Abstract
Achyranthes coynei is a rare, endemic perennial shrub reported from Karnataka and Maharashtra states of India. The plant is used to treat various disorders by folk healers and was proven to have antimicrobial and antioxidant properties. The present study was undertaken to evaluate microscopic and macroscopic characters of A. coynei stem, along with its physicochemical parameters. ProgRes® CapturePro and Microsoft Excel were used for statistical analysis. Perennial, shrubby nature and woody stem were the distinguishing morphological characters observed. Transverse section (TS) illustrated quadrangular outline of the stem and showed the presence of two types of trichomes on the thick-walled epidermis. TS also showed number of rosette calcium oxalates crystals; prismatic and microsphenoid crystals; conjoint, collateral open secondary vascular bundles; and two amphixylic medullary bundles in the pith. Ash and extractive values, micro and macro elements and nutritive factors were estimated in the present study. The presence of alkaloids, saponins and triterpenoids were observed in preliminary phytochemical screening. High-performance thin layer chromatographic analysis yielded different bands and also indicated the presence of oleanolic acid. The studied parameters for A. coynei stem will be useful for identification and authentication of the plant material.
Keywords: Achyranthes coynei, Amaranthaceae, endemic, high-performance thin layer chromatography, pharmacognosy, physicochemistry
INTRODUCTION
Ayurveda prescribes the use of medicinal plants in a holistic approach to human health. “Apamarga” (Achyranthes aspera L.) is one such medicinal plant used in Ayurveda for treating various diseases.[1,2] Achyranthes coynei Santapau (family Amaranthaceae) is other species from the same genus and shows morphological similarities with A. aspera. This rare, endemic plant is locally known as “Kempu Uttarani” (Kannada) or “Lal Aghada” (Marathi).[3,4,5] It is used in the treatment of fever, cough, piles etc., similar to or in place of A. aspera by local folklore practitioners.[3,4]
Each plant as a medicine shows distinctive characters in terms of its botany, chemistry and healing potentials, and hence the changes in their morphology and structures can be the quality indicators of the drug materials.[6] Kokate et al., suggested that quality control of a crude drug can be achieved by studying the pharmacognostic features of the plant materials.[7] As the quality of the plant material determines the efficacy of the drug, it is essential to study every medicinal plant for its differentiating characters from the others.[6] In this context, it was found that, no pharmacognostic studies have been carried out on stem of A. coynei. Hence, the present study was aimed to screen the stem of A. coynei for its preliminary pharmacognostic parameters.
MATERIALS AND METHODS
Collection of plant material
The Stem of A. coynei was collected from Madhanbhavi, Belgaum and authenticated herbarium was deposited at Regional Medical Research Centre (Indian Council of Medical Research), Belgaum, Karnataka (Voucher Specimen No. RMRC 784).
Chemicals, reagents, and solvents
All chemicals, reagents and solvents used during the experimentation were of analytical grade.
Macroscopic and microscopic analysis
Key morphological features of the stem were observed using a dissecting microscope (Labomed, India). Transverse section (TS) of the stem was taken using LEICA CM (1850) cryostat. Fresh plant material was mounted on the specimen disk covered with tissue freezing medium (Jung). The specimen disks were kept for freezing at − 18°C ± 2°C (30 min), and sections were taken at a thickness of 20 ± 2 μ. Histochemical and powder microscopy were carried out by using standard reagents and stains.[8] Various sensory parameters (color, odor, and taste) of the stem were studied by organoleptic evaluation.[8]
Microphotographs were taken using a microscope (Olympus BX 41) at different magnifications (×4, ×10 and ×40) with the inbuilt analog camera (ProgRessC3-JENOPTIK) using software ProgRes®CapturePro2.1.1-JENOPTIK laser optical system manufactured by JENOPTIK.
Preliminary phytochemical analysis
The powdered material was extracted by a continuous shaking method using methanol and water for overnight. The filtrate was evaporated to dryness and stored at 4°C for further use. These extracts were subjected to preliminary phytochemical analysis following the tests given by Khandelwal.[8]
Physico-chemical and nutritive content analysis
Physico-chemical parameters were determined as per standard procedures.[1,8] Determination of macro and microelements were estimated using atomic absorption techniques.[9,10] Nitrogen content was estimated by Kjeldahl method.[9,10] Nutritive contents were estimated by standard methodologies.[6,9,10]
High-performance thin layer chromatography analysis
Extraction method and solvent system given by Tandon,[7] was employed for high-performance thin-layer chromatographic (HPTLC) analysis. The method given by Pai et al.,[11] were used for other general parameters. The data were generated using CAMAG TLC scanner (540 nm) and visualizer.
Statistical and data analysis
Cell dimensions were represented as RDS (Radius for circle), DST (Length of line) and Maj (Length of large half axis for an ellipse) as defined in ProgRes® CapturePro2.1.1-JENOPTIK, software. The Standard deviation was calculated as a mean of three replicates using Microsoft Excel (2007).
RESULTS
Morphological description
Achyranthes coynei is a tall (2–4.5 m), perennial shrub [Figure 1a] growing wild on bunds of small streams and canals in agricultural lands. The Stem is woody at the base with long, opposite branches. Branches are striate, terete or obsoletely quadrangular. Young branches tinged with purple color, pubescent with swollen nodes [Figure 1b].
Figure 1.

(a) Achyranthes coynei habit; (b) stem; (c) stem powder; (d) dried stem; (e) transverse section (TS) of stem (entire); (f) TS of stem (portion); (g) medullary bundle; (h) unstained TS of stem; (i) rosette calcium oxalate crystals; (j) cluster of prismatic calcium oxalate crystals; (k) trichomes; (l) long, multicellular warty trichome; (m) short, glandular trichome; (n and o) xylem vessels; (p) high performance thin layer chromatography (HPTLC) densitogram; (q) HPTLC image; (r) peak values magnification: As given above for each image; stain used: Phloroglucinol with concentrated HCl (1:1). cam: Cambium, chl: Chlorenchyma cells, col: Collenchyma cells, cu: Cuticle, end: Endodermis, epi: Epidermis, g-tri: Glandular trichomes with blunt apex, mb: Medullary bundle, mr: Medullary rays, par: Parenchyma, per: Pericycle, ph: Phloem, rcc: Rosette calcium oxalate crystals, tri: Trichome, t-tri: Long; multicellular warty trichome, vb: Vascular bundle, x: Xylem
Morphological differences between Achyranthes coynei and Achyranthes aspera
Morphologically both the species were similar in vegetative conditions. However, A. coynei is perennial shrub [Figure 1a] whereas A. aspera is annual herb [Figure 2a]. Flowers of A. coynei were rosy-purplish [Figure 1a and b] whereas flowers of A. aspera were greenish white in nature [Figure 2a and b]. Young branches of A. aspera show greenish white [Figure 2c] nature whereas A. coynei exhibit purple color [Figure 1b]. The dried stem of both the species appears morphologically similar but the bark of A. coynei exhibit hard nature [Figures 1c and 2d].
Figure 2.
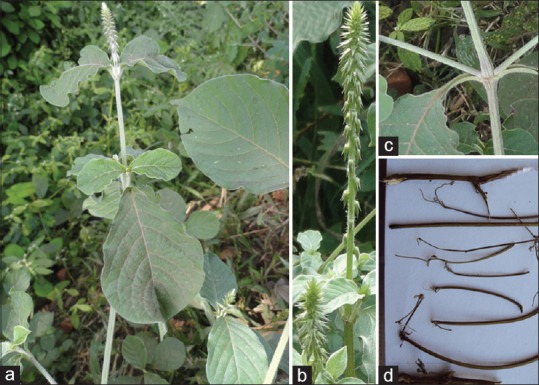
(a) Achyranthes aspera habit; (b) inflorescence; (c) stem; (d) dried stem
Organoleptic characters
The dried stem was brown in color [Figure 1d]. Stem powder was asparagus green [Figure 1c], without any specific odor and taste. The dried stem was hard in nature whereas the powder was coarse in texture.
Anatomical description and powder microscopy
The TS of the young stem of A. coynei showed quadrangular outline [Figure 1e, f, and h] with wavy margins whereas mature stem was cylindrical in nature. Epidermis was single layered, showing rectangular cells (size WD: 5.724–14.24 square μm) with convex nature on the upper and lower margins covered by thick cuticle (thickness of DST: 1.213–5.387 μm). Small glandular trichomes and few long, pointed apex multicellular (2–4 cells) warty trichomes (DST: 249.4–443.7 μm) were present on the epidermis. The glandular trichomes were short, irregularly bent with globular heads (DST: 28.47–127.8 μm) containing 2–4 cells.
Cortex was 5–12 layered, comprising collenchyma and parenchyma layers. Both collenchyma and parenchyma showed the presence of large number of rosette calcium oxalates crystals [Figure 2h] of varied sizes (RDS: 30.45–9.283 μm). Few clusters of prismatic calcium oxalate crystals (DST: 2.307–19.8 μm) and idioblast with microsphenoid crystals were also observed.
The transverse section showed the presence of distinct endodermis with a ring of well-developed vascular bundles. Vascular bundles were situated opposite to discontinuous pericycle made up of lignified pericyclic fibers. Pericycle was followed by a layer of parenchymatous cells, intermittently merged with phloem. Vascular tissues showed the presence of secondary growth with incomplete rings of xylem and phloem [Figure 1e, f, and h]. Vascular bundles were conjoint, collateral, open with centrifugal phloem and centripetal xylem. Secondary xylems followed by distinct cambial cells were seen in TS. Xylem consisted of annular, spiral, reticulate, pitted radial rows of vessels; lignified, elongated fibers and tracheids. Medullary rays were observed in continuation of phloem. Pith showed different sized, round, oval and/or polygonal types of parenchymatous cells with intercellular spaces. Two diametrically opposite, round or oval shaped medullary bundles were situated in the ground tissue [Figure 1e]. The medullary bundles were amphixylic in nature [Figure 1g].
Powder microscopy revealed presence of rosette crystals of calcium oxalate [Figure 1i], prismatic crystals [Figure 1j], long, pointed and short glandular trichomes [Figure 1k-m], epidermal cells [Figure 1i], sclernchyma and parenchyma cells [Figure 1i] and fragmented, annular [Figure 1n], reticulate and pitted [Figure 1o] xylem vessels.
Histochemical analysis
Stem showed the presence of calcium oxalate crystals, starch grains and cellulose after reacting with the standard stains used.
Physicochemical parameters
The results of physiochemical analysis such as total ash, acid soluble ash, moisture content, extractive values, macro elements (N, P, K, S, Ca, Mg, Na), micro elements (Fe, Zn, Cu, Mn), nutritive values (reducing, nonreducing sugars, total carbohydrates, starch, protein) are represented in Table 1.
Table 1.
Physico chemical parameters and nutritive content

Phytochemical analysis
Water and ethanol extracts showed the presence of triterpenoids, alkaloids, glycosides, steroids, and saponins.
High-performance thin layer chromatography analysis
For fast screening of the triterpenoids, the better separation was observed with chloroform: methanol (9:1 v/v). The densitogram, HPTLC image and peak values were presented as Figure 1p-r. In all, 12 peaks were identified in the densitogram. Among the 12 peaks, peak 3, 8 and 10 showed an area under curve more than 15%. Peak 10 had a highest percent area (27.73%) and height at Rf (max) 0.57.
DISCUSSION
Achyranthes coynei was first reported by Santapau from wet areas of Khandala, later the species was recorded from Raigad, Sindhudurg, Thane and Amaravati districts of Maharashtra.[3,5] The habitat of A. coynei was reported to be the damp environment.[3,5]
Similar morphological differences were observed by Pai et. al., between A. coynei and A. aspera from the present study area.[3] The bigger size and hard nature of old stem in A. coynei can be used to differentiate it from A. aspera. Dastur[12] and Joshi[13] observed two medullary bundles at the internodes, converging to form one amphixylic medullary bundles towards the inflorescence in TS of A. aspera stem. In the present study, TS of A. coynei stem showed two amphixylic medullary bundles in the ground tissue. The TS and powder study revealed similarities in both the species in terms of prismatic and rosette shaped calcium oxalate crystals, long, multicellular, warty, pointed apex and short, blunt, glandular trichomes and spiral and annular xylem vessels.[1,2] The microscopic analysis of A. coynei leaves also showed similar cellular structures.[14]
The ash content of A. coynei lies within the range, as reported for A. aspera in Ayurvedic pharmacopeia.[1] The results of water and ethanol extractive studies of A. coynei revealed the presence of secondary metabolites in the powdered sample.[15] Upadhya et al., reported considerable amount of total phenolic content from stem of A. coynei, which was further reported to be responsible for in vitro antioxidant activities[16] and also may be to the antimicrobial activities.[17] Among the macro and micro elements estimated, calcium and iron content were high respectively in the stem of A. coynei [Table 1].
The results of HPTLC analysis for triterpenoids from A. coynei stem extract were in accordance with the reports of Tondon, for A. aspera, where the band with Rf value 0.57 was attributed to oleanolic acid for A. aspera.[2] In the present study, similar observations for Rf value of 0.57 with the intense blue color band was recorded. This indicated the presence of oleanolic acid, as also reported by Upadhya et al., using HPLC method.[18]
This is the first report on microscopic and physicochemical evaluation of A. coynei stem. The results of the present study will be helpful in quantitative and qualitative standardization of A. coynei. However, detailed differential studies using molecular and chemical markers are required for A. coynei and other species of genus Achyranthes, for their authentication especially in their drug form. Studies on various biological activities, similar to that of A. aspera, are needed to be conducted to understand the basis for the traditional usage of A. coynei.
ACKNOWLEDGMENT
Authors are grateful to the Director-in-Charge, Regional Medical Research Centre (ICMR), Belgaum for the facilities and ANCHROM, Mumbai for providing HPTLC facility. Authors are also thankful to Mr. Bhoopal Talawar, lab attendant for his assistance and Mr. Manjunath S. Kolkar, Bailhongal for his help in plant collection. Vinayak Upadhya is indebted to Indian Council of Medical Research, New Delhi for Senior Research Fellowship during the study.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Part 2. Vol. 2. New Delhi: Department of AYUSH Ministry of Health and Family Welfare; 2007. Anonymous. The Ayurveda Pharmacopeia of India; pp. 7–9. [Google Scholar]
- 2.Tandon N, editor. 1st ed. Vol. 9. New Delhi: Indian Council of Medical Research; 2011. Quality Standards of Indian Medicinal Plants; pp. 18–31. [Google Scholar]
- 3.Pai SR, Upadhya V, Hegde HV, Kholkute SD. Achyranthes coynei Santapau (Amranthaceae) – An addition of endemic taxon to Flora of Karnataka, India. J Threat Taxa. 2011;3:1875–9. [Google Scholar]
- 4.Gurudeva MR. 2nd ed. Bangalore: Divyachandra Prakashana; 2001. Karnatakada Aushadhiya Sasyagalu (Kannada) pp. 36–9. [Google Scholar]
- 5.Sharma BD, Kulkarni BG. Achyranthes coynei Santapau, Amaranthaceae. In: Nayar MP, Sastry AR, editors. Red Data Book of Indian Plants. Vol. 2. Calcutta: Botanical Survey of India; 1987. pp. 8–9. [Google Scholar]
- 6.Hegde SV, Hegde GR, Mulgund GS, Upadhya V. Pharmacognostic evaluation of leaf and fruit of Capsicum frutescens (Solanaceae) Pharmacognsy J. 2014;6:14–22. [Google Scholar]
- 7.Kokate CK, Purohit AP, Gokhale SB. 36th ed. Pune: Niarali Publications; 2006. Pharmacognosy. [Google Scholar]
- 8.Khandelwal KR. 10th ed. Pune: Nirali Publication; 2003. Practical Pharmacognosy. [Google Scholar]
- 9.Thimmaiah SK. 1st ed. Calcutta: Kalyani Publisher; 1999. Standard Methods of Biochemical analysis. [Google Scholar]
- 10.Chopra SL, Kanwar JS. 1st ed. Calcutta: Kalyani Publishers; 1991. Analytical Agricultural Chemistry. [Google Scholar]
- 11.Pai SR, Upadhya V, Hegde HV, Joshi RK, Kholkute SD. New report of triterpenoid betulinic acid along with oleanolic acid from Achyranthes aspera by reversed-phase-ultra flow liquid chromatographic analysis and confirmation using high-performance thin-layer chromatographic and fourier transform-infrared spectroscopic techniques. J Planar Chromatogr. 2014;27:38–41. [Google Scholar]
- 12.Dastur RH. The origin and course of vascular bundles in Achyranthes aspera L. Ann Bot. 1925;39:539–45. [Google Scholar]
- 13.Joshi AC. Variations in the medullary bundles of Achyranthes apera L. and the original hone of the species. New Phytol. 1934;33:53–7. [Google Scholar]
- 14.Ankad GM, Pai SR, Upadhya V, Hurkadale PJ, Hegde HV. Pharmacognostic evaluation of Achyranthes coynei: Leaf. Egypt J Basic Appl Sci. 2014;2:25–31. [Google Scholar]
- 15.Bigoniya P, Singh CS, Srivastava B. Pharmacognostical and physic-chemical standardization of Syzgium cumini and Azadirachta indica seed. Asian Pac J Trop Biomed. 2012;2:S290–5. [Google Scholar]
- 16.Upadhya V, Pai SR, Ankad G, Hurkadale PJ, Hegde HV. Phenolic contents and antioxidant properties from aerial parts of Achyranthes coynei Sant. Indian J Pharm Sci. 2013;75:483–6. doi: 10.4103/0250-474X.119812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ankad G, Upadhya V, Pai SR, Hegde HV, Roy S. In vitro antimicrobial activity of Achyranthes coynei Sant. Asian Pac J Trop Dis. 2013;3:930–5. [Google Scholar]
- 18.Upadhya V, Ankad GM, Pai SR, Hegde HV, Kholkute SD. Accumulation and trends in distribution of three triterpenoids in various parts of Achyranthes coynei determined using RP-UFLC analysis. Pharmacogn Mag. 2014;10:398–401. doi: 10.4103/0973-1296.141761. [DOI] [PMC free article] [PubMed] [Google Scholar]


