Abstract
Alzheimer’s disease (AD) is a progressive neurodegenerative disorder and the leading cause of senile dementia all over the world. Still no existing drugs can effectively reverse the cognitive impairment. However, Sigma-1 (σ-1) receptors have been long implicated in multiple neurological and psychiatric conditions over these years. In this review, we discuss the current understanding of σ-1 receptor functions. Through regulation of lipid rafts, secretases, kinases, neuroceptors and ion channels, σ-1 receptors can influence cellular signal transduction, TCA cycle, oxidative stress, neuron plasticity and neurotransmitter release etc. Based on this, we suggest the key cellular mechanisms linking σ-1 receptor to Alzheimer’s disease. Besides, we detail the evidences showing that σ-1 receptors agonists, being the promising compounds for treatment of cognitive dysfunction, exhibit robust neuroprotection and anti-amnesia effect against Aβ neurotoxicity in the progress of Alzheimer’s disease. The evidence comes from animal models, preclinical studies in humans and full clinical trials. In addition, the questions to be solved regarding this receptor are also presented. When concerned with NMDAR, σ-1 receptor activation may result in two totally different influences on AD. Utilization of σ-1 agents early in AD remains an overlooked therapeutic opportunity. This article may pave the way for further studies about sigma-1 receptor on Alzheimer’s disease.
Keywords: Sigma-1 receptor, Alzheimer’s disease, pathogenesis, Aβ neurotoxicity, NMDA receptor
Introduction
Characterized by progressive cognitive dysfunction and behavioral impairment, Alzheimer’s disease (AD) is a neurodegenerative disorder with insidious onset. So far the most widely accepted pathology of AD comprises amyloid-β deposition and neurofibrillary tangles of hyperphosphorylated tau protein. But no existing drugs can effectively reverse the cognitive impairment. Recently, σ-1 receptor has shown an emerging new look of improving cognitive function, especially its anti-amnesic and neuroprotective effects [1]. An early postmortem study reported that σ-1 receptor were decreased in hippocampus CA1 region of AD patients [2]. Later M. Mishina found σ-1 receptor loss in the early phase of AD using Positron emission tomography (PET) with (11C) SA4503. The binding potential was significantly decreased by 44-60% in the frontal, temporal, and occipital lobe, cerebellum and thalamus [3]. Based on changes of σ-1 receptor density, the following research over these years observed that σ-1 receptor agonists can significantly reduce AD induced cognitive dysfunction. Thus, we aim at highlighting the prospect of sigma-1 receptor effects and treatment in the progress of Alzheimer’s disease.
Characteristics and biological effects of σ receptor
Sigma (σ) receptor was first identified as subtype of opioid receptor [4]. It independently established a receptor family after Quirion R proposing its difference from opioid receptor and phencyclidine binding site [5]. σ receptors can be divided into 2 subtypes: σ-1 and σ-2. Still there are disputes over the existence of σ-3 subtype. σ receptors are abundant in the body, especially in the central nervous system. It has high density distribution in the spinal cord, pons, medulla oblongata, red nucleus, cerebellum, hippocampus , medium density distribution in the cerebral cortex and hypothalamus and low density distribution in the basal ganglia and thalamus [6]. Study comparing σ1 versus σ2 receptor found their dramatic difference in size, distribution and ligand affinity [7]. To date, σ-1 receptor has been cloned g in guinea-pig and human [8,9] and, then, in rat and mouse [10,11]. Its gene encodes a protein of 223 amino acid with two transmembrane domains and a typical endoplasmic reticulum localized signal near the short N terminus [12]. But so far there is no mammalian protein can specifically bind to this receptor. σ-2 receptor has not yet been cloned and little knowledge is known about its relationship with AD. Recently, Izzol et al. found that Aβ1-42 exhibits synaptic toxicity after binding to the σ-2/PGRMC1 receptor [13]. However, it is generally believed that σ-1 receptor plays a more important role in the progression of Alzheimer’s disease.
In normal times, σ-1 receptors mainly localize on the mitochondrial associated endoplasmic reticulum membrane (MAM), forming a Bip chaperone structure with high sensitivity to the calcium ion. When activated by agonists such as cocaine or analgesic, σ-1 receptors separate from BiP and translocate from MAM to other parts of the cell. Through regulation of inositol triphosphate (IP3) receptors, N-methyl-D-aspartic acid receptor (NMDA) receptors, dopamine (DA) receptors and ion channels, σ-1 receptors can influence TCA cycle, oxidative stress [14], mitochondrial function, neuron plasticity and neurotransmitter release such as 5-hydroxy tryptamine, glutamate, dopamine, norepinephrine, acetylcholine, γ-aminobutyric acid and so on [15].
Potential mechanisms of σ-1 receptor in the progression of Alzheimer’s disease
Despite of the mounting evidence on the etiology and pathogenesis of AD over these decades, the exact cause has not been fully elucidated, which may be attributed to the complexity and multiple factors related to it. Here, we suggest the key cellular mechanisms linking σ-1 receptor to Alzheimer’s disease (Figure 1).
Figure 1.
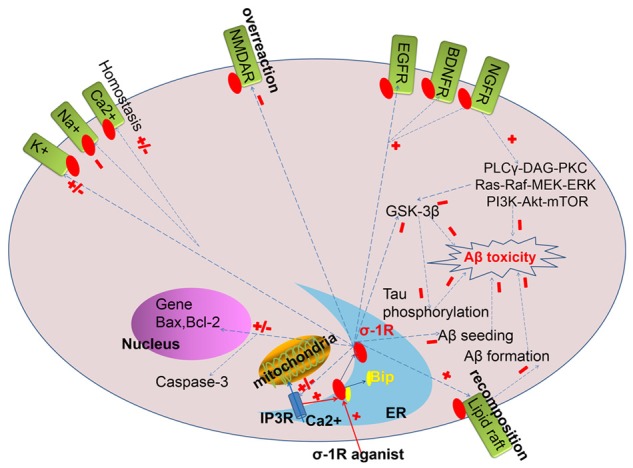
The possible mechanism of σ-1 receptor in the profession of Alzheimer’s Disease.
Aβ cascade hypothesis
Considered as multi-gene inherited disease with genetic heterogeneity, AD can be generally divided into familial AD and sporadic AD. Now three different autosomal dominant gene have been found to be related to early-onset AD: presenilin-1 (PS-1), presenilin-2 (PS-2) and amyloid precursor protein (APP). However, only one predisposing gene for late-onset AD is universally recognized, namely APOE epsilon 4 (APOEε4). Recently, emerging technologies to analyze the entire genome in large data sets have revealed new genes associated with late-onset AD risk, including ABCA7, BIN1, CASS4, CD33, CD2AP, HLA-DRB5-DBR1and so on [16]. Unfortunately, to date there is no evidence that combines AD related genes to σ-1 receptors. Only a few researches on correlation between risk for developing AD and σ-1 receptor gene mutation polymorphism have been reported. Some typical studies are discussed in subsequent sections of this paper. Among the 4 discovered AD genes, mutation of APP, PS-1 and PS-2 has been found to increase Aβ generation while APOEε4 results in Aβ deposition. Substantial evidence suggests the imbalance of amyloid beta production and clearance is the initial event in neuronal degeneration and dementia, which is also the common pathway of other cause in the pathogenesis leading to AD.
Different mechanisms could be evoked to describe the nature of σ-1 receptor alleviating Aβ neurotoxicity: (i) σ-1 receptors may have effective protection against Aβ toxicity by modulating recomposition of lipid rafts, inhibiting Aβ fibrin formation [17]. Substantial amounts of the aspartyl protease β-secretase (BACE-1) and γ-secretase [18] localize in lipid rafts, where Aβ production occurs preferentially. What’s more, GM1 ganglioside-bound amyloid β protein (GM1/Aβ), a seed proposed to be involved in initiation of amyloid fibril formation, is also associated within lipid rafts on the plasma membrane [19,20]. Chronic activation of σ-1 receptor results in its translocation from the endoplasmic reticulum (ER), within lipid droplets, towards the lipid rafts on cytomembrane where it may involve in recomposition of lipid rafts and modifying cellular functions including signal transduction of protein coupled receptor, biosynthetic or endocytic traffic of protein [21]. Therefore, sustained σ-1 receptor activation may preferentially translocate to lipid rafts, on which binding sites are actively involved in Aβ production and transportation.
(ii) Attenuate Aβ25-35-induced Aβ1-42 seeding in hippocampal neurons [22]. Administration of Aβ25-35 can provoke a significant increase in APP expression and activation of the β-secretase pathway resulting in the endogenous Aβ peptide content in hippocampus, which is called Aβ seeding [23]. σ-1 receptors can block this process by regulation the Akt and glycogen synthase kinase-3β (GSK-3β) activity [22].
(iii) Facilitate cognition protection against Aβ through NMDAR response in various brain areas such as hippocampus and prefrontal cortex [24-26]. Pharmacology inhibition of NMDA receptor function, either by systemic administration of compounds directly into the brain or by crossing the blood brain barrier, results in impaired spatial learning and passive avoidance learning [27,28]. σ receptors can potentiate pyramidal neurons in CA3 [29] region of dorsal hippocampus to NMDA (excitatory activation) and NMDA-dependent CA1 synapses [30], which is indispensable for learning ability and spatial memory storage. Besides, σ-1 receptors can prevent Aβ-associated NMDAR neurotoxicity [31].
(iv) Potentiate ATP/IP3-induced Ca2+ influx on endoplasmic reticulum and plasma membranes, thus protecting the cognitive performance of rodents impaired by Aβ neurotoxicity or antagonists of voltage-dependent calcium channels in various learning tests [1]. σ-1 receptors not only localize in endoplasmatic reticulum, but also in nuclear and plasma membranes and on mitochondria [32-36], on which binding sites are involved in the regulation of calcium mobilization. Furthermore, σ-1 receptor protects mitochondria and neural viability by maintaining intracellular calcium homeostasis.
(v) Overexpression of σ-1 receptors potentiate lipid rafts-associated NGF, EGF and BDNF effect [1] and NGF-induced neuroprotection through regulating PLCγ-DAG-PKC, Ras-Raf-MEK-ERK and PI3K-Akt-mTOR signaling pathways [37]; Since the discovery of growth factor receptors localizing in lipid rafts [21], sustained activation of σ-1 receptors may be beneficial for cell survival rate and, therefore, facilitate neuroprotection or neuronal recovery against Aβ neurotoxicity. In addition, σ-1 receptors can enhance the axonal and dendritic growth in hippocampus [38].
(vi) Mitigate Aβ25-35-induced apoptosis by decreasing expression of proapoptotic gene Bax and the death protease caspase-3 whereas preserving antiapoptotic gene Bcl-2 levels, resulting in a concomitant enhancement in cell survival.
Tau protein hypothesis
Widely expressed in the nervous system, tau protein is a microtubule-associated protein, to catalyze microtubule assembly and stabilize microtubule structure. Being a defining pathological characteristic of AD, tauopathies may consists of increased resistance to proteolytic enzymes and the subsequent formation of hyperphosphorylation tau, paired helical filaments (PHF-tau), neurofibrillary tangles (NFTs) deposits and, consequently, neuron death. Glycogen synthase kinase-3 (GSK-3) has been shown to be the key kinase that mediates tau hyperphosphorylation [22]. However, the molecular process underlying over activation of GSK-3 and its potential linkage to AD pathologies in vivo remain unclear. σ-1 receptor could prevent alterations in GSK-3β activity: (i) block the reduction of P(Ser473)-Akt/Akt ratio; (ii) phosphorylate Ser9 residue of GSK-3β by enhancing PI3K/Akt and PLCγ/PKC signaling pathway [39] suppressed in Aβ incubation. Thus, Tau hyperphosphorylation and neurofibrillary tangles could be alleviated to maintain the neuronal cytoskeleton stability.
Neurotransmitter hypothesis
Modulation of acetylcholine release
Cholinergic neurotransmitter is a principal process underlying the cognitive function, especially for memory storage. The cholinergic neurons in basal forebrain, nucleus basalis, cerebral cortex, amygdaloid complex and hippocampus were observed to be indispensable for learning and memory formation [40,41]. In normal aging, some cortical cholinergic activity can be slightly lost whereas patients suffering from AD or related dementias present a severe decline in acetylcholine levels, which can be attributed to a corresponding reduction in choline acetyltransferase (chAT) and acetylcholinesterase (AchE) [42-44].
Both in vitro and in vivo, σ-1 receptors are potent modulators of acetylcholine release. These proteins can upregulate the KCl-evoked or electrically evoked release of 3H-acetylcholine from rat frontal cortex and hippocampus [45-48]. In the meanwhile, acetylcholine release in the striatum was marginally affected [49-51]. Therefore, σ-1 receptors, with less undesired side effects seen in AChE inhibitors [50], alleviate the Aβ-induced dysfunction of cholinergic that are implicated in AD [52].
Modulation of glutamate release
Besides the well-known deficits of cholinergic activity, the neurotransmitter glutamate can also be reduced in AD. Both neurotransmitters are supposed to play key roles in memory [53,54]. σ-1 receptor represents a strategy for modulating glutamatergic levels: (i) Brain-derived neurotrophic factor (BDNF) associated glutamate release are potentiated by not only pharmacological activation but also overexpression of σ-1 receptor. It seems to occur via the involvement of PLCγ/IP3 pathway [55]. (ii) Elevation of the intracellular Ca2+ levels, which is released from the endoplasmatic reticulum, plays a vital role in spontaneous glutamate release [56,57]. (iii) The effect also appears to be mediated via alpha-1 adrenergic and dopamine receptor [57,58].
Thus, regulation of glutamate levels by σ-1 receptor in the frontal cortex and hippocampus could be an additional mechanism underlying the anti-amnesic action.
Inflammation hypothesis
Since McGeer et al. [59] put forward that AD probably results from the inappropriate activation of immune and inflammatory response, a window of opportunity appears for introductions of disease-modifying regimens in AD. The inflammation pathology may arise from the extracellular Aβ deposits and become later enhanced by aggregates of tau. Driven by activated microglia [60], astrocytes and inflammatory factors, the overactive response increases as the disease progresses and, finally, results in the “wrong direction” to attack the normal nervous tissue, causing synapses damage and neurons death.
Abundantly expressed in immunocyte of central immune system, σ-1 receptor activation can not only maintain pro-inflammatory and anti-inflammatory homeostasis [61], but also preserve the neuronal viability [62]. The possible mechanism is as follows: (i) reduce activation and damage of microglial from Aβ25-35-evoked apoptosis; Microglial is the only immune cell type present in the organotypic hippocampal slice. By blocking intracellular calcium signals, many aspects of microglial activations such as cytoskeleton rearrangement, migration, and cytokines production were suppressed. In addition, the activators like lipopolysaccharide (LPS), monocyte chemoattractant protein 1 (MCP-1) and adenosine triphosphate (ATP) can loss the enhancing effect on microglia [63]. (ii) Prevent T-cell mediated immunity by potentiating the anti-inflammatory cytokine IL-10 [64]; (iii) the neuroprotective effects against inflammation at delayed time points maybe mutually adjusted by central and peripheral immune system [65].
In short, lack of σ receptors, neurocyte will be more vulnerable to Aβ-mediated amyloid toxicity. The intracellular lipid metabolism, cell skeleton network and immune response will be more easily damaged, thus accelerating the neural degeneration and oxidative stress-caused neural death, which contribute greatly to the etiology of AD.
Research on σ-1 receptor and Aβ neurotoxicity
The hallmark changes of AD comprise Aβ deposition and neurofibrillary tangles due to tau protein hyperphosphorylation. Aβ neurotoxicity comes both from the aggregation state and soluble oligomeric β. With the ability to stimulating hyperphosphorylation of tau protein, the extracellular Aβ aggregation is considered to be the main molecular mechanisms of AD. However, it is still unclear how Aβ acting on the σ receptors expression. At present, studies on σ receptor with AD are limited to receptor ligands, mainly including endogenous and exogenous ligand.
Endogenous ligand
Neurosteroids might be the endogenous ligands for σ receptors such as dehydroepiandrosterone and luteal hormone [66]. With insufficient level and lower affinity for σ receptors, the title of endogenous ligands has been controversial [67]. Pregnenolone (PREG) and dehydroepiandrosterone (DHEA), being σ-1 receptor ligands under physiological conditions, can improve the memory and learning ability in a cholinergic and NMDAR-dependent way [56]. It has been early demonstrated that levels of PREG in the hippocampus is strongly correlated to the memory performance of rodents or aged rats [52]. For instance, post-training injection of PREGS into the hippocampus and amygdala of mice enhances the recollection for foot shock active avoidance training [68]. On the other hand, administration of DHEA could also ameliorate memory deficits induced by Aβ25-35. In particular, DHEA improves the axonal, dendritic growth [38] or even neural stem cell survival rate [69].
Although the exact mechanisms in positive cognitive effects by neurosteroids are not fully understood, it is suggest that the endogenous agents regulates a series of ion channels such as γ-aminobutyric acid-type A(GABAA) receptors [70], N-methyl-daspartate (NMDA) receptors [71] and voltage-gated Ca2+ channels [72]. Meyer DA [56] found that PREGS selectively leads to a robust increase in the frequency of mEPSCs and glutamate release from presynaptic terminals, which may rely on an elevation in intracellular Ca2+ levels triggered by activation of presynaptic Gi/o protein coupled σ-1 like receptors. Moreover, PREG has been recently shown to rescue the reduction of PI3K/Akt and Ras/ERK signals in Aβ25-35-mice [31] while DHEA activates σ-1 receptors, enhancing the growth of neuronal projections through a modulation of PI3K-Akt-mTOR-p70S6k signaling in Aβ25-35-impaired newborn neurons [37].
Exogenous ligand
Exogenous σ-1 receptor agonists show potential anti-amnesia and neuroprotection effect on both pharmacological and pathological models. Marrazzo, A first proved σ-1 receptor agonist PRE-084 and MR-22 (-), without involvement of NMDA receptors blockade, can reduce Aβ-mediated cortical neurons toxicity [73] to slow down the progression of Alzheimer’s disease. They projected the σ-1 proteins may obstruct the neurodegenerative process other than excitotoxic death. Based on this, Donepezil, licensed for symptomatic treatment of mild to moderate AD, was found to attenuate Aβ and glutamate toxicity [74] and potentiate the axonal growth [1] with its cholinergic and σ-1 agonistic properties. Additionally, afobazole mitigate neuron apoptosis through modulation of gene Bax, Bcl-2 expression and death protease caspase-3 levels in response to Aβ. Furthermore, treatment with afobazole decreased microglial activation and prevented disruption of ATP signaling in microglia incubated in Aβ25-35, indicating its potent preservation of microglial function after Aβ exposure. Last but not least, afobazole maintains intracellular proinflammatory and anti-inflammatory homeostasis [61] and potentiate NGF-induced neurite outgrowth [75]. Recently, it is clearly proposed that ANAVEX2-73, a mixed σ-1/muscarinic receptor ligand, can efficiently decrease the hyper-activation of GSK-3β in AD and prevent both the Tau hyperphosphorylation and Aβ1-42 Seeding [22]. Still there are (+) pentazocine and SA4503 alleviating amyloid load in pharmacology of a biphasic bell-shaped dose response curve. The protective effects of σ-1 receptors mentioned above can be mostly blocked by σR antagonists such as haloperidol and BMY-14802.
As for roles of exogenous ligand in cognitive improvement, with vivo microdialysis in freely moving rats, (+)-SKF10,047 was demonstrated to increase extracellular acetylcholine in both frontal cortex and hippocampus in a stereoselective way, which could also be blocked by haloperidol [48,76]. Besides, σ-1 agonists have shown anti-amnesic efficacy in both pharmacological and pathological models, which include cholinergic destruction, Aβ administration, normal aging, senescence accelerated mouse (SAM), glutamatergic/serotonergic or calcium channel deficits [1]. Intriguingly, in most behavioral tests, σ-1 receptor ligands do not facilitate or impede the learning ability in the control groups, suggesting that it is under pathological conditions that σ-1 receptors are activated [1].
Overall, σ-1 receptor agonists, being the promising compounds for the treatment of cognitive dysfunction, exhibit robust neuroprotection and anti-amnesic effect against Aβ neurotoxicity.
Research on σ-1 receptor and psychotic symptoms
In addition to the character of acquired impairment in cognitive function, AD exert a gradual bad impact on the patient’s professional social and family activities. The earliest non-cognitive expressions such as various types of depressive and anxiety disorder may develop [77] whereas behavioral disorders, aggression, hallucinations occur in late AD. There is still no effective clinical drug against these psychiatric symptoms. The role of σ receptors, especially σ-1 subtype, has been long identified as a target for pathophysiology in neuropsychiatric disorders. However, the preclinical and clinical evidence of σ-1 receptor on AD psychotic symptoms is meager at present.
Behavioral models have suggested some ligands that bind to σ receptors possess “antidepressant and anxiolytic” like properties [78]. Urani A [79] demonstrated that when AD rats were submitted to the conditioned fear stress test, igmesine and (+)-SKF-10,047 can significantly reduce the stress-induced motor suppression, indicating exogenous σ-1 receptor agonists may alleviate AD-associated depressive symptoms. Besides, BMY-14802, a σ-1 antagonist with potential antipsychotic activity, shows its potential anxiolytic properties by reducing dorsal raphe and hippocampal release of 5-HT in a direct interaction with somatodendritic 5-HT (1A) receptors in the raphe nuclei [80]. On the other hand, synergistic stimulation of σ and 5-HT (1A) receptors is requested in acute antidepressant-like action of OPC-14523 [81].
Early clinical trials of some antipsychotic drugs have exhibited a certain affinity for σ-1 proteins [82]. For example, haloperidol [83], a σ-1 receptor antagonist, have better effect on controlling the agitation, hostility and aggression. Panamesine (EMD 57445) [84], with high affinity for σ-1 receptor, has antipsychotic effects and is free of side effects related to the extrapyramidal motoric system (EPMS). What’s more, memantine (10 μM) [85], licensed for use in moderate to severe AD [86], had been demonstrated to improve the bradykinin induced mobilization of intracellular Ca2+ in NG108-15 neuroblastoma cells, mimicking effect of σ-1 against PRE-084 (1 μM). Still there are many antipsychotics like Chlorpromazine and Nemonapride with high affinity for σ-1 proteins.
Thus, σ-1 receptor ligands may presents a promising effect either as individual or adjuvant on the accompanying psychotic symptoms in AD. However, large double-blind randomized placebo-controlled clinical trials are needed to confirm its treatment prospect.
Clinical research and application of σ-1 receptor in Alzheimer’s disease
Research on correlation between risk for developing AD and σ-1 receptor gene mutation polymorphism is not much. The first study, a Japanese case-control sample [87], showed TT-P haplotype a protective factor for AD. The subsequent Polish study, however, did not validate the findings [88]. Recently, A. Feher [89] found TT-P gene mutation of σ-1 receptor to be the risk factors against AD. With no consistency, these observations suggest that a clinical study with larger sample size and greater ethnic similarity is necessary.
Fluvoxamine, as a selective serotonin reuptake inhibitor (SSRI) and σ-1 receptor against, is considered by Izzo, N.J to be an alternative approach in alleviating delirium [90] in patients with Alzheimer’s disease. However, there are no clinical evidence showing fluvoxamine has any therapeutic effect in cognitive disturbance of patients with AD though some case reports exist in Depression and Schizophrenia [91,92]. In contrast to other SSRIs including sertraline and paroxetine, it has been suggested that Fluvoxamine, as a potent sigma-1 receptor agonist, may reduce Aβ-mediated neurotoxicity through increasing phosphorylation of Akt-1 [93]. To date, only a few σ-1 receptor agonists (SA4503 and ANAVEX2-73) [22,94,95] have entered phase II clinical trials of acute/chronic neurodegenerative disorders.
Therefore, despite the increasing positive experimental results, utilization of σ-1 agents early in the disease process remains an overlooked therapeutic opportunity.
Dispute over σ-1 receptor role in Alzheimer’s disease
Recently, Tackenberg et al. holds that Aβ induces Tau-dependent neurodegeneration and dendritic spine loss via pathway involving NR2B/NR2A-containing NMDAR [96], considering NMDAR to be closely linked with the progression of AD. Soon after this, Sha et al. proved that NMDAR action is downregulated through reducing NR2B phosphorylation in σ-1 receptors knockout mice [97]. Therefore, it is projected that the σ-1 receptors deficits in AD brain, by decreasing NR2B phosphorylation, can reduce the Aβ-enhanced Ca2+ influx across NMDAR and prevent NMDAR-mediated neurotoxicity, which is proved by Yin, J. lately. Yin, J. et al. provides, for the first time, in vivo evidence that σ-1 receptor deficiency can attenuate Aβ25-35-induced hippocampal neuronal death and spatial cognitive deficits. Either σ-1 receptor deficiency or the blockade of σ-1 receptor in this study can significantly reduce the Aβ25-35-induced neuronal death. Paradoxically, there have been enormous reports describing the neuroprotection of σ-1 agonists in Aβ25-35/1-42 mice [98-100]. Although PREGS had also been observed to amplify NMDA-induced excitotoxicity in cultured hippocampal neurons [101], the discrepancy is hard to be reconciled. Exact timing of σ-1 receptors activation was raised to explain the contradictory effects. Administration of σ-1 receptor antagonists within 48 h post-Aβ25-35 can block the Aβ-neurotoxicity through suppressing NMDAR. However, after 72 h of Aβ25-35-injection, neuronal injury can be mitigated by σ-1 activation through enhancing ERK/PI3K-Akt signaling cascade [31] or decreasing levels of oxidative stress [102]. They also proposed that downregulation of PKC and reorganization of lipid rafts by σ-1 receptor deficiency can suppress NR2B subunit-containing NMDAR [103].
However, evidence in adverse effect of σ-1 receptor on AD is insufficient. Besides, NMDAR-mediated responses, traditionally been regarded as a double-edged sword, controversially interacted with σ-1 receptors over these decades. For example, σ ligands, including haloperidol, (+)-pentazocine, (+)-SKF-10,047 and DTG, depress NMDAR currents in Xenopus oocytes [104]. In addition, Kume et al. provided that σ-1 receptor ligands with affinity for NMDAR may act as neuron protector through reducing Ca2+ influx through NMDAR [105]. However, neurosteroids PREG as σ-1 agonist, have two-ways regulation of NMDAR function. Yang found σ-1 receptor activation by PREGS can suppress NMDAR response to resist neurocyte death in Aβ25-35-mice [31] Whereas Chen et al. suggested PREGS lead to NR2B tyrosine phosphorylation and Ca2+ influx through NMDAR, which cascaded the ERK/CREB pathway crucial for NMDAR-dependent long-term potentiation (LTP) involving in synaptic plasticity [106]. Contrarily to the results above, a large number of evidences consider the σ-1 receptor to be an accelerator for NMDAR response. It has been early demonstrated that σ-1 receptor can reverse OBX (olfactory bulbectomy)-induced NMDA-impaired behaviors [107]. Moreover, σ receptors facilitate pyramidal neurons in CA3 region of dorsal hippocampus to NMDA (excitatory activation) [29] and potentiate NMDA-dependent CA1 synapses [30], which is indispensable for learning ability and spatial memory storage. Nonsteroid hormones like progesterone and testosterone act as antagonists of σ-1 and, consequently, of NMDA-mediated responses [1]. Generally, σ-1 receptor exerts dual-directional regulation on NMDAR function. Nevertheless, the mechanism how σ-1 receptors act on NMDAR-mediated responses has not yet been explained. Martina supposed that σ-1 modulates NMDAR synaptic transmission and plasticity via blocking the small conductance Ca2+-activated K+ current (SK channels) in rat hippocampus [108]. On the other hand, it is possibly attributed to the additional diversity of NMDAR responses arising from the complexity of subunit composition and variations in localization. Synaptic NMDAR, with numbers of subunits NR2A>NR2B [109], can activate the nuclear calcium signaling pathways to promote the synaptic plasticity and improve the neural excitatory. Instead, activation of extrasynaptic NMDAR, with numbers of subunits NR2A<NR2B, causes calcium overload in neurons and starts the apoptosis or death pathway [110,111]. Therefore, as far as the NMDAR itself, the imbalance between the two different pathways can cause pathogenesis in neural degenerative diseases, especially for AD.
Thus, we propose that when concerned with NMDAR, σ-1 receptor activation may result in two totally different influences on AD: (a) facilitation of cognition improvement through modulating NMDAR-dependent learning and memory; (b) aggravation of the Aβ-mediated neurotoxicity by NR2B subunit-containing NMDAR in apoptosis or death pathway. Further studies are urgently needed to evaluate the exact process of its dual effect on both AD and NMDAR subtypes response.
Conclusion
An increasing studies show that σ receptor is implicated in cellular differentiation, neuroprotection, neuroplasticity and anti-amnesia of the brain, which suggests its potential prospects in the treatment of cognitive deficits. But several questions regarding this receptor are still open. There is no σ receptor ligands applied in current clinic. Only a few σ-1 agonists have entered phase II clinical trials of neurodegenerative disorders. Results of correlation studies between AD and variation of σ receptor gene polymorphism are not yet unified. When it comes to the NMDAR response, further studies are needed to evaluate whether σ receptors aggravate the NMDAR-dependent neural apoptosis. What’s more, cause of lower density of σ-1 receptors in early phase of AD is still unknown. With little knowledge of how Aβ acting on the σ receptors expression, the existing research on σ receptor is limited to endogenous and exogenous ligands. Besides, specific endogenous ligands have not been deeply studied. However, either as individual or adjuvant agent, σ receptor ligand is bound to be beneficial for degenerative disease of CNS, especially for AD-related cognitive impairment, and may provide an alternative to AchEIs/memantine which is currently available. The mechanism of how Aβ acting on the σ receptors expression is becoming a new direction for pathogenesis of AD.
Acknowledgements
This study was supported by grants from the National Natural Science Foundation of China (No. 81171163, No. 81371212).
Disclosure of conflict of interest
None.
References
- 1.van Waarde A, Ramakrishnan NK, Rybczynska AA, Elsinga PH, Ishiwata K, Nijholt IM, Luiten PG, Dierckx RA. The cholinergic system, sigma-1 receptors and cognition. Behav Brain Res. 2011;221:543–554. doi: 10.1016/j.bbr.2009.12.043. [DOI] [PubMed] [Google Scholar]
- 2.Jansen K, Faull R, Storey P, Leslie R. Loss of sigma binding sites in the CA1 area of the anterior hippocampus in Alzheimer’s disease correlates with CA1 pyramidal cell loss. Brain Res. 1993;623:299–302. doi: 10.1016/0006-8993(93)91441-t. [DOI] [PubMed] [Google Scholar]
- 3.Mishina M, Ohyama M, Ishii K, Kitamura S, Kimura Y, Oda KI, Kawamura K, Sasaki T, Kobayashi S, Katayama Y, Ishiwata K. Low density of sigma (1) receptors in early Alzheimer’s disease. Ann Nucl Med. 2008;22:151–156. doi: 10.1007/s12149-007-0094-z. [DOI] [PubMed] [Google Scholar]
- 4.Martin WR, Eades C, Thompson J, Huppler R, Gilbert P. The effects of morphine-and nalorphine-like drugs in the nondependent and morphine-dependent chronic spinal dog. J Pharmacol Exp Ther. 1976;197:517–532. [PubMed] [Google Scholar]
- 5.Quirion R, Bowen WD, Itzhak Y, Junien JL, Musacchio J, Rothman RB, Tsung-Ping S, Tam SW, Taylor DP. A proposal for the classification of sigma binding sites. Trends Pharmacol Sci. 1992;13:85–86. doi: 10.1016/0165-6147(92)90030-a. [DOI] [PubMed] [Google Scholar]
- 6.Gundlach AL, Largent BL, Snyder SH. Autoradiographic localization of sigma receptor binding sites in guinea pig and rat central nervous system with (+) 3H-3-(3-hydroxyphenyl)-N-(1-propyl) piperidine. J Neurosci. 1986;6:1757–1770. doi: 10.1523/JNEUROSCI.06-06-01757.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Banister SD, Manoli M, Kassiou M. The development of radiotracers for imaging sigma (sigma) receptors in the central nervous system (CNS) using positron emission tomography (PET) J Labelled Comp Radiopharm. 2013;56:215–224. doi: 10.1002/jlcr.3010. [DOI] [PubMed] [Google Scholar]
- 8.Hanner M, Moebius FF, Flandorfer A, Knaus HG, Striessnig J, Kempner E, Glossmann H. Purification, molecular cloning, and expression of the mammalian sigma1-binding site. Proc Natl Acad Sci U S A. 1996;93:8072–8077. doi: 10.1073/pnas.93.15.8072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kekuda R, Prasad PD, Fei YJ, Leibach FH, Ganapathy V. Cloning and functional expression of the human type 1 sigma receptor (hSigmaR1) Biochem Biophys Res Commun. 1996;229:553–558. doi: 10.1006/bbrc.1996.1842. [DOI] [PubMed] [Google Scholar]
- 10.Seth P, Leibach FH, Ganapathy V. Cloning and structural analysis of the cDNA and the gene encoding the murine type 1 sigma receptor. Biochem Biophys Res Commun. 1997;241:535–540. doi: 10.1006/bbrc.1997.7840. [DOI] [PubMed] [Google Scholar]
- 11.Seth P, Fei YJ, Li HW, Huang W, Leibach FH, Ganapathy V. Cloning and functional characterization of a sigma receptor from rat brain. J Neurochem. 1998;70:922–931. doi: 10.1046/j.1471-4159.1998.70030922.x. [DOI] [PubMed] [Google Scholar]
- 12.Kourrich S, Su TP, Fujimoto M, Bonci A. The sigma-1 receptor: roles in neuronal plasticity and disease. Trends Neurosci. 2012;35:762–771. doi: 10.1016/j.tins.2012.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Izzo NJ, Xu J, Zeng C, Kirk MJ, Mozzoni K, Silky C, Rehak C, Yurko R, Look G, Rishton G, Safferstein H, Cruchaga C, Goate A, Cahill MA, Arancio O, Mach RH, Craven R, Head E, LeVine H 3rd, Spires-Jones TL, Catalano SM. Alzheimer’s Therapeutics Targeting Amyloid Beta 1-42 Oligomers II: Sigma-2/PGRMC1 Receptors Mediate Abeta 42 Oligomer Binding and Synaptotoxicity. PLoS One. 2014;9:e111899. doi: 10.1371/journal.pone.0111899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tsai SY, Rothman RK, Su TP. Insights into the Sigma-1 receptor chaperone’s cellular functions: a microarray report. Synapse. 2012;66:42–51. doi: 10.1002/syn.20984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hindmarch I, Hashimoto K. Cognition and depression: the effects of fluvoxamine, a sigma-1 receptor agonist, reconsidered. Hum Psychopharmacol. 2010;25:193–200. doi: 10.1002/hup.1106. [DOI] [PubMed] [Google Scholar]
- 16.Karch CM, Goate AM. Alzheimer’s disease risk genes and mechanisms of disease pathogenesis. Biol Psychiatry. 2015;77:43–51. doi: 10.1016/j.biopsych.2014.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Maurice T, Gregoire C, Espallergues J. Neuro (active) steroids actions at the neuromodulatory sigma (1) (sigma (1)) receptor: biochemical and physiological evidences, consequences in neuroprotection. Pharmacol Biochem Behav. 2006;84:581–597. doi: 10.1016/j.pbb.2006.07.009. [DOI] [PubMed] [Google Scholar]
- 18.Hutter-Paier B, Huttunen HJ, Puglielli L, Eckman CB, Kim DY, Hofmeister A, Moir RD, Domnitz SB, Frosch MP, Windisch M, Kovacs DM. The ACAT inhibitor CP-113,818 markedly reduces amyloid pathology in a mouse model of Alzheimer’s disease. Neuron. 2004;44:227–238. doi: 10.1016/j.neuron.2004.08.043. [DOI] [PubMed] [Google Scholar]
- 19.Kakio A, Nishimoto SI, Yanagisawa K, Kozutsumi Y, Matsuzaki K. Cholesterol-dependent formation of GM1 ganglioside-bound amyloid beta-protein, an endogenous seed for Alzheimer amyloid. J Biol Chem. 2001;276:24985–24990. doi: 10.1074/jbc.M100252200. [DOI] [PubMed] [Google Scholar]
- 20.Kakio A, Nishimoto S, Yanagisawa K, Kozutsumi Y, Matsuzaki K. Interactions of amyloid beta-protein with various gangliosides in raft-like membranes: importance of GM1 ganglioside-bound form as an endogenous seed for Alzheimer amyloid. Biochemistry. 2002;41:7385–7390. doi: 10.1021/bi0255874. [DOI] [PubMed] [Google Scholar]
- 21.Simons K, Ikonen E. Functional rafts in cell membranes. Nature. 1997;387:569–572. doi: 10.1038/42408. [DOI] [PubMed] [Google Scholar]
- 22.Lahmy V, Meunier J, Malmstrom S, Naert G, Givalois L, Kim SH, Villard V, Vamvakides A, Maurice T. Blockade of Tau hyperphosphorylation and Abeta (1) (-) (4) (2) generation by the aminotetrahydrofuran derivative ANAVEX2-73, a mixed muscarinic and sigma (1) receptor agonist, in a nontransgenic mouse model of Alzheimer’s disease. Neuropsychopharmacology. 2013;38:1706–1723. doi: 10.1038/npp.2013.70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Meunier J, Villard V, Givalois L, Maurice T. The gamma-secretase inhibitor 2-[(1R)-1-[(4-chlorophenyl)sulfonyl] (2,5-difluorophenyl) amino] ethyl-5-fluorobenzenebutanoic acid (BMS-299897) alleviates Abeta1-42 seeding and short-term memory deficits in the Abeta25-35 mouse model of Alzheimer’s disease. Eur J Pharmacol. 2013;698:193–199. doi: 10.1016/j.ejphar.2012.10.033. [DOI] [PubMed] [Google Scholar]
- 24.Hayashi T, Kagaya A, Takebayashi M, Shimizu M, Uchitomi Y, Motohashi N, Yamawaki S. Modulation by sigma ligands of intracellular free Ca++ mobilization by N-methyl-D-aspartate in primary culture of rat frontal cortical neurons. J Pharmacol Exp Ther. 1995;275:207–214. [PubMed] [Google Scholar]
- 25.Klette KL, Lin Y, Clapp LE, DeCoster MA, Moreton JE, Tortella FC. Neuroprotective sigma ligands attenuate NMDA and trans-ACPD-induced calcium signaling in rat primary neurons. Brain Res. 1997;756:231–240. doi: 10.1016/s0006-8993(97)00142-x. [DOI] [PubMed] [Google Scholar]
- 26.Monnet FP, Morin-Surun MP, Leger J, Combettes L. Protein kinase C-dependent potentiation of intracellular calcium influx by sigma1 receptor agonists in rat hippocampal neurons. J Pharmacol Exp Ther. 2003;307:705–712. doi: 10.1124/jpet.103.053447. [DOI] [PubMed] [Google Scholar]
- 27.Cory-Slechta DA. The impact of NMDA receptor antagonists on learning and memory functions. Psychopharmacol Bull. 1994;30:601–612. [PubMed] [Google Scholar]
- 28.Morris RG, Steele RJ, Bell JE, Martin SJ. N-methyl-d-aspartate receptors, learning and memory: chronic intraventricular infusion of the NMDA receptor antagonist d-AP5 interacts directly with the neural mechanisms of spatial learning. Eur J Neurosci. 2013;37:700–717. doi: 10.1111/ejn.12086. [DOI] [PubMed] [Google Scholar]
- 29.Couture S, Debonnel G. Modulation of the neuronal response to N-methyl-D-aspartate by selective sigma2 ligands. Synapse. 1998;29:62–71. doi: 10.1002/(SICI)1098-2396(199805)29:1<62::AID-SYN5>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- 30.Tsien JZ, Huerta PT, Tonegawa S. The essential role of hippocampal CA1 NMDA receptor-dependent synaptic plasticity in spatial memory. Cell. 1996;87:1327–1338. doi: 10.1016/s0092-8674(00)81827-9. [DOI] [PubMed] [Google Scholar]
- 31.Yang R, Chen L, Wang H, Xu B, Tomimoto H, Chen L. Anti-amnesic effect of neurosteroid PREGS in Abeta25-35-injected mice through sigma1 receptor- and alpha7nAChR-mediated neuroprotection. Neuropharmacology. 2012;63:1042–1050. doi: 10.1016/j.neuropharm.2012.07.035. [DOI] [PubMed] [Google Scholar]
- 32.Itzhak Y, Stein I, Zhang SH, Kassim CO, Cristante D. Binding of sigma-ligands to C57BL/6 mouse brain membranes: effects of monoamine oxidase inhibitors and subcellular distribution studies suggest the existence of sigma-receptor subtypes. J Pharmacol Exp Ther. 1991;257:141–148. [PubMed] [Google Scholar]
- 33.Jiang G, Mysona B, Dun Y, Gnana-Prakasam JP, Pabla N, Li W, Dong Z, Ganapathy V, Smith SB. Expression, subcellular localization, and regulation of sigma receptor in retinal muller cells. Invest Ophthalmol Vis Sci. 2006;47:5576–5582. doi: 10.1167/iovs.06-0608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.McCann DJ, Su TP. Haloperidol-sensitive (+) [3H] SKF-10,047 binding sites (sigma sites) exhibit a unique distribution in rat brain subcellular fractions. Eur J Pharmacol. 1990;188:211–218. doi: 10.1016/0922-4106(90)90004-h. [DOI] [PubMed] [Google Scholar]
- 35.Phan VL, Urani A, Sandillon F, Privat A, Maurice T. Preserved sigma1 (sigma1) receptor expression and behavioral efficacy in the aged C57BL/6 mouse. Neurobiol Aging. 2003;24:865–881. doi: 10.1016/s0197-4580(02)00231-2. [DOI] [PubMed] [Google Scholar]
- 36.Samovilova NN, Vinogradov VA. Subcellular distribution of (+)-[3H] SKF 10,047 binding sites in rat liver. Eur J Pharmacol. 1992;225:69–74. doi: 10.1016/0922-4106(92)90041-s. [DOI] [PubMed] [Google Scholar]
- 37.Li L, Xu B, Zhu Y, Chen L, Sokabe M, Chen L. DHEA prevents Abeta25-35-impaired survival of newborn neurons in the dentate gyrus through a modulation of PI3K-Akt-mTOR signaling. Neuropharmacology. 2010;59:323–333. doi: 10.1016/j.neuropharm.2010.02.009. [DOI] [PubMed] [Google Scholar]
- 38.Brinton RD. The neurosteroid 3 alpha-hydroxy-5 alpha-pregnan-20-one induces cytoarchitectural regression in cultured fetal hippocampal neurons. J Neurosci. 1994;14:2763–2774. doi: 10.1523/JNEUROSCI.14-05-02763.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Liu SJ, Zhang AH, Li HL, Wang Q, Deng HM, Netzer WJ, Xu H, Wang JZ. Overactivation of glycogen synthase kinase-3 by inhibition of phosphoinositol-3 kinase and protein kinase C leads to hyperphosphorylation of tau and impairment of spatial memory. J Neurochem. 2003;87:1333–1344. doi: 10.1046/j.1471-4159.2003.02070.x. [DOI] [PubMed] [Google Scholar]
- 40.Matsuoka N, Aigner TG. Cholinergic-glutamatergic interactions in visual recognition memory of rhesus monkeys. Neuroreport. 1996;7:565–568. doi: 10.1097/00001756-199601310-00045. [DOI] [PubMed] [Google Scholar]
- 41.Gallagher M, Colombo PJ. Ageing: the cholinergic hypothesis of cognitive decline. Curr Opin Neurobiol. 1995;5:161–168. doi: 10.1016/0959-4388(95)80022-0. [DOI] [PubMed] [Google Scholar]
- 42.Bartus RT, Dean RL 3rd, Beer B, Lippa AS. The cholinergic hypothesis of geriatric memory dysfunction. Science. 1982;217:408–414. doi: 10.1126/science.7046051. [DOI] [PubMed] [Google Scholar]
- 43.Coyle JT, Price DL, DeLong MR. Alzheimer’s disease: a disorder of cortical cholinergic innervation. Science. 1983;219:1184–1190. doi: 10.1126/science.6338589. [DOI] [PubMed] [Google Scholar]
- 44.Davies P, Maloney AJ. Selective loss of central cholinergic neurons in Alzheimer’s disease. Lancet. 1976;2:1403. doi: 10.1016/s0140-6736(76)91936-x. [DOI] [PubMed] [Google Scholar]
- 45.Horan B, Gifford AN, Matsuno K, Mita S, Ashby CR Jr. Effect of SA4503 on the electrically evoked release of (3)H-acetylcholine from striatal and hippocampal rat brain slices. Synapse. 2002;46:1–3. doi: 10.1002/syn.10107. [DOI] [PubMed] [Google Scholar]
- 46.Junien JL, Roman FJ, Brunelle G, Pascaud X. JO1784, a novel sigma ligand, potentiates [3H] acetylcholine release from rat hippocampal slices. Eur J Pharmacol. 1991;200:343–345. doi: 10.1016/0014-2999(91)90593-f. [DOI] [PubMed] [Google Scholar]
- 47.Matsuno K, Matsunaga K, Mita S. Increase of extracellular acetylcholine level in rat frontal cortex induced by (+)N-allylnormetazocine as measured by brain microdialysis. Brain Res. 1992;575:315–319. doi: 10.1016/0006-8993(92)90096-r. [DOI] [PubMed] [Google Scholar]
- 48.Matsuno K, Matsunaga K, Senda T, Mita S. Increase in extracellular acetylcholine level by sigma ligands in rat frontal cortex. J Pharmacol Exp Ther. 1993;265:851–859. [PubMed] [Google Scholar]
- 49.Matsuno K, Senda T, Kobayashi T, Okamoto K, Nakata K, Mita S. SA4503, a novel cognitive enhancer, with sigma 1 receptor agonistic properties. Behav Brain Res. 1997;83:221–224. doi: 10.1016/s0166-4328(97)86074-3. [DOI] [PubMed] [Google Scholar]
- 50.Kobayashi T, Matsuno K, Nakata K, Mita S. Enhancement of acetylcholine release by SA4503, a novel sigma 1 receptor agonist, in the rat brain. J Pharmacol Exp Ther. 1996;279:106–113. [PubMed] [Google Scholar]
- 51.Kobayashi T, Matsuno K, Mita S. Regional differences of the effect of sigma receptor ligands on the acetylcholine release in the rat brain. J Neural Transm. 1996;103:661–669. doi: 10.1007/BF01271226. [DOI] [PubMed] [Google Scholar]
- 52.Zvejniece L, Vavers E, Svalbe B, Vilskersts R, Domracheva I, Vorona M, Veinberg G, Misane I, Stonans I, Kalvinsh I, Dambrova M. The cognition-enhancing activity of E1R, a novel positive allosteric modulator of sigma-1 receptors. Br J Pharmacol. 2014;171:761–771. doi: 10.1111/bph.12506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Nakazato E, Yamamoto T, Ohno M, Watanabe S. Cholinergic and glutamatergic activation reverses working memory failure by hippocampal histamine H1 receptor blockade in rats. Life Sci. 2000;67:1139–1147. doi: 10.1016/s0024-3205(00)00713-x. [DOI] [PubMed] [Google Scholar]
- 54.Aigner TG. Pharmacology of memory: cholinergic-glutamatergic interactions. Curr Opin Neurobiol. 1995;5:155–160. doi: 10.1016/0959-4388(95)80021-2. [DOI] [PubMed] [Google Scholar]
- 55.Yagasaki Y, Numakawa T, Kumamaru E, Hayashi T, Su TP, Kunugi H. Chronic antidepressants potentiate via sigma-1 receptors the brain-derived neurotrophic factor-induced signaling for glutamate release. J Biol Chem. 2006;281:12941–12949. doi: 10.1074/jbc.M508157200. [DOI] [PubMed] [Google Scholar]
- 56.Meyer DA, Carta M, Partridge LD, Covey DF, Valenzuela CF. Neurosteroids enhance spontaneous glutamate release in hippocampal neurons. Possible role of metabotropic sigma1-like receptors. J Biol Chem. 2002;277:28725–28732. doi: 10.1074/jbc.M202592200. [DOI] [PubMed] [Google Scholar]
- 57.Dong Y, Fu YM, Sun JL, Zhu YH, Sun FY, Zheng P. Neurosteroid enhances glutamate release in rat prelimbic cortex via activation of alpha1-adrenergic and sigma1 receptors. Cell Mol Life Sci. 2005;62:1003–1014. doi: 10.1007/s00018-005-5004-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Dong LY, Cheng ZX, Fu YM, Wang ZM, Zhu YH, Sun JL, Dong Y, Zheng P. Neurosteroid dehydroepiandrosterone sulfate enhances spontaneous glutamate release in rat prelimbic cortex through activation of dopamine D1 and sigma-1 receptor. Neuropharmacology. 2007;52:966–974. doi: 10.1016/j.neuropharm.2006.10.015. [DOI] [PubMed] [Google Scholar]
- 59.McGeer PL, McGeer EG. The amyloid cascade-inflammatory hypothesis of Alzheimer disease: implications for therapy. Acta Neuropathol. 2013;126:479–497. doi: 10.1007/s00401-013-1177-7. [DOI] [PubMed] [Google Scholar]
- 60.Cai Z, Hussain MD, Yan LJ. Microglia, neuroinflammation, and beta-amyloid protein in Alzheimer’s disease. Int J Neurosci. 2014;124:307–321. doi: 10.3109/00207454.2013.833510. [DOI] [PubMed] [Google Scholar]
- 61.Behensky AA, Yasny IE, Shuster AM, Seredenin SB, Petrov AV, Cuevas J. Stimulation of sigma receptors with afobazole blocks activation of microglia and reduces toxicity caused by amyloid-beta25-35. J Pharmacol Exp Ther. 2013;347:458–467. doi: 10.1124/jpet.113.208348. [DOI] [PubMed] [Google Scholar]
- 62.Vagnerova K, Hurn PD, Bhardwaj A, Kirsch JR. Sigma 1 receptor agonists act as neuroprotective drugs through inhibition of inducible nitric oxide synthase. Anesth Analg. 2006;103:430–434. doi: 10.1213/01.ane.0000226133.85114.91. [DOI] [PubMed] [Google Scholar]
- 63.Hall AA, Herrera Y, Ajmo CT Jr, Cuevas J, Pennypacker KR. Sigma receptors suppress multiple aspects of microglial activation. Glia. 2009;57:744–754. doi: 10.1002/glia.20802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Zhu LX, Sharma S, Gardner B, Escuadro B, Atianzar K, Tashkin DP, Dubinett SM. IL-10 mediates sigma 1 receptor-dependent suppression of antitumor immunity. J Immunol. 2003;170:3585–3591. doi: 10.4049/jimmunol.170.7.3585. [DOI] [PubMed] [Google Scholar]
- 65.Hall AA, Leonardo CC, Collier LA, Rowe DD, Willing AE, Pennypacker KR. Delayed treatments for stroke influence neuronal death in rat organotypic slice cultures subjected to oxygen glucose deprivation. Neuroscience. 2009;164:470–477. doi: 10.1016/j.neuroscience.2009.08.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Moriguchi S, Yamamoto Y, Ikuno T, Fukunaga K. Sigma-1 receptor stimulation by dehydroepiandrosterone ameliorates cognitive impairment through activation of CaM kinase II, protein kinase C and extracellular signal-regulated kinase in olfactory bulbectomized mice. J Neurochem. 2011;117:879–891. doi: 10.1111/j.1471-4159.2011.07256.x. [DOI] [PubMed] [Google Scholar]
- 67.Elfverson M, Johansson T, Zhou Q, Le Greves P, Nyberg F. Chronic administration of the anabolic androgenic steroid nandrolone alters neurosteroid action at the sigma-1 receptor but not at the sigma-2 or NMDA receptors. Neuropharmacology. 2011;61:1172–1181. doi: 10.1016/j.neuropharm.2011.01.005. [DOI] [PubMed] [Google Scholar]
- 68.Flood JF, Morley JE, Roberts E. Pregnenolone sulfate enhances post-training memory processes when injected in very low doses into limbic system structures: the amygdala is by far the most sensitive. Proc Natl Acad Sci U S A. 1995;92:10806–10810. doi: 10.1073/pnas.92.23.10806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Suzuki M, Wright LS, Marwah P, Lardy HA, Svendsen CN. Mitotic and neurogenic effects of dehydroepiandrosterone (DHEA) on human neural stem cell cultures derived from the fetal cortex. Proc Natl Acad Sci U S A. 2004;101:3202–3207. doi: 10.1073/pnas.0307325101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Lambert JJ, Belelli D, Hill-Venning C, Callachan H, Peters JA. Neurosteroid modulation of native and recombinant GABAA receptors. Cell Mol Neurobiol. 1996;16:155–174. doi: 10.1007/BF02088174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Park-Chung M, Wu FS, Purdy RH, Malayev AA, Gibbs TT, Farb DH. Distinct sites for inverse modulation of N-methyl-D-aspartate receptors by sulfated steroids. Mol Pharmacol. 1997;52:1113–1123. doi: 10.1124/mol.52.6.1113. [DOI] [PubMed] [Google Scholar]
- 72.ffrench-Mullen JM, Danks P, Spence KT. Neurosteroids modulate calcium currents in hippocampal CA1 neurons via a pertussis toxin-sensitive G-protein-coupled mechanism. J Neurosci. 1994;14:1963–1977. doi: 10.1523/JNEUROSCI.14-04-01963.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Marrazzo A, Caraci F, Salinaro ET, Su TP, Copani A, Ronsisvalle G. Neuroprotective effects of sigma-I receptor agonists against beta-amyloid-induced toxicity. Neuroreport. 2005;16:1223–1226. doi: 10.1097/00001756-200508010-00018. [DOI] [PubMed] [Google Scholar]
- 74.Meunier J, Ieni J, Maurice T. The anti-amnesic and neuroprotective effects of donepezil against amyloid beta25-35 peptide-induced toxicity in mice involve an interaction with the sigma1 receptor. Br J Pharmacol. 2006;149:998–1012. doi: 10.1038/sj.bjp.0706927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Nishimura T, Ishima T, Iyo M, Hashimoto K. Potentiation of nerve growth factor-induced neurite outgrowth by fluvoxamine: role of sigma-1 receptors, IP3 receptors and cellular signaling pathways. PLoS One. 2008;3:e2558. doi: 10.1371/journal.pone.0002558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Matsuno K, Senda T, Kobayashi T, Mita S. Involvement of sigma 1 receptor in (+)-N-allylnormetazocine-stimulated hippocampal cholinergic functions in rats. Brain Res. 1995;690:200–206. doi: 10.1016/0006-8993(95)00618-z. [DOI] [PubMed] [Google Scholar]
- 77.Bierman EJ, Comijs HC, Jonker C, Beekman AT. Symptoms of anxiety and depression in the course of cognitive decline. Dement Geriatr Cogn Disord. 2007;24:213–219. doi: 10.1159/000107083. [DOI] [PubMed] [Google Scholar]
- 78.Hayashi T, Su TP. Sigma-1 receptor ligands: potential in the treatment of neuropsychiatric disorders. CNS Drugs. 2004;18:269–284. doi: 10.2165/00023210-200418050-00001. [DOI] [PubMed] [Google Scholar]
- 79.Urani A, Romieu P, Roman FJ, Yamada K, Noda Y, Kamei H, Manh Tran H, Nagai T, Nabeshima T, Maurice T. Enhanced antidepressant efficacy of sigma1 receptor agonists in rats after chronic intracerebroventricular infusion of beta-amyloid-(1-40) protein. Eur J Pharmacol. 2004;486:151–161. doi: 10.1016/j.ejphar.2003.12.018. [DOI] [PubMed] [Google Scholar]
- 80.Matos FF, Korpinen C, Yocca FD. 5-HT1A receptor agonist effects of BMY-14802 on serotonin release in dorsal raphe and hippocampus. Eur J Pharmacol. 1996;317:49–54. doi: 10.1016/s0014-2999(96)00699-1. [DOI] [PubMed] [Google Scholar]
- 81.Tottori K, Miwa T, Uwahodo Y, Yamada S, Nakai M, Oshiro Y, Kikuchi T, Altar CA. Antidepressant-like responses to the combined sigma and 5-HT1A receptor agonist OPC-14523. Neuropharmacology. 2001;41:976–988. doi: 10.1016/s0028-3908(01)00147-2. [DOI] [PubMed] [Google Scholar]
- 82.Tam SW, Cook L. Sigma opiates and certain antipsychotic drugs mutually inhibit (+)-[3H] SKF 10,047 and [3H] haloperidol binding in guinea pig brain membranes. Proc Natl Acad Sci U S A. 1984;81:5618–5621. doi: 10.1073/pnas.81.17.5618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Verachai V, Rukngan W, Chawanakrasaesin K, Nilaban S, Suwanmajo S, Thanateerabunjong R, Kaewkungwal J, Kalayasiri R. Treatment of methamphetamine-induced psychosis: a double-blind randomized controlled trial comparing haloperidol and quetiapine. Psychopharmacology (Berl) 2014;231:3099–3108. doi: 10.1007/s00213-014-3485-6. [DOI] [PubMed] [Google Scholar]
- 84.Frieboes RM, Murck H, Wiedemann K, Holsboer F, Steiger A. Open clinical trial on the sigma ligand panamesine in patients with schizophrenia. Psychopharmacology. 1997;132:82–88. doi: 10.1007/s002130050323. [DOI] [PubMed] [Google Scholar]
- 85.Peeters M, Romieu P, Maurice T, Su TP, Maloteaux JM, Hermans E. Involvement of the sigma 1 receptor in the modulation of dopaminergic transmission by amantadine. Eur J Neurosci. 2004;19:2212–2220. doi: 10.1111/j.0953-816X.2004.03297.x. [DOI] [PubMed] [Google Scholar]
- 86.Zemek F, Drtinova L, Nepovimova E, Sepsova V, Korabecny J, Klimes J, Kuca K. Outcomes of Alzheimer’s disease therapy with acetylcholinesterase inhibitors and memantine. Expert Opin Drug Saf. 2014;13:759–774. doi: 10.1517/14740338.2014.914168. [DOI] [PubMed] [Google Scholar]
- 87.Uchida N, Ujike H, Tanaka Y, Sakai A, Yamamoto M, Fujisawa Y, Kanzaki A, Kuroda S. A variant of the sigma receptor type-1 gene is a protective factor for Alzheimer disease. Am J Geriatr Psychiatry. 2005;13:1062–1066. doi: 10.1176/appi.ajgp.13.12.1062. [DOI] [PubMed] [Google Scholar]
- 88.Maruszak A, Safranow K, Gacia M, Gabryelewicz T, Slowik A, Styczynska M, Peplonska B, Golan MP, Zekanowski C, Barcikowska M. Sigma receptor type 1 gene variation in a group of Polish patients with Alzheimer’s disease and mild cognitive impairment. Dement Geriatr Cogn Disord. 2007;23:432–438. doi: 10.1159/000101990. [DOI] [PubMed] [Google Scholar]
- 89.Feher A, Juhasz A, Laszlo A, Kalman J Jr, Pakaski M, Kalman J, Janka Z. Association between a variant of the sigma-1 receptor gene and Alzheimer’s disease. Neurosci Lett. 2012;517:136–139. doi: 10.1016/j.neulet.2012.04.046. [DOI] [PubMed] [Google Scholar]
- 90.Furuse T, Hashimoto K. Sigma-1 receptor agonist fluvoxamine for delirium in patients with Alzheimer’s disease. Ann Gen Psychiatry. 2010;9:6. doi: 10.1186/1744-859X-9-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Iyo M, Shirayama Y, Watanabe H, Fujisaki M, Miyatake R, Fukami G, Shiina A, Nakazato M, Shiraishi T, Hashimoto K, Ookami T. Fluvoxamine as a sigma-1 receptor agonist improved cognitive impairments in a patient with schizophrenia. Prog Neuropsychopharmacol Biol Psychiatry. 2008;32:1072–1073. doi: 10.1016/j.pnpbp.2008.01.005. [DOI] [PubMed] [Google Scholar]
- 92.Mandelli L, Serretti A, Colombo C, Florita M, Santoro A, Rossini D, Zanardi R, Smeraldi E. Improvement of cognitive functioning in mood disorder patients with depressive symptomatic recovery during treatment: an exploratory analysis. Psychiatry Clin Neurosci. 2006;60:598–604. doi: 10.1111/j.1440-1819.2006.01564.x. [DOI] [PubMed] [Google Scholar]
- 93.Nakano M, Osada K, Misonoo A, Fujiwara K, Takahashi M, Ogawa Y, Haga T, Kanai S, Tanaka D, Sasuga Y, Yanagida T, Asakura M, Yamaguchi N. Fluvoxamine and sigma-1 receptor agonists dehydroepiandrosterone (DHEA)-sulfate induces the Ser473-phosphorylation of Akt-1 in PC12 cells. Life Sci. 2010;86:309–314. doi: 10.1016/j.lfs.2009.11.017. [DOI] [PubMed] [Google Scholar]
- 94.Urfer R, Moebius HJ, Skoloudik D, Santamarina E, Sato W, Mita S, Muir KW. Phase II trial of the Sigma-1 receptor agonist cutamesine (SA4503) for recovery enhancement after acute ischemic stroke. Stroke. 2014;45:3304–3310. doi: 10.1161/STROKEAHA.114.005835. [DOI] [PubMed] [Google Scholar]
- 95.Collina S, Gaggeri R, Marra A, Bassi A, Negrinotti S, Negri F, Rossi D. Sigma receptor modulators: a patent review. Expert Opin Ther Pat. 2013;23:597–613. doi: 10.1517/13543776.2013.769522. [DOI] [PubMed] [Google Scholar]
- 96.Tackenberg C, Grinschgl S, Trutzel A, Santuccione AC, Frey MC, Konietzko U, Grimm J, Brandt R, Nitsch RM. NMDA receptor subunit composition determines beta-amyloid-induced neurodegeneration and synaptic loss. Cell Death Dis. 2013;4:e608. doi: 10.1038/cddis.2013.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Sha S, Qu WJ, Li L, Lu ZH, Chen L, Yu WF, Chen L. Sigma-1 receptor knockout impairs neurogenesis in dentate gyrus of adult hippocampus via down-regulation of NMDA receptors. CNS Neurosci Ther. 2013;19:705–713. doi: 10.1111/cns.12129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Maurice T, Lockhart BP. Neuroprotective and anti-amnesic potentials of sigma (sigma) receptor ligands. Prog Neuropsychopharmacol Biol Psychiatry. 1997;21:69–102. doi: 10.1016/s0278-5846(96)00160-1. [DOI] [PubMed] [Google Scholar]
- 99.Luedtke RR, Perez E, Yang SH, Liu R, Vangveravong S, Tu Z, Mach RH, Simpkins JW. Neuroprotective effects of high affinity Sigma1 receptor selective compounds. Brain Res. 2012;1441:17–26. doi: 10.1016/j.brainres.2011.12.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Griesmaier E, Posod A, Gross M, Neubauer V, Wegleiter K, Hermann M, Urbanek M, Keller M, Kiechl-Kohlendorfer U. Neuroprotective effects of the sigma-1 receptor ligand PRE-084 against excitotoxic perinatal brain injury in newborn mice. Exp Neurol. 2012;237:388–395. doi: 10.1016/j.expneurol.2012.06.030. [DOI] [PubMed] [Google Scholar]
- 101.Weaver CE, Land MB, Purdy RH, Richards KG, Gibbs TT, Farb DH. Geometry and charge determine pharmacological effects of steroids on N-methyl-D-aspartate receptor-induced Ca (2+) accumulation and cell death. J Pharmacol Exp Ther. 2000;293:747–754. [PubMed] [Google Scholar]
- 102.Hyrskyluoto A, Pulli I, Tornqvist K, Ho TH, Korhonen L, Lindholm D. Sigma-1 receptor agonist PRE084 is protective against mutant huntingtin-induced cell degeneration: involvement of calpastatin and the NF-kappaB pathway. Cell Death Dis. 2013;4:e646. doi: 10.1038/cddis.2013.170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Yin J, Sha S, Chen T, Wang C, Hong J, Jie P, Zhou R, Li L, Sokabe M, Chen L. Sigma-1 (sigma) receptor deficiency reduces beta-amyloid-induced hippocampal neuronal cell death and cognitive deficits through suppressing phosphorylation of the NMDA receptor NR2B. Neuropharmacology. 2015;89:215–24. doi: 10.1016/j.neuropharm.2014.09.027. [DOI] [PubMed] [Google Scholar]
- 104.Whittemore ER, Ilyin VI, Woodward RM. Antagonism of N-methyl-D-aspartate receptors by sigma site ligands: potency, subtype-selectivity and mechanisms of inhibition. J Pharmacol Exp Ther. 1997;282:326–338. [PubMed] [Google Scholar]
- 105.Kume T, Nishikawa H, Taguchi R, Hashino A, Katsuki H, Kaneko S, Minami M, Satoh M, Akaike A. Antagonism of NMDA receptors by sigma receptor ligands attenuates chemical ischemia-induced neuronal death in vitro. Eur J Pharmacol. 2002;455:91–100. doi: 10.1016/s0014-2999(02)02582-7. [DOI] [PubMed] [Google Scholar]
- 106.Chen L, Miyamoto Y, Furuya K, Mori N, Sokabe M. PREGS induces LTP in the hippocampal dentate gyrus of adult rats via the tyrosine phosphorylation of NR2B coupled to ERK/CREB [corrected] signaling. J Neurophysiol. 2007;98:1538–1548. doi: 10.1152/jn.01151.2006. [DOI] [PubMed] [Google Scholar]
- 107.Bermack JE, Debonnel G. The role of sigma receptors in depression. J Pharmacol Sci. 2005;97:317–336. doi: 10.1254/jphs.crj04005x. [DOI] [PubMed] [Google Scholar]
- 108.Martina M, Turcotte ME, Halman S, Bergeron R. The sigma-1 receptor modulates NMDA receptor synaptic transmission and plasticity via SK channels in rat hippocampus. J Physiol. 2007;578:143–157. doi: 10.1113/jphysiol.2006.116178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Li JH, Wang YH, Wolfe BB, Krueger KE, Corsi L, Stocca G, Vicini S. Developmental changes in localization of NMDA receptor subunits in primary cultures of cortical neurons. Eur J Neurosci. 1998;10:1704–1715. doi: 10.1046/j.1460-9568.1998.00169.x. [DOI] [PubMed] [Google Scholar]
- 110.Petralia RS, Al-Hallaq RA, Wenthold RJ. Trafficking and Targeting of NMDA Receptors. In: Van Dongen AM, editor. Biology of the NMDA Receptor. Boca Raton (FL): CRC Press; 2009. [PubMed] [Google Scholar]
- 111.Li S, Jin M, Koeglsperger T, Shepardson NE, Shankar GM, Selkoe DJ. Soluble Abeta oligomers inhibit long-term potentiation through a mechanism involving excessive activation of extrasynaptic NR2B-containing NMDA receptors. J Neurosci. 2011;31:6627–6638. doi: 10.1523/JNEUROSCI.0203-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]


