Abstract
Background:
At present, the treatment options available to delay the onset or slow down the progression of Alzheimer's disease (AD) are not effective. Recent studies have suggested that diet and lifestyle factors may represent protective strategies to minimize the risk of developing AD. Date palm fruits are a good source of dietary fiber and are rich in total phenolics and natural antioxidants, such as anthocyanins, ferulic acid, protocatechuic acid and caffeic acid. These polyphenolic compounds have been shown to be neuroprotective in different model systems.
Objective:
We investigated whether dietary supplementation with 2% and 4% date palm fruits (grown in Oman) could reduce cognitive and behavioral deficits in a transgenic mouse model for AD (amyloid precursor protein [APPsw]/Tg2576).
Materials and Methods:
The experimental groups of APP-transgenic mice from the age of 4 months were fed custom-mix diets (pellets) containing 2% and 4% date fruits. We assessed spatial memory and learning ability, psychomotor coordination, and anxiety-related behavior in all the animals at the age of 4 months and after 14 months of treatment using the Morris water maze test, rota-rod test, elevated plus maze test, and open-field test. We have also analyzed the levels of amyloid beta (Aβ) protein (1–40 and 1–42) in plasma of control and experimental animals.
Results:
Standard diet-fed Tg mice showed significant memory deficits, increased anxiety-related behavior, and severe impairment in spatial learning ability, position discrimination learning ability and motor coordination when compared to wild-type on the same diet and Tg mice fed 2% and 4% date supplementation at the age of 18 months. The levels of both Aβ proteins were significantly lowered in date fruits supplemented groups than the Tg mice without the diet supplement. The neuroprotective effect offered by 4% date fruits diet to AD mice is higher than 2% date fruits diet.
Conclusions:
Our results suggest that date fruits dietary supplementation may have beneficial effects in lowering the risk, delaying the onset or slowing down the progression of AD.
Keywords: Alzheimer's disease, amyloid beta, behavior study, dates, Oman, Tg2576 mice, water maze and rota-rod test
INTRODUCTION
Alzheimer's disease (AD) is characterized by a progressive loss of cognitive function, occurring parallel to granulovacuolar degeneration, the deposition of β-amyloid amyloid beta (Aβ) in extracellular plaques and in the cerebrovasculature, and the formation of intracellular neurofibrillary tangles containing hyperphosphorylated tau protein in neurons. AD is the third leading cause of death after cardiovascular disease and cancer and affects more than 25 million individuals world-wide. At present, the number of patients suffering from AD is rising continually world-wide and represents one of the biggest challenges for most societies throughout the world.[1] Although the etiology of AD remains unclear, increased oxidative stress have been reported to be an important underlying cause of neurodegeneration in AD.[2] Brain structures supporting memory are uniquely sensitive to oxidative stress due to their high demand for oxygen.[3,4] Chronic oxidative stress increases with age, and ageing is one of the major risk factor for AD.
Numerous studies have suggested that a diet rich in fruits and vegetables may represent an important alternative therapeutic options by improving age-related memory decline, and cognitive dysfunction observed in AD.[5,6] These beneficial “antiageing” effects are associated with their high antioxidant capacity. Antioxidants such as Vitamin E could reduce β-amyloid-induced neurotoxicity in cultured hippocampal neurons.[7] And can slow the progression of AD significantly.[8] Similarly, Ginkgo biloba extract improves cognitive function in AD patients.[9] Walnut extracts have been reported to offer benefits to Aβ induced cytotoxicity in PC12 cells[10] and pomegranate could improve memory in AD transgenic mice.[11]
Fruits of the date palm (Phoenix dactylifera L. Arecaceae) are commonly consumed in several parts of the world and represent a vital component of the diet and a staple food in most of the Arabian countries. The date fruit is listed in folk remedies for the treatment of various diseases including cancer[12] and has been demonstrated to show immunomodulatory activity.[13] Moreover, studies have also shown the antibacterial activity[14] antihyperlipidemic activity,[15] hepatoprotective activity,[16] nephroprotective activity,[17] anticancer activity,[18] antifungal[19,20] properties and antimutagenic activity[21] of date palm fruits.
Dates are a good source of energy, vitamins, and important elements such as phosphorus, iron, potassium, and a significant amount of calcium.[22] Besides nutritional value, date fruits are rich in phenolic compounds possessing free radical scavenging and antioxidant activity. Several studies have reported such activity of date fruits cultivated in Algeria,[23] Kuwait,[21] Oman,[24,25] Iran,[26] Bahrain[27] and the USA.[23] These studies show that fresh and dried dates varied quantitatively and qualitatively in their phenolic acids content. Studies with three varieties of Omani dates have shown the presence of both free (protocatechuic acid, vanillic acid, syringic acid, and ferulic acid) and bound phenolic acids (gallic acid [GA], protocatechuic acid, p-hydroxybenzoic acid, vanillic acid, caffeic acid, syringic acid, p-coumaric acid, ferulic acid, and coumaric acid).[25] The potent antioxidant activity of dates might be due to its phenolic compounds and flavonoid constituents.[23,28] Date varieties from different regions of Oman had different levels and patterns of phenolic acids. Nine phenolic acids (gallic, protocatechuic, p-hydroxybenzoic, vanillic, caffeic, syringic, p-coumaric, ferulic, and o-coumaric acid) have been tentatively identified. It has been reported that ferulic acid is the one of the major phenolic acids found in high amounts in all date varieties of Oman.[29] We have recently reported that the date palm fruits growing in Oman can inhibit the Aβ fibrils formation under in vitro conditions.[30] Consequently, we asked whether dietary supplementation with dates could improve AD-like cognitive and behavioral deficits in the amyloid precursor protein (APPsw)/Tg2576 Transgenic mice model of AD.
MATERIALS AND METHODS
Collection and diet preparation
The plant was identified by the Crop Science Department people from Sultan Qaboos University, Oman and fresh date fruits khalas variety were collected from Al-Jabal Al-Akdhar farms, Oman. The flesh were isolated manually and rinsed with water and dried for 18 h in a drying cabinet at 40°C. The dried fruits were crushed and extracted with acetone (1:1 ratio, weight to volume) under agitation at room temperature. After 48 h, the extract was then filtered, and the filtrate was evaporated to dryness in a drying cabinet at 40°C. After that, the samples were ground into a fine powder using a coffee grinder.
Total phenolics of dates were measured by the modified Folin-Ciocalteu assay as previously described.[31] Briefly, 250μL Folin-Ciocalteu reagent was mixed with 10μL of date juice extract. After a short incubation of 5 min, 750μL of sodium carbonate (1.9 M) was added and incubated for 2/h at 25°C. The absorbance at 765nm was measured and compared with that from GA standards. The concentration of phenolics in date juice extracts was expressed as gallic acid equivalents (GAE). The total phenolic content was found to be 231.2 ± 0.50 (mg of GAE/100 g). The date extract was sent to Research Diet Inc. (NJ, USA) for preparation of the 2% and 4% date with standard chow.
Animals
Transgenic mice expressing a form of the APP that leads to early onset familial AD (APPsw/Tg2576) were purchased from Taconic form, NY, USA. Twenty four female mice were required for this study, 18 mice were transgenic and 6 mice were wild-type (nontransgenic) control littermates of the APPsw. These animals were free from pathogens and viruses. Female APPsw/Tg2576 and wild-type mice were housed in individual cages under a 12:12 h light-dark cycle (7 am light on) with ad libitum access to standard chow (Harlan Teklad Laboratory Diets, Wisconsin, USA), and the 2% and 4% date diet (D04112303 Research Diets Inc., New Jersey, USA) and tap water. The study was approved by the Animal Care and Use Committee of the Sultan Qaboos University, Oman (SQU/AEC/2010–11/3).
Behavioral analysis
To find out the effect of dates, we have chosen two different doses (2% and 4%) for this study. The experimental period commenced from the age of 4 months. The animals were divided into four groups: Group 1: Wild type (nontransgenic) control of the APPsw mice fed with standard chow diet, Group 2: APPsw/Tg2576 mice also fed with standard chow diet, Group 3: APPsw/Tg2576 mice fed with 2% date fruit diet, and Group 4: APPsw/Tg2576 mice fed with 4% date fruit diet. The cognitive and behavioral function were assessed in these experimental and control mice at the age of 4 months, and after 14 months of dietary supplementation using the Morris water maze test (for spatial memory and learning ability), T-maze test (for position discrimination learning ability), rota-rod test (for psychomotor coordination), elevated plus maze (EPM) test (for anxiety-related behavior) and open-field test to determine the potential effect of a diet rich in dates, on memory, anxiety and learning skills.
Open field test
Locomotors activity was measured in the open-field of a white Plexiglas chamber (45 cm × 45 cm × 40 cm). Illumination in the chamber was adjusted to 70 lux. The mice were placed in the same environment as that of the chamber 30 min before exposure to the open-field. Each mouse was placed individually at the center of the open-field, and locomotion was recorded for 60 min. Horizontal locomotors activity was judged according to the distance the animal moved. The inner 30% of the open-field was defined as the center in the current study.
Rota-rod test
Motor coordination and motor learning were measured by means of rota-rod tests. The rota-rod consisted of a rotating cylinder (diameter 4.5 cm, Ugo Basil, Italy) with a speed controller. The mice were placed on top of the cylinder with the coarse surface for a firm grip. The rota-rod was accelerated from 5 to 20 rpm and maintained at the indicated speed for 5 min, and then the mice were subjected to tests at successively higher speeds. A cutoff time of 5 min and an inter-trial interval of 60 min were used. The time spent on the rod without falling down was recorded.
Morris water maze test
The water maze consisted of a metal pool (170 cm in diameter × 58 cm tall, Ugo Basil, Italy) filled with tap water (25°C, 40 cm deep) divided into four quadrants. A removable escape platform below the water level and covered with a nontoxic milk powder was placed in the center of one quadrant. The pool was divided into four quadrants (northeast, northwest, southeast, and southwest) by two imaginary lines crossing the center of the pool. For each animal, the location of the invisible platform was placed at the center of one quadrant and remained there throughout training. The mice must memorize the platform location in relation to various environmental cues, and there was nothing directly indicative of the location of the escape platform in and out of the pool. Therefore, the placement of the water tank and platform were the same in all acquisition trials. Each mouse was gently placed in the water facing the wall of the pool from one of the four starting points (north, east, south, or west) along the perimeter of the pool, and the animal was allowed to swim until it found and climbed onto the platform. During the training session, the mouse was gently placed on the platform by an experienced investigator when it could not reach the platform in 60 s. In either case, the subject was left on the platform for 15 s and removed from the pool. The time for animals to climb onto the hidden platform was recorded as escape latency or acquisition time. In order to determine the capability of the animals to retrieve and retain information, the platform was removed 24 h later, and the mouse was released into the quadrant diagonally opposite to that which contained the platform. Time spent in the region that previously contained the platform was recorded as retention time. In each trial, the animal was quickly dried with a towel before being returned to the cage. All tests were carried out after 14 months following 2% and 4% date fruit dietary supplementation.
Elevated plus maze test
The EPM was made of black Plexiglas. The apparatus consisted of four arms (30 cm × 7 cm) elevated 50 cm from the floor and placed at right angles to each other. Two of the arms had 20 cm high walls (enclosed arms), whereas two had no walls (open arms). The illumination at the center was adjusted to 40 lux. For the test, the mouse was initially placed at the center of the platform and left to explore the arms for 5 min. The number of entries closed arms and the time spent in open arms was recorded. Entry into arm was scored as an event if the animal placed all four paws in the arm.
Left-right discrimination learning
The T-maze (length of stem 64 cm, length of arms 30 cm, width 12 cm, and height of walls 16 cm) was made of clear plexiglass and filled with water (23 ± 1°C) at a height of 12 cm. A platform (11 cm × 11 cm) was submerged 1 cm below at the end of the target arm. During the first two trials, platforms were placed on both arms to test turning preferences. Afterwards, only the least chosen arm, if any, was reinforced, with approximately the same number of mice being reinforced on either side. APPsw and control mice were placed in the stem of the T-maze and swam either to the left or the right until finding the submerged platform up to a maximum of 60 s. The animals were gently guided to the platform if they failed to find it. After reaching the platform, the mice remained on it for 20 s and then placed back in the maze for up to a maximum of 48 trials, except for a 10 min rest period after each 10-trial block. A mouse was considered to have achieved the criterion after 5 consecutive errorless trials. The reversal learning phase was then conducted 2 days later, with the protocol repeated except that the mice were trained to find the escape platform on the opposite side. Escape latencies and errors were recorded.
Sample collection
The day after completion of the behavioral tests, blood samples were collected drawn from the orbital sinus in 2 ml Eppendorf tubes. Collected blood samples were centrifuged at 4000 rpm for 15 min at 4°C, and the plasma were collected and stored at −80°C until measurement.
Determination of plasma amyloid beta (1–40) and amyloid beta (1–42)
At the time of sacrifice, 200–500 μL of blood was collected and centrifuged, at 5000 rpm for 15 min at 40°C, to separate out plasma. Plasma samples, stored at 80°C with a preservative solution, were analyzed in duplicate. Human Aβ1–40 and Aβ1–42 plasma levels were determined through two specific Sandwich ELISA kits, AB test 40 and AB test 42 (Araclon Biotech Ltd. Zaragoza, Spain) following the supplier's instructions. Aβ1–40 and Aβ1–42 levels were calculated from a standard curve developed with optical density at 450 nm versus serial dilutions of known concentration.
Statistical analysis
Data analysis was performed by using the Graph Pad Prism software. All values are mean ± standard deviation one-way analysis of variance and post-hoc Tukey's multiple comparison tests were used to determine statistical significance between treatment groups. Differences between treatment groups were considered as significant if P < 0.05.
RESULTS
Impaired coordinated motor movement were improved in aged amyloid precursor protein sw/Tg2576 mice exposed to a date rich diet
In this study, we characterized the behavioral properties of the APPsw/Tg2576 and wild-type control mice, with and without date fruit supplementation. In the open-field test, the locomotors activity of APPsw/Tg2576 mice was significantly impaired compared to the wild-type control mice after 14 months of supplementation of the normal diet [Figure 1a and b]. Similarly, the motor coordination of the APPsw/Tg2576 mice on the rota-rod was significantly lower compared to wild-type controls at 18 months of age [Figure 1]. Supplementation with 2% and 4% dates to APPsw/Tg2576 mice restored their locomotors activity in both the open-field and rota-rod test after 14 months of supplementation [Figure 1c and d].
Figure 1.
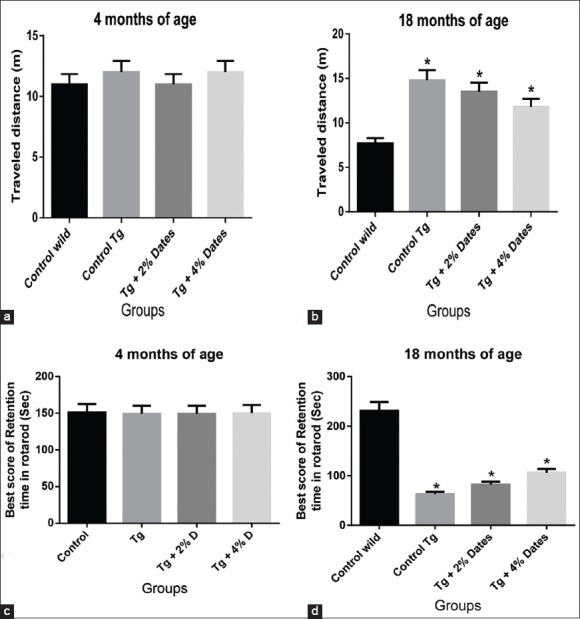
Impaired coordinated motor movement of amyloid precursor protein (APPsw)/Tg2576 mice was attenuated by 2% and 4% date supplementation. (a). Locomotors activity in the open-field test of APPsw/Tg2576 mice age of 4 months. (b). Locomotors activity in the open-field test of supplementation of dates diet for 14 months in APPsw/Tg2576 mice. (c). Motor coordination on the rota-rod of APPsw/Tg 2576 mice age of 4 months. (d). Motor coordination on the rota-rod of APPsw/Tg 2576 mice after 14 months of date diet supplementation. Data are presented as mean ± standard deviation, n = 6/group. *P<0.05 compared to wild-type mice
2% and 4% date rich diet improved spatial memory in aged amyloid precursor protein sw/Tg2576 mice
The cognitive ability of the APPsw/Tg2576 mice was assessed by applying the Morris water maze test. Wild-type control mice at 18 months of age, were given the task of learning how to find the hidden platform in the Morris water maze, and their performance was found to improve in an experience-dependent manner. In contrast, the APPsw/Tg2576 mice at 18 months of age showed a significantly delayed latency to finding the hidden platform compared with the wild control mice [Figure 2a and b]. Supplementation with dates in the diet of APPsw/Tg2576 mice for 14 months, improved the escape latency in finding a platform in a dose-dependent manner. The escape latency in the 4% group was not significantly different to APPsw/Tg2576 not fed with the date diet APPsw/Tg2576 controls [Figure 2c and d]. These data indicate that 4% date dietary supplementation in APPsw/Tg2576 mice may improve spatial memory.
Figure 2.
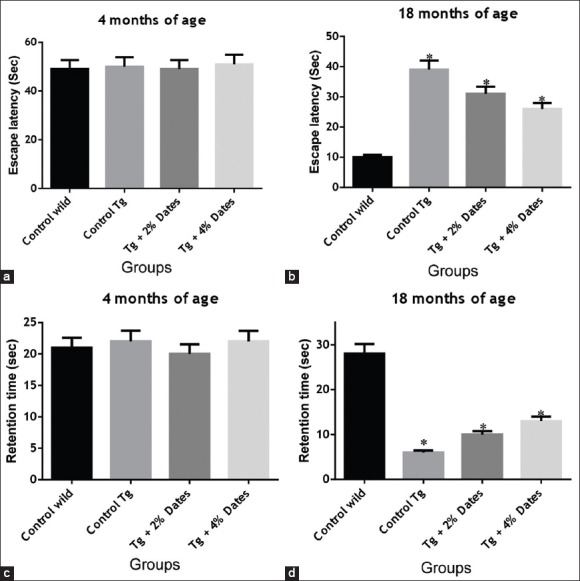
Supplementation with 2% and 4% dates ameliorated the decline in spatial memory and learning ability of amyloid precursor protein sw/Tg2576 mice. (a). Escape latency or the time to reach the platform in Morris water maze test at 4 months. (b). Escape latency or the time to reach the platform in Morris water maze test after 14 months. (c). Retention time in Morris water maze test at 4 months. (d). Retention time in Morris water maze test after 14 months of supplementation. Data are presented as mean ± standard deviation, n = 6/group. *P<0.05 compared to wild-type mice
Amyloid precursor protein sw/Tg2576 displayed severely increased anxiety which was attenuated with date supplementation
Our data also showed that the level of anxiety in APPsw/Tg2576 mice was significantly reduced in mice receiving 2% and 4% date dietary supplementation. The APPsw/Tg2576 mice receiving the 4% date diet, and the nontransgenic controls showed a greater preference for the duration of time spent in open arm [Figure 3a and b], and a lower number of entries closed arm in the EPM at 18 months of age compared to APPsw/Tg2576 mice control mice receiving the standard chow, and APPsw/Tg2576 mice exposed to 2% date supplementation [Figure 3c and d].
Figure 3.
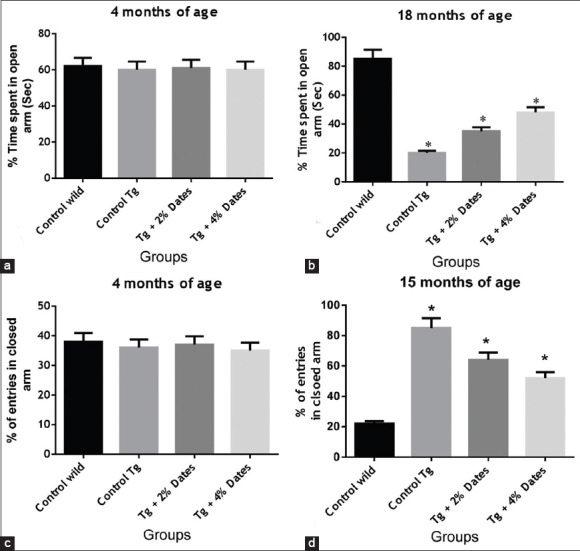
Antianxiolytic effect of 2% and 4% date supplementation in amyloid precursor protein (APPsw)/Tg2576 mice. (a). Time spent in open arm in APPsw/Tg2576 mice aged 4 months. (b). Time spent in open arm in APPsw/Tg2576 mice aged 18 months. (c). The number of entries into closed arms of APPsw/Tg2576 mice at 4 months. (d). The number of entries into closed arms of APPsw/Tg2576 mice at 18 months. Data are presented as mean ± standard deviation, n = 6/group. *P<0.05 compared to wild-type mice
Position discrimination learning ability was improved following date supplementation in amyloid precursor proteinsw/Tg2576
We also showed that date supplementation improved position discrimination learning ability in APPsw/Tg2576 mice [Figure 4]. The time to reach the platform was significantly increased at 18 months of age in APPsw/Tg2576 mice receiving standard chow compared to wild-type control mice, and was reduced in APPsw/Tg2576 mice receiving the 2% and 4% dates supplementation [Figure 4a]. Similarly, the number of errors was significantly increased in APPsw/Tg2576 mice fed standard chow compared to wild-type controls. Similarly, treatment with 2% and 4% date supplementation significantly reduced the number of errors.
Figure 4.
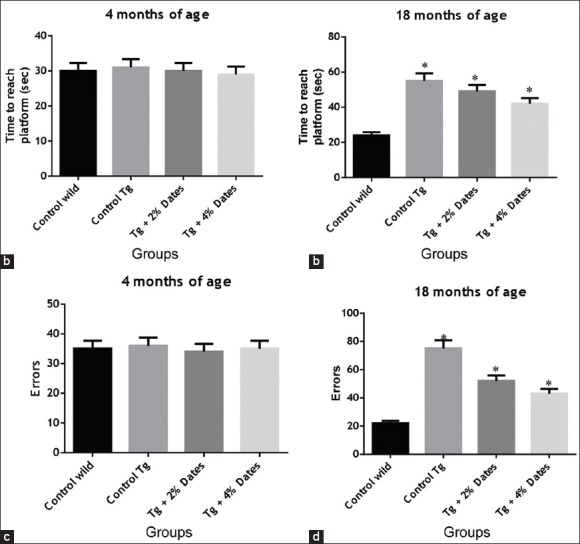
2% and 4% date supplementation improved T-water maze left-right discrimination learning. (a). T-water maze left-right discrimination at 4 months of age. (b). T-water maze left-right discrimination after 14 months of supplementation. (c). T-water maze errors at 4 months of age. (d). T-water maze errors at 18 months of age. Data are presented as mean ± standard deviation, n = 6/group. *P< 0.05 compared to wild-type mice
All the above said behavioral assessments APPsw/Tg2576 mice treated with 4% date supplementation showed better results than 2% dates.
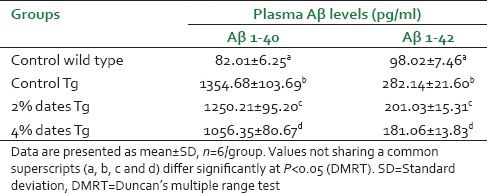
Amyloid beta content in Alzheimer's disease model mice
Amyloid beta 1–40 and Aβ1–42 levels were measured in plasma from control wild, control APPsw/Tg2576 and dietary supplementation of 2% or 4% dates for APPsw/Tg2576 mice, using two independent specific ELISA. Plasma Aβ 1–40 and Aβ 1–42 were detected in samples taken from the APPsw/Tg2576 and control wild mice. Circulating levels of both Aβ1–40 and Aβ1–42 were significantly reduced in 2% or 4% dates diet-fed APPsw/Tg2576 mice after 14 months of dietary supplementation versus the control APPsw/Tg2576 mice values [Table 1]. The more effect was found in 4% dates supplemented animals than 2% ones (P < 0.05).
Table 1.
Effect of dietary supplementation of 2 or 4% dates on Aβ (1-40, 1-42) content of mice
DISCUSSION
The main goal of this study was to examine the effects of long-term dietary supplementation of date fruit on spatial memory, learning ability and anxiety in APPsw/Tg2576 mice model of AD. Our results clearly demonstrated that date palm fruits dietary supplementation significantly improved the learning and memory deficits, motor coordination and reduced the anxiety along with plasma Aβ1–40 and Aβ1–42 reduction.
Date fruits supplementation in diet improved reversal learning of left-right discrimination, implying better cognitive flexibility. We also observed a tendency of the date diet to improve the performance over the acquisition phase of the spatial learning, as was indicated by the residual decrease of latencies in 18 month in APPsw/Tg2576 mice. Our data also suggests that the date diet was able to improve the performance of the animals during the probe test of water maze. In which, the date-fed animals spent more time in the target quadrant and made more annulus crossings than the animals fed with the standard chow diet, which demonstrates significant cognitive and learning improvement. Moreover, APPsw/Tg2576 control mice had significantly higher number of entries into the closed arms of the EPM and spent less time in the open arms of the EPM, and they have locomotors problems as evidenced by open-field test and rota-rod than wild control mice and APPsw/Tg2576 mice receiving the date diet. These behaviors were related to an anxiogenic effect induced by deposition Aβ [Table 1]. Given that increased anxiety is a problematic symptom of human AD patients, the date diet may represent as an adjunct treatment to reduce anxiety symptoms in AD.[32] An increasing amount of studies have reported beneficial effects of other diets supplemented with different extracts acting as potential antioxidants on improving neuropathology and behavior in transgenic mouse models of AD.[33,34]
The increased levels of plasma Aβ previously documented with AD.[35,36] Furthermore, in experimental mouse model of AD it has been observed that after a few months of birth they secreted more Aβ1–40, and Aβ1–42 than their wild control littermates throughout their life.[37,38] Herein, we report as well the presence of Aβ in plasma, but the decreasing effect of dates diet on Aβ1–40 and Aβ1–42 plasma levels in APPsw/Tg2576 mice compared with Control diet-fed Tg2576 mice, when applied for 14 months before Aβ plaque formation. Although this decrease is not large, taking into account the dynamic nature of Aβ present in plasma samples, the reduction observed is promising.
Regarding this aspect, dates have been revealed as a promising source of phytochemicals with a broad array of health benefits. Date products containing high antioxidant activity, directly related to their phenolic content. Ferulic acid is a natural molecule abundantly present in all date varieties in Oman. It has antioxidant[39,40] and antiinflammatory activities.[41] Previous reports have shown that long-term (4 weeks) administration of ferulic acid protects against intracerebroventricularly administered Aβ 1–42 induced learning and memory impairment, and inhibits astrocyte and microglial activation[42,43] in mice. Ferulic acid was also reported to destabilize preformed Aβ fibrils in vitro.[44,45] Administration of ferulic acid showed protection in APP/PS1 transgenic mice, including a decrease in Aβ deposition in the brain.[46] Intravenous administration of ferulic acid has also been shown to protect against neuronal cell death induced by cerebral ischemia.[47,48,49] Interestingly, ferulic acid could ameliorate stress-induced depression-like behavior in mice by promoting neural progenitor cell proliferation in vitro and in vivo conditions,[50] which is consistent with the antianxiolytic effect of dates in this study. Treatment with ferulic acid, remediates behavioral impairment, reduces amyloidogenic APP metabolism by modulating β-secretase, and mitigates AD-like pathology in the PSAPP transgenic mouse[51] also supports our current findings.
Apart from the protective effects of ferulic acid,[52] it also demonstrated the neuroprotective effect of protocatechuic acid, another important polyphenolic constituent in dates, against Aβ-induced toxicity in cultured neurons. Similarly, there were reports[53] that caffeic acid protected the PC12 cells against Aβ-induced toxicity by the inhibition of calcium influx and tau phosphorylation, which are important pathological hallmarks of AD. Another report[54] showed the protective effects of caffeic acid against acrolein-induced neurotoxicity in HT22 mouse hippocampal cells. Acrolein-induced oxidative stress is hypothesized to be involved in the etiology of AD. Recently we have reported that the long-term dietary supplementation of date and other fruits could counteract oxidative stress related changes in AD transgenic mice[55,56,57,58] which also support our current findings. The long-term supplementation of date palm fruits from Oman could be able to improve behavior performances in AD transgenic mice could be due to the high presence of active phytochemicals in dates and the exact mechanism is still unclear. Further extensive studies are warranted.
CONCLUSION
This study highlights the beneficial effect of dates on cognition and locomotors activity in the APPsw/Tg2576 mice model of AD and the same was presented recently in experimental biology 2014 conference in USA.[59] Further studies are necessary to validate and determine the mechanism of action of dates in the brain, and whether dates can prevent or slow down the progression of clinical AD.
ACKNOWLEDGMENTS
The project that was supported by The Research Council, Oman (Grant no. RC/AGR/FOOD/11/01), to M. M. Essa is gratefully acknowledged. The help rendered for performing animal studies by Dr. Maha Samuoi from College of Science and Mr. Sultan Al-Maskari and Mr. Seyad Farook from small animal house, Sultan Qaboos University is highly acknowledged. Moreover, a postdoctoral fellowship offered to Selvaraju Subash from The Research Council, Oman (RC/AGR/FOOD/11/01), is gratefully acknowledged.
Footnotes
Source of Support: Supported by a research grant from The Research Council, Oman (RC/AGR/FOOD/11/01)
Conflict of Interest: None declared.
REFERENCES
- 1.Francis PT, Palmer AM, Snape M, Wilcock GK. The cholinergic hypothesis of Alzheimer's disease: A review of progress. J Neurol Neurosurg Psychiatry. 1999;66:137–47. doi: 10.1136/jnnp.66.2.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Markesbery WR, Carney JM. Oxidative alterations in Alzheimer's disease. Brain Pathol. 1999;9:133–46. doi: 10.1111/j.1750-3639.1999.tb00215.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Coyle JT, Puttfarcken P. Oxidative stress, glutamate, and neurodegenerative disorders. Science. 1993;262:689–95. doi: 10.1126/science.7901908. [DOI] [PubMed] [Google Scholar]
- 4.Floyd RA. Antioxidants, oxidative stress, and degenerative neurological disorders. Proc Soc Exp Biol Med. 1999;222:236–45. doi: 10.1046/j.1525-1373.1999.d01-140.x. [DOI] [PubMed] [Google Scholar]
- 5.Andres-Lacueva C, Shukitt-Hale B, Galli RL, Jauregui O, Lamuela-Raventos RM, Joseph JA. Anthocyanins in aged blueberry-fed rats are found centrally and may enhance memory. Nutr Neurosci. 2005;8:111–20. doi: 10.1080/10284150500078117. [DOI] [PubMed] [Google Scholar]
- 6.Jabeen B, Badaruddin M, Ali R, Haleem DJ. Attenuation of restraint-induced behavioral deficits and serotonergic responses by stabilized rice bran in rats. Nutr Neurosci. 2007;10:11–6. doi: 10.1080/10284150601153967. [DOI] [PubMed] [Google Scholar]
- 7.Yamada K, Tanaka T, Han D, Senzaki K, Kameyama T, Nabeshima T. Protective effects of idebenone and alpha-tocopherol on beta-amyloid-(1-42)-induced learning and memory deficits in rats: Implication of oxidative stress in beta-amyloid-induced neurotoxicity in vivo. Eur J Neurosci. 1999;11:83–90. doi: 10.1046/j.1460-9568.1999.00408.x. [DOI] [PubMed] [Google Scholar]
- 8.Sano M, Ernesto C, Thomas RG, Klauber MR, Schafer K, Grundman M, et al. A controlled trial of selegiline, alpha-tocopherol, or both as treatment for Alzheimer's disease. The Alzheimer's Disease Cooperative Study. N Engl J Med. 1997;336:1216–22. doi: 10.1056/NEJM199704243361704. [DOI] [PubMed] [Google Scholar]
- 9.Oken BS, Storzbach DM, Kaye JA. The efficacy of Ginkgo biloba on cognitive function in Alzheimer disease. Arch Neurol. 1998;55:1409–15. doi: 10.1001/archneur.55.11.1409. [DOI] [PubMed] [Google Scholar]
- 10.Muthaiyah B, Essa MM, Chauhan V, Chauhan A. Protective effects of walnut extract against amyloid beta peptide-induced cell death and oxidative stress in PC12 cells. Neurochem Res. 2011;36:2096–103. doi: 10.1007/s11064-011-0533-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hartman RE, Shah A, Fagan AM, Schwetye KE, Parsadanian M, Schulman RN, et al. Pomegranate juice decreases amyloid load and improves behavior in a mouse model of Alzheimer's disease. Neurobiol Dis. 2006;24:506–15. doi: 10.1016/j.nbd.2006.08.006. [DOI] [PubMed] [Google Scholar]
- 12.Duke JA. Boca Raton, FL: CRC Press; 1992. Handbook of Phytochemicals of GRAS Herbs and Other Economic Plants. [Google Scholar]
- 13.Puri A, Sahai R, Singh KL, Saxena RP, Tandon JS, Saxena KC. Immunostimulant activity of dry fruits and plant materials used in Indian traditional medical system for mothers after child birth and invalids. J Ethnopharmacol. 2000;71:89–92. doi: 10.1016/s0378-8741(99)00181-6. [DOI] [PubMed] [Google Scholar]
- 14.Salah A, Al-Maiman SA. Effect of date palm (Phoenix dactylifera) seed fibers on plasma lipids in rats. J King Saud Univ. 2005;17:117–23. [Google Scholar]
- 15.Saafi EB, Louedi M, Elfeki A, Zakhama A, Najjar MF, Hammami M, et al. Protective effect of date palm fruit extract (Phoenix dactylifera L.) on dimethoate induced-oxidative stress in rat liver. Exp Toxicol Pathol. 2011;63:433–41. doi: 10.1016/j.etp.2010.03.002. [DOI] [PubMed] [Google Scholar]
- 16.Al Qarawi AA, Abdel-Rahman H, Mousa HM, Ali BH, El-Mougy SA. Nephroprotective action of Phoenix dactylifera in gentamicin-induced nephrotoxicity. Pharm Biol. 2008;46:227–30. [Google Scholar]
- 17.Ishurda O, John FK. The anti-cancer activity of polysaccharide prepared from Libyan dates (Phoenix dactylifera L.) Carbohydr Polym. 2005;59:531–5. [Google Scholar]
- 18.Sallal AK, El-Teen KH, Abderrahman S. Effect of date extract on growth and morphology of Candida albicans. Biomed Lett. 1996;53:179–84. [Google Scholar]
- 19.Shraideh ZA, Abu-Elteen KH, Sallal AK. Ultrastructural effects of date extract on Candida albicans. Mycopathologia. 1998;142:119–23. doi: 10.1023/a:1006901019786. [DOI] [PubMed] [Google Scholar]
- 20.Sallal AK, Ashkenani A. Effect of date extract on growth and spore germination of Bacillus subtilis. Microbios. 1989;59:203–10. [PubMed] [Google Scholar]
- 21.Vayalil PK. Antioxidant and antimutagenic properties of aqueous extract of date fruit (Phoenix dactylifera L. Arecaceae) J Agric Food Chem. 2002;50:610–7. doi: 10.1021/jf010716t. [DOI] [PubMed] [Google Scholar]
- 22.Gamil-Abdel-Hafez M, Fouad-Shalaby A, Akhal I. Chemical composition of 15 varieties of dates grown in Saudi Arabia. Fourth Symposium on Biological Aspects of Saudi Arabia. 1980:89–91. [Google Scholar]
- 23.Mansouri A, Embarek G, Kokkalou E, Kefalas P. Phenolic profile and antioxidant activity of the Algerian ripe date palm fruit (Phoenix dactylifera) Food Chem. 2005;89:411–20. [Google Scholar]
- 24.Al-Farsi M, Alasalvar C, Morris A, Baron M, Shahidi F. Compositional and sensory characteristics of three native sun-dried date (Phoenix dactylifera L.) varieties grown in Oman. J Agric Food Chem. 2005;53:7586–91. doi: 10.1021/jf050578y. [DOI] [PubMed] [Google Scholar]
- 25.Al-Farsi M, Alasalvar C, Morris A, Baron M, Shahidi F. Comparison of antioxidant activity, anthocyanins, carotenoids, and phenolics of three native fresh and sun-dried date (Phoenix dactylifera L.) varieties grown in Oman. J Agric Food Chem. 2005;53:7592–9. doi: 10.1021/jf050579q. [DOI] [PubMed] [Google Scholar]
- 26.Biglari F, AlKarkhi, Abbas FM, Easa AM. Antioxidant activity and phenolic content of various date palm (Phoenix dactylifera) fruits from Iran. Food Chem. 2008;107:1636–41. [Google Scholar]
- 27.Allaith AA. Antioxidant activity of Bahraini date palm (Phoenix dactylifera L.) fruit of various cultivars. Int J Food Sci Technol. 2008;43:1033–40. [Google Scholar]
- 28.Vinson JA, Zubik L, Bose P, Samman N, Proch J. Dried fruits: Excellent in vitro and in vivo antioxidants. J Am Coll Nutr. 2005;24:44–50. doi: 10.1080/07315724.2005.10719442. [DOI] [PubMed] [Google Scholar]
- 29.Al-Farsi M, Morris A, Baron M. Abu Dhabi, United Arab Emirates: 2006. Functional properties of Omani dates (Phoenix dactylifera). The Third International Date Palm Conference; pp. 19–21. [Google Scholar]
- 30.Essa MM, Guillemin GJ, Al-Adawi S, Al-Asmi, Vaishnav R, Ramachandiran N, et al. Manickavasagan A, Essa MM, Sukumar E. The Dates – Genous Phoneix. UK: CRC Press; 2012. Anti amyloidogenic effect of dates with reference to their protection against Alzheimer's disease; pp. 397–403. [Google Scholar]
- 31.Berker KI, Ozdemir Olgun FA, Ozyurt D, Demirata B, Apak R. Modified Folin-Ciocalteu antioxidant capacity assay for measuring lipophilic antioxidants. J Agric Food Chem. 2013;61:4783–91. doi: 10.1021/jf400249k. [DOI] [PubMed] [Google Scholar]
- 32.Kennedy JS, Bymaster FP, Schuh L, Calligaro DO, Nomikos G, Felder CC, et al. A current review of olanzapine's safety in the geriatric patient: From pre-clinical pharmacology to clinical data. Int J Geriatr Psychiatry. 2001;16 Suppl 1:S33–61. doi: 10.1002/1099-1166(200112)16:1+<::aid-gps571>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- 33.Hartman D. Aging and oxidative stress. JIFFC. 1998;10:24–7. [PubMed] [Google Scholar]
- 34.Essa MM, Vijayan RK, Castellano-Gonzalez G, Memon MA, Braidy N, Guillemin GJ. Neuroprotective effect of natural products against Alzheimer's disease. Neurochem Res. 2012;37:1829–42. doi: 10.1007/s11064-012-0799-9. [DOI] [PubMed] [Google Scholar]
- 35.Cosentino SA, Stern Y, Sokolov E, Scarmeas N, Manly JJ, Tang MX, et al. Plasma ß-amyloid and cognitive decline. Arch Neurol. 2010;67:1485–90. doi: 10.1001/archneurol.2010.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Laske C, Sopova K, Gkotsis C, Eschweiler GW, Straten G, Gawaz M, et al. Amyloid-ß peptides in plasma and cognitive decline after 1 year follow-up in Alzheimer's disease patients. J Alzheimers Dis. 2010;21:1263–9. doi: 10.3233/jad-2010-100510. [DOI] [PubMed] [Google Scholar]
- 37.Kuo YM, Kokjohn TA, Beach TG, Sue LI, Brune D, Lopez JC, et al. Comparative analysis of amyloid-beta chemical structure and amyloid plaque morphology of transgenic mouse and Alzheimer's disease brains. J Biol Chem. 2001;276:12991–8. doi: 10.1074/jbc.M007859200. [DOI] [PubMed] [Google Scholar]
- 38.Kawarabayashi T, Younkin LH, Saido TC, Shoji M, Ashe KH, Younkin SG. Age-dependent changes in brain, CSF, and plasma amyloid (beta) protein in the Tg2576 transgenic mouse model of Alzheimer's disease. J Neurosci. 2001;21:372–81. doi: 10.1523/JNEUROSCI.21-02-00372.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Graf E. Antioxidant potential of ferulic acid. Free Radic Biol Med. 1992;13:435–48. doi: 10.1016/0891-5849(92)90184-i. [DOI] [PubMed] [Google Scholar]
- 40.Scott BC, Butler J, Halliwell B, Aruoma OI. Evaluation of the antioxidant actions of ferulic acid and catechins. Free Radic Res Commun. 1993;19:241–53. doi: 10.3109/10715769309056512. [DOI] [PubMed] [Google Scholar]
- 41.Ozaki Y. Antiinflammatory effect of tetramethylpyrazine and ferulic acid. Chem Pharm Bull (Tokyo) 1992;40:954–6. doi: 10.1248/cpb.40.954. [DOI] [PubMed] [Google Scholar]
- 42.Kim HS, Cho JY, Kim DH, Yan JJ, Lee HK, Suh HW, et al. Inhibitory effects of long-term administration of ferulic acid on microglial activation induced by intracerebroventricular injection of beta-amyloid peptide (1-42) in mice. Biol Pharm Bull. 2004;27:120–1. doi: 10.1248/bpb.27.120. [DOI] [PubMed] [Google Scholar]
- 43.Cho JY, Kim HS, Kim DH, Yan JJ, Suh HW, Song DK. Inhibitory effects of long-term administration of ferulic acid on astrocyte activation induced by intracerebroventricular injection of beta-amyloid peptide (1-42) in mice. Prog Neuropsychopharmacol Biol Psychiatry. 2005;29:901–7. doi: 10.1016/j.pnpbp.2005.04.022. [DOI] [PubMed] [Google Scholar]
- 44.Ono K, Hirohata M, Yamada M. Ferulic acid destabilizes preformed beta-amyloid fibrils in vitro. Biochem Biophys Res Commun. 2005;336:444–9. doi: 10.1016/j.bbrc.2005.08.148. [DOI] [PubMed] [Google Scholar]
- 45.Ono K, Hamaguchi T, Naiki H, Yamada M. Anti-amyloidogenic effects of antioxidants: Implications for the prevention and therapeutics of Alzheimer's disease. Biochim Biophys Acta. 2006;1762:575–86. doi: 10.1016/j.bbadis.2006.03.002. [DOI] [PubMed] [Google Scholar]
- 46.Yan JJ, Jung JS, Kim TK, Hasan A, Hong CW, Nam JS, et al. Protective effects of ferulic acid in amyloid precursor protein plus presenilin-1 transgenic mouse model of Alzheimer disease. Biol Pharm Bull. 2013;36:140–3. doi: 10.1248/bpb.b12-00798. [DOI] [PubMed] [Google Scholar]
- 47.Cheng CY, Su SY, Tang NY, Ho TY, Chiang SY, Hsieh CL. Ferulic acid provides neuroprotection against oxidative stress-related apoptosis after cerebral ischemia/reperfusion injury by inhibiting ICAM-1 mRNA expression in rats. Brain Res. 2008;1209:136–50. doi: 10.1016/j.brainres.2008.02.090. [DOI] [PubMed] [Google Scholar]
- 48.Cheng CY, Su SY, Tang NY, Ho TY, Lo WY, Hsieh CL. Ferulic acid inhibits nitric oxide-induced apoptosis by enhancing GABA (B1) receptor expression in transient focal cerebral ischemia in rats. Acta Pharmacol Sin. 2010;31:889–99. doi: 10.1038/aps.2010.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Koh PO. Ferulic acid prevents the cerebral ischemic injury-induced decrease of Akt and Bad phosphorylation. Neurosci Lett. 2012;507:156–60. doi: 10.1016/j.neulet.2011.12.012. [DOI] [PubMed] [Google Scholar]
- 50.Yabe T, Hirahara H, Harada N, Ito N, Nagai T, Sanagi T, et al. Ferulic acid induces neural progenitor cell proliferation in vitro and in vivo. Neuroscience. 2010;165:515–24. doi: 10.1016/j.neuroscience.2009.10.023. [DOI] [PubMed] [Google Scholar]
- 51.Mori T, Koyama N, Guillot-Sestier MV, Tan J, Town T. Ferulic acid is a nutraceutical ß-secretase modulator that improves behavioral impairment and alzheimer-like pathology in transgenic mice. PLoS One. 2013;8:e55774. doi: 10.1371/journal.pone.0055774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ban JY, Cho SO, Jeon SY, Bae K, Song KS, Seong YH. 3,4-dihydroxybenzoic acid from Smilacis chinae rhizome protects amyloid beta protein (25-35)-induced neurotoxicity in cultured rat cortical neurons. Neurosci Lett. 2007;420:184–8. doi: 10.1016/j.neulet.2007.05.009. [DOI] [PubMed] [Google Scholar]
- 53.Sul D, Kim HS, Lee D, Joo SS, Hwang KW, Park SY. Protective effect of caffeic acid against beta-amyloid-induced neurotoxicity by the inhibition of calcium influx and tau phosphorylation. Life Sci. 2009;84:257–62. doi: 10.1016/j.lfs.2008.12.001. [DOI] [PubMed] [Google Scholar]
- 54.Huang Y, Jin M, Pi R, Zhang J, Chen M, Ouyang Y, et al. Protective effects of caffeic acid and caffeic acid phenethyl ester against acrolein-induced neurotoxicity in HT22 mouse hippocampal cells. Neurosci Lett. 2013;535:146–51. doi: 10.1016/j.neulet.2012.12.051. [DOI] [PubMed] [Google Scholar]
- 55.Subash S, Essa MM, Al-Asmi A, Al-Adawi S, Vaishnav R, Guillemin GJ. Effect of dietary supplementation of dates in Alzheimer's disease APPsw/2576 transgenic mice on oxidative stress and antioxidant status. Nutr Neurosci. 2014 doi: 10.1179/1476830514Y.0000000134. [DOI] [PubMed] [Google Scholar]
- 56.Subash S, Essa MM, Al-Asmi A, Al-Adawi S, Vaishnav R. Chronic dietary supplementation of 4% figs on the modification of oxidative stress in Alzheimer's disease transgenic mouse model. Biomed Res Int 2014. 2014:546357. doi: 10.1155/2014/546357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Subash S, Braidy N, Essa MM, Al-Buraiki Z, Vaishnav R, Al-Adawi S, et al. Long term (15 months) dietary supplementation with pomegranates from Oman attenuates cognitive and behavioural deficts in a transgenic mice model of Alzheimer's disease. Nutrition. 2014 doi: 10.1016/j.nut.2014.06.004. [DOI] [PubMed] [Google Scholar]
- 58.Subash S, Essa MM, Braidy N, Al-Jabri A, Vaishnav R, Al-Adawi S, et al. Consumption of fig fruits grown in Oman can improve memory, anxiety, and learning skills in a transgenic mice model of Alzheimer's disease. Nutr Neurosci. 2014 doi: 10.1179/1476830514Y.0000000131. [DOI] [PubMed] [Google Scholar]
- 59.Subash S, Essa MM, Awlad-Thani K, Al-Adawi S, Al-Asmi A, Vaishnav R, et al. Memory deficits and learning skills improved in transgenic mouse model of Alzheimer's disease after date-rich diet supplementation. FASEB J. 2014;28 Suppl 1:8454. [Google Scholar]


