degree, clinical stage, lymph node metastasis, recurrence and overall survival of the cancer. Therefore, it’s firmly established the potential of MTDH/AEG-1 to be an independent prognostic indicator of multiple cancers such as GBC [46,58], tongue carcinoma [59], bladder cancer [60], breast cancer [61,62], metastatic ovarian tumors [63], endometrial cancer [64] and oral squamous cell carcinoma [65]. Moreover, the first and only FDA-approved individualized metastasis risk assessment assay for breast cancer is MammaPrint which contains a unique 70-gene signature, including MTDH/AEG-1. In the search for new biomarkers for early diagnosis of cancer, serum samples from 483 different cancer patients and 230 normal blood donors were analyzed by ELISA to detect anti-MTDH/AEG-1 antibodies. The results indicated that anti-MTDH/AEG-1 antibodies were present in a broad range of cancers, compared with 0 of 230 (0%) in normal individuals, and the levels of anti-MTDH/AEG-1 antibodies have an appreciable correlation with the age and stage of patients, thereby, may be used as a novel marker for the diagnosis and prognostic evaluation of cancer patients with MTDH/AEG-1-positive expression [66].
Since accumulating evidences elucidated that MTDH/AEG-1 is an important regulator in multiple aspects of cancer progression and prognosis, the relation of MTDH/AEG-1 to treatment has already aroused the concern of researchers. In the treatment of HCC, MTDH/AEG-1 confers resistance to 5-fluorouracil (5-FU) by increasing expression of the transcription factor LSF (late SV40 Factor) that regulates the expression of thymidylate synthase, the target of 5-FU, and enhancing the expression of dihydropyrimidine dehydrogenase (DPYD) which is the initial and rate-limiting enzyme in the catabolism of 5-FU [67]. Additionally, MTDH/AEG-1 induces doxorubicin resistance in the way of augmenting the expression of multidrug resistance gene 1 (MDR1) protein, leading to increased efflux and decreased accumulation of doxorubicin. Further study documents that MTDH/AEG-1 facilitates association of MDR1 mRNA to polysomes, increases translation, and inhibits ubiquitination and proteasome-mediated degradation of MDR1 protein [68]. Another mechanism of MTDH/AEG-1-mediated drug resistance is inhibition of apoptosis. In MTDH/AEG-1 knockdown breast cancer cells, two cell death genes inducing TRAIL and BINP3 are upregulated, while ALDH3A1 and MET genes which are implicated in clinical resistance decrease, resulting in enhancing the sensitivity of cancer cells to doxorubicin, cisplatin, etoposide and paclitaxel [43]. In a breast cancer therapeutic model, MTDH/AEG-1 attenuated vaccine is proved to augment chemosensitivity to doxorubicin and inhibit the lung metastasis of breast cancer [69]. Meng et al found that knockdown of MTDH/AEG-1 could increase the response of endometrial cancer cells to combinatorial treatment with TRAIL and the histone deacetylase inhibitor LBH589 via upregulating pro-apoptotic BH3-only protein Bim and inhibiting antiapoptotic gene X-linked inhibitor of apoptosis protein (XIAP) [70].
Moreover, MTDH/AEG-1 can induce drug resistance by regulating microRNAs. Elevated MTDH/AEG-1 levels inversely correlate with miRNA-375 expression in breast cancer cells. Re-expression of miRNA-375 in tamoxifen-resistant cancer cells makes cells regain the sensitivity to tamoxifen, and depletion of MTDH/AEG-1 which is the target of miRNA-375 also partly reverses resistance to tamoxifen [53]. Functional and clinical analyses suggest that MTDH/AEG-1 is a potentially valuable target in cancer treatments, and application of RNA interference technology (mediated by siRNAs and microRNAs) targeting MTDH/AEG-1 in combination with chemotherapy may be a novel strategy for the treatment of cancer [35,71,72].
Conclusions
MTDH/AEG-1, as a novel gene, has represented an important role in multiple aspects of cancer progression by modulating a series of signaling pathways. Clinical and functional analyses have established that MTDH/AEG-1 could be considered as a potentially valuable target in the treatments of malignant neoplasm. However, the precise mechanisms of this multifunctional gene in regulating carcinogenesis remain poorly understood, and the findings are still required to be validated in broader model systems. Meanwhile, in-depth study of canonical functional domains of MTDH/AEG-1 is expected to facilitate the development of small-molecule target inhibitors. The identification of anti- MTDH/AEG-1 antibody titer as a serum biomarker for malignancy is an encouraging approach, whereas comparative studies need to be carried out to verify its specificity and sensitivity versus other currently employed serum biomarkers. Ultimately, more extensive future investigation might elevate MTDH/AEG-1 as a pivotal tool that could be routinely employed in diagnosing diverse cancers, monitoring cancer progression, and evaluating efficacy of cancer treatment.
Acknowledgements
This study was partly supported by: National Natural Science Foundation (No. 81270598, No. 81473486, and No. 31340009), National Public Health Grand Research Foundation (No. 201202017), Natural Science Foundations of Shandong Province (No. 2009ZRB14176, No. ZR2011HQ009, and No. ZR2012HZ003), Technology Development Projects of Shandong Province (No. 2010GSF10250, and No. 2014GSF118021), Program of Shandong Medical Leading Talent, and Taishan Scholar Foundation of Shandong Province.
Disclosure of conflict of interest
None.
References
- 1.Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144:646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 2.Su ZZ, Kang DC, Chen Y, Pekarskaya O, Chao W, Volsky DJ, Fisher PB. Identification and cloning of human astrocyte genes displaying elevated expression after infection with HIV-1 or exposure to HIV-1 envelope glycoprotein by rapid subtraction hybridization, RaSH. Oncogene. 2002;21:3592–3602. doi: 10.1038/sj.onc.1205445. [DOI] [PubMed] [Google Scholar]
- 3.Kang DC, Su ZZ, Sarkar D, Emdad L, Volsky DJ, Fisher PB. Cloning and characterization of HIV-1-inducible astrocyte elevated gene-1, AEG-1. Gene. 2005;353:8–15. doi: 10.1016/j.gene.2005.04.006. [DOI] [PubMed] [Google Scholar]
- 4.Huang Y, Li LP. Progress of cancer research on astrocyte elevated gene-1/Metadherin (Review) Oncol Lett. 2014;8:493–501. doi: 10.3892/ol.2014.2231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brown DM, Ruoslahti E. Metadherin, a cell surface protein in breast tumors that mediates lung metastasis. Cancer Cell. 2004;5:365–374. doi: 10.1016/s1535-6108(04)00079-0. [DOI] [PubMed] [Google Scholar]
- 6.Britt DE, Yang DF, Yang DQ, Flanagan D, Callanan H, Lim YP, Lin SH, Hixson DC. Identification of a novel protein, LYRIC, localized to tight junctions of polarized epithelial cells. Exp Cell Res. 2004;300:134–148. doi: 10.1016/j.yexcr.2004.06.026. [DOI] [PubMed] [Google Scholar]
- 7.Sutherland HG, Lam YW, Briers S, Lamond AI, Bickmore WA. 3D3/lyric: a novel transmembrane protein of the endoplasmic reticulum and nuclear envelope, which is also present in the nucleolus. Exp Cell Res. 2004;294:94–105. doi: 10.1016/j.yexcr.2003.11.020. [DOI] [PubMed] [Google Scholar]
- 8.Emdad L, Sarkar D, Su ZZ, Randolph A, Boukerche H, Valerie K, Fisher PB. Activation of the nuclear factor kappaB pathway by astrocyte elevated gene-1: implications for tumor progression and metastasis. Cancer Res. 2006;66:1509–1516. doi: 10.1158/0008-5472.CAN-05-3029. [DOI] [PubMed] [Google Scholar]
- 9.Emdad L, Sarkar D, Su ZZ, Lee SG, Kang DC, Bruce JN, Volsky DJ, Fisher PB. Astrocyte elevated gene-1: recent insights into a novel gene involved in tumor progression, metastasis and neurodegeneration. Pharmacol Ther. 2007;114:155–170. doi: 10.1016/j.pharmthera.2007.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Thirkettle HJ, Girling J, Warren AY, Mills IG, Sahadevan K, Leung H, Hamdy F, Whitaker HC, Neal DE. LYRIC/AEG-1 is targeted to different subcellular compartments by ubiquitinylation and intrinsic nuclear localization signals. Clin Cancer Res. 2009;15:3003–3013. doi: 10.1158/1078-0432.CCR-08-2046. [DOI] [PubMed] [Google Scholar]
- 11.Lee SG, Su ZZ, Emdad L, Sarkar D, Fisher PB. Astrocyte elevated gene-1 (AEG-1) is a target gene of oncogenic Ha-ras requiring phosphatidylinositol 3-kinase and c-Myc. Proc Natl Acad Sci U S A. 2006;103:17390–17395. doi: 10.1073/pnas.0608386103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Emdad L, Das SK, Dasgupta S, Hu B, Sarkar D, Fisher PB. AEG-1/MTDH/LYRIC: signaling pathways, downstream genes, interacting proteins, and regulation of tumor angiogenesis. Adv Cancer Res. 2013;120:75–111. doi: 10.1016/B978-0-12-401676-7.00003-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lee SG, Su ZZ, Emdad L, Sarkar D, Franke TF, Fisher PB. Astrocyte elevated gene-1 activates cell survival pathways through PI3K-Akt signaling. Oncogene. 2008;27:1114–1121. doi: 10.1038/sj.onc.1210713. [DOI] [PubMed] [Google Scholar]
- 14.Kikuno N, Shiina H, Urakami S, Kawamoto K, Hirata H, Tanaka Y, Place RF, Pookot D, Majid S, Igawa M, Dahiya R. Knockdown of astrocyte-elevated gene-1 inhibits prostate cancer progression through upregulation of FOXO3a activity. Oncogene. 2007;26:7647–7655. doi: 10.1038/sj.onc.1210572. [DOI] [PubMed] [Google Scholar]
- 15.Li J, Yang L, Song L, Xiong H, Wang L, Yan X, Yuan J, Wu J, Li M. Astrocyte elevated gene-1 is a proliferation promoter in breast cancer via suppressing transcriptional factor FOXO1. Oncogene. 2009;28:3188–3196. doi: 10.1038/onc.2009.171. [DOI] [PubMed] [Google Scholar]
- 16.Ke ZF, Mao X, Zeng C, He S, Li S, Wang LT. AEG-1 expression characteristics in human non-small cell lung cancer and its relationship with apoptosis. Med Oncol. 2013;30:383. doi: 10.1007/s12032-012-0383-9. [DOI] [PubMed] [Google Scholar]
- 17.Song L, Li W, Zhang H, Liao W, Dai T, Yu C, Ding X, Zhang L, Li J. Over-expression of AEG-1 significantly associates with tumour aggressiveness and poor prognosis in human non-small cell lung cancer. J Pathol. 2009;219:317–326. doi: 10.1002/path.2595. [DOI] [PubMed] [Google Scholar]
- 18.Zheng J, Li C, Wu X, Liu M, Sun X, Yang Y, Hao M, Sheng S, Sun Y, Zhang H, Long J, Liang Y, Hu C. Astrocyte elevated gene-1 (AEG-1) shRNA sensitizes Huaier polysaccharide (HP)-induced anti-metastatic potency via inactivating downstream P13K/Akt pathway as well as augmenting cell-mediated immune response. Tumour Biol. 2014;35:4219–4224. doi: 10.1007/s13277-013-1552-y. [DOI] [PubMed] [Google Scholar]
- 19.Sarkar D, Park ES, Emdad L, Lee SG, Su ZZ, Fisher PB. Molecular basis of nuclear factor-kappaB activation by astrocyte elevated gene-1. Cancer Res. 2008;68:1478–1484. doi: 10.1158/0008-5472.CAN-07-6164. [DOI] [PubMed] [Google Scholar]
- 20.Yoo BK, Emdad L, Su ZZ, Villanueva A, Chiang DY, Mukhopadhyay ND, Mills AS, Waxman S, Fisher RA, Llovet JM, Fisher PB, Sarkar D. Astrocyte elevated gene-1 regulates hepatocellular carcinoma development and progression. J Clin Invest. 2009;119:465–477. doi: 10.1172/JCI36460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Robertson CL, Srivastava J, Siddiq A, Gredler R, Emdad L, Rajasekaran D, Akiel M, Shen XN, Guo C, Giashuddin S, Wang XY, Ghosh S, Subler MA, Windle JJ, Fisher PB, Sarkar D. Genetic deletion of AEG-1 prevents hepatocarcinogenesis. Cancer Res. 2014;74:6184–93. doi: 10.1158/0008-5472.CAN-14-1357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Alexia C, Poalas K, Carvalho G, Zemirli N, Dwyer J, Dubois SM, Hatchi EM, Cordeiro N, Smith SS, Castanier C, Le Guelte A, Wan L, Kang Y, Vazquez A, Gavard J, Arnoult D, Bidere N. The endoplasmic reticulum acts as a platform for ubiquitylated components of nuclear factor kappaB signaling. Sci Signal. 2013;6:ra79. doi: 10.1126/scisignal.2004496. [DOI] [PubMed] [Google Scholar]
- 23.Khuda II, Koide N, Noman AS, Dagvadorj J, Tumurkhuu G, Naiki Y, Komatsu T, Yoshida T, Yokochi T. Astrocyte elevated gene-1 (AEG-1) is induced by lipopolysaccharide as toll-like receptor 4 (TLR4) ligand and regulates TLR4 signalling. Immunology. 2009;128:e700–706. doi: 10.1111/j.1365-2567.2009.03063.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhao Y, Kong X, Li X, Yan S, Yuan C, Hu W, Yang Q. Metadherin mediates lipopolysaccharide-induced migration and invasion of breast cancer cells. PLoS One. 2011;6:e29363. doi: 10.1371/journal.pone.0029363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jian-bo X, Hui W, Yu-long H, Chang-hua Z, Long-juan Z, Shi-rong C, Wen-hua Z. Astrocyte-elevated gene-1 overexpression is associated with poor prognosis in gastric cancer. Med Oncol. 2011;28:455–462. doi: 10.1007/s12032-010-9475-6. [DOI] [PubMed] [Google Scholar]
- 26.Zhang F, Yang Q, Meng F, Shi H, Li H, Liang Y, Han A. Astrocyte elevated gene-1 interacts with beta-catenin and increases migration and invasion of colorectal carcinoma. Mol Carcinog. 2013;52:603–610. doi: 10.1002/mc.21894. [DOI] [PubMed] [Google Scholar]
- 27.Ge X, Lv X, Feng L, Liu X, Gao J, Chen N, Wang X. Metadherin contributes to the pathogenesis of diffuse large B-cell lymphoma. PLoS One. 2012;7:e39449. doi: 10.1371/journal.pone.0039449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kong X, Moran MS, Zhao Y, Yang Q. Inhibition of metadherin sensitizes breast cancer cells to AZD6244. Cancer Biol Ther. 2012;13:43–49. doi: 10.4161/cbt.13.1.18868. [DOI] [PubMed] [Google Scholar]
- 29.Wei J, Li Z, Chen W, Ma C, Zhan F, Wu W, Peng Y. AEG-1 participates in TGF-beta1-induced EMT through p38 MAPK activation. Cell Biol Int. 2013;37:1016–1021. doi: 10.1002/cbin.10125. [DOI] [PubMed] [Google Scholar]
- 30.Yu C, Chen K, Zheng H, Guo X, Jia W, Li M, Zeng M, Li J, Song L. Overexpression of astrocyte elevated gene-1 (AEG-1) is associated with esophageal squamous cell carcinoma (ESCC) progression and pathogenesis. Carcinogenesis. 2009;30:894–901. doi: 10.1093/carcin/bgp064. [DOI] [PubMed] [Google Scholar]
- 31.Nikpour M, Emadi-Baygi M, Fischer U, Niegisch G, Schulz WA, Nikpour P. MTDH/AEG-1 contributes to central features of the neoplastic phenotype in bladder cancer. Urol Oncol. 2014;32:670–677. doi: 10.1016/j.urolonc.2013.11.005. [DOI] [PubMed] [Google Scholar]
- 32.Emdad L, Sarkar D, Lee SG, Su ZZ, Yoo BK, Dash R, Yacoub A, Fuller CE, Shah K, Dent P, Bruce JN, Fisher PB. Astrocyte elevated gene-1: a novel target for human glioma therapy. Mol Cancer Ther. 2010;9:79–88. doi: 10.1158/1535-7163.MCT-09-0752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Liu H, Song X, Liu C, Xie L, Wei L, Sun R. Knockdown of astrocyte elevated gene-1 inhibits proliferation and enhancing chemo-sensitivity to cisplatin or doxorubicin in neuroblastoma cells. J Exp Clin Cancer Res. 2009;28:19. doi: 10.1186/1756-9966-28-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Su P, Zhang Q, Yang Q. Immunohisto-chemical analysis of Metadherin in proliferative and cancerous breast tissue. Diagn Pathol. 2010;5:38. doi: 10.1186/1746-1596-5-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhang N, Wang X, Huo Q, Sun M, Cai C, Liu Z, Hu G, Yang Q. MicroRNA-30a suppresses breast tumor growth and metastasis by targeting metadherin. Oncogene. 2014;33:3119–3128. doi: 10.1038/onc.2013.286. [DOI] [PubMed] [Google Scholar]
- 36.Carmeliet P, Jain RK. Angiogenesis in cancer and other diseases. Nature. 2000;407:249–257. doi: 10.1038/35025220. [DOI] [PubMed] [Google Scholar]
- 37.Emdad L, Lee SG, Su ZZ, Jeon HY, Boukerche H, Sarkar D, Fisher PB. Astrocyte elevated gene-1 (AEG-1) functions as an oncogene and regulates angiogenesis. Proc Natl Acad Sci U S A. 2009;106:21300–21305. doi: 10.1073/pnas.0910936106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Srivastava J, Siddiq A, Emdad L, Santhekadur PK, Chen D, Gredler R, Shen XN, Robertson CL, Dumur CI, Hylemon PB, Mukhopadhyay ND, Bhere D, Shah K, Ahmad R, Giashuddin S, Stafflinger J, Subler MA, Windle JJ, Fisher PB, Sarkar D. Astrocyte elevated gene-1 promotes hepatocarcinogenesis: novel insights from a mouse model. Hepatology. 2012;56:1782–1791. doi: 10.1002/hep.25868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Li C, Li R, Song H, Wang D, Feng T, Yu X, Zhao Y, Liu J, Wang Y, Geng J. Significance of AEG-1 expression in correlation with VEGF, microvessel density and clinicopathological characteristics in triple-negative breast cancer. J Surg Oncol. 2011;103:184–192. doi: 10.1002/jso.21788. [DOI] [PubMed] [Google Scholar]
- 40.Lee SG, Jeon HY, Su ZZ, Richards JE, Vozhilla N, Sarkar D, Van Maerken T, Fisher PB. Astrocyte elevated gene-1 contributes to the pathogenesis of neuroblastoma. Oncogene. 2009;28:2476–2484. doi: 10.1038/onc.2009.93. [DOI] [PubMed] [Google Scholar]
- 41.Sun S, Ke Z, Wang F, Li S, Chen W, Han A, Wang Z, Shi H, Wang LT, Chen X. Overexpression of astrocyte-elevated gene-1 is closely correlated with poor prognosis in human non-small cell lung cancer and mediates its metastasis through up-regulation of matrix metalloproteinase-9 expression. Hum Pathol. 2012;43:1051–1060. doi: 10.1016/j.humpath.2011.08.017. [DOI] [PubMed] [Google Scholar]
- 42.Wang F, Ke ZF, Sun SJ, Chen WF, Yang SC, Li SH, Mao XP, Wang LT. Oncogenic roles of astrocyte elevated gene-1 (AEG-1) in osteosarcoma progression and prognosis. Cancer Biol Ther. 2011;12:539–548. doi: 10.4161/cbt.12.6.16301. [DOI] [PubMed] [Google Scholar]
- 43.Hu G, Chong RA, Yang Q, Wei Y, Blanco MA, Li F, Reiss M, Au JL, Haffty BG, Kang Y. MTDH activation by 8q22 genomic gain promotes chemoresistance and metastasis of poor-prognosis breast cancer. Cancer Cell. 2009;15:9–20. doi: 10.1016/j.ccr.2008.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zhou Z, Deng H, Yan W, Huang H, Deng Y, Li Y, Tian D. Expression of metadherin/AEG-1 gene is positively related to orientation chemotaxis and adhesion of human hepatocellular carcinoma cell lines of different metastatic potentials. J Huazhong Univ Sci Technolog Med Sci. 2012;32:353–357. doi: 10.1007/s11596-012-0061-3. [DOI] [PubMed] [Google Scholar]
- 45.Jiang T, Zhu A, Zhu Y, Piao D. Clinical implications of AEG-1 in liver metastasis of colorectal cancer. Med Oncol. 2012;29:2858–2863. doi: 10.1007/s12032-012-0186-z. [DOI] [PubMed] [Google Scholar]
- 46.Liu DC, Yang ZL. MTDH and EphA7 are markers for metastasis and poor prognosis of gallbladder adenocarcinoma. Diagn Cytopathol. 2013;41:199–205. doi: 10.1002/dc.21821. [DOI] [PubMed] [Google Scholar]
- 47.Chen W, Ke Z, Shi H, Yang S, Wang L. Overexpression of AEG-1 in renal cell carcinoma and its correlation with tumor nuclear grade and progression. Neoplasma. 2010;57:522–529. doi: 10.4149/neo_2010_06_522. [DOI] [PubMed] [Google Scholar]
- 48.Wan L, Hu G, Wei Y, Yuan M, Bronson RT, Yang Q, Siddiqui J, Pienta KJ, Kang Y. Genetic ablation of metadherin inhibits autochthonous prostate cancer progression and metastasis. Cancer Res. 2014;74:5336–5347. doi: 10.1158/0008-5472.CAN-14-1349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Li C, Liu J, Lu R, Yu G, Wang X, Zhao Y, Song H, Lin P, Sun X, Yu X, Zhang Y, Ning X, Geng J. AEG-1 overexpression: a novel indicator for peritoneal dissemination and lymph node metastasis in epithelial ovarian cancers. Int J Gynecol Cancer. 2011;21:602–608. doi: 10.1097/IGC.0b013e3182145561. [DOI] [PubMed] [Google Scholar]
- 50.Turley EA, Veiseh M, Radisky DC, Bissell MJ. Mechanisms of disease: epithelial-mesenchymal transition--does cellular plasticity fuel neoplastic progression? Nat Clin Pract Oncol. 2008;5:280–290. doi: 10.1038/ncponc1089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zeisberg M, Neilson EG. Biomarkers for epithelial-mesenchymal transitions. J Clin Invest. 2009;119:1429–1437. doi: 10.1172/JCI36183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Li X, Kong X, Huo Q, Guo H, Yan S, Yuan C, Moran MS, Shao C, Yang Q. Metadherin enhances the invasiveness of breast cancer cells by inducing epithelial to mesenchymal transition. Cancer Sci. 2011;102:1151–1157. doi: 10.1111/j.1349-7006.2011.01919.x. [DOI] [PubMed] [Google Scholar]
- 53.Ward A, Balwierz A, Zhang JD, Kublbeck M, Pawitan Y, Hielscher T, Wiemann S, Sahin O. Re-expression of microRNA-375 reverses both tamoxifen resistance and accompanying EMT-like properties in breast cancer. Oncogene. 2013;32:1173–1182. doi: 10.1038/onc.2012.128. [DOI] [PubMed] [Google Scholar]
- 54.Liu K, Guo L, Miao L, Bao W, Yang J, Li X, Xi T, Zhao W. Ursolic acid inhibits epithelial-mesenchymal transition by suppressing the expression of astrocyte-elevated gene-1 in human nonsmall cell lung cancer A549 cells. Anticancer Drugs. 2013;24:494–503. doi: 10.1097/CAD.0b013e328360093b. [DOI] [PubMed] [Google Scholar]
- 55.Zhu K, Dai Z, Pan Q, Wang Z, Yang GH, Yu L, Ding ZB, Shi GM, Ke AW, Yang XR, Tao ZH, Zhao YM, Qin Y, Zeng HY, Tang ZY, Fan J, Zhou J. Metadherin promotes hepatocellular carcinoma metastasis through induction of epithelial-mesenchymal transition. Clin Cancer Res. 2011;17:7294–7302. doi: 10.1158/1078-0432.CCR-11-1327. [DOI] [PubMed] [Google Scholar]
- 56.Tang J, Shen L, Yang Q, Zhang C. Overexpression of metadherin mediates metastasis of osteosarcoma by regulating epithelial-mesenchymal transition. Cell Prolif. 2014;47:427–434. doi: 10.1111/cpr.12129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Yu C, Liu Y, Tan H, Li G, Su Z, Ren S, Zhu G, Tian Y, Qiu Y, Zhang X. Metadherin regulates metastasis of squamous cell carcinoma of the head and neck via AKT signalling pathway-mediated epithelial-mesenchymal transition. Cancer Lett. 2014;343:258–267. doi: 10.1016/j.canlet.2013.09.033. [DOI] [PubMed] [Google Scholar]
- 58.Sun W, Fan YZ, Xi H, Lu XS, Ye C, Zhang JT. Astrocyte elevated gene-1 overexpression in human primary gallbladder carcinomas: an unfavorable and independent prognostic factor. Oncol Rep. 2011;26:1133–1142. doi: 10.3892/or.2011.1387. [DOI] [PubMed] [Google Scholar]
- 59.Ke ZF, He S, Li S, Luo D, Feng C, Zhou W. Expression characteristics of astrocyte elevated gene-1 (AEG-1) in tongue carcinoma and its correlation with poor prognosis. Cancer Epidemiol. 2013;37:179–185. doi: 10.1016/j.canep.2012.10.007. [DOI] [PubMed] [Google Scholar]
- 60.Zhou J, Li J, Wang Z, Yin C, Zhang W. Metadherin is a novel prognostic marker for bladder cancer progression and overall patient survival. Asia Pac J Clin Oncol. 2012;8:e42–48. doi: 10.1111/j.1743-7563.2012.01541.x. [DOI] [PubMed] [Google Scholar]
- 61.Li J, Zhang N, Song LB, Liao WT, Jiang LL, Gong LY, Wu J, Yuan J, Zhang HZ, Zeng MS, Li M. Astrocyte elevated gene-1 is a novel prognostic marker for breast cancer progression and overall patient survival. Clin Cancer Res. 2008;14:3319–3326. doi: 10.1158/1078-0432.CCR-07-4054. [DOI] [PubMed] [Google Scholar]
- 62.Tokunaga E, Nakashima Y, Yamashita N, Hisamatsu Y, Okada S, Akiyoshi S, Aishima S, Kitao H, Morita M, Maehara Y. Overexpression of metadherin/MTDH is associated with an aggressive phenotype and a poor prognosis in invasive breast cancer. Breast Cancer. 2014;21:341–349. doi: 10.1007/s12282-012-0398-2. [DOI] [PubMed] [Google Scholar]
- 63.Li C, Chen K, Cai J, Shi QT, Li Y, Li L, Song H, Qiu H, Qin Y, Geng JS. Astrocyte elevated gene-1: a novel independent prognostic biomarker for metastatic ovarian tumors. Tumour Biol. 2014;35:3079–3085. doi: 10.1007/s13277-013-1400-0. [DOI] [PubMed] [Google Scholar]
- 64.Song H, Li C, Lu R, Zhang Y, Geng J. Expression of astrocyte elevated gene-1: a novel marker of the pathogenesis, progression, and poor prognosis for endometrial cancer. Int J Gynecol Cancer. 2010;20:1188–1196. doi: 10.1111/igc.0b013e3181ef8e21. [DOI] [PubMed] [Google Scholar]
- 65.Xia X, Du R, Zhao L, Sun W, Wang X. Expression of AEG-1 and microvessel density correlates with metastasis and prognosis of oral squamous cell carcinoma. Hum Pathol. 2014;45:858–865. doi: 10.1016/j.humpath.2013.08.030. [DOI] [PubMed] [Google Scholar]
- 66.Chen X, Dong K, Long M, Lin F, Wang X, Wei J, Ren J, Zhang H. Serum anti-AEG-1 auto-antibody is a potential novel biomarker for malignant tumors. Oncol Lett. 2012;4:319–323. doi: 10.3892/ol.2012.734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Yoo BK, Gredler R, Vozhilla N, Su ZZ, Chen D, Forcier T, Shah K, Saxena U, Hansen U, Fisher PB, Sarkar D. Identification of genes conferring resistance to 5-fluorouracil. Proc Natl Acad Sci U S A. 2009;106:12938–12943. doi: 10.1073/pnas.0901451106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Yoo BK, Chen D, Su ZZ, Gredler R, Yoo J, Shah K, Fisher PB, Sarkar D. Molecular mechanism of chemoresistance by astrocyte elevated gene-1. Cancer Res. 2010;70:3249–3258. doi: 10.1158/0008-5472.CAN-09-4009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Qian BJ, Yan F, Li N, Liu QL, Lin YH, Liu CM, Luo YP, Guo F, Li HZ. MTDH/AEG-1-based DNA vaccine suppresses lung metastasis and enhances chemosensitivity to doxorubicin in breast cancer. Cancer Immunol Immunother. 2011;60:883–893. doi: 10.1007/s00262-011-0997-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Meng X, Brachova P, Yang S, Xiong Z, Zhang Y, Thiel KW, Leslie KK. Knockdown of MTDH sensitizes endometrial cancer cells to cell death induction by death receptor ligand TRAIL and HDAC inhibitor LBH589 co-treatment. PLoS One. 2011;6:e20920. doi: 10.1371/journal.pone.0020920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Huang S, Wu B, Li D, Zhou W, Deng G, Zhang K, Li Y. Knockdown of astrocyte elevated gene-1 inhibits tumor growth and modifies microRNAs expression profiles in human colorectal cancer cells. Biochem Biophys Res Commun. 2014;444:338–345. doi: 10.1016/j.bbrc.2014.01.046. [DOI] [PubMed] [Google Scholar]
- 72.Yang Y, Wu J, Guan H, Cai J, Fang L, Li J, Li M. MiR-136 promotes apoptosis of glioma cells by targeting AEG-1 and Bcl-2. FEBS Lett. 2012;586:3608–3612. doi: 10.1016/j.febslet.2012.08.003. [DOI] [PubMed] [Google Scholar]
- 73.Gong Z, Liu W, You N, Wang T, Wang X, Lu P, Zhao G, Yang P, Wang D, Dou K. Prognostic significance of metadherin overexpression in hepatitis B virus-related hepatocellular carcinoma. Oncol Rep. 2012;27:2073–2079. doi: 10.3892/or.2012.1749. [DOI] [PubMed] [Google Scholar]
- 74.Ahn S, Hyeon J, Park CK. Metadherin is a prognostic predictor of hepatocellular carcinoma after curative hepatectomy. Gut Liver. 2013;7:206–212. doi: 10.5009/gnl.2013.7.2.206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Baygi ME, Nikpour P. Deregulation of MTDH gene expression in gastric cancer. Asian Pac J Cancer Prev. 2012;13:2833–2836. doi: 10.7314/apjcp.2012.13.6.2833. [DOI] [PubMed] [Google Scholar]
- 76.Zhang CF, Xia YH, Zheng QF, Li ZJ, Guo XH, Zhou HC, Zhang LL, Dong LP, Han Y, Liu ZE, Wang WJ, Luo YL. [Effect of silencing AEG-1 with small interfering RNA on the proliferation and cell cycle of gastric carcinoma SGC-7901 cells] . Zhonghua Zhong Liu Za Zhi. 2013;35:22–27. doi: 10.3760/cma.j.issn.0253-3766.2013.01.005. [DOI] [PubMed] [Google Scholar]
- 77.Huang W, Yang L, Liang S, Liu D, Chen X, Ma Z, Zhai S, Li P, Wang X. AEG-1 is a target of perifosine and is over-expressed in gastric dysplasia and cancers. Dig Dis Sci. 2013;58:2873–2880. doi: 10.1007/s10620-013-2735-5. [DOI] [PubMed] [Google Scholar]
- 78.Song H, Li C, Li R, Geng J. Prognostic significance of AEG-1 expression in colorectal carcinoma. Int J Colorectal Dis. 2010;25:1201–1209. doi: 10.1007/s00384-010-1009-3. [DOI] [PubMed] [Google Scholar]
- 79.Gnosa S, Shen YM, Wang CJ, Zhang H, Stratmann J, Arbman G, Sun XF. Expression of AEG-1 mRNA and protein in colorectal cancer patients and colon cancer cell lines. J Transl Med. 2012;10:109. doi: 10.1186/1479-5876-10-109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Wang N, Du X, Zang L, Song N, Yang T, Dong R, Wu T, He X, Lu J. Prognostic impact of Metadherin-SND1 interaction in colon cancer. Mol Biol Rep. 2012;39:10497–10504. doi: 10.1007/s11033-012-1933-0. [DOI] [PubMed] [Google Scholar]
- 81.Casimiro S, Fernandes A, Oliveira AG, Franco M, Pires R, Peres M, Matias M, Tato-Costa J, Guerra N, Ramos M, Cruz J, Costa L. Metadherin expression and lung relapse in patients with colorectal carcinoma. Clin Exp Metastasis. 2014;31:689–696. doi: 10.1007/s10585-014-9659-0. [DOI] [PubMed] [Google Scholar]
- 82.Liao WT, Guo L, Zhong Y, Wu YH, Li J, Song LB. Astrocyte elevated gene-1 (AEG-1) is a marker for aggressive salivary gland carcinoma. J Transl Med. 2011;9:205. doi: 10.1186/1479-5876-9-205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Erdem H, Oktay M, Yildirim U, Uzunlar AK, Kayikci MA. Expression of AEG-1 and p53 and their clinicopathological significance in malignant lesions of renal cell carcinomas: a microarray study. Pol J Pathol. 2013;64:28–32. doi: 10.5114/pjp.2013.34600. [DOI] [PubMed] [Google Scholar]
- 84.Xu C, Kong X, Wang H, Zhang N, Ding X, Li X, Yang Q. MTDH mediates estrogen-independent growth and tamoxifen resistance by down-regulating PTEN in MCF-7 breast cancer cells. Cell Physiol Biochem. 2014;33:1557–1567. doi: 10.1159/000358719. [DOI] [PubMed] [Google Scholar]
- 85.Wan L, Lu X, Yuan S, Wei Y, Guo F, Shen M, Yuan M, Chakrabarti R, Hua Y, Smith HA, Blanco MA, Chekmareva M, Wu H, Bronson RT, Haffty BG, Xing Y, Kang Y. MTDH-SND1 interaction is crucial for expansion and activity of tumor-initiating cells in diverse oncogene- and carcinogen-induced mammary tumors. Cancer Cell. 2014;26:92–105. doi: 10.1016/j.ccr.2014.04.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Meng F, Luo C, Ma L, Hu Y, Lou G. Clinical significance of astrocyte elevated gene-1 expression in human epithelial ovarian carcinoma. Int J Gynecol Pathol. 2011;30:145–150. doi: 10.1097/PGP.0b013e3181ffd2f7. [DOI] [PubMed] [Google Scholar]
- 87.Li C, Li Y, Wang X, Wang Z, Cai J, Wang L, Zhao Y, Song H, Meng X, Ning X, Xu C, Lin M, Li L, Geng J. Elevated expression of astrocyte elevated gene-1 (AEG-1) is correlated with cisplatin-based chemoresistance and shortened outcome in patients with stages III-IV serous ovarian carcinoma. Histopathology. 2012;60:953–963. doi: 10.1111/j.1365-2559.2012.04182.x. [DOI] [PubMed] [Google Scholar]
- 88.Yuan C, Li X, Yan S, Yang Q, Liu X, Kong B. The MTDH (-470G>A) polymorphism is associated with ovarian cancer susceptibility. PLoS One. 2012;7:e51561. doi: 10.1371/journal.pone.0051561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Liu L, Wu J, Ying Z, Chen B, Han A, Liang Y, Song L, Yuan J, Li J, Li M. Astrocyte elevated gene-1 upregulates matrix metalloproteinase- 9 and induces human glioma invasion. Cancer Res. 2010;70:3750–3759. doi: 10.1158/0008-5472.CAN-09-3838. [DOI] [PubMed] [Google Scholar]
- 90.Xia Z, Zhang N, Jin H, Yu Z, Xu G, Huang Z. Clinical significance of astrocyte elevated gene-1 expression in human oligodendrogliomas. Clin Neurol Neurosurg. 2010;112:413–419. doi: 10.1016/j.clineuro.2010.02.007. [DOI] [PubMed] [Google Scholar]
- 91.Liu HY, Liu CX, Han B, Zhang XY, Sun RP. AEG-1 is associated with clinical outcome in neuroblastoma patients. Cancer Biomark. 2012;11:115–121. doi: 10.3233/CBM-2012-0268. [DOI] [PubMed] [Google Scholar]
- 92.Yao Y, Gu X, Liu H, Wu G, Yuan D, Yang X, Song Y. Metadherin regulates proliferation and metastasis via actin cytoskeletal remodelling in non-small cell lung cancer. Br J Cancer. 2014;111:355–364. doi: 10.1038/bjc.2014.267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Liu B, Wu Y, Peng D. Astrocyte elevated gene-1 regulates osteosarcoma cell invasion and chemoresistance via endothelin-1/endothelin A receptor signaling. Oncol Lett. 2013;5:505–510. doi: 10.3892/ol.2012.1056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Yan J, Zhang M, Chen Q, Zhang X. Expression of AEG-1 in human T-cell lymphoma enhances the risk of progression. Oncol Rep. 2012;28:2107–2114. doi: 10.3892/or.2012.2055. [DOI] [PubMed] [Google Scholar]
- 95.Li PP, Feng LL, Chen N, Lu K, Meng XH, Ge XL, Lv X, Wang X. Metadherin interference inhibits proliferation and enhances chemo-sensitivity to doxorubicin in diffuse large B cell lymphoma. Int J Clin Exp Med. 2014;7:2081–2086. [PMC free article] [PubMed] [Google Scholar]
- 96.Nohata N, Hanazawa T, Kikkawa N, Mutallip M, Sakurai D, Fujimura L, Kawakami K, Chiyomaru T, Yoshino H, Enokida H, Nakagawa M, Okamoto Y, Seki N. Tumor suppressive microRNA- 375 regulates oncogene AEG-1/MTDH in head and neck squamous cell carcinoma (HNSCC) J Hum Genet. 2011;56:595–601. doi: 10.1038/jhg.2011.66. [DOI] [PubMed] [Google Scholar]
- 97.Wang YP, Liu IJ, Chiang CP, Wu HC. Astrocyte elevated gene-1 is associated with metastasis in head and neck squamous cell carcinoma through p65 phosphorylation and upregulation of MMP1. Mol Cancer. 2013;12:109. doi: 10.1186/1476-4598-12-109. [DOI] [PMC free article] [PubMed] [Google Scholar]


