Abstract
The Ayurvedic literature during the medieval period suggests the use of Musta (Cyperus rotundus), a common weed, as a pratinidhi dravya (substitute) for Ativisha (Aconitum heterophyllum), an endangered species. Contemporary Ayurvedic practice also uses Cryptocoryne spiralis, (known as Naattu Atividayam in South India) and Nagaramusta (Cyperus scariosus) as substitutes for Ativisha and Musta, respectively. This article reviews published literature on the pharmacology of the above four species. Both A. heterophyllum and C. rotundus are reported to possess antiinflammatory, antipyretic, antibacterial and antidiarrhoeal properties, while antiinflammatory and antibacterial activities are attributed to C. scariosus. No reports exist on the bioactivity of Cryptocoryne spiralis. It is interesting to note that other than the veerya which is different, the biological properties of Ativisha and Musta are similar according to Ayurvedic classification of dravyaguna. This is also supported by modern pharmacological studies, which show that, both A. heterophyllum and C. rotundus have antidiarrheal, antipyretic, antiinflammatory, antihyperlipidemic and hypoglycemic activities. However, the similarities between the discussed species cannot be attributed to their phytochemical composition or taxonomical classification as these are quite distinct. The dravyaguna method of classifying materials, which we are calling as “pharmaco-taxonomy”, offers a unique way of classifying those plant materials which lack similarity at the botanical or chemical level, but are similar at the level of biological functions.
Keywords: Aconitum, Ativisha, Ayurveda, Cryptocoryne, Cyperus, Musta, pharmacology
INTRODUCTION
As per the Ayurvedic concept of abhava-pratinidhi dravya, a rare or unavailable medicinal plant (abhava dravya) is substituted by a more readily available species (pratinidhi dravya). Although this concept dates back to Caraka,[1] it was systematically codified by Bhavamishra.[2] Later works such as Bhaishajya Ratnavali[3] and Yogaratnakara[4] give an elaborate list of substitutes for unavailable drugs.
Ativisha (Aconitum heterophyllum Wall. ex Royle) is a commonly prescribed herb in Ayurveda for diarrhea, fever and inflammation. Having a natural habitat only in sub alpine Himalayas, Ativisha is an endangered species.[5] Trade in Ativisha far exceeds the natural availability of this plant, leading to rampant substitution/adulteration. The solution to this problem of nonavailability of Ativisha is suggested in Ayurvedic books like Bhavaprakasha[6] and Yogaratnakara,[4] where Ativisha (abhava) is substituted by Musta (pratinidhi). Musta (Cyperus rotundus L.) is a common weed, with a widespread distribution[7] Naattu Athividayam [Cryptocoryne spiralis (Retz.) Fisch ex Wydler] and Nagara Musta (Cyperus scariosus R. Br.) are other traded species as substitutes for Ativisha and Musta, respectively.[7]
This article reviews the published literature on the pharmacology of botanical entities used as Ativisha and Musta in order to compare the similarities and differences between the species. Though it would be ideal to present the phytochemical and pharmacological details of the species together in a single review article, the enormous information available on the phytochemistry of the species discussed, necessitated a separate presentation of the same.
The scope of this review covers the published literature on the pharmacology information on the selected species till November 2013 as available in the databases PubMed, SciFinder, and Agricola. Ayurveda details were obtained from classical Ayurveda texts of Brhattrayi and Nighantus.
PHARMACOLOGY
Aconitum heterophyllum Wall. ex Royle
Use of Aconitum heterophyllum Wall. ex Royle (Ativisha) in Ayurveda and other traditional systems of medicine
Aconitum species are used in Ayurveda and Chinese systems of medicine. A brief review of the use of A. heterophyllum in Ayurveda has been published.[8] Aconitine, the most abundant alkaloid in most Aconitum species, is a known cardiotoxin.[9] Therefore, Aconitum species are used only after due process of detoxification.[10,11] A. heterophyllum, on the other hand, contains atisine as the principal alkaloid, and the species is considered to be nonpoisonous.[12] Ayurveda does not mandate and practice detoxification of A. heterophyllum.
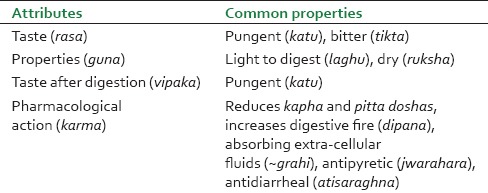
As per Ayurvedic pharmacology, Ativisha (A. heterophyllum) has tikta (bitter) and katu (pungent) taste; laghu (light) and ruksha (dry) properties; ushna veerya (hot potency) and katu vipaka (attains pungency after digestion). In terms of actions, it is kapha-pittahara (reduces kapha and pitta doshas), dipana (increases digestive fire), pachana (digests undigested material), grahi (prevents water loss from the body), shotahara (antiinflammatory), vishaghna (antipoisonous), krimihara (anthelmintic), arshoghna (antihemorrhoid), jwarahara (antipyretic), kasahara (antitussive) and atisaraghna (antidiarrhoeal). In the classical Ayurvedic text Caraka Samhita, Ativisha is listed in the following categories: Tikta skandha (bitter tasting drugs), lekhaneeya (has scraping action on tissues and kapha), arshoghna (treating hemorrhoids) sirovirechana (clearing morbid doshas from head and neck) [Table 1].[1,2,7]
Table 1.
Summary of properties and actions (rasapanchakas) of Ativisha-Musta
The drug finds common use in the treatment of fevers, diarrhea, indigestion, inflammation, helminthiasis, hyperlipidemia and as an antiemetic in children.[13,14] Sudarshana Churna, Balachaturbhadra Churna, Rasnerandadi Kwatha and Panchatiktaka Guggulu Ghrta are some of the popular multi-drug formulations in which Ativisha is one of the main ingredients.[13]
Similar uses for A. heterophyllum are also found in Unani and Siddha systems of medicines as well.[15,16]
Although antidiarrheal activity is one of the major indications for which A. heterophyllum is used in traditional medicine, modern scientific studies are missing, except for one preliminary report. Venkatasubramanian et al. showed the antidiarrheal activity of alcoholic extract of A. heterophyllum tubers in castor oil induced diarrhea and gastric transit time in mice. However, the active molecule responsible for this activity has not been reported.[17]
Antiinflammatory and antipyretic activity
In order to assess the antiinflammatory activity of A. heterophyllum, Verma et al. employed the widely used cotton-pellet induced granuloma method. Their investigations showed that A. heterophyllum tuber (ethanolic extract) has significant antiinflammatory activity, thereby providing scientific evidence for a traditional medicinal claim as shotha/shophahara karma (antiinflammatory action).[18] The antipyretic effects of roots of A. heterophyllum in the form of aqueous, chloroform and hexane extracts were examined using the method of yeast induced pyrexia, with aspirin as a standard antipyretic agent for comparison. These studies, by Ikrum, showed that the extracts were nontoxic (up to 1.6 g/kg) and had no significant antipyretic activity. However, in Ayurveda A. heterophyllum is administered as a powder (churna) and kashaya (decoction) for controlling fever.[19]
Antibacterial activity
The new aconitine type nor-diterpenoid alkaloids 6-dehydroacetylsepaconitine and 13-hydroxylappaconitine, isolated from the tubers of A. heterophyllum along with the known alkaloids lycoctonine, delphatine and lappaconitine, were screened for antibacterial activity against different bacterial strains. They showed antibacterial activity against gram negative (diarrhea causing) bacteria Escherichia coli, Shigella flexineri, Pseudomonas aeruginosa and Salmonella typhi.[20] This reports strengthens the prescription of Ativisha as Krimihara (antimicrobial/anthelmintic). These tests were however not carried out using the plant extracts.
Immunomodulatory activity
The immunomodulatory activity of ethanolic extract of A. heterophyllum tubers along with other medicines of the Ayurveda and Unani systems of medicine were investigated on delayed type hypersensitivity (DTH), humoral responses to sheep red blood cells, skin allograft rejection and phagocytic activity of the reticuloendothelial system in mice. It was found that the extract appeared to enhance the phagocytic function and to inhibit the humoral component of the immune system. The results obtained from these preliminary studies show that, A. heterophyllum has immunomodulatory activity, which could possibly lead to new immunomodulating agents of herbal origin.[21]
Action on the nervous system
Hamet showed that, A. heterophyllum has the ability to make the sympathetic nervous system more sensitive to physiological stimuli. He found that while atisine had a hypotensive effect at every tested dose, the plant extract as a whole showed hypertensive properties. Hypertension produced by high doses of aqueous extract was attributed to the excitement of the sympathetic nervous system.[22,23]
Two new diterpenoid alkaloids heterophyllinines A and B, isolated from the roots of A. heterophyllum were about 13 times more selective in inhibiting the enzyme butyrylcholinesterase than acetylcholinesterase. These enzymes are involved in the transmission of nerve impulses.[24]
Anthelminthic activity
Aqueous and alcoholic extracts of tubers of A. heterophyllum gave encouraging results when evaluated against Pheritema postuma (earthworm), using piperazine citrate as standard. Time required for initial three paralytic attacks and death was used as parameters to evaluate the drug.[25] Though Ativisha is considered to have krmihara (anthelmintic) property as per Ayurveda, the results obtained here need to be compared with other standard Ayurvedic anthelmintic agents like Vidanga (Emblia ribes) to establish the utility of this drug in practice.
Antihyperlipidemic activity
The methanolic extract of tubers of A. heterophyllum had a hypolipidemic effect on diet induced obese rats. It was observed that the pharmacological effect was due to two factors; (i) inhibition of Hydroxymethylglutarate-Coenzyme A reductase (HMGR) and (ii) activation of Lecithin-cholesterol acyltransferase. This resulted in lowering of total cholesterol, low-density lipoprotein cholesterol (LDL-c), triglycerides and apolipoprotein B in blood serum, decrease in intestinal fat absorption and increase of high-density lipoprotein cholesterol (HDL-c) and apolipoprotein A, supporting the classification of Ativisha as a drug having “lekaneeya” (scraping) action with antihyperlipidemic properties.[26] It is worth mentioning here that, two common classes of compounds used in modern medicine to control hyperlipidemia are statins and fibrates. The former act on HMGR and the latter regulate HDL-LDL ratios. A. hetrophyllum is active at both levels and hence could prove to be a valuable antihyperlipidemic agent.
Cyperus rotundus L.
Use of Cyperus rotundus L. in Ayurveda and other traditional systems of medicine
Musta (C. rotundus) is widely used in Ayurveda. It has katu (pungent), tikta (bitter) and kashaya (astringent) taste; laghu (light) and ruksha (dry) properties; sita (cold) potency and katu (pungent) taste after digestion. It is kapha-pittahara (reduces kapha and pitta dosha), dipana (increases digestive fire), pachana (digests undigested material), grahi (water absorbing), jwarahara (antipyretic), atisaraghna (antidiarrhoeal) and kanduhara (antiitching).[1,2,7] The similarity of properties and activity between Ativisha and Musta as elucidated in Ayurveda is both unique and striking and forms the cornerstone of the concept of abhava-pratinidhi dravya in Ayurveda[3,4] [Table 1].
Musta is categorized as lekhaneeya (scraping action on body fat and kapha), trishnanigrahana (alleviating morbid thirst), kandughna (reducing itch) and stanyashodhana (clearing the problems of breast/breast milk).[1,7,13]
Musta is used extensively in the management of fevers, diarrhea, thirst, inflammation, tastelessness, helminthiasis, indigestion and obesity.[2,7,27,28] The commonly prescribed forms are powder, decoction and hot water infusion. It is also a component of formulations such as Mustakadi Churna, Mustakarishta, Mustakadikwatha, Ashokarishta, Shadangapaniya and Balachaturbhadra Churna.[4,19]
Similar uses for C. rotundus are found in the Siddha system as well.[16] A review of the phyto-pharmacotherapeutics of C. rotundus has been published recently, detailing its uses in Ayurveda.[29]
Cyperus rotundus is also used extensively in traditional Chinese medicine. Its popularity in that system is evident from the over 500 patents issued in the past decade, governing its use in various formulations.
Antidiarrhoeal activity
Daswani et al. examined the action of C. rotundus rhizome on adherence and enterotoxin production of 2 groups of enteropathogenic E. coli, and enterotoxigenic E. coli ETEC. The aqueous decoction did not affect the adherence; however, there was significant inhibition in labile toxin and stable toxin production. An important observation was that there was an inverse correlation observed between stable toxin production and concentration of the decoction that is, maximum inhibition was seen at 1:1000 dilution.[30] Their results suggest that C. rotundus exhibits limited antibacterial/antirotaviral activity. Musta's antidiarrheal effect is probably due to the action on some feature of bacterial virulence such as colonization, production of cholera toxin or labile toxin rather than killing the bacteria.[31]
The antidiarrheal effect of the methanol extract of C. rotundus rhizome against castor oil induced diarrhea in mice was also established by Uddin. The methanol extract was found to significantly suppress the frequency of the diarrheal episodes as well as prolong the latent period for the onset of diarrhea when compared with standard drug loperamide. They, however, did not identify the active principles responsible for the activity.[32] The aqueous extracts also exhibited antidiarrheal activity in the same model.[17] Musta is a well-known atisaraghna (antidiarrheal) and grahi (absorbing/preventing water loss from the body) drug of Ayurveda. It is mentioned in Nighantus (lexicons) of Ayurveda that, it is increasing agni and thereby cure the problems of gastro intestinal tract.
Antiinflammatory activity
Biradar et al. studied the antiinflammatory, antiarthritic, analgesic and anticonvulsant (for treatment of epilepsy) effect of essential oils of C. rotundus. The antiinflammatory activity was determined using carrageenan-induced paw edema in Swiss albino rats. A dosage of 500 mg/kg was found to be comparable to the control (indomethacin 10 mg/kg). The aqueous, ethanol and ether extracts of C. rotundus showed good activity at 400 mg/kg with the ethanol extract exhibiting best inhibitory activity at 65.4%. These studies validate the use of C. rotundus as an antiinflammatory drug in traditional medicine.[33]
Tsoyi et al. have studied the role of heme oxygenase-1(HO-1) in systemic inflammatory disorders such as sepsis. As part of their research to unearth potential HO-1 inducible agents from traditional medicinal herbs, they studied the extracts of C. rotundus rhizome, use of which is mentioned against inflammatory diseases. Their work on RAW264.7 cells led them to conclude that the antiinflammatory mechanism of the extracts of C. rotundus is due to HO-1 induction and inhibition of inducible nitric oxide synthase. They established that the sesquiterpenes (+)-nootkatone and (+)-valencene present in the extract were particularly active.[34]
Similar results were obtained by Korean investigators who observed that the hexane soluble portion of the hydro-alcoholic extract of C. rotundus inhibited nitric oxide formation induced by lipopolysaccharides in RAW264.7 cells. Once again, sesquiterpenes were shown to be responsible for the activity.[35] The same authors also established that in the same cell line the sesquiterpene cyperone present in C. rotundus, inhibited lipopolysaccharide induced COX-2 expression and PGE-2 production through negative regulation of NF-κB signaling.[36]
The extracts of C. rotundus were tested in rodents for antiinflammatory, analgesic and antigenotoxic activity, and positive results were obtained in each case. The activities were attributed to the flavonoids, tannins and polyphenols present in the extract.[37]
The wide spectrum of antiinflammatory activity displayed by C. rotundus in the above mentioned examples establishes it as a useful drug in keeping with its role as a shotha hara (antiinflammatory) drug in traditional medicinal systems.
Antimicrobial activity
In 2004, antimicrobial studies were done using the essential oil of C. rotundus rhizomes prepared by hydro distillation. From the antimicrobial study, it was concluded that essential oil was active against gram positive micro-organisms but completely inactive against gram negative species, the test used being the disc agar diffusion method.[38] Similarly, sequential cold extraction of C. rotundus rhizomes was done by Sini et al. using hexane, chloroform and water and the extracts tested for antibacterial activity against Bacillus pumilis and E. coli by the disc diffusion method. The hexane and water extracts of C. rotundus showed inhibition against B. pumilis but not E. coli.[39]
Streptococcus mutans is known as the primary causative bacteria in the formation of dental plaque and dental caries. S. mutans multiplies in plaque and generates organic acids such as lactic, propionic, formic and butyric acids which demineralize the tooth surfaces and thereby induce dental caries. Studies by Yu et al. in 2007 showed that the ethanol extract of C. rotundus tubers repressed the growth of S. mutans and also inhibited the production of organic acids by the bacteria. Thus, it shows good anticariogenic properties.[40]
In their work on rhizomes of C. rotundus sourced from Tunisia, Kilani et al. reported on the antibacterial activity of the extracts against food related bacteria. The strains used were: Staphylococcus aureus, Enterococcus faecalis, E. coli, Salmonella enteritidis, and Salmonella typhimurium. Various levels of antibacterial effect were seen against all strains tested. The total oligomers flavonoids (TOFs) enriched extract was the most effective against S. enteritidis and S. aureus. The ethyl acetate extract showed remarkable activity against the gram positive bacteria S. aureus and E. faecalis.[41]
Antibacterial, antifungal and analgesic activities of ethanolic extract of C. rotundus rhizomes were reported by several authors.[42,43,44] Bisht et al. reported good activity of the oil as compared to the standard chloramphenicol especially against Bacillus subtilis, S. aureus, E. coli and P. aeruginosa, while, on eight fold dilution, inhibition of S. aureus alone was seen. Good antifungal activity as compared to the standard drug nystatin was observed against C. parapsilosis and A. fumigatus while inhibition of spore formation was seen in F. oxysporum and A. flavus.[45] Similar results were also obtained by Biradar et al., who showed that the essential oil of C. rotundus was active against S. aureus, Staphylococcus albus, P. aeruginosa, E. coli, Candida albicans and Aspergillus niger.[46]
Sharma and Singh examined the effect of various extracts of the rhizomes of C. rotundus in order to evaluate their antimicrobial activity. Different gram positive (Staphylococcus epidermidis, Bacillus cereus) and gram negative (P. aeruginosa, E. coli) bacteria along with fungal strains (C. albicans, A. niger) were used in this study. It was found that none of the extracts exhibited antifungal activity against the strains used. However, the ethanolic extract was found to be most effective against the gram positive as well as one of the gram negative (P. aeruginosa – nosocomoal diarrhea causing agent) bacteria though it was ineffective against E. coli.[47]
Extracts of the aerial parts of C. rotundus showed antibacterial activity against S. aureus, E. faecalis, Salmonella enteridis and S. typhimurium.[48]
Essential oil of C. rotundus exhibited anthelmintic activity against P. postuma and Ascardia galli.[46]
C. rotundus has a broad spectrum antimicrobial activity corresponding to its classification as a krimihara in Ayurveda. It is more effective against gram positive bacteria than gram negative species. In addition, it also displays antifungal and anthelmintic properties.
Cytotoxic activity
The essential oil of C. rotundus obtained from the rhizomes by hydro distillation was also tested for cytotoxic activity against human tumor cell lines (U 251 and Hela), and Ehrlich ascites carcinoma cells. A positive result was obtained against Ehrlich ascites carcinoma cells with 100% inhibition at all concentrations tested. Negative results were seen when tested against human tumor cell lines.[38] However, the phytoconstituents responsible for this action was not recognized.
Antioxidant activity
Several studies established the antioxidant potential of Musta. Using in vitro models Nagulendran et al. showed that C. rotundus hydro-alcoholic extracts exhibit free radical scavenging, reducing power and metal chelating activity. Their study on the superoxide anion scavenging activity and hydroxyl radical scavenging activity gave good results namely IC50 values of 0.031–0.021 mg/ml respectively. They did not, however, identify the constituents responsible for the activities.[49]
The TOF(total oligomeric flavonoids) and ethyl acetate extracts of C. rotundus rhizomes have strong antimutagenic and antigenotoxic activities. The same extracts are potent radical scavengers, display cytotoxic effects and induce apoptic DNA fragmentation.[50]
The antioxidant activity of the extract of C. rotundus has also been correlated to its anticataract activity.[51]
Several antioxidant assays such as xanthine oxidase inhibition, superoxide anion inhibition and protection against hydrogen peroxide/UV induced DNA damage were correlated with the ability of C. rotundus rhizome extracts to exert an antiproliferative effect toward K 562 erythroleukemia cells. Flavonoids were shown to be important for this activity.[52]
In their search for novel antioxidants, Dutta and Pal in 2006 investigated the rhizomes of C. rotundus. They carried out the evaluation by nonenzymatic glycosylation of hemoglobin measured colorimetrically and found that at concentrations of 1 mg/ml the extracts displayed inhibition with ethanol showing maximum activity at 35.3% followed by aqueous (14.1%), chloroform (12%) and petroleum ether (7.9%).[53]
Bashir et al. compared the activity of methanolic and ethanolic extracts of C. rotundus rhizomes. The percentage yield, as well as total phenolic and flavonoid content, were found to be more in roots followed by leaves and then stem. Furthermore, the methanolic extracts were found to be better in terms of activity and yield as compared to ethanolic extracts, suggesting that it is a better solvent for extraction. The tests used for determination of antioxidant activity were 1,1-diphenyl-2-picrylhydrazyl(DPPH) assay and inhibition of linoleic acid lipid peroxidation.[54]
It is well known that the presence of reactive oxidation species(ROS) causes a number of undesirable effects on the body including ischemia and reperfusion. The effect of this can be studied by the damage to the stomach tissues. In Turkey, Guldur and others studied the effect of the extracts of C. rotundus rhizomes on gastric mucosal damage induced in rats. They reported that the treatment with the extracts showed a significant decrease in the mucosal damage and suggested that the effective free radical scavenging activity of the C. rotundus extracts helped in the protection against damage caused by free radicals.[55]
Extracts of the aerial parts of C. rotundus reduced genotoxicity induced by nifuroxazide and aflatoxin B1.[48]
C. rotundus, therefore, has widely distributed antioxidant activity and its use for this purpose deserves more attention.
Antiallergic activity
Release of histamine and β-hexosaminidase by degranulation during an allergic reaction can be used as a biomarker of allergic response of mast cells. Furthermore, inhibition of 5 – LOX (lipoxygenase) enzyme which produces the mediators of allergic reactions; leukotrienes (LT) can be used as a measure of antiallergic action. Jin et al. carried out in vitro tests for immediate-type hypersensitivity by inhibition of β-hexosaminidase and 5 – LOX enzyme as well as in vivo study examining the delayed-type hypersensitivity (DTH). Jin worked on the ethanol extracts of C. rotundus rhizomes as well as isolated sesquiterpenes, monoterpenes and 4-cymene. In the case of 5-LOX inhibition, the extract inhibited LT production by 66–91% at 30–300 μg/ml. At 100 μM, the sesquiterpenes valencene, nootkatone and caryophyllene α-oxide showed 60%, 93%, and 99% inhibition while the monoterpenes and 4-cymene did not show any effect. Furthermore, at the same concentration, valencene, nootkatone, and caryophyllene α-oxide showed 88%, 44% and 28% inhibition of β-hexosaminidase while the monoterpenes and 4-cymene showed less than 15% inhibition. In the case of in vivo studies also, the sesquiterpenes showed the most significant activity.[56]
Activity on central nervous system
The first report on the central nervous system (CNS) activity of C. rotundus rhizome was by Pal, who studied the action of the extracts of this plant against different activities. Preliminary work showed that the ethanol extract displayed marked CNS depressant action compared to the other extracts. They studied the ethanol extract in detail including the chemical constituents. Toxicity study, effect on sleeping time and analgesic activity study using different models, were carried out. The results showed that the extract enhanced sleeping time, has analgesic and anticonvulsant activities and reduced different behavioral reflexes. The researchers thereby concluded that the extract exhibited strong CNS depressant action.[57]
Sunil et al. carried out a detailed investigation into the neuroprotective effects of the TOFs obtained from rhizomes of C. rotundus, a nootropic and nervine tonic according to traditional medicine. There was a significant improvement in excitotoxicity, oxidative stress, neurological, and behavioral alterations in male Sprague Dawley rats subjected to middle cerebral artery occlusion and reperfusion. These findings led the researchers to suggest that TOFs should be studied further for development of drugs for the treatment of cerebral stroke.[58]
The ethanolic extract of C. rotundus rhizomes exhibited neuroprotective activity as shown by inhibition of peroxynitrite induced neurotoxicity in human neuroblastoma SH-SY5Y cells.[59] It was also reported to ameliorate hydrogen peroxide induced human neuronal cell damage via its antioxidative and antiapoptotic activity. High content of phenolics and flavonoids in rhizomes of C. rotundus is related to this activity. In this context, Kumar and Khanum also suggest the possibility to develop C. rotundus as preventive therapy against neurodegeneration.[60] The above research indicates that C. rotundus is a potent neuroprotective agent. Further research in this direction may bring out a herbal nootropic drug, which is the need of the hour.
Antiplatelet activity
Seo et al. in 2011 studied the antiplatelet effects of C. rotundus rhizome ethanolic extract as well as some of its components especially nootkatone. Their in vitro and in vivo studies showed that the extract inhibited platelet aggregation, thereby exhibiting antiplatelet activity. This was further confirmed by in vivo studies showing prolongation effects in bleeding time in rats. Of the different constituents examined, nootkatone in particular was found to inhibit platelet aggregation as well as prolong bleeding time in in vivo studies. The studies suggest that the extract and nootkatone in particular can be useful in the treatment of atherothrombotic diseases in which inhibition of platelet aggregation plays an important role.[61]
Antiobesity and the antihyperlipidemic activity
Ayurveda claim Musta to reduce muscle fat (meda). It has been categorized under lekhaneeya gana (group of drugs able to scrape off excess fat and kapha) in Caraka Samhita.[1] Studies using obese Zucker rats showed that supplementation of the diet with the hexane extracts of C. rotundus rhizomes at 220 mg/kg body weight reduced weight gain by 10%.[62] It was suggested that the effect was due to activation of the β3-AR (adrenoreceptor) by the extract, supporting “lekhaneeya” property indicated in Ayurveda.
Raut et al. reported the antihyperglycemic effect of the hydro-alcoholic extract of C. rotundus rhizomes on alloxan induced hyperglycemia in rats. The results showed that the extract at a dosage of 500 mg/kg was as effective as metformin standard at 450 mg/kg.[63] Similarly, studies by Ardestani on advanced glycation end products formation as a result of persistent hyperglycemia showed that C. rotundus extracts have protein glycation inhibitory and antioxidant activity.[64]
The methanolic extract of C. rotundus rhizomes showed inhibitory activity against alpha-glucosidase and alpha-amylase, enzymes involved in carbohydrate digestion. Compounds responsible for the activity were identified as a new flavone, namely (2RS, 3SR)-3,4’,5,6,7,8-hexahydroxyflavane as well as the known stilbene dimers cassigerol E and scirpusins A and B.[65]
C. rotundus has been mentioned as very effective in the treatment of lipid disorders that is, hyperlipidemia. Chandratre's research group has studied the lipid lowering activity of the aqueous as well as alcoholic extracts of the rhizomes of C. rotundus in rats. In acute toxicity studies, the two extracts were found to be safe up to a dosage of 2000 mg/kg. They found significant lowering of total cholesterol, triglyceride and low density lipoprotein levels in serum, although the effect on high density lipoprotein was found to be statistically insignificant. It was concluded that the studies validated the claims made in traditional medicine regarding the use of C. rotundus in hyperlipidemia although the specific constituents responsible for the activity were not identified.[66,67]
Ayurvedic literature including brhattrai (Caraka, Susruta and Vagbhata samhita) and nighantus (lexicons) and practices use Musta as a medohara (antilipidemic) drug. The studies mentioned above support the traditional usage of Musta as a lipid lowering drug.
Insecticidal and plasmodicidal activity
The essential oil of C. rotundus showed ovicidal and larvicidal activities against Aedes albopictus (Skuse) (the “Asian tiger mosquito”).[68] Similarly, Singh et al. in 2009 reported that the hexane extract of C. rotundus had repellent activity against three mosquito species (two malaria vectors and one filarial vector). It showed comparatively better activity than that of DEET (N, N diethyl- 3-methylbenzamide). Therefore, the extract can be an effective and safe personal protective measure against mosquito bites.[69]
The hexane extract of C. rotundus rhizomes also exhibited high potency against Plasmodium falciparum, a malarial parasite. Qualitative assessment of the antimalarial activity in vitro was determined by means of microculture radioisotope technique.[70]
The toxicity of different constituents of C. rotundus against the German cockroach Blatella germanica L was examined by Chang et al. in 2012. The encouraging results obtained suggest C. rotundus as a potential alternative to widely used synthetic insecticides.[71] Green insect repellants, safe for use in indoor environments are highly desired.
Cyperus scariosus R. Br.
Cyperus scariosus is known as Nagaramusta in Ayurveda. It is used in various traditional systems of medicine to cure fever, diarrhoea, thirst and burning sensation all over the body. The essential oil of C. scariosus has hypotensive, antiinflammatory, antimicrobial as well as CNS stimulating properties while the rhizome is used as a diuretic, stomachic and antidiarrheal.[27,72,73]
In Bangladesh, C. scariosus is used in tribal areas as a phytotherapeutic agent against dysentery, prompting Rahman et al. to investigate the ethanol extract of the rhizomes of this herb for antibacterial and cytotoxic activity. The petroleum ether extract showing high antimicrobial activity was subjected to fractionation using column chromatography. One of the fractions showing high antibacterial and high antifungal activity was subjected to various spectroscopic analyses, and a key compound was identified as longiverbenone, a sesquiterpene. Specifically, against Vibrio cholerae it showed minimum inhibitory concentration at 20 μg/ml and minimum bactericidal concentration at 80 μg/ml. In the brine shrimp assay for cytotoxic activity, the LC50 of longiverbenone was found to be 14.38 μg/ml.[74]
Gupta et al. studied the antiinflammatory activity of the essential oil of C. scariosus. They used 3 different techniques in their studies: Carrageenan-induced edema, cotton-pellet granuloma pouch test and adjuvant-induced arthritis. The oil at 100 mg/kg, i.p., dose showed 66.2% inhibition in the carrageenan-induced edema study and significant inhibition of granulation tissue formation comparable to that of hydrocortisone in the cotton-pellet induced granuloma pouch test and also suppressed adjuvant-induced arthritis. Thus, the studies indicated that C. scariosus oil possesses potent antiinflammatory activity against the exudative and proliferative phases of inflammation.[75]
Hepatoprotective activity of the aqueous-methanolic extract of C. scariosus against acetaminophen and carbon tetrachloride induced liver damage in rats was studied by Gilani and Janbaz. They found that pretreatment of rats with the plant extract at a dosage of 500 mg/kg was able to restore to normal the serum levels of alkaline phosphatase, oxaloacetate transaminase and glutamate pyruvate transaminase and reduced morbidity by 70%.[76]
Bhagwat investigated the immune-modulation potential of the aqueous-alcoholic extract of rhizomes of C. scariosus and its therapeutical potential as an antiinflammatory agent. C. scariosus inhibited Th1 cytokines suggesting its immunosuppressive potential. This indicates its potential use as an antiinflammatory agent and also in the treatment of rheumatoid arthritis.[77]
The methanolic extract of C. scariosus leaves showed significant antinociceptive and antihyperglycemic activity. The antinociceptive activity was determined using the model of acetic acid induced gastric pain in mice. At a dose of 200 mg/kg the extracts gave results comparable to that of aspirin at the same dose. Antihyperglycemic activity determined through glucose tolerance test in mice showed that a dose of 400 mg/kg extract was as effective as 10 mg/kg of glibenclamide.[78]
Cyperus scariosus is not used as extensively as C. rotundus and deserves to be investigated in greater detail.
Cryptocoryne spiralis (Retz.) Fisch ex Wydler
Cryptocoryne spiralis (Indian Ipecacuanha) is used widely in Sri Lanka as a traditional remedy for infantile vomiting and cough, and in adults for the treatment of abdominal complaints and fever.[27] However, a search of the literature has not revealed any studies carried out on the bioactivity of this plant. It is used as a substitute for Ipecacuanha root in the treatment of dysentery.[79] Usually, larger doses of Ipecacuanha are administered in the case of drug intolerance, but only after alkaloids have been removed from the drug (de-emetinised Ipecacuanha). Cryptocoryne spiralis, which does not contain emetine and cephaeline, is often used as a substitute. It is interesting to observe that C. rotundus, which is used in the treatment of diarrhea, contains only trace amount of alkaloids.
DISCUSSION
This review shows that both A. heterophyllum and C. rotundus have several similar pharmacological activities such as antiinflammatory, antipyretic, antibacterial and antidiarrheal. C. scariosus also possesses antiinflammatory and antibacterial activities.
Table 2 present the summary of the phytochemical and pharmacological activities of the reviewed species.
Table 2.
Summary of the reported phytochemical and pharmacological profiles of Ativisha, Musta and related species

It is a well-known fact that alkaloids like morphine have antidiarrheal activity, and alkaloids may be the key constituents responsible for the activity in the case of A. heterophyllum as well. While modern studies have shown that A. heterophyllum has good antimicrobial activity against gram-negative bacteria (diarrhea causing), the same does not seem to be the case with gram positive and other microbes.
In C. rotundus, especially, the mode of action studies carried out suggest that its antidiarrheal activity may be due to the effect on cell adherence and toxin production rather than by antimicrobial. The alkaloids reported as present by Jeong[81] are in such miniscule amounts that it is very unlikely that they are wholly responsible for the antidiarrheal action of the plant. Not much work has been done on the remaining two species on the antidiarrheal activity thus leaving a big lacuna as far as our understanding of their mode of activity and scientific legitimacy of substitution.
Report of the presence of alkaloids in C. rotundus in 2000[81] was the first hint of any possible similarities in the constituents of the two species. However, no comparative studies have been reported bringing out any possible similarities between the plants except for the experimental work based on abhava dravya initiated by Venkatasubramanian et al. Phytochemical studies and preliminary HPLC showed some similarities in the constituents of A. heterophyllum and C. rotundus. They have also reported the antidiarrheal activity of both drugs.[17]
The fact that C. rotundus and C. scariosus belong to the same Cyperaceae family could account for their substitution along with the fact that just visual observation makes their differentiation difficult for the uninformed user. Cryptocoryne spiralis shows presence of tannins which are known to be used in the treatment of diarrhea. The prominent South Indian name Naattu Athividayam for Cryptocoryne hints at the possibility of a potential and genuine substitute for A. heterophyllum, which needs to be further supported with scientific studies.
Comparative studies between the four species are warranted to throw more clarity on any commonalities in the phytoconstituents and bioactivities of the four species. This could pave the way for understanding the concept of abhava-pratinidhi dravya and also help identify legitimate substitutes for unavailable species. It is indeed striking that despite differences in morphological, botanical, phytochemical details, there are similarities in Ayurvedic pharmacologic classification and bioactivities of Ativisha and Musta [Table 2].
CONCLUSION
Identification of the substitute species appears to be mainly based on dravya karmukatva (pharmaco-dynamics) and karma (pharmacological action). There are striking similarities in the rasa (taste), guna (properties), vipaka (taste after digestion), doshakarma (action on dosha) and rogaghnata (therapeutical indications) of Ativisha and Musta [Table 1]. The difference in their veerya (potency), do not seems to alter the therapeutic actions. This indicates that similar bioactivities could be obtained from unrelated species even when the chemical composition and botanical identities may not be the same. This way of classification of materials based on the biological effect on the body could be called as pharmaco-taxonomy or functional taxonomy.
Many questions remain unanswered since details are not available in Ayurvedic literature about the drug/substitute identification processes. E.g., in formulations like chaturbhadra churna that contain both the abhava (Ativisha) as well as the pratinidhi (Musta) dravya, it is not clear if it is permitted to use Musta alone in excess as a substitute for Ativisha. Systematic research on abhava-pratinidhi dravya is still in its fledgling stage. Probing deeper into the Ayurvedic way of classifying a drug can help understand the pharmaco-dynamics of drugs and also how to identify new drugs.
ACKNOWLEDGMENTS
Thanks are due to Mr. DK Ved, Dr. GS Goraya, Dr. K Ravikumar and Dr. K Haridasan for authentic plant material collections. This project was funded by the Centre of Excellence, Ministry of Environment and Forestry (MoEF), which is gratefully acknowledged. Subrahmanya Kumar K acknowledges affiliation by Manipal University, for his PhD research on the topic of abhava pratinidhi dravya and to ETC-CAPTURED, The Netherlands for fellowship.
Footnotes
Source of Support: Centre of Excellence, Ministry of Environment and Forestry (MoEF), Government of India.
Conflicts of Interest: None declared.
REFERENCES
- 1.Sastri K, editor. 5th ed. I. Varanasi: Chaukhambha Sanskrit Sansthan; 1997. Caraka Samhita of Agnivesa, Sootrasthana. [Google Scholar]
- 2.Chunekar KC, editor. 1st ed. Varanasi: Chaukhambha Bharati Academy; 2004. Bhavaprakasa Nighantu of Sri Bhavamisra; p. 63. (127-8, 243-4). [Google Scholar]
- 3.Mishra SN, editor. 1st ed. Varanasi: Chaukhamba Surbharti Prakashan; 2007. Bhaishajyaratnavali of Kaviraj Govind Das Sen; pp. 80–2. [Google Scholar]
- 4.Shastry L, editor. 7th ed. Varanasi: Chaukhamba Sanskrit Sansthan; 2002. Yogaratnakara, with Vidyotini Hindi Commentary; p. 171. [Google Scholar]
- 5.Ved DK, Goraya GS. Dehradun: Bishen Singh Mahendrapal Singh and Bangalore, FRLHT; 2008. Demand and Supply of Medicinal Plants in India; p. 125. [Google Scholar]
- 6.Misra BS, editor. 2nd ed. Varanasi: Chaukhamba Sanskrit Sansthan; 2002. Bhavaprakasha; p. 234. [Google Scholar]
- 7.Sastry JL. 2nd ed. II. Varanasi: Chaukhambha Orientalia; 2005. Dravyaguna Vijnana; pp. 23–32. (551-7). [Google Scholar]
- 8.Ukani MD, Mehta NK, Nanavati DD. Aconitum heterophyllum (ativisha) in ayurveda. Anc Sci Life. 1996;16:166–71. [PMC free article] [PubMed] [Google Scholar]
- 9.O’Neil MJ, Smith A, Hecklman PE, Obenchain JR, Gallipeau JAR, D’Arecca MA. New Jersey: Merck and Co, Inc; 2001. The Merck Index; p. 118. [Google Scholar]
- 10.Hiremath SG. 1st ed. Bangalore: IBH Prakashana; 2000. Text Book of Bhaishajya Kalpna; p. 75. [Google Scholar]
- 11.Tripathi I, editor. 1st ed. Varanasi: Krishnadas Academy; 1982. Rajanighantu; p. 163. [Google Scholar]
- 12.Khare CP, editor. Heidelberg: Springer; 2004. Indian Herbal Remedies: Rational Western Therapy, Ayurvedic, and Other Traditional Usage, Botany; p. 16. [Google Scholar]
- 13.1st ed. Part-I. I. New Delhi: Government of India, Ministry of Health and Family Welfare, Department of Indian Systems of Medicine and Homoeopathy; 2001. Anonymous. The Ayurvedic Pharmacopoeia of India; pp. 22–3. [Google Scholar]
- 14.Kirtikar KR, Basu BD. 2nd ed. I. Dehradun: Bishen Singh Mahendra Pal Singh; 1993. Indian Medicinal Plants; pp. 34–6. [Google Scholar]
- 15.Part-I. I. New Delhi: Ministry of Health and Family Welfare, Dept of Indian Systems of Medicine and Homoeopathy, Government of India; 1981. Anonymous. The Unani Pharmacopoeia of India; pp. 11–2. [Google Scholar]
- 16.Madras: The Indian Medical Practitioners’ Co-operative Pharmacy and Stores Ltd; 1993. Anonymous. Formulary of Siddha Medicines; p. 331. (444-5). [Google Scholar]
- 17.Venkatasubramanian P, Kumar SK, Nair VS. Cyperus rotundus, a substitute for Aconitum heterophyllum: Studies on the ayurvedic concept of Abhava Pratinidhi Dravya (drug substitution) J Ayurveda Integr Med. 2010;1:33–9. doi: 10.4103/0975-9476.59825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Verma S, Ojha S, Raish M. Anti-inflammatory activity of Aconitum heterophyllum on cotton pellet-induced granuloma in rats. J Med Plants Res. 2010;4:1566–9. [Google Scholar]
- 19.1st ed. Part-I. Vol. 3. New Delhi: Government of India, Ministry of Health and Family Welfare, Department of Indian Systems of Medicine and Homoeopathy; 2001. Anonymous. The Ayurvedic Pharmacopoeia of India; pp. 129–30. [Google Scholar]
- 20.Ahmad M, Ahmad W, Ahmad M, Zeeshan M, Obaidullah, Shaheen F. Norditerpenoid alkaloids from the roots of Aconitum heterophyllum Wall with antibacterial activity. J Enzyme Inhib Med Chem. 2008;23:1018–22. doi: 10.1080/14756360701810140. [DOI] [PubMed] [Google Scholar]
- 21.Atal CK, Sharma ML, Kaul A, Khajuria A. Immunomodulating agents of plant origin. I: Preliminary screening. J Ethnopharmacol. 1986;18:133–41. doi: 10.1016/0378-8741(86)90025-5. [DOI] [PubMed] [Google Scholar]
- 22.Hamet R. Physiological effect of atisine, an alkaloid of Aconitum heterophyllum Wallich. C R Seances Soc Biol Fil. 1938;128:479–82. [Google Scholar]
- 23.Hamet R. Hypertensive effect of Aconitum heterophyllum Wallich. C R Seances Soc Biol Fil. 1954;148:1221–4. [PubMed] [Google Scholar]
- 24.Nisar M, Ahmad M, Wadood N, Lodhi MA, Shaheen F, Choudhary MI. New diterpenoid alkaloids from Aconitum heterophyllum Wall: Selective butyrylcholinestrase inhibitors. J Enzyme Inhib Med Chem. 2009;24:47–51. doi: 10.1080/14756360801906202. [DOI] [PubMed] [Google Scholar]
- 25.Pattewar AM, Pandharkar TM, Yerawar PP, Patawar VA. Evaluation of in-vitro antihelminthic activity of Aconitum heterophyllum. J Chem Biol Phys Sci. 2012;2:2401–7. [Google Scholar]
- 26.Subash AK, Augustine A. Hypolipidemic effect of methanol fraction of Aconitum heterophyllum wall ex Royle and the mechanism of action in diet-induced obese rats. J Adv Pharm Technol Res. 2012;3:224–8. doi: 10.4103/2231-4040.104713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kirtikar KR, Basu BD. 2nd ed. IV. Dehradun: Bishen Singh Mahendra Pal Singh; 1993. Indian Medicinal Plants; pp. 2637–9. [Google Scholar]
- 28.Sharma PV, editor. 1st ed. III. Varanasi: Chaukhambha Visvabharati; 2001. Susruta Samhita. [Google Scholar]
- 29.Singh N, Pandey BR, Varma P, Bhalla M, Gilca M. Phyto-pharmacotherapeutics of Cyperus rotundus Linn. (Motha): An overview. Indian J Nat Prod Resour. 2012;3:467–6. [Google Scholar]
- 30.Daswani PG, Birdi TJ, Antia NH. Study of the action of Cyperus rotundus root decoction on the adherence and enterotoxin production of diarrhoeagenic Escherichia coli. Indian J Pharmacol. 2001;33:116–7. [Google Scholar]
- 31.Daswani PG, Brijesh S, Tetali P, Birdi TJ. Studies on the activity of Cyperus rotundus Linn. Tubers against infectious diarrhea. Indian J Pharmacol. 2011;43:340–4. doi: 10.4103/0253-7613.81502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Uddin SJ, Mondal K, Shilpi JA, Rahman MT. Antidiarrhoeal activity of Cyperus rotundus. Fitoterapia. 2006;77:134–6. doi: 10.1016/j.fitote.2004.11.011. [DOI] [PubMed] [Google Scholar]
- 33.Biradar S, Kangralkar VA, Mandavkar Y, Thakur M, Chougule N. Antiinflammatory, antiarthritic, analgesic and anticonvulsant activity of Cyperus essential oils. Int J Pharm Pharm Sci. 2010;2:112–5. [Google Scholar]
- 34.Tsoyi K, Jang HJ, Lee YS, Kim YM, Kim HJ, Seo HG, et al. (+)-Nootkatone and (+)-valencene from rhizomes of Cyperus rotundus increase survival rates in septic mice due to heme oxygenase-1 induction. J Ethnopharmacol. 2011;137:1311–7. doi: 10.1016/j.jep.2011.07.062. [DOI] [PubMed] [Google Scholar]
- 35.Kim SJ, Ryu B, Kim HY, Yang YI, Ham J, Choi JH, et al. Sesquiterpenes from the rhizomes of Cyperus rotundus and their potential to inhibit LPS- induced nitric oxide production. Bull Korean Chem Soc. 2013;34:2207–10. [Google Scholar]
- 36.Jung SH, Kim SJ, Jun BG, Lee KT, Hong SP, Oh MS, et al. A-Cyperone, isolated from the rhizomes of Cyperus rotundus, inhibits LPS-induced COX-2 expression and PGE2 production through the negative regulation of NF?B signalling in RAW 264?B signalling in RAW 2647 cells. J Ethnopharmacol. 2013;147:208–14. doi: 10.1016/j.jep.2013.02.034. [DOI] [PubMed] [Google Scholar]
- 37.Soumaya KJ, Dhekra M, Fadwa C, Zied G, Ilef L, Kamel G, et al. Pharmacological, antioxidant, genotoxic studies and modulation of rat splenocyte functions by Cyperus rotundus extracts. BMC Complement Altern Med. 2013;13:28. doi: 10.1186/1472-6882-13-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.El-Gohary HM. Study of essential oils of the tubers of Cyperus rotundus L. and Cyperus alopecuroides Rottb. Bull Fac Pharm Cairo Univ. 2004;42:157–64. [Google Scholar]
- 39.Sini S, Malathy NS. Antimicrobial properties of roots of medicinal plants. Anc Sci Life. 2005;25:62–5. [PMC free article] [PubMed] [Google Scholar]
- 40.Yu HH, Lee DH, Seo SJ, You YO. Anticariogenic properties of the extract of Cyperus rotundus. Am J Chin Med. 2007;35:497–505. doi: 10.1142/S0192415X07005016. [DOI] [PubMed] [Google Scholar]
- 41.Kilani S, Bouhlel I, BenAmmar R, BenSghaiar M, Skandrani I, Boubaker J, et al. Chemical investigation of different extracts and essential oil from the tubers of (Tunisian) Cyperus rotundus. Correlation with their antiradical and antimutagenic properties. Ann Microbiol. 2007;57:657–64. [Google Scholar]
- 42.Ahmad M, Mahayrookh, Mehjabeen, Rehman AB, Jahan N. Analgesic, antimicrobial and cytotoxic effect of Cyperus rotundus alcoholic extract. Pak J Pharm. 2012;29:7–13. [Google Scholar]
- 43.Nima ZA, Jabier MS, Wagi RI, Hussain HA. Extraction, identification and antibacterial activity of Cyperus oil from Iraqi C. rotundus. Eng Technol. 2008;26:1156. [Google Scholar]
- 44.Balpande SM, Cherian KJ. Phytochemical analysis of aqueous methanol extract of Cyperus rotundus and Vetiveria zizanoides and its antifungal activities on indoor airborne fungi of some Schools in Nagpur City, India. J Environ Res Dev. 2013;7:1597–601. [Google Scholar]
- 45.Bisht A, Bisht GR, Singh M, Gupta R, Singh V. Chemical composition and antimicrobial activity of essential oil of tubers of C. rotundus Linn. Collected from Dehradun (Uttarakhand) Int J Res Pharm Biomed Sci. 2011;2:661–5. [Google Scholar]
- 46.Biradar SS, Rasal VP, Biradar SS, Thakur M. Antimicrobial and anthelmintic effect of Cyperus esculentus Linn. and Cyperus rotundus Linn. Essential oils. Pharm Online. 2010;1:19–24. [Google Scholar]
- 47.Sharma SK, Singh AP. Morphological microscopical and physicochemical investigations on the rhizomes of Cyperus rotundus Linn. Res J Pharm Biol Chem Sci. 2011;2:798–806. [Google Scholar]
- 48.Kilani-Jaziri S, Bhouri W, Skandrani I, Limem I, Chekir-Ghedira L, Ghedira K. Phytochemical, antimicrobial, antioxidant and antigenotoxic potentials of Cyperus rotundus extracts. S Afr J Bot. 2011;77:767–76. [Google Scholar]
- 49.Nagulendran KR, Velavan S, Mahesah R, Begum HV. In vitro antioxidant activity and total polyphenolic content of Cyperus rotundus rhizomes. E-J Chem. 2007;4:440–9. [Google Scholar]
- 50.Kilani S, Ben Sghaier M, Limem I, Bouhlel I, Boubaker J, Bhouri W, et al. In vitro evaluation of antibacterial, antioxidant, cytotoxic and apoptotic activities of the tubers infusion and extracts of Cyperus rotundus. Bioresour Technol. 2008;99:9004–8. doi: 10.1016/j.biortech.2008.04.066. [DOI] [PubMed] [Google Scholar]
- 51.Seema S, Priya CN, Vijayalakshmi K. Comparative study on antioxidant potential and anticataract activity of Cyperus rotundus and Cyamopsis tetragonolobus. Bioscon. 2011;6:61–6. [Google Scholar]
- 52.Kilani-Jaziri S, Neffati A, Limem I, Boubaker J, Skandrani I, Sghair MB, et al. Relationship correlation of antioxidant and antiproliferative capacity of Cyperus rotundus products towards K562 erythroleukemia cells. Chem Biol Interact. 2009;181:85–94. doi: 10.1016/j.cbi.2009.04.014. [DOI] [PubMed] [Google Scholar]
- 53.Pal DK, Dutta S. Evaluation of the antioxidant activity of the roots and rhizomes of Cyperus rotundus L. Indian J Pharm Sci. 2006;68:256–8. [Google Scholar]
- 54.Bashir A, Bushra S, Akhtar FH, Munir A, Amjad M, Hassan Q. Investigation on the antioxidant activity of Dheela Grass (Cyperus rotundus) Afr J Basic Appl Sci. 2012;4:1–6. [Google Scholar]
- 55.Guldur ME, Ozgonul A, Kilic IH, Sogut O, Ozaslan M. Gastroprotective effect of Cyperus rotundus extract against gastric mucosal injury induced by ischemia and reperfusion in rats. Int J Pharm. 2010;6:104–10. [Google Scholar]
- 56.Jin JH, Lee DU, Kim YS, Kim HP. Anti-allergic activity of sesquiterpenes from the rhizomes of Cyperus rotundus. Arch Pharm Res. 2011;34:223–8. doi: 10.1007/s12272-011-0207-z. [DOI] [PubMed] [Google Scholar]
- 57.Pal D, Dutta S, Sarkar A. Evaluation of CNS activities of ethanol extract of roots and rhizomes of Cyperus rotundus in mice. Acta Pol Pharm. 2009;66:535–41. [PubMed] [Google Scholar]
- 58.Sunil AG, Kesavanarayanan KS, Kalaivani P, Sathiya S, Ranju V, Priya RJ, et al. Total oligomeric flavonoids of Cyperus rotundus ameliorates neurological deficits, excitotoxicity and behavioral alterations induced by cerebral ischemic-reperfusion injury in rats. Brain Res Bull. 2011;84:394–405. doi: 10.1016/j.brainresbull.2011.01.008. [DOI] [PubMed] [Google Scholar]
- 59.Hemanth Kumar K, Tamatam A, Pal A, Khanum F. Neuroprotective effects of Cyperus rotundus on SIN-1 induced nitric oxide generation and protein nitration: Ameliorative effect against apoptosis mediated neuronal cell damage. Neurotoxicology. 2013;34:150–9. doi: 10.1016/j.neuro.2012.11.002. [DOI] [PubMed] [Google Scholar]
- 60.Kumar KH, Khanum F. Hydroalcoholic extract of Cyperus rotundus ameliorates H2O2-induced human neuronal cell damage via its anti-oxidative and anti-apoptotic machinery. Cell Mol Neurobiol. 2013;33:5–17. doi: 10.1007/s10571-012-9865-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Seo EJ, Lee DU, Kwak JH, Lee SM, Kim YS, Jung YS. Antiplatelet effects of Cyperus rotundus and its component (+)-nootkatone. J Ethnopharmacol. 2011;135:48–54. doi: 10.1016/j.jep.2011.02.025. [DOI] [PubMed] [Google Scholar]
- 62.Lemaure B, Touché A, Zbinden I, Moulin J, Courtois D, Macé K, et al. Administration of Cyperus rotundus tubers extract prevents weight gain in obese Zucker rats. Phytother Res. 2007;21:724–30. doi: 10.1002/ptr.2147. [DOI] [PubMed] [Google Scholar]
- 63.Raut NA, Gaikwad NJ. Antidiabetic activity of hydro-ethanolic extract of Cyperus rotundus in alloxan induced diabetes in rats. Fitoterapia. 2006;77:585–8. doi: 10.1016/j.fitote.2006.09.006. [DOI] [PubMed] [Google Scholar]
- 64.Ardestani A, Yazdanparast R. Cyperus rotundus suppresses AGE formation and protein oxidation in a model of fructose-mediated protein glycoxidation. Int J Biol Macromol. 2007;41:572–8. doi: 10.1016/j.ijbiomac.2007.07.014. [DOI] [PubMed] [Google Scholar]
- 65.Tran HH, Nguyen MC, Le HT, Nguyen TL, Pham TB, Chau VM, et al. Inhibitors of a-glucosidase and a-amylase from Cyperus rotundus. Pharm Biol. 2014;52:74–7. doi: 10.3109/13880209.2013.814692. [DOI] [PubMed] [Google Scholar]
- 66.Chandratre RS, Chandarana S, Mengi SA. Lipid lowering activity of alcoholic extract of Cyperus rotundus. Int J Res Pharm Chem. 2011;1:1042–5. [Google Scholar]
- 67.Chandratre RS, Chandarana S, Mengi SA. Effect of aqueous extract of Cyperus rotundus on hyperlipidaemia in rat model. Int J Pharm Biol Arch. 2012;3:598–600. [Google Scholar]
- 68.Kempraj V, Bhat SK. Ovicidal and larvicidal activities of Cyperus giganteus Vahl and Cyperus rotundus Linn. Essential oils against Aedes albopictus (Skuse) Nat Prod Radiance. 2008;7:416–9. [Google Scholar]
- 69.Singh SP, Raghavendra K, Dash AP. Evaluation of hexane extract of tuber of root of Cyperus rotundus Linn (Cyperaceae) for repellency against mosquito vectors. J Parasitol Res 2009. 2009:1–5. doi: 10.1155/2009/908085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Thebtaranonth C, Thebtaranonth Y, Wanauppathamkul S, Yuthavong Y. Antimalarial sesquiterpenes from tubers of Cyperus rotundus: Structure of 10,12-peroxycalamenene, a sesquiterpene endoperoxide. Phytochemistry. 1995;40:125–8. doi: 10.1016/0031-9422(95)00260-e. [DOI] [PubMed] [Google Scholar]
- 71.Chang KS, Shin EH, Park C, Ahn YJ. Contact and fumigant toxicity of Cyperus rotundus steam distillate constituents and related compounds to insecticide-susceptible and -resistant Blattella germanica. J Med Entomol. 2012;49:631–9. doi: 10.1603/me11060. [DOI] [PubMed] [Google Scholar]
- 72.Khare CP. New York: Springer; 2007. Indian Medicinal Plants an Illustrated Dictionary; pp. 195–6. [Google Scholar]
- 73.Khare CP. Berlin: Springer; 2004. Indian Herbal Remedies-Rational Western Therapy, Ayurvedic and Other Traditional Usage, Botany; pp. 15–6. [Google Scholar]
- 74.Rahman MS, Anwar MN. Antibacterial and cytotoxic activity of Longiverbenone Isolated from the rhizome of Cyperus scariosus. Bangladesh J Microbiol. 2008;25:82–4. [Google Scholar]
- 75.Gupta SK, Sharma RC, Aggarwal OP, Arora RB. Anti-inflammatory activity of the oil isolated from Cyperus scariosus (R. Br.) Indian J Exp Biol. 1972;10:41–2. [PubMed] [Google Scholar]
- 76.Gilani AU, Janbaz KH. Studies on protective effect of Cyperus scariosus extract on acetaminophen and CCl4-induced hepatotoxicity. Gen Pharmacol. 1995;26:627–31. doi: 10.1016/0306-3623(94)00200-7. [DOI] [PubMed] [Google Scholar]
- 77.Bhagwat D, Kharya MD, Bani S, Pandey A, Chauhan PS, Kour K, et al. Cyperus scariosus chloroform fraction inhibits T cell responses in Balb/C mice. Trop J Pharm Res. 2009;8:399–408. [Google Scholar]
- 78.Alam MA, Jahan R, Rahman S, Das AK, Rahmatullah M. Antinociceptive and anti-hyperglycemic activity of methanol leaf extract of Cyperus scariosus. Pak J Pharm Sci. 2011;24:53–6. [PubMed] [Google Scholar]
- 79.Greenish HG. 3rd ed. Jodhpur: Scientific Publishers (India); 1999. Materia Medica; pp. 323–9. [Google Scholar]
- 80.Ikram M, Khattak SG, Gilani SN. Antipyretic studies on some indigenous Pakistani medicinal plants: II. J Ethnopharmacol. 1987;19:185–92. doi: 10.1016/0378-8741(87)90040-7. [DOI] [PubMed] [Google Scholar]
- 81.Jeong SJ, Miyamoto T, Inagaki M, Kim YC, Higuchi R. Rotundines A-C, three novel sesquiterpene alkaloids from Cyperus rotundus. J Nat Prod. 2000;63:673–5. doi: 10.1021/np990588r. [DOI] [PubMed] [Google Scholar]


