Abstract
Background:
Jyotishmati, scientifically known as Celastrus paniculatus Wild (Celastraceae) is one of the most important medicinal plants in Ayurveda. The plant has shown significant pharmacological activities like anti-arthritic, wound healing, hypolipidemic, and antioxidant.
Objective:
To study possible effects of alcoholic extract of Celastrus paniculatus seeds (AlcE) in experimentally induced pain and inflammation in mice.
Materials and Methods:
The antinociceptive activity was evaluated in Swiss albino mice by tail immersion, hot plate, and acetic-acid-induced writhing tests at doses of 250, 500, and 1,000 mg/kg. Anti-inflammatory activity was evaluated in model of carrageenan-induced acute plantar inflammation in Wistar rats.
Results:
In tail immersion test, AlcE showed significant (P < 0.05) increase in tail withdrawal response at dose of 250 mg/kg with maximum possible effect of 15.71%. The maximum possible effect of 23.32% and 30.16% (P < 0.001) was seen at dose of 500 and 1000 mg/kg at 3 hours after administration of extract, respectively. In hot plate test, increase in paw licking time was reported at dose of 500 and 1000 mg/kg. AlcE (1,000 mg/kg) showed maximum response (6.23 ± 0.46) when compared with control (3.20 ± 0.18) at 90 min. In acetic acid induced writhings, AlcE at dose of 250, 500, and 1,000 mg/kg body weight showed 32.35%, 49.01%, and 58.82% inhibition in writhings, respectively. AlcE treated animals (500 and 1,000 mg/kg) showed significant decrease in paw edema at 3 hours and 4 hours, when compared with control animals.
Conclusion:
Jyotishmati seed extract possesses significant antinociceptive and anti-inflammatory activity.
Keywords: Acetic acid, analgesic, anti-inflammatory, carrageenan, hot plate, tail immersion
INTRODUCTION
Nociception is a noxious stimulus, which is processed through neural path. Mechanical, thermal, or chemical stimuli that have potential to damage the tissue, activate the primary afferent nociceptors in the peripheral and central nervous system. After stimulation, a nociceptor transmits a signal to the brain via the spinal cord which causes perception of pain.[1]
Inflammatory responses in the peripheral and central nervous systems play key roles in the development and persistence of many pathological pains. Inflammatory responses occur in acute and chronic phase. An early acute phase is characterized by migration of leukocytes and phagocytic cells into inflamed site under control of endogenous cytokines, chemokines, cell adhesion molecules, and pro-inflammatory stimuli.[2,3] Chronic phase is characterized by infiltration of leukocytes and phagocytic cells.[4] Certain proinflammatory cytokines are involved in the development of inflammatory and neuropathic pain.[5]
Opioids and non-steroidal anti-inflammatory drugs (NSAIDS) are used for management of pain and inflammation. Though these drugs are effective, they are having limitations because of their side effects. Non-steroidal anti-inflammatory drugs are responsible for gastric mucosal damage and renal damage while opioids cause tolerance, dependence, euphoria, and respiratory depression, etc.[6]
Traditionally, varieties of herbal drugs have been used to get relief from the pain and inflammation. Scientific validation of the activities of herbal drugs could provide a phytomedicine which is effective in management of pain and inflammation with minimum or no side effects. Literatures shows that the herbal drugs having phyto-constituents like curcumin, resveratrol, kaempferol, quercetin, catechine, and sesquiterpenes have the antinociceptive and anti-inflammatory activity.[7]
Celastrus paniculatus Wild (Celastraceae) is a woody liana, popularly known as “Jyotishmati.” Tribal system of medicine in different regions of India has claimed the use of Celastrus paniculatus in treatment of wide range of disorders.[8] The seed oil of Jyotishmati is used as brain tonic due to its beneficial effect on memory and intellect.[9]
The phytochemicals present in Celastrus paniculatus are sesquiterpenes, alkaloids celastrine, celapanine, celapanigine, celapagine,[10] polyalcohol (malangunin, malkanginnol, malkanguniol and paniculatusdiol).[11] It contains triterpenoid pristimerin[12] and sterols (β-amyrin and β-sitosterol), sesquiterpeniod polyol esters have also been isolated from Celastrus paniculatus.[13]
Different parts of C. paniculatus have been evaluated for various pharmacological activities. Leaves of the plant have shown the wound healing activity.[14] C. paniculatus seeds were found to have hypolipidemic[15] and antioxidant activity.[16,17] Seed oil of plant reversed the scopolamine induced deficits in navigational memory performance.[18] Seeds also showed potent relaxant effect on ileum.[19] Biogenic amines were found to be decreased in rats after treatment with C. paniculatus seed oil.[20] Extract of whole plant of C. paniculatus have anti-epileptic activity.[21]
Ayurveda mentions use of Jyotishmati, i.e. C. paniculatus seeds in treatment of vatavyadhi.[22] Vatavyadhi includes diseases like arthritis, gout, etc., All such diseases shows characteristic symptom-pida, i.e. pain.[23] Pre-clinical evaluation of the alcoholic extract of seeds of C. paniculatus showed significant effects in adjuvant induced arthritis in rats.[24] Also flower extract of the plant has been reported for its significant analgesic and anti-inflammatory activities.[25]
In Ayurveda, seed oil is used topically and internally for treatment of various disorders. The literature search related with the plant showed that the maximum pharmacological activities were tested on extracts obtained from polar solvents like methanol and ethanol. The extractive value of the seed for alcohol was found to about 52% w/w. So we selected alcoholic extract for the in vivo study in animals. Preliminary phytochemical screening of the extract indicated presence of alkaloids and oil has been reported for fatty acids.
Though the seeds of Celastrus paniculatus have been screened for different pharmacological activities, no systematic study has been reported to explore its effect in nociception and inflammation. So, in present study the alcoholic extract (AlcE) of seeds of Celastrus paniculatus was evaluated for its anti-nociceptive and anti-inflammatory activity.
MATERIALS AND METHODS
Drugs and reagents
Carrageenan was obtained from SD Fine-Chem Ltd., Mumbai, India. Solvents for high performance thin layer chromatography (HPTLC) were obtained from Thermo Fisher Scientific India Pvt. Ltd., Mumbai, India. Aspirin and Diclofenac were purchased from Welcome Chemist, Mumbai, India.
Plant material
Seeds of the Celastrus paniculatus were purchased from local vendor in Mumbai and were authenticated by scientists of Agharkar Research Institute, Pune, India. The voucher specimen (S-02) was deposited in department for future reference. Seeds were powdered using grinder. Powdered seeds were used to prepare extract.
Preparation of alcoholic extract
Alcoholic extract of powdered seeds was prepared by Soxhlet extraction technique. Powdered seeds (100 g) were packed in extractor and extracted with absolute alcohol. Extract was concentrated by using rotary evaporator under reduced pressure and was dried in vacuum dryer.
Preliminary phytochemical screening
Preliminary phytochemical tests were carried out to find out presence of various phytoconstituents. The extract was screened for the presence of secondary metabolites such as alkaloids, steroidal compounds, phenolic compounds, flavonoids, saponins, glycosides using standard procedures.[26]
HPTLC profiling of alcoholic extract
HPTLC studies were carried out by using DESEGA (Germany) HPTLC system with sample applicator (AS30), thin layer chromatography (TLC) scanner DESEGA CD60, and ProQuant software. CAMAG twin trough TLC plate development chamber was used for plate development. TLC plate (10 × 6 cm) coated with 0.2 mm layer of silica gel 60 F254 (Merck, Germany) was used for the study. Solution of extract was prepared in methanol to get concentration of l50 mg/ml. Sample was applied in three lanes (10, 20, and 30 μl) as 8 mm bands at spraying rate of 10 s/μl. Ascending development of plate was done in TLC chamber which was saturated with mobile phase previously for 30 min. The plate was developed by using the mobile phase-toluene: Ethyl acetate: Acetic acid (9:1:0.1). Densitometric scanning was performed at 254 nm.
Experimental animals
Swiss albino mice (18-25 g) and Wistar rats (150-180 g) were used for experiments. The animals were procured from Haffkine Institute, Mumbai, India and housed under standard laboratory conditions (temperature 25 ± 2°C, relative humidity 75 ± 5% and 12 hours light and dark cycle). Animals were fed with standard pellet diet (Nutrimix Laboratory Animal Feed, Maharashtra, India) and had free access to the water. The study protocol was approved by Institutional Animal Ethics Committee which was formed in accordance with norms of Committee for the Purpose of Control and Supervision of Experiments on Animals (CPCSEA), India and complied with the National Institutes of Health (NIH) guidelines on handling of experimental animals.
Toxicity study
Acute oral toxicity study for the extract was conducted in accordance with Organization for Economic Cooperation and Development (OECD) guidelines No. 423.[27] The female mice were used for the study. Twelve female mice were divided in four groups containing three in each. Group I received 1% carboxymethyl cellulose (CMC) solution. Group II, III, IV received AlcE orally, at dose of 300, 2,000, and 5,000 mg/kg body weight, respectively. After dosing, each animal was observed carefully, at least once during the first 30 min, periodically during the first 24 hours. Special attention was given during the first four hours and daily thereafter, for a total of 14 days. Changes in skin and fur, eyes, and mucous membranes, and also respiratory, circulatory, autonomic, and central nervous systems, and somatomotor activity and behavior pattern were also considered during observation. Animals were also carefully observed for tremors, convulsions, salivation, diarrhea, lethargy, sleep, and coma.[28] The body weight, food, and water intake were monitored weekly.
Selection of doses
Three dose levels 250, 500, and 1,000 mg/kg were selected for the study. One-fifth, 1/10th and 1/20th of the safe dose of the extract was selected for screening of AlcE in animal models of pain and inflammation. Extract was prepared in 1% CMC solution for its oral administration in experimental animals. The volume of extract administered to the experimental animals was not greater than 2 ml/100 g.
Antinociceptive activity
Antinociceptive activity of alcoholic extract was tested in three animal models, tail immersion test, hot plate, and writhing test.
Tail immersion test
Tail immersion test was carried out in Swiss albino mice as described by Ben-Bassat et al.[29] Animals were adapted for restrainers 30 min before the study. Animals were divided in five groups containing five in each. Group I received vehicle (1% CMC solution), Group II received standard drug, pentazocine (17 mg/kg body weight), Group III, IV, V received AlcE orally, at dose of 250, 500, and 1,000 mg/kg, respectively. Animals were kept in restrainers leaving the tail hanging out freely. Tails were marked and immersed in cup filled with water having temperature of 55 ± 0.5°C. The reaction time was recorded in seconds by using stopwatch. Cut of time of 10 sec was fixed to avoid the skin damage. The response was recorded at intervals of 0.5, 1, 1.5, 2, 3, 4, 5, and 6 hours after administration of vehicle or AlcE.
Hot plate test
Hot plate test was carried out in Swiss albino mice by using hot plate analgesia meter (IITC, USA). Animals were accustomed twice to the hot plate in advance. Response was defined as licking or biting of a paw, or jumping (where all four paws leave the plate). The time in seconds between the platform and reaction was recorded as the response latency. To prevent tissue damage, cut off time of 15 sec was determined. Animals were divided in five group containg five in each. Group I received vehicle (1% CMC solution), Group II received standard drug, pentazocine p.o. (17 mg/kg body weight), Group III, IV, V received AlcE orally, at dose of 250, 500, and 1000 mg/kg, respectively. The animals were placed on the hot plate maintained at 55 ± 0.5°C.[30] Latency time until either licking or jumping occurs was recorded periodically at 0, 20, 60, 90, and 120 min after administration of vehicle or AlcE.
Writhing test
Writhing test was performed in Swiss albino mice as described by Koster et al.[31] Mice were divided in five groups containing five in each. Group I received vehicle (1% CMC solution). Group II received standard drug- aspirin at a dose of 30 mg/kg p.o. Group III, IV, and V received AlcE p.o. at dose of 250, 500, and 1,000 mg/kg body weight, respectively. After 30 min of administration of vehicle or AlcE writhings were induced by intraperitonial injection of 0.1 ml of 0.6% v/v of acetic acid solution. Numbers of writhings were counted over the period of 20 min after acetic acid injection.[32] The percentage inhibition was calculated by the formula:
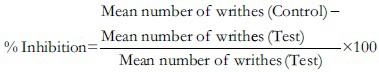
Anti-inflammatory activity
Edema was induced in paw of male Wistar rats (120-150 g) by subcutaneous injection of 0.1 ml of 1% carrageenan solution under subplantar aponeurosis.[33] The test groups were administered three doses of 250, 500, and 1,000 mg/kg of AlcE, 30 min before carrageenan injection. At the same time, the control group received 1% CMC solution and the reference group received 13.5 mg/kg Diclofenac by oral route.[34] Paw volume was measured by using plethysmometer (IITC, USA) after injection for the duration of next 5 hours at the interval 1 hour.
Percentage reduction in edema volume was calculated by using the formula:
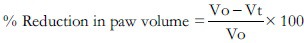
Where Vo = Volume of the paw of control at time ‘t’, Vt = Volume of the paw of drug treated at time ‘t’.
Statistical analysis
The differences among experimental and control groups were determined using the statistical software graph pad prism 5.0 for Windows. Significant differences between control and experimental groups were assessed by analysis of variance (ANOVA) followed by Dunnett's multiple comparison test. All data are expressed as mean ± standard error of mean (SEM); P values less than 0.05 was considered to be significant.
RESULTS
Phytochemical screening of the extract indicated presence of alkaloids and phenolics.
HPTLC profiling
The HPTLC profile of the alchoholic extract indicated eleven spots with various Rf values after densitometric scanning and Figure 1.
Figure 1.
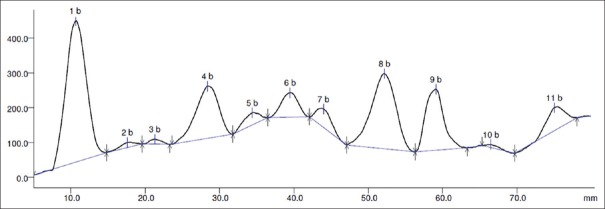
Densitogram of alcoholic extract of Celastrus paniculatus at 254 nm
Acute oral toxicity study
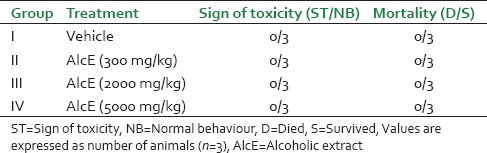
Single oral administration of different dose levels of AlcE produced no mortality or morbidity in the animals during observation period of 14 days [Table 1]. Cage side observations showed no change in general appearance and morphological characteristics of animals. During the observations no tremors, convulsion, salivation, diarrhea, lethargy, or unusual behaviors were noticed in treated animals, throughout study tenure. There were no significant changes in body weight, food, and water intake in extract treated animals when compared with control animals (data not shown).
Table 1.
Effect of alcoholic extract of seeds of Celastrus paniculatus in acute toxicity study
Antinociceptive activity
Tail immersion test
AlcE has significantly increased the time for tail withdrawal response in tail immersion test at all selected doses [Figure 2]. AlcE at dose 250 mg/kg did not show significant increase in tail withdrawal response up to 1.5 hours after dosing. It indicated significant (P < 0.05) increase in response at 2 hours which was continued up to 4 hours. AlcE at dose of 500 and 1,000 mg/kg showed significant increase tail withdrawal effect throughout study period, i.e. up to 6 hours. AlcE (1,000 mg/kg) showed maximum effect (P < 0.001) which was comparable to that of standard drug pentazocine.
Figure 2.
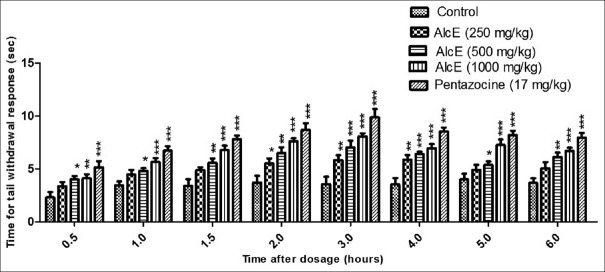
Effect of alcoholic extract of seeds of Celastrus paniculatus in tail immersion test. *P < 0.05, **P < 0.01, ***P < 0.001 compared with control
Hot plate test
The results of the hot plate test are presented in Figure 3. Alcoholic extract failed to increase the paw licking response at dose of 250 mg/kg throughout the study duration. The extract significantly (P < 0.05) delayed response at 500 mg/kg at 90 min after treatment. AlcE showed maximum effect at 1,000 mg/kg at 90 min, it indicated increase in paw licking response of 6.23 ± 0.46 (P < 0.01) when compared with response of control animals (3.20 ± 0.18).
Figure 3.
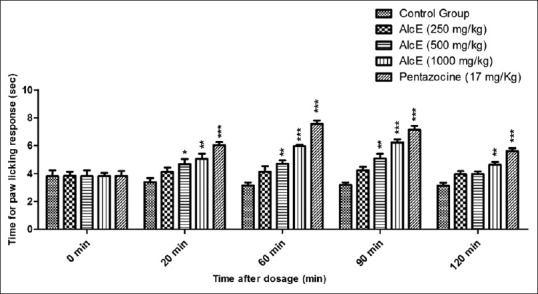
Effect of alcoholic extract of seeds of Celastrus paniculatus hot plate test. *P < 0.05, **P < 0.01, ***P < 0.001 compared with control
Writhing test
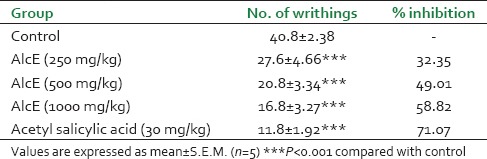
The results of writhing test are tabulated in Table 2. When compared with control, AlcE at all the three doses has shown significant (P < 0.01) decrease in writhing count. AlcE 250, 500, and 1,000 mg/kg body weight showed 32.35%, 49.01%, and 58.82% inhibition in writhing response. For the standard treatment, i.e. acetyl salicylic acid 30 mg/kg, 71.07% inhibition in writhing count was observed.
Table 2.
Effect of alcoholic extract of seeds of Celastrus paniculatus in writhing test
Anti-inflammatory activity
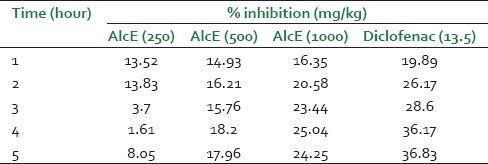
Alcoholic extract at dose 250 mg/kg did not show significant effect at any time interval throughout the study span. The extract at dose of 500 and 1000 mg/kg produced significant (P < 0.05 and P < 0.01) inhibition of paw edema on 3 h which was continued up to 5 hours [Figure 4]. The standard drug diclofenac showed significant inhibition of inflammation throughout the study period. The extract showed maximum inhibition of 25.04% at 4 hours at dose of 1,000 mg/kg [Table 3].
Figure 4.
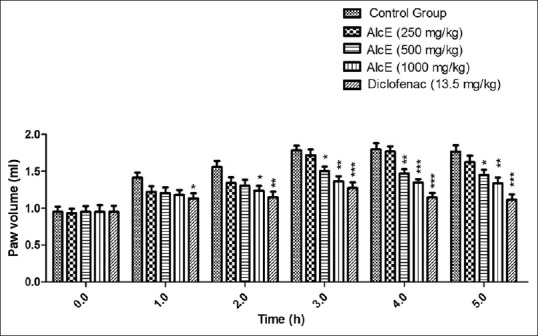
Effect of alcoholic extract of seeds of Celastrus paniculatus in Carrageenan induced rat paw edema. *P < 0.05, **P < 0.01, ***P < 0.001 compared with control
Table 3.
Effect of alcoholic extract of Celastrus paniculatus in carrageenan induced rat paw edema
DISCUSSION
It is necessary to generate the toxicity data for a new drug. It helps in determining the oral dose of the drug which can be used safely in the animals. As no systematic reports were present on evaluation of toxicity of alcoholic extract of Celastrus paniculatus seeds, toxicity study was started with dose of 300 mg/kg. The extract did not indicate any toxic symptoms and found to be well tolerated after single oral administration at doses of 300, 2,000, and 5,000 mg/kg body weight. Thus maximally tolerated dose (MTD) of alcoholic extract may be considered as 5,000 mg/kg. The AlcE was found nontoxic after single administration.
The tail immersion and hot plate tests are commonly used to screen compounds for their central antinociceptive activities. Though these two tests are linked with central activity, they are distinguished by their inclination to respond to the pain stimuli conducting through neuronal pathways. Tail immersion test mediates spinal reflexes to nociceptive stimuli, whereas the hot plate represents supraspinally organized response of pain.[35,36] The analgesic effect is shown by opioid agents through supraspinal (μ1, κ3, δ1, σ2) and spinal (μ2, κ1, δ2) receptors.[37] Alcoholic extract of Celastrus paniculatus exhibited significant antinociceptive effects in hot plate and tail immersion tests, showing possible involvement of the opioid mechanisms.
The acetic acid-induced writhing test is a visceral pain model widely accepted and has frequently been used to screen new agents for their peripheral antinociceptive activity.
The acetic acid administration into the peritoneal cavity leads to an increased level of cyclooxygenase (COX) products like eicosanoids (prostaglandins) in peritoneal fluid. It is also responsible for the release of various inflammatory mediators like serotonin, bradykinin, histamine, and TNF-α, interleukin (IL).[38,39,40] These released mediators eventually excite the primary afferent nociceptors entering the dorsal horn of the central nervous system leading to sensation of pain.[41,42] The alcoholic extract inhibited the acetic acid induced writhings significantly, so its antinociceptive effect may be linked to inhibition cyclooxygenase and inflammatory mediators.
As, the extract showed effect in central and peripheral nociception models in animals, it may be suggested extract is nonspecific in its effect against pain.
Carrageenan induced rat paw inflammation represents formation of edema in two phases. Inflammatory mediators like histamine and serotonin are released and this represents the early stage. The second phase of edema is represented by the release of protease, prostaglandins.[43,44] Most of the drugs show anti-inflammatory effect by inhibiting the second phase.[45] Bradykinin liberated as response to injected carrageenan, later induces the biosynthesis of prostaglandin and other autacoids, which are responsible for the formation of the inflammatory exudates. The cytokines constitute a link between cellular injuries or immunological recognition and the local or systemic signs of inflammation, e.g. cell migration, edema, fever, and hyperalgesia.[46] IL-1β, produced by various cells like macrophages, monocytes, and glial cells induces the production of other inflammatory mediators involved with acute phase protein release, fever and increase of vascular permeability.[47] It is possible that the mechanism of anti-inflammatory action of Celastrus paniculatus extract may be related to inhibition of prostaglandin synthesis and the release of IL-1β.
CONCLUSION
Celastrus paniculatus seeds have significant antinociceptive and anti-inflammatory activity. Further investigations may be focused on bioactivity guided fractionation and isolation to find out the possible phytoconstituents responsible for the mentioned activities.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Loeser JD, Treede RD. The Kyoto protocol of IASP basic pain terminology. Pain. 2008;137:473–7. doi: 10.1016/j.pain.2008.04.025. [DOI] [PubMed] [Google Scholar]
- 2.Gilroy DW, Lawrence T, Perretti M, Rossi AG. Inflammatory resolution: New opportunities for drug discovery. Nat Rev Drug Discov. 2004;3:401–16. doi: 10.1038/nrd1383. [DOI] [PubMed] [Google Scholar]
- 3.Serhan CN, Chiang N, Van Dyke TE. Resolving inflammation: Dual anti-inflammatory and pro-resolution lipid mediators. Nat Rev Immunol. 2008;8:349–61. doi: 10.1038/nri2294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hochberg MC, Silman AJ, Smolen JS, Weinblatt ME, Weisman MH. Rheumatology. Edinburgh: Mosby-Elsevier; 2008. [Google Scholar]
- 5.Zhang JM, An J. Cytokines, inflammation and pain. Int Anesthesiol Clin. 2007;45:27–37. doi: 10.1097/AIA.0b013e318034194e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tripathi KD. 5th ed. New Delhi: Jaypee Brothers Medical Publisher Ltd; 2009. Essentials of Medical Pharmacology; pp. 453–68. [Google Scholar]
- 7.Maroon JC, Bost JW, Maroon A. Natural anti-inflammatory agents for pain relief. Surg Neurol Int. 2010;1:80. doi: 10.4103/2152-7806.73804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Monojit D, Reshmi P, Harshita K, Nishteswar K. Medico ethnobotanical perspectives of jyotismati (Celastrus paniculatus Willd): A herbal tranquilizer. J Drug Deliv Ther. 2012;2:8–11. [Google Scholar]
- 9.Nadkarni KM. Mumbai: Popular Prakashan; 2007. Indian materia medica; p. 296. [Google Scholar]
- 10.Basu NK, Pabrai PR. Chemical investigation of Celastrus paniculatus Willd. J Am Pharm Assoc. 2006;35:272–3. doi: 10.1002/jps.3030350906. [DOI] [PubMed] [Google Scholar]
- 11.Patel DK, Amin KS, Nanavati DD. Chemistry and pharmacology of Celastrus paniculatus Willd. Indian Drugs. 1995;32:566–73. [Google Scholar]
- 12.Yasu LU, Yang S, Zou Z, Chen H, Zhen X, Zhongemei Z, et al. Evoninoate sesquiterpene alkaloids from the stem of Celastrus paniculatus. Heterocycl. 2006;68:1241–7. [Google Scholar]
- 13.Katekhaye S, Duggal S, Singh AP. An inside preview of nutritional and pharmacological profile of Celastrus paniculatus. Int J Recent Adv Pharm Res. 2011;1:19–24. [Google Scholar]
- 14.Harish BG, Krishna V, Santosh Kumar HS, Khadeer Ahamed BM, Sharath R, Kumara Swamy HM. Wound healing activity and docking of glycogen-synthase-kinase-3-beta-protein with isolated triterpenoid lupeol in rats. Phytomedicine. 2008;15:763–7. doi: 10.1016/j.phymed.2007.11.017. [DOI] [PubMed] [Google Scholar]
- 15.Mathur NT, Varma M, Dixit VP. Hypolipidaemic and antiatherosclerotic effect of Celastrus paniculatus seed extract in cholesterol fed rabbits. Indian Drugs. 1993;30:76–82. [Google Scholar]
- 16.Godkar PB, Gordon RK, Ravindran A, Doctor BP. Celastrus paniculatus seed oil and organic extracts attenuate hydrogen peroxide- and glutamate-induced injury in embryonic rat forebrain neuronal cells. Phytomedicine. 2006;13:29–36. doi: 10.1016/j.phymed.2003.11.011. [DOI] [PubMed] [Google Scholar]
- 17.Kumar MH, Gupta YK. Antioxidant property of Celastrus paniculatus willd: A possible mechanism in enhancing cognition. Phytomedicine. 2002;9:302–11. doi: 10.1078/0944-7113-00136. [DOI] [PubMed] [Google Scholar]
- 18.Gattu M, Boss KL, Terry AV, Jr, Buccafusco JJ. Reversal of scopolamine-induced deficits in navigational memory performance by the seed oil of Celastrus paniculatus. Pharmacol Biochem Behav. 1997;57:793–9. doi: 10.1016/s0091-3057(96)00391-7. [DOI] [PubMed] [Google Scholar]
- 19.Borrelli F, Borboneb N, Capassoa R, Montesanoc D, Marinob S, Aviello G, et al. Potent relaxant effect of a Celastrus paniculatus extract in the rat and human ileum. J Ethnopharmacol. 2009;122:434–8. doi: 10.1016/j.jep.2009.02.003. [DOI] [PubMed] [Google Scholar]
- 20.Nalini K, Karanth KS, Rao A, Aroor AR. Effects of Celastrus paniculatus on passive avoidance performance and biogenic amine turnover in albino rats. J Ethnopharmacol. 1995;47:101–8. doi: 10.1016/0378-8741(95)01264-e. [DOI] [PubMed] [Google Scholar]
- 21.Atigari DV, Gundamaraju R, Sabbithi S, Chaitanya KB, Ramesh C. Evaluation of antiepileptic activity of methanolic extract of Celastrus paniculatus willd whole plant in rodents. Int J Pharm Phytopharmacol Res. 2012;2:20–5. [Google Scholar]
- 22.1st ed. Part 1. II. New Delhi: Government of India, Ministry of Health and Family Welfare, Department of AYUSH; 2004. The Ayurvedic Pharmacopeia of India; pp. 62–3. [Google Scholar]
- 23.Sharma P. IV. Varanasi: Chaukhambha Orientalia; 2012. Charak Samhita; p. 201. [Google Scholar]
- 24.Patil KS, Suryavanshi J. Effect of Celastrus paniculatus, willd seed on adjuvant induced arthritis in rats. Pharmacogn Mag. 2007;3:177–81. [Google Scholar]
- 25.Ahmad F, Khan RA, Rasheed S. Preliminary screening of methanolic extracts of Celastrus paniculatus and Tecomella undulata for analgesic and anti-inflammatory activities. J Ethnopharmacol. 1994;42:193–8. doi: 10.1016/0378-8741(94)90085-x. [DOI] [PubMed] [Google Scholar]
- 26.Gokhale SB, Kulkarni YA, Gokhale A, Yele S. Pune: Nirali Prakashan; 2011. Experimental Pharmacognosy; pp. 21–28. [Google Scholar]
- 27.No. 423 Paris, France: Organization for Economic Cooperation and Development; 2001. OECD: Acute oral toxicity test method. In: OECD Guidelines for testing of Chemicals. [Google Scholar]
- 28.Kulkarni YA, Veeranjaneyulu A. Toxicological studies on aqueous extract of Gmelina arborea in rodents. Pharm Biol. 2010;48:1413–20. doi: 10.3109/13880209.2010.489228. [DOI] [PubMed] [Google Scholar]
- 29.Ben-Bassat J, Peretz E, Sulman FG. Analgesimetry and ranking of analgesic drugs by the receptacle method. Arch Int Pharmacodyn Ther. 1959;122:434–47. [PubMed] [Google Scholar]
- 30.Vogel GH. New York: Springer; 2002. Drug Discovery and Evaluation: Pharmacological Assays; pp. 696–8. 716. [Google Scholar]
- 31.Koster R, Anderson M, De Beer EJ. Acetic acid for analgesic screening. Fed Proc. 1959;18:412–6. [Google Scholar]
- 32.Kulkarni YA, Panjabi R, Patel V, Tawade A, Gokhale A. Effect of Gmelina arborea Roxb. in experimentally induced inflammation and nociception. J Ayurveda Integr Med. 2013;4:152–7. doi: 10.4103/0975-9476.118697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Winter CA, Risley EA, Nuss GW. Carrageenan-induced edema in hind paw of the rats as an assay for anti-inflammatory drugs. Proc Soc Exp Biol Med. 1962;111:544–7. doi: 10.3181/00379727-111-27849. [DOI] [PubMed] [Google Scholar]
- 34.Kulkarni YA, Gokhale SB, Veeranjaneyulu A, Surana SJ, Tatiya AU. Effect of Persea macrantha against acute inflammation and adjuvant-induced arthritis in rats. Pharm Biol. 2009;47:304–8. [Google Scholar]
- 35.Chapman CR, Casey KL, Dubner R, Foley KM, Gracely RH, Reading AE. Pain measurement: An overview. Pain. 1985;22:1–31. doi: 10.1016/0304-3959(85)90145-9. [DOI] [PubMed] [Google Scholar]
- 36.Morales L, Perez-Garcia C, Alguacil LF. Effects of yohimbine on the antinociceptive and place conditioning effects of opioid agonists in rodents. Br J Pharmacol. 2001;133:172–8. doi: 10.1038/sj.bjp.0704057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Arslan R, Bektas N, Ozturk Y. Antinociceptive activity of methanol extract of fruits of Capparis ovata in mice. J Ethnopharmacol. 2010;131:28–32. doi: 10.1016/j.jep.2010.05.060. [DOI] [PubMed] [Google Scholar]
- 38.Deraedt R, Jouquey S, Delevallée F, Flahaut M. Release of prostaglandins E and F in an algogenic reaction and its inhibition. Eur J Pharmacol. 1980;61:17–24. doi: 10.1016/0014-2999(80)90377-5. [DOI] [PubMed] [Google Scholar]
- 39.Shang X, Wang CJ, Li M, Miao X, Pan H, Yang Y, et al. Antinociceptive and anti-inflammatory activities of Phlomis umbrosa Turcz extract. Fitoterapia. 2011;82:716–21. doi: 10.1016/j.fitote.2011.03.001. [DOI] [PubMed] [Google Scholar]
- 40.Czeschik JC, Hagenacker T, Schäfers M, Büsselberg D. TNF-alpha differentially modulates ion channels of nociceptive neurons. Neurosci Lett. 2008;434:293–8. doi: 10.1016/j.neulet.2008.01.070. [DOI] [PubMed] [Google Scholar]
- 41.Ikeda A, Ueno H, Naraba H, Oh-ishi S. Involvement of vanilloid receptor VR1 and prostanoids in the acid induced writhing responses of mice. Life Sci. 2001;69:2911–9. doi: 10.1016/s0024-3205(01)01374-1. [DOI] [PubMed] [Google Scholar]
- 42.Gomez R, Por ED, Berg KA, Clarke WP, Glucksman MJ, Jeske NA. Metallopeptidase inhibition potentiates bradykinin-induced hyperalgesia. Pain. 2011;152:1548–54. doi: 10.1016/j.pain.2011.02.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Vinegar R, Schreiber W, Hugo R. Biphasic development of carrageenan edema in rats. J Pharmacol Exp Ther. 1969;166:96. [PubMed] [Google Scholar]
- 44.Crunkhorn P, Meacock SC. Mediators of the inflammation induced in the rat paw by carrageenin. Br J Pharmacol. 1971;42:392. doi: 10.1111/j.1476-5381.1971.tb07124.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Di Rosa M, Giroud JP, Willoughby DA. Studies of the acute inflammatory response induced in rats in different sites by carrageenan and turpentine. J Pathol. 1971;104:15. doi: 10.1002/path.1711040103. [DOI] [PubMed] [Google Scholar]
- 46.Magaji M, Anuka J, Abdu-Aguye I, Yaro A, Hussaini I. Preliminary studies on anti-inflammatory and analgesic activities of Securinega virosa (Euphorbiaceae) in experimental animal models. J Med Plant Res. 2008;2:39. [Google Scholar]
- 47.Besra SE, Sharma RM, Gomes A. Anti-inflammatory effect of petroleum ether extract of leaves of Litchi chinensis Gaertn (Sapindaceae) J Ethnopharmacol. 1996;54:1. doi: 10.1016/0378-8741(96)01440-7. [DOI] [PubMed] [Google Scholar]


