Abstract
Background
The prevalence of metabolic diseases rises rapidly with an ageing population. Recent studies suggest the potential involvement of environmental chemicals in insulin resistance (IR) that plays a core role in the development of metabolic diseases. Polycyclic aromatic hydrocarbons (PAHs) are ubiquitous components of outdoor and indoor air pollution. The influence of PAHs on IR may differ depending on sex and weight.
Objectives
We examined the association between exposure to environmental PAHs and IR in Korean urban elderly adults controlling for major risk factors that contribute to an increase in IR.
Methods
Between 2008 and 2010, PAH metabolite levels (urinary 1-hydroxypyrene (1-OHP)) and the homoeostatic model assessment index (HOMA-IR) were repeatedly measured in 502 adults aged ≥60 years. Linear mixed effect models were fit to evaluate the associations of 1-OHP concentration with HOMA-IR. Subgroups were modelled by sex and weight.
Results
After adjusting for sociodemographics, air pollution and metabolic disease status, the highest (vs lowest) quartile of 1-OHP was associated with an 0.57 (95% CI 0.10 to 1.04) increase in the HOMA-IR score (p trend=0.037). When stratified by sex, women presented a significantly dose-dependent trend of 1-OHP with HOMA-IR (p trend=0.013), whereas no association was observed in men (p trend=0.904). When further stratified by weight (body mass index ≥25 vs <25 kg/m2), a significant association was found only in overweight women (p trend=0.023).
Conclusions
Our results suggest that environmental exposure to PAHs is associated with increased IR in elderly adults and that the association may be limited to overweight women.
Keywords: Biomonitoring, Environmental epidemiology, ELDERLY
Introduction
Environmental pollutants are a worldwide public health issue affecting various health outcomes.1 Over recent years, the potential involvement of environmental chemicals in ‘metabolic diseases’ (eg, metabolic syndrome, diabetes mellitus (DM), cardiovascular disease and obesity) is of particular concern in the research community2 3 because of the increasing prevalence of metabolic diseases. Despite the extensive investigation of metabolic diseases, responsible mechanisms and a causative role of chemicals have not been fully elucidated.4 Hectors et al suggested that the implication of environmental chemicals in contributing to metabolic diseases may provide novel preventive strategies different from traditional methods, including lifestyle modification. They also emphasised the need for research on the link between environmental chemicals and insulin resistance (IR)2 because of IR's core role in the development of metabolic diseases.4–6
The polycyclic aromatic hydrocarbons (PAHs) and their derivatives are known to be carcinogenic and mutagenic7 and to be associated with an increase in oxidative stress.8 PAHs are ubiquitous components of outdoor air pollution (from motor vehicles, generation of electricity and residential heating) and of indoor air pollution (from environmental tobacco smoke (ETS) and cooking fumes).9 10 In East Asian (ie, Korean and Japanese) cities, major sources were in traffic exhaust and industrial production. Transboundary air pollution from neighbouring countries such as China also accounts for considerable amounts of PAHs.11 Several East Asian studies reported that PAHs are present chiefly in atmospheric particulate matter (PM).12–14 A Chinese study of five cities observed high PAH concentrations in an urban background and suggested that 90% of the total PAHs in ambient air were bound to PM2.5.12
While obesity is known to be inter-related with other metabolic conditions, the prevalence of obesity in the Asia-Pacific region is low compared with that in Europe and North America, although diabetes and metabolic syndrome are commonly experienced by Asians.15 16 Thus, identification of factors such as environmental chemicals that may trigger IR development would be particularly important for Asian people.
Given the ubiquitous high levels of PAHs in East Asian cities and the fact that IR is a central mechanism in the development of metabolic diseases, we focused on PAHS as potentially responsible for elevated IR. Owing to a report that women are more susceptible to the influence of PAHs17 and that obesity modifies associations between air pollution exposures and metabolic diseases,18 19 this study aimed to investigate the associations between IR and exposure to environmental PAHs and to evaluate the influences of sex-dependence and body weight-dependence in a longitudinal panel of elderly adults in Seoul.
Methods
Study participants
This study is a part of the Korean Elderly Environmental Panel (KEEP) study. Between August 2008 and August 2010, 560 non-institutionalised elderly adults (60 years of age or more) who regularly attended the Seongbuk-gu Community Center in Seoul were invited to participate in the study. Information on sociodemographic, environmental and behavioural factors and medical history was obtained at the time of enrolment. Participants underwent physical examination and laboratory testing at each follow-up visit (visits ranging from 1 to 5; 152, 70, 138, 124 and 76 participants visited 5, 4, 3, 2 and 1 times, respectively).
Of the initial 560 eligible participants (1856 observations), those lacking data regarding IR measures (n=14), urinary metabolites (ie, PAHs and cotinine; n=9), sociobehavioural factors (ie, education and physical activity; n=18), air pollution (n=23) and medical history (n=18; 9, 6, and 12 for diabetes, hypertension and cholesterol only) were excluded from the analysis, leaving a total of 502 individuals (996 observation).
Urinary metabolites
Participants came to the study centre between 10:00 and 12:00 at every visit. Spot urine samples, collected from each participant, were shipped to the NeoDIN Medical Institute (Seoul, Korea) for storage at −20°C until laboratory analysis.
PAHs exposure was estimated by measuring concentration of urinary 1-hydroxypyrene (1-OHP, CAS 5315-79-7, metabolite of pyrene), a biomarker commonly used to indicate the extent of short-term exposure to PAH.20 The elimination half-life of urinary 1-OHP was estimated to be 3.9 h.21 1-OHP was measured using a high performance liquid chromatograph (HPLC) system (Agilent 6410 triple Quad LCMS; Agilent, Santa Clara, California, USA) with a fluorescence detector (Model FP-2020; JASCO.co., Tokyo, Japan).22
The lower limit of detection (LODL) was 0.004 µg/L. At baseline, 1.59% of study participants had 1-OHP concentrations below the LODL.
Exposure to tobacco smoke was estimated using urinary cotinine concentration; metabolites were determined using urine cotinine test strips (Accutest NicAlert Strip; Jant Pharmaceutical Co., Encino, California, USA). The detection range for cotinine was 1–10 000 µg/L. At baseline, 31.7% of study participants had cotinine concentrations below the LODL, and 0.8% had concentrations above the upper LOD (LODU).
For urinary metabolites of 1-OHP and cotinine, those values below the LODL were imputed a value of LODL/2, and those over the LODU were imputed a value of LODU×2. Metabolite measurements were creatinine (Cr) corrected by dividing 1-OHP and cotinine concentrations by urinary Cr concentration (1-OHP/Cr and cotinine/Cr µg/g) in order to capture variation in urine concentration.23
Insulin resistance
Overnight fasting serum samples were collected and sent to the laboratory (NeoDIN Medical Institute) for storage at −70°C until analysis. The fasting blood glucose (FBG) level was determined by the hexokinase method using a Pureauto S GLU kit (Daiichi Pure Chemicals, Tokyo, Japan), and fasting insulin level by the double-antibody batch method using radio immunoassay (Elecsys Insulin and Elecsys 2010 Immunoanalyzers, Roche Diagnostics, Mannheim, Germany).
IR was then estimated by determination of the homoeostatic model assessment (HOMA-IR) score using the equation developed by Matthews et al24: HOMA-IR score=(fasting insulin level in µU/mL×FBG level in mmol/L)/22.5.
Covariates
We considered age, sex, education, physical activity, overweight, hypertension, DM, cholesterol level, exposure to tobacco smoke and to ambient PM ≤10 μm in diameter (PM10), and the length of time elapsed from the first visit as potential confounders. Covariates to be controlled were identified a priori, based on biological consideration and the literature review.25–27
Education was self-identified (≤elementary school, ≤high school, higher). Physical activity was defined as moderate to vigorous leisure activity versus none (yes/no).
Exposure to active and passive tobacco smoke was determined using urinary cotinine (log-transformed for normalisation; continuous). Exposure to ambient air pollution was estimated by daily mean levels of PM10 on the day immediately prior to an examination visit (continuous), using data from the monitoring site closest to each participant's residence, obtained from the Korea National Institute of Environmental Research (NIER, Incheon, Korea).
Body mass index (BMI) was computed as weight in kilograms divided by height in metres squared (kg/m2); overweight was defined as BMI ≥25 kg/m2 (yes/no). High-density lipoprotein cholesterol level (HDL-C) was defined according to whether it was lower or higher than the threshold value of 40 mg/dL for men and 50 mg/dL for women (low/high). Hypertension was defined as an average systolic blood pressure ≥140 mm Hg; diastolic blood pressure ≥90 mm Hg; self-reported physician diagnosis; or the use of antihypertensive medication (yes/no). DM was defined as FBG ≥100 mg/dL; self-reported physician diagnosis or the use of medication including antihyperglycaemic agents or insulin therapy (yes/no).
Statistical analysis
All statistical analyses were performed using SAS V.9.3 software (SAS Institute Inc, Cary, North Carolina, USA). Multivariate analysis using the linear mixed effect models was performed to estimate the effects of urinary 1-OHP concentrations on glucose level, insulin level and HOMA-IR score. Because 1-OHP metabolite was right-skewed, we used natural log-transformed levels to normalise the data. The influence of potential confounders was identified by the development of five sequential models: (1) model A adjusted for demographic factors (age, sex) and the time elapsed from the first visit; (2) model B adjusted for model A variables plus sociobehavioural factors (education, physical activity); (3) model C adjusted for model B plus ambient exposures (tobacco smoke, PM); (4) model D adjusted for model C plus metabolic syndrome components (overweight, hypertension, DM, HDL-C) and (5) model E adjusted for model D minus DM. In all models, we used a random intercept and a slope for the duration of time from the study baseline to account for the heterogeneity across subjects and subject-specific variability of IR over time. Follow-up observations were weighted to capture potential selection bias from the loss to follow-up by imputing the inverse predicted probability of having a follow-up response.28–30
We also modelled quartiles of 1-OHP. Values of 1-OHP concentration for all observations were categorised into four groups using the cut-off values for each quartile for 1-OHP concentration at the first visit (0.0753, 0.1237 and 0.2039 µg/g Cr). The models of 1-OHP quartiles with HOMA-IR were evaluated in all participants and subgroups stratified by sex (men vs women) and weight (BMI ≥25 vs <25 kg/m2). We estimated the change in HOMA-IR by comparing each of the upper three quartiles with the lowest quartile (reference).
We conducted sensitivity analyses for those who did not have abnormal liver function and for subgroups stratified by the presence of DM. We set the cut-off values for elevated aspartate aminotransferase, alanine aminotransferase and γ-glutamyl transferase at 35, 45 and 60 IU/L, respectively. Then we set the criteria for clinically significant abnormality for liver function test as >1.5 times the cut-off value of any one of the liver enzymes.31
Results
A total of 502 individuals (132 men and 370 women) were included in the final study population. Compared with individuals excluded, those included reported no significant differences regarding any variable except a diagnosis of current hypertension (see online supplementary table S1). Table 1 shows the general characteristics of the study participants at enrolment. Overall, the means of age and HOMA-IR were 70.8 (±SD 5.3) years and 1.7 (±1.7). The geometric mean of urinary 1-OHP was 0.12 (95% CI 0.11 to 0.13) µg/g Cr. Women were more likely to be less educated and more exposed to ambient PM10 and more likely to have higher BMI, higher fasting insulin levels, lower cotinine levels and low HDL-C.
Table 1.
General characteristics (mean±SD) at enrolment (n=502)
| Characteristic | All (n=502) | Male (n=132) | Female (n=370) | p Value* |
|---|---|---|---|---|
| Number of visits | 1.7±0.5 | 1.6±0.5 | 1.7±1.5 | 0.104 |
| Age (years) | 70.8±5.3 | 71.3±4.5 | 70.6±5.5 | 0.126 |
| BMI (kg/m2) | 24.8±2.9 | 24.3±3.2 | 24.9±2.9 | 0.038 |
| Fasting insulin (µU/mL) | 7.0±6.0 | 6.1±4.5 | 7.3±6.4 | 0.018 |
| Fasting glucose (mmol/L) | 5.34±1.15 | 5.4±1.2 | 5.3±1.2 | 0.529 |
| HOMA-IR† | 1.7±1.7 | 1.5±1.3 | 1.8±1.8 | 0.059 |
| Urinary PAH, 1-OHP (µg/g Cr)‡ | 0.12 (0.11 to 0.13) | 0.11 (0.09 to 0.13) | 0.12 (0.11 to 0.14) | 0.234 |
| Urinary cotinine (µg/g Cr)‡ | 3.23 (2.69 to 3.86) | 8.79 (5.20 to 14.84) | 2.26 (1.96 to 2.60) | <0.001 |
| Ambient PM10 (µg/m3 on lag day 1) | 44.4±25.1 | 39.2±21.5 | 46.3±26.1 | 0.005 |
| Education (n (%)) | <0.001 | |||
| ≤Elementary School | 284 (56.6) | 37 (28.0) | 247 (66.8) | |
| ≤High School | 164 (32.7) | 57 (43.2) | 107 (28.9) | |
| >High School | 54 (10.8) | 38 (28.8) | 16 (4.3) | |
| Physical activity (moderate to vigorous leisure; n (%)) | 313 (62.4) | 82 (62.1) | 231 (62.4) | 1.000 |
| Current hypertension (n (%)) | 334 (66.5) | 92 (69.7) | 242 (65.4) | 0.392 |
| Current diabetes mellitus (n (%)) | 168 (33.5) | 50 (37.9) | 118 (31.9) | 0.237 |
| Low HDL-C§ (n (%)) | 199 (39.6) | 34 (25.8) | 165 (44.6) | <0.001 |
*Continuous variables: t test; categorical variables: 2×2 table (Fisher’s exact test) or 2×C table (Mantel-Haenszel χ2 test).
†HOMA index: fasting insulin (µU/mL) × fasting glucose (mmol/L)/22.5.
‡Geometric means (95% CI) were presented.
§Male: HDL-C <40 mg/dL; female: HDL-C <50 mg/dL.
BMI, body mass index; Cr, creatinine; HDL-C, high-density lipoprotein cholesterol; HOMA, homoeostatic model assessment; IR, insulin resistance; PAH, polycyclic aromatic hydrocarbons; PM10, particulate matter ≤10 μm in diameter.
Table 2 presents distributions of the three IR measures by quartiles of urinary 1-OHP concentration. Female participants with higher 1-OHP levels were more likely to have higher HOMA-IR. No significant pattern by 1-OHP levels was found in male participants.
Table 2.
Characteristics of study population by urinary 1-OHP quartile
| 1-OHP metabolite (µg/g Cr) (n=502) | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Q1(0.0011–0.075) | Q2(0.075–0.124) | Q3(0.124–0.203) | Q4(0.204–4.461) | p Trend | |||||
| Number of participants (n (%)) | |||||||||
| All | 125 | 126 | 126 | 125 | |||||
| Male | 40 | (32.0) | 37 | (29.4) | 27 | (21.4) | 28 | (22.4) | |
| Female | 85 | (68.0) | 89 | (70.6) | 99 | (78.6) | 97 | (77.6) | |
| Glucose (mmol/L) | |||||||||
| All | 5.27 | (±1.03) | 5.37 | (±1.06) | 5.19 | (±0.71) | 5.54 | (±1.60) | 0.183 |
| Male | 5.32 | (±1.13) | 5.59 | (±1.25) | 5.10 | (±0.64) | 5.54 | (±1.39) | 0.842 |
| Female | 5.25 | (±0.98) | 5.28 | (±0.96) | 5.22 | (±0.73) | 5.54 | (±1.67) | 0.131 |
| Insulin (µU/mL) | |||||||||
| All | 6.58 | (±4.97) | 6.61 | (±5.04) | 7.11 | (±5.97) | 7.59 | (±7.71) | 0.143 |
| Male | 6.36 | (±5.19) | 6.22 | (±4.59) | 6.00 | (±4.79) | 5.50 | (±3.07) | 0.436 |
| Female | 6.68 | (±4.88) | 6.78 | (±5.24) | 7.41 | (±6.24) | 8.19 | (±8.51) | 0.084 |
| HOMA-IR (score)* | |||||||||
| All | 1.60 | (±1.42) | 1.61 | (±1.28) | 1.71 | (±1.62) | 1.98 | (±2.36) | 0.068 |
| Male | 1.57 | (±1.49) | 1.57 | (±1.25) | 1.45 | (±1.38) | 1.43 | (±1.10) | 0.601 |
| Female | 1.61 | (±1.40) | 1.62 | (±1.30) | 1.79 | (±1.68) | 2.14 | (±2.60) | 0.039 |
*HOMA index: fasting insulin (µU/mL) × fasting glucose (mmol/L)/22.5.
1-OHP, 1-hydroxypyrene; Cr, creatinine; HOMA, homoeostatic model assessment; IR, insulin resistance.
Table 3 shows estimated associations between urinary 1-OHP concentration, as a continuous variable, and glucose level, insulin level and HOMA-IR score in different covariate-adjusted models. 1-OHP concentration was positively associated with HOMA-IR score in all models with borderline significance. Adjustments made in models B, C and D did not alter those associations. In the fully adjusted model (model D), a unit increase of log-transformed 1-OHP was significantly associated with a 0.16 (95% CI 0.00 to 0.31) score increase in HOMA-IR. When subtracted DM from a fully adjusted model, their association was similar but less significant (see model E). In all of the following analyses, we used the model E because development of DM is highly correlated with IR and controlling for such a DM may result in over-adjustment.
Table 3.
Regression coefficients (β (95% CIs)) for change in glucose, insulin and HOMA-IR score by a unit increase in ln-transformed urinary 1-OHP
| 1-OHP (ln)a | Glucose (mmol/L) | p Value | Insulin (µU/mL) | p Value | HOMA-IR | ||||
|---|---|---|---|---|---|---|---|---|---|
| β | (95% CIs) | β | (95% CIs) | β | (95% CIs) | p Value | |||
| Model A | 0.03 | (−0.03, 0.10) | 0.332 | 0.34 | (−0.13, 0.81) | 0.154 | 0.14 | (−0.01, 0.30) | 0.077 |
| Model B | 0.03 | (−0.03, 0.10) | 0.332 | 0.33 | (−0.13, 0.80) | 0.164 | 0.14 | (−0.02, 0.30) | 0.079 |
| Model C | 0.03 | (−0.03, 0.10) | 0.290 | 0.38 | (−0.09, 0.84) | 0.117 | 0.16 | (0.00, 0.31) | 0.052 |
| Model D | 0.04 | (−0.02, 0.10) | 0.160 | 0.36 | (−0.10, 0.82) | 0.130 | 0.16 | (0.00, 0.31) | 0.049 |
| Model E | 0.03 | (−0.03, 0.10) | 0.336 | 0.34 | (−0.12, 0.80) | 0.152 | 0.15 | (−0.01, 0.30) | 0.069 |
Model A was adjusted for age, sex and elapsed time since the first visit.
Model B: Model A+further adjusted for education and physical activity.
Model C: Model B+further adjusted for urinary cotinine and ambient PM10 concentrations.
Model D: Model C+further adjusted for obesity, diabetes, hypertension and HDL-cholesterol.
Model E: Model D—deductible adjustment for diabetes.
Change in glucose, insulin and HOMA for 1 unit ln-1-OHP increase.
1-OHP, 1-hydroxypyrene; HDL, high-density lipoprotein; HOMA, homoeostatic model assessment; IR, insulin resistance; PM10, particulate matter ≤10 μm in diameter.
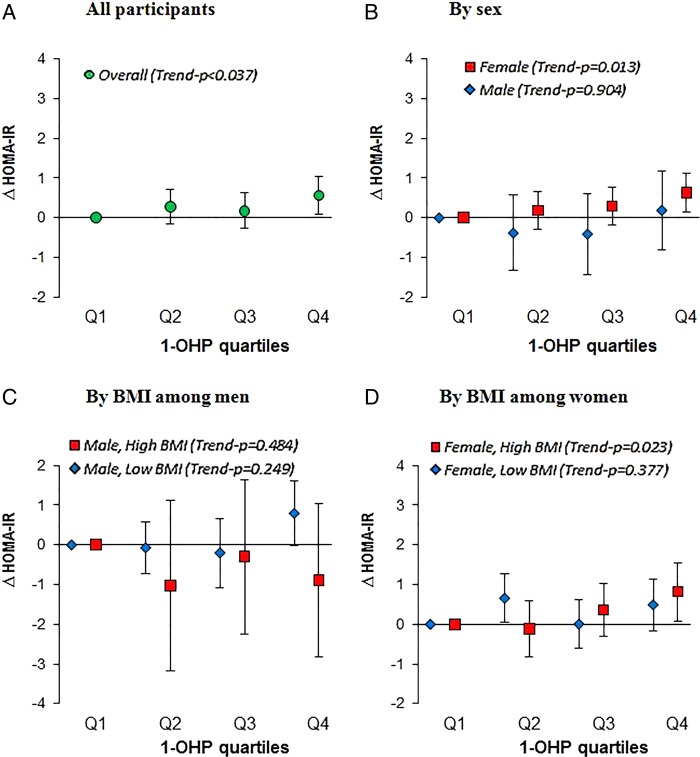
Figure 1 (online supplementary table S2) shows the estimated changes in HOMA-IR with urinary 1-OHP concentration as quartiles. Overall, there was a significant trend of 1-OHP quartiles with HOMA-IR (p=0.037). After controlling for potential confounders, individuals in the highest quartile (1-OHP >0.204 µg/g Cr) had 0.57 (95% CI 0.10 to 1.04) higher HOMA-IR scores than those in the lowest quartile. We also evaluated associations of HOMA-IR with urinary 1-OHP as quartiles in four groups stratified by a combination of sex and overweight (BMI ≥25 kg/m2 or not). In particular, overweight women presented a significant trend of 1-OHP with HOMA-IR (p=0.023), whereas no trends were observed in men and non-overweight women.
Figure 1.
Fully-adjusted changes (95% CI) in HOMA-IR score in relation to 1-OHP quartiles (quartile 1 is reference) in (A) overall participants, (B) different sex, and in subgroups stratified by overweight in (C) men and (D) women. Values were adjusted for age, sex, elapsed time, education, physical activities, BMI, hypertension, HDL-cholesterol, cotinine, and ambient PM10. Overweight was defined as BMI≥25kg/m2. 1-OHP quartiles cut-off points: 0.0753, 0.1237, 0.203 µg/g Cr” BMI, body mass index; 1-OHP, 1-hydroxypyrene; HOMA-IR, homoeostatic model assessment of insulin resistance)
Owing to a possibility that poor functional enzymes in the liver may increase IR and delay metabolism of PAHs, we evaluated associations of HOMA-IR with urinary 1-OHP in the study population that excluded participants who had abnormal liver function (see online supplementary table S3). However, this exclusion did not alter our findings. We also conducted subgroup analyses stratified by the presence of DM and found an enhanced effect of 1-OHP on HOMA-IR in individuals with DM, whereas we did not observe the significance in the relations in those without DM (see online supplementary table S4).
Discussion
In this panel study of urban elderly adults in Korea, environmental exposure to PAHs was associated with increased IR, even after adjusting for sociodemographic factors and potential risk factors including other environmental exposures and metabolic conditions. In addition, the present data suggest that the influence of PAHs exposure may be limited to overweight women.
To the best of our knowledge, this is the first investigation to examine dose–response associations between IR and PAHs. We found that log-transformed concentrations of urinary 1-OHP reflecting PAHs were positively correlated with HOMA-IR scores with borderline significance. Categorical analysis of 1-OHP modelled as quartiles yields a significant dose–dependent trend with elevated HOMA-IR. Several epidemiological studies suggested that elevated oxidative stress is related to increased PAHs exposure.8 32 A recent study of 1333 male coke oven workers monitored the levels of environmental PAHs and oxidative biomarkers and indicated significant dose-related increases in oxidative damage to DNA and lipids with urinary metabolites (1-OHP) and plasma adducts (BPDE-Alb) of PAHs.32 Another study of 120 Korean schoolchildren reported a significant association of urinary 1-OHP with an oxidative stress biomarker using urinary malondialdehyde (MDA).8 These reports could provide useful biological interpretation of our finding an association between urinary 1-OHP and IR. In fact, we examined associations between urinary 1-OHP and MDA levels and were able to confirm a significant relationship (online supplementary table S5) consistent with those from previous studies. From the observation that 1-OHP is positively associated with oxidative stress and that oxidative stress may be involved in IR pathogenesis,33 the associations of PAHs with IR may be explained by a pathway in which oxidative stress is increased by PAHs exposure.
Another possible explanation for the observed associations of PAHs with IR is that PAHs are potentially related to oestrogenic activity,34 35 a relationship that could mediate the effect of PAHs in development of IR. In our analysis, when modelled subgroups stratified by sex, a significant dose–dependent association for PAHs with IR was observed in women only, but not in men. Previous research has suggested that the correlation of fasting glucose and insulin is greater with the oestradiol/testosterone ratio than with oestradiol or testosterone alone.36 Since the oestradiol/testosterone ratio is higher in women, even old women, than in men, these research suggestions support our finding that women are susceptible to the influence of oestrogen-like PAHs on IR.
We also examined subgroups further stratified by weight. Overweight women were observed to have a significant association of HOMA-IR with PAHs exposure, whereas men and non-overweight women had no significant associations. The weight-dependent effects are consistent with previous studies indicating obesity as an effect modifier on the associations of air pollution and metabolic diseases.18 19 It is still unclear how weight modifies the relationship between PAHs exposure and IR in women. Overweight may cause metabolic disturbance in itself via oxidative stress/oestrogenic activity,18 19 which may potentially act in an additive manner with PAHs exposure, particularly in sensitive women.
Casals-Casas and Desvergne suggest a ‘metabolic disruptor hypothesis’, that is, the potential involvement of environmental chemicals in metabolic disease aetiology.37 Since the worldwide prevalence of diabetes and metabolic syndrome has been increasing with an ageing population, identifying non-traditional risk factors for metabolic diseases could provide a better strategy than traditionally known factors, and many researchers emphasise the need to understand the particular role of environmental chemicals in the development of IR.2 3 Owing to this context, our study focused on PAHs, a ubiquitous environmental chemical with potential risk, and our findings suggest that exposure to PAHs contributes to the development of IR, after adjusting for traditional risk factors.
The current study suggests that even low-level exposure to environmental PAHs observed in an urban population is associated with IR and so supports efforts to reduce current levels of environmental PAHs. Compared with participants in the lowest quartile, those in the highest quartile of urinary 1-OHP (0.204–4.461 µg/g Cr) are at risk for significantly higher IR. Unfortunately, there is no standard for acceptable concentrations of urinary 1-OHP resulting from PAHs exposure, although the US Environmental Protection Agency (EPA) has designated 32 PAH compounds as priority pollutants.38 A recent report using data from the Korean National Environmental Health Survey, a representative sample of Korean adults aimed at estimating nationwide environmental exposures, showed that the national geometric mean of urinary 1-OHP was estimated to be 0.15 (95% CI 0.05 to 0.25) µg/g Cr.39 Therefore, our findings show that a considerable number of Korean adults may be exposed to PAHs in ranges of risk. The primary sources of PAHs producing high 1-OHP concentrations are motor vehicles, generation of electricity and residential heating of outdoor air; ETS and cooking of indoor air; and burned food of dietary intake in the general population.9 10 40
This study also examined associations between IR and other PAHs biomarker (urinary 2-naphthol) but failed to observe a meaningful relationship (data not shown), although 2-naphthol is known to be highly correlated with exposure to PAHs specific to tobacco smoking.41 Our observations of 1-OHP but not of 2-naphthol associations suggest that PAHs responsible for IR may not have their source in tobacco smoke but from other exposures (eg, air pollution from motor vehicles and heating, grilled foods). Further investigation in a large general population is required, however, to elucidate the source-dependent role of PAHs for IR.
The main strengths of this study include (A) the adjustment for potential confounders of traditional risk factors including environmental exposures as well as metabolic conditions, which enabled us to observe independent effects of PAHs exposure in IR; (B) the repeated measures design of the panel study, which enabled the capture of between-subjects and within-subjects variations and (C) the use of short-term biomarkers from each participant for PAHs exposure and IR outcome that accounted for variation in individual daily levels.
Several limitations must be considered in interpreting the results. First, because of the nature of cross-sectional measures, our results may not necessarily indicate a causative role for PAHs exposure in elevating IR. Second, given that the number of participants is not large enough to observe a statistically reliable finding; our finding with borderline significance would be improved when the sample size is bigger. Third, this study used HOMA-IR score, a surrogate marker to evaluate IR. One might argue, therefore, that HOMA modelling may be less accurate than direct measures using the gold standard method (ie, the glucose clamp technique),42 and HOMA-IR response may be altered on patients with DM treated with insulin.43 In fact, we observed an enhanced effect of 1-OHP on IR in DM-diagnosed participants while we did not find a significant relationship in the participants excluding patients with DM. However, a simpler tool, HOMA-IR, may be more appropriate for use in large-scale epidemiological studies43 44 such as this study with repeated measures within subjects, although the gold standard is a good tool for intensive physiological studies. The HOMA-IR score is strongly correlated with the gold standard-IR and has proved to be a robust epidemiological tool for IR assessment.45 In addition, most of the study subjects with DM may be managed by oral antihyperglycaemic agents or lifestyle change (ie, diet, exercise) without insulin treatment (see online supplementary text). Several studies also support that HOMA-IR is a useful method to evaluate insulin sensitivity even in patients with DM treated with insulin.46 47 Nevertheless, our observation on enhanced effect in patients with DM still needs to be interpreted carefully. Fourth, we cannot rule out selection bias in finding the association between PAHs and IR, which may be different for participants included in our analysis and those excluded (for absence of information). The included participants were more likely to have hypertension than those excluded (see online supplementary table S1). In sensitivity analysis for subgroups by diagnosis of hypertension, a stronger influence of 1-OHP on IR was found in hypertension-diagnosed participants than in non-diagnosed participants, although the difference was only marginally significant (data not shown). We expect, therefore, that associations in the excluded participants and/or in a general population may be smaller than our observed effect.
In conclusion, this study supports the contention that exposure to environmental PAHs currently observed in the Korean urban elderly is a risk factor for development of IR, independent of traditionally known risk factors, and that its risk may be limited to women, particularly overweight women, suggesting sex-dependent and body weight-dependent effects. Given the increasing prevalence of metabolic disease, efforts in reducing exposure to environmental PAHs is important for improving public health.
What is already known on this subject.
Recent studies suggest the potential involvement of environmental chemicals in insulin resistance, which plays a core role in the development of metabolic diseases.
Polycyclic aromatic hydrocarbons (PAHs) are ubiquitous environmental chemicals of outdoor and indoor air pollution, with a potential risk of elevated oxidative stress.
To the best of our knowledge, there has been no investigation to examine dose–response associations between insulin resistance and PAHs.
What this study adds.
The exposure to environmental PAHs currently observed in the Korean urban elderly is associated with increased insulin resistance.
The association is limited to overweight women, suggesting sex-dependent and body weight-dependent effects.
Given the prevalence of metabolic disease, efforts in reducing exposure to environmental PAHs is important for improving public health.
Supplementary Material
Acknowledgments
KM Kim, GH Cho and YM Choi were responsible for interviewing participants and cleaning the collected data.
Footnotes
Contributors: Y-HC and Y-CH designed the study; JHK acquired the data; Y-HC analysed data and wrote the manuscript; Y-CH critically revised the manuscript.
Funding: This work was supported primarily by the Susceptible Population Research Program (0411-20080013, 0411-20090007, 0411-20100016) from the Korea Ministry of Environment, and the Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Korea Ministry of Education (2012018364 and 2013R1A6A3A04059556).
Competing interests: None.
Patient consent: Obtained.
Ethics approval: This study was approved by the Institutional Review Board of Seoul National University Hospital/College of Medicine (IRB no. H-0804-045-241).
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Cohen AJ, Ross Anderson H, Ostro B, et al. . The global burden of disease due to outdoor air pollution. J Toxicol Environ Health A 2005;68:1301–7. 10.1080/15287390590936166 [DOI] [PubMed] [Google Scholar]
- 2.Hectors TL, Vanparys C, Van Gaal LF, et al. . Insulin resistance and environmental pollutants: experimental evidence and future perspectives. Environ Health Perspect 2013;121:1273–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Thayer KA, Heindel JJ, Bucher JR, et al. . Role of environmental chemicals in diabetes and obesity: a National Toxicology Program workshop review. Environ Health Perspect 2012;120:779–89. 10.1289/ehp.1104597 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Probst-Hensch NM. Chronic age-related diseases share risk factors: do they share pathophysiological mechanisms and why does that matter? Swiss Med Wkly 2010;1:13072. [DOI] [PubMed] [Google Scholar]
- 5.Stahlhut RW, van Wijngaarden E, Dye TD, et al. . Concentrations of urinary phthalate metabolites are associated with increased waist circumference and insulin resistance in adult U.S. males. Environ Health Perspect 2007;115:876–82. 10.1289/ehp.9882 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gunji T, Matsuhashi N, Sato H, et al. . Alcohol consumption is inversely correlated with insulin resistance, independent of metabolic syndrome factors and fatty liver diseases. J Clin Gastroenterol 2011;45:808–13. 10.1097/MCG.0b013e318223bd53 [DOI] [PubMed] [Google Scholar]
- 7.IARC. Polynuclear aromatic compounds. Part l. Chemical, environmental, and experimental data. International Agency for Research on Cancer, 1983. [PubMed] [Google Scholar]
- 8.Bae S, Pan X-C, Kim S-Y, et al. . Exposures to particulate matter and polycyclic aromatic hydrocarbons and oxidative stress in schoolchildren. Environ Health Perspect 2009;118:579–83. 10.1289/ehp.0901077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lewtas J. Human exposure to complex mixtures of air pollutants. Toxicol Lett 1994;72:163–9. 10.1016/0378-4274(94)90024-8 [DOI] [PubMed] [Google Scholar]
- 10.Nethery E, Wheeler AJ, Fisher M, et al. . Urinary polycyclic aromatic hydrocarbons as a biomarker of exposure to PAHs in air: a pilot study among pregnant women. J Expo Sci Environ Epidemiol 2012;22:70–81. 10.1038/jes.2011.32 [DOI] [PubMed] [Google Scholar]
- 11.Tamamura S, Sato T, Ota Y, et al. . Long-range transport of polycyclic aromatic hydrocarbons (PAHs) from the eastern Asian continent to Kanazawa, Japan with Asian dust. Atmospheric Environ 2007;41:2580–93. 10.1016/j.atmosenv.2006.11.021 [DOI] [Google Scholar]
- 12.Kong S, Ding X, Bai Z, et al. . A seasonal study of polycyclic aromatic hydrocarbons in PM(2.5) and PM(2.5–10) in five typical cities of Liaoning Province, China. J Hazard Mater 2010;183:70–80. 10.1016/j.jhazmat.2010.06.107 [DOI] [PubMed] [Google Scholar]
- 13.Yang D, Qi S, Devi N, et al. . Characterization of polycyclic aromatic hydrocarbons in PM2.5 and PM10 in Tanggu District, Tianjin Binhai New Area, China. Front Earth Sci 2012;6:324–30. 10.1007/s11707-012-0326-y [DOI] [Google Scholar]
- 14.Kazuichi H, Ning T, Takayuki K, et al. . Atmospheric behaviors of polycyclic aromatic hydrocarbons and nitropolycyclic aromatic hydrocarbons in East Asia. Asian J Atmospheric Environ 2007;1:19–27. 10.5572/ajae.2007.1.1.019 [DOI] [Google Scholar]
- 15.APCSC. The burden of overweight and obesity in the Asia-Pacific region. Obes Rev 2007;8:191–6. 10.1111/j.1467-789X.2006.00292.x [DOI] [PubMed] [Google Scholar]
- 16.KCDC. 2008 KNHANES statistics. Seoul: Korea Centers for Disease Control & Prevention, Korea Ministry for Health, Welfare, and Family Affairs, 2009. [Google Scholar]
- 17.Uppstad H, Osnes GH, Cole KJ, et al. . Sex differences in susceptibility to PAHs is an intrinsic property of human lung adenocarcinoma cells. Lung Cancer 2011;71:264–70. 10.1016/j.lungcan.2010.09.006 [DOI] [PubMed] [Google Scholar]
- 18.Baja ES, Schwartz JD, Wellenius GA, et al. . Traffic-related air pollution and QT interval: modification by diabetes, obesity, and oxidative stress gene polymorphisms in the normative aging study. Environ Health Perspect 2010;118:840–6. 10.1289/ehp.0901396 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Park SK, Auchincloss AH, O'Neill MS, et al. . Particulate air pollution, metabolic syndrome, and heart rate variability: the multi-ethnic study of atherosclerosis (MESA). Environ Health Perspect 2010;118:1406–11. 10.1289/ehp.0901778 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jongeneelen FJ. Methods for routine biological monitoring of carcinogenic PAH-mixtures. Sci Total Environ 1997;199:141–9. 10.1016/S0048-9697(97)00064-8 [DOI] [PubMed] [Google Scholar]
- 21.Li Z, Romanoff L, Bartell S, et al. . Excretion profiles and half-lives of ten urinary polycyclic aromatic hydrocarbon metabolites after dietary exposure. Chem Res Toxicol 2012;25:1452–61. 10.1021/tx300108e [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jongeneelen F, Anzion R, Henderson P. Determination of hydroxylated metabolites of polycyclic aromatic hydrocarbons in urine. J Chromatogr 1987;413:227–32. 10.1016/0378-4347(87)80230-X [DOI] [PubMed] [Google Scholar]
- 23.Barr DB, Wilder LC, Caudill SP, et al. . Urinary creatinine concentrations in the U.S. population: implications for urinary biologic monitoring measurements. Environ Health Perspect 2005;113:192–200. 10.1289/ehp.7337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Matthews DR, Hosker JP, Rudenski AS, et al. . Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 1985;28:412–19. 10.1007/BF00280883 [DOI] [PubMed] [Google Scholar]
- 25.Choi YH, Kim JH, Lee BE, et al. . Urinary benzene metabolite and insulin resistance in elderly adults. Sci Total Environ 2014;482–483:260–8. 10.1016/j.scitotenv.2014.02.121 [DOI] [PubMed] [Google Scholar]
- 26.James-Todd T, Stahlhut R, Meeker JD, et al. . Urinary phthalate metabolite concentrations and diabetes among women in the National Health and Nutrition Examination Survey (NHANES) 2001–2008. Environ Health Perspect 2012;120:1307–13. 10.1289/ehp.1104717 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kim JH, Choi YH, Bae S, et al. . eNOS gene polymorphisms modify the association of PM(10) with oxidative stress. Toxicol Lett 2012;214:263–7. 10.1016/j.toxlet.2012.09.006 [DOI] [PubMed] [Google Scholar]
- 28.Rubin DB. Inference and missing data. Biometrika 1976;63:581–92. 10.1093/biomet/63.3.581 [DOI] [Google Scholar]
- 29.Robins JM, Rotnitzky A, Zhao LP. Analysis of semiparametric regression models for repeated outcomes in the presence of missing data. J Am Stat Assoc 1995;90:106–21. 10.1080/01621459.1995.10476493 [DOI] [Google Scholar]
- 30.McCracken J, Baccarelli A, Hoxha M, et al. . Annual ambient black carbon associated with shorter telomeres in elderly men: veterans affairs normative aging study. Environ Health Perspect 2010;118:1564–70. 10.1289/ehp.0901831 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lee MR, Park H, Bae S, et al. . Urinary bisphenol a concentrations are associated with abnormal liver function in the elderly: a repeated panel study. J Epidemiol Community Health 2014;68:312–17. 10.1136/jech-2013-202548 [DOI] [PubMed] [Google Scholar]
- 32.Kuang D, Zhang W, Deng Q, et al. . Dose-response relationships of polycyclic aromatic hydrocarbons exposure and oxidative damage to DNA and lipid in coke oven workers. Environ Sci Technol 2013;47:7446–56. 10.1021/es304492j [DOI] [PubMed] [Google Scholar]
- 33.Houstis N, Rosen ED, Lander ES. Reactive oxygen species have a causal role in multiple forms of insulin resistance. Nature 2006;440:944–8. 10.1038/nature04634 [DOI] [PubMed] [Google Scholar]
- 34.Verma Y, Rana SV. Endocrinal toxicity of industrial solvents—a mini review. Indian J Exp Biol 2009;47:537–49. [PubMed] [Google Scholar]
- 35.Kummer V, Maskova J, Zraly Z, et al. . Estrogenic activity of environmental polycyclic aromatic hydrocarbons in uterus of immature Wistar rats. Toxicol Lett 2008;180:212–21. 10.1016/j.toxlet.2008.06.862 [DOI] [PubMed] [Google Scholar]
- 36.Phillips GB, Jing T, Heymsfield SB. Relationships in men of sex hormones, insulin, adiposity, and risk factors for myocardial infarction. Metabolism 2003;52:784–90. 10.1016/S0026-0495(03)00072-6 [DOI] [PubMed] [Google Scholar]
- 37.Casals-Casas C, Desvergne B. Endocrine disruptors: from endocrine to metabolic disruption. Annu Rev Physiol 2011;73:135–62. 10.1146/annurev-physiol-012110-142200 [DOI] [PubMed] [Google Scholar]
- 38.EPA. Priority pollutants. Washington DC: Agency UEP, 2012. [Google Scholar]
- 39.NIER. Annual Report on Korean National Environmental Health Survey—The First Stage (2009∼2011) 3rd Year. Incheon, Korea: National Institute of Environmental Research, 2011. [Google Scholar]
- 40.Viau C, Diakité A, Ruzgyté A, et al. . Is 1-hydroxypyrene a reliable bioindicator of measured dietary polycyclic aromatic hydrocarbon under normal conditions? J Chromatogr B Analyt Technol Biomed Life Sci 2002;778: 165–77. 10.1016/S0378-4347(01)00465-0 [DOI] [PubMed] [Google Scholar]
- 41.Yang M, Koga M, Katoh T, et al. . A study for the proper application of urinary naphthols, new biomarkers for airborne polycyclic aromatic hydrocarbons. Arch Environ Contam Toxicol 1999;36:99–108. 10.1007/s002449900447 [DOI] [PubMed] [Google Scholar]
- 42.DeFronzo RA, Tobin JD, Andres R. Glucose clamp technique: a method for quantifying insulin secretion and resistance. Am J Physiol 1979;237:E214–23. [DOI] [PubMed] [Google Scholar]
- 43.Wallace TM, Levy JC, Matthews DR. Use and abuse of HOMA modeling. Diabetes Care 2004;27:1487–95. 10.2337/diacare.27.6.1487 [DOI] [PubMed] [Google Scholar]
- 44.Singh B, Saxena A. Surrogate markers of insulin resistance: a review. World J Diabetes 2010;1:36–47. 10.4239/wjd.v1.i2.36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Muniyappa R, Lee S, Chen H, et al. . Current approaches for assessing insulin sensitivity and resistance in vivo: advantages, limitations, and appropriate usage. Am J Physiol Endocrinol Metab 2008;294:E15–26. 10.1152/ajpendo.00645.2007 [DOI] [PubMed] [Google Scholar]
- 46.Katsuki A, Sumida Y, Gabazza EC, et al. . Homeostasis model assessment is a reliable indicator of insulin resistance during follow-up of patients with type 2 diabetes. Diabetes Care 2001;24:362–5. 10.2337/diacare.24.2.362 [DOI] [PubMed] [Google Scholar]
- 47.Okita K, Iwahashi H, Kozawa J, et al. . Homeostasis model assessment of insulin resistance for evaluating insulin sensitivity in patients with type 2 diabetes on insulin therapy. Endocr J 2013;60:283–90. 10.1507/endocrj.EJ12-0320 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.