Abstract
Objective
To evaluate surveillance methods and their utility in detecting recurrence of disease in a high grade endometrial cancer population.
Methods
We performed a multi-institutional retrospective chart review of women diagnosed with high grade endometrial cancer between the years 2000 and 2011. Surveillance data was abstracted and analyzed. Surveillance method leading to detection of recurrence was identified and compared by stage of disease and site of recurrence.
Results
Two hundred and fifty-four patients met the criteria for inclusion. Vaginal cytology was performed in the majority of early stage patients, but was utilized less in advanced stage patients. CA-125 and CT imaging were used more frequently in advanced stage patients compared to early stage. Thirty-six percent of patients experienced a recurrence and the majority of initial recurrences (76%) had a distant component. Modalities that detected cancer recurrences were: symptoms (56%), physical exam (18%), surveillance CT (15%), CA-125 (10%), and vaginal cytology (1%). All local recurrences were detected by symptoms or physical exam findings. While the majority of loco-regional and distant recurrences (68%) were detected by symptoms or physical exam, 28% were detected by surveillance CT scan or CA 125. One loco-regional recurrence was identified by vaginal cytology but no recurrences with a distant component detected by this modality.
Conclusions
Symptoms and physical examination identify the majority of high grade endometrial cancer recurrences, while vaginal cytology is the least likely surveillance modality to identify a recurrence. The role of CT and CA-125 surveillance outside of a clinical trial needs to be further reviewed
Keywords: Surveillance, High grade, Endometrial cancer, Recurrence, Survival
Introduction
Endometrial cancer is subdivided into two types based on histopathology, molecular profile and clinical prognosis. Type I encompasses the low grade (grades 1 and 2) endometrioid tumors. Type II endometrial cancers include the more aggressive grade 3 endometrioid, clear cell, papillary serous and carcinosarcomas [1]. The recurrence rate of all histologic subtypes of endometrial cancer ranges from 13%–17% in large reported studies [2,3]. While the Type II, or high grade, endometrial cancers represent only a small proportion of all endometrial cancers, they are disproportionately responsible for 75% of deaths [4]. In general, the anatomic location of endometrial cancer recurrences is equally divided between local and distant sites [5–9]. While low grade and early stage endometrial cancers generally have a low recurrence rate and often present with a local recurrence, high grade endometrial cancers will more frequently recur with a distant component. There have been several studies that have focused on the best practices for followup and surveillance but most have focused on Type I and early stage cancers [10–13]. In this subgroup, vaginal cytology and imaging studies have not been shown to be cost effective. Additionally, these modalities have not identified recurrences earlier or improved survival compared with a thorough clinical evaluation [14]. The smaller number of high grade endometrial cancers has made it more difficult to define an optimal surveillance strategy that balances the detection of salvageable or treatable recurrences, psychosocial reassurance for the patient and cost effectiveness. The National Comprehensive Cancer Network (NCCN) guidelines, updated in 2015 (version 2.2015), recommend that a physical exam be performed every 3 to 6 months for 2–3 years then every 6 months or annually. In addition, providing patient education regarding symptoms of recurrence is encouraged while vaginal cytology has been designated category 3, inappropriate for incorporation into surveillance. Imaging studies are recommended as clinically indicated and CA-125 is optional [15]. Despite general awareness of these recommendations, practitioners continue to practice a variety of surveillance methods. The objective of this retrospective multi-institutional study was to evaluate contemporary surveillance methods and the utility of vaginal cytology, imaging studies, and tumor markers in detecting sites of recurrent disease that are unique to high grade endometrial cancer.
Methods
Study population
The cancer registries, pathology database and multidisciplinary tumor board notes at the University of Chicago and NorthShore University HealthSystem were searched for the high grade endometrial cancer subtypes (grade 3 endometrioid, papillary serous, carcinosarcoma and clear cell) between the years 2000–2011. The endometrial cancer registry at the University of Oklahoma was similarly searched for these high grade endometrial cancer histologies. Institutional Review Board approval was obtained at each of these institutions. All the patients included in this retrospective study underwent comprehensive surgical staging, which included hysterectomy, bilateral salpingo-oophorectomy, lymph node dissection and omentectomy, when appropriate, to determine stage. Pathology was reviewed at each institution's multidisciplinary tumor board conference. The patients were excluded if they did not undergo comprehensive surgical staging or if no followup data was available. The medical record and pathology reports were abstracted for age, histology, stage, adjuvant therapy, months of followup, number of appointments, symptoms reported, physical exam, Pap tests and results, type and results of imaging studies, CA-125, sites of recurrence and modality that detected a recurrence.
Recurrence of disease
Information regarding a subject's first recurrence was abstracted from the medical record. If multiple sites of recurrence were identified, the most distant site was used to define the type of recurrence. A local recurrence was defined as a vaginal recurrence. Regional recurrences (regional and local/regional) were defined as pelvic or nodal recurrences. Distant recurrences (distant, regional/distant, local/regional/distant and local/distant) were defined as recurrences outside of the pelvis. If a patient developed a recurrence, the surveillance method used to detect the recurrence was identified and categorized. If more than one surveillance technique identified a recurrence, the method that first detected the recurrence and initiated a workup was defined as the method of detection for the recurrence.
Statistical analysis and data review
Clinicopathologic, followup data, and surveillance techniques were examined and compared utilizing SAS version 9.2. Fisher's exact test was used for categorical variables and Student's t-test for continuous variables. We analyzed the surveillance tests utilized and compared them by stage of disease. The patients with a recurrence of disease were separately identified and the site of recurrence was stratified by stage. The overall utilization of each surveillance technique that detected a recurrence was noted and then categorized by stage and site. Progression free survival (PFS) was calculated from the date of surgery until first recurrence. Overall survival (OS) was calculated from date of surgery until last known followup or death. Survival was analyzed using the Kaplan–Meier method and log-rank analysis. The chi-square test or Fisher exact test was used to evaluate categorical variables. Statistical significance was set at a P value < 0.05. All statistical analyses were performed using SAS version 9.1 software (SAS Institute Inc., Cary, NC, USA, 2003).
Results
Study population
We identified a total of 324 patients with grade 3 endometrioid, papillary serous, carcinosarcoma and clear cell carcinoma of the uterus who underwent primary surgical staging at the 3 designated institutions between 2000 and 2011. Of these, 254 patients had adequate followup data available for analysis and are the subject of this study. The patient demographics and clinicopathologic characteristics are summarized in Table 1 and are consistent with other high grade endometrial cancer patient populations [16–18]. The median followup time was 25 months (range 1–178). The patient population was evenly divided between early Stage (I and II) and advanced Stage (III and IV) patients. The most common histologic types were grade 3 endometrioid (34%), papillary serous (34%) and carcinosarcoma (22%). The majority of patients (80%) received adjuvant treatment post-operatively: 22% received chemotherapy alone, 31% radiation alone, and 27% had a combination of chemotherapy and radiation. The median number of followup appointments was 7 (1–43). The median number of Pap tests, imaging studies and CA-125 performed was 2 (range 0–24), 2 (range 0–20) and 2 (range 0–37) respectively.
Table 1.
Demographics and clinicopathologic characteristics.
| Demographics | ||
|---|---|---|
| Number of patients | 254 | |
| Age at diagnosis (median) | 67 (36–107) | |
| Stage N (%) | ||
| I | 103 (41) | |
| II | 33 (13) | |
| III | 83 (33) | |
| IV | 35 (14) | |
| Histology N (%) | ||
| Grade 3 Endometrioid | 86 (34) | |
| Papillary Serous | 87 (34) | |
| Carcinosarcoma | 57 (22) | |
| Clear Cell | 14 (6) | |
| Mixed | 10 (4) | |
| Post-operative therapy N (%) | ||
| Chemotherapy | 57 (22) | |
| Radiation | 79 (31) | |
| Chemo/radiation | 68 (27) | |
| None | 38 (15) | |
| Unknown | 12 (5) | |
| Months follow-up (median) | 25 (1–178) | |
| Number of follow-up appointments | 7 (1–43) | |
| Number of Pap smears per patient | 2 (0–24) | |
| Number of imaging studies per patient | 2 (0–20) | |
| Number of CA-125 tests | 2 (0–37) |
Surveillance methods
Table 2 identifies the surveillance techniques used for the patients stratified by stage of disease. All the patients, regardless of stage, underwent a physical exam that included a pelvic exam as part of their routine followup visit. Vaginal cytology was performed in the majority of early stage (Stage I and Stage II) patients (82%), but was utilized less frequently in advanced stage (Stage III and Stage IV) patients (57%) (P < 0.0001). CA-125 and CT imaging were used more frequently in advanced stage patients as compared to early stage patients: 78% vs. 43%; P < 0.0001 and 82% vs. 56%; P = 0.001, respectively.
Table 2.
Surveillance technique utilized by stage.
| Stage I N=103 |
Stage II N=33 |
Stage III N=83 |
Stage IV N=35 |
Mean % | P value | |
|---|---|---|---|---|---|---|
| History (evaluation of symptoms) & physical exam (%) | 103 (100) | 33 (100) | 83 (100) | 35 (100) | 100 | |
| Vaginal cytology (%) | 83 (81) | 27 (82) | 58 (70) | 15 (43) | 72 | .007 |
| CA-125 (%) | 31 (30) | 18 (55) | 56 (67) | 31 (89) | 54 | <.0001 |
| CT imaging (%) | 58 (56) | 18 (55) | 64 (77) | 30 (86) | 67 | .19 |
Recurrence
With a median followup of 25 months, 36% (n = 92) of all the patients experienced a recurrence: 20% of Stage I patients, 30% of Stage II patients, 45% of Stage III patients and 69% of Stage IV patients. Overall, the majority of initial recurrences had a distant component (76%) even when stratified by the stage of disease: 57% (12/21) of Stage I cancers, 80% (8/10) of Stage II cancers, 78% (29/37) of Stage III cancers and 88% (21/24) of Stage IV cancers. Table 3 outlines these results as well as the distribution of local, regional and distant recurrences.
Table 3.
First site of recurrence by stage.
| Recurrence (%) | Stage I |
Stage II |
Stage III |
Stage IV |
Total |
|---|---|---|---|---|---|
| 21/103 (20%) |
10/33 (30%) |
37/83 (45%) |
24/35 (69%) |
92/254 (36%) |
|
| Local | 3 | 1 | 6 | 1 | 11 (12%) |
| Regional | 6 | 1 | 2 | 2 | 11 (12%) |
| Distant | 12 | 8 | 29 | 21 | 70 (76%) |
The modalities that detected cancer recurrences were: symptoms (56%), isolated physical exam findings without symptoms (18%), surveillance CT scan imaging without concordant symptoms (15%), and routine CA-125 blood tests (10%), followed by vaginal cytology without accompanying symptoms or exam findings (1%).
The location of disease recurrence was associated with particular surveillance techniques. Local recurrences (n = 11, 100%) were all detected by either symptoms (vaginal bleeding) or exam findings (tumor suspected on speculum or pelvic exam). There were a total of 11 regional recurrences. Of these, 6 (55%) were detected by symptoms or physical exam findings while only 1 (9%) was detected by cytology alone. CT imaging (n = 3, 27%) and CA-125 (n= 1, 9%) diagnosed the remaining regional recurrences. The vast majority of the patients who experienced a distant recurrence had disease detected by the patient reported symptoms including pain, abdominal discomfort, swelling and fatigue (n=46, 66%). Surveillance CT imaging detected recurrence in 11 patients (16%) followed by an asymptomatic rise in the CA-125 (n = 8, 11%) and physical examination (n = 5, 7%). No recurrences with a distant component were detected by vaginal cytology. Fig. 1 depicts the association between the method of detection and site of recurrence. The symptoms and physical examination identified the majority of local and distant recurrences. A combination of symptoms and physical examination identified over 50% of regional recurrences.
Fig. 1.
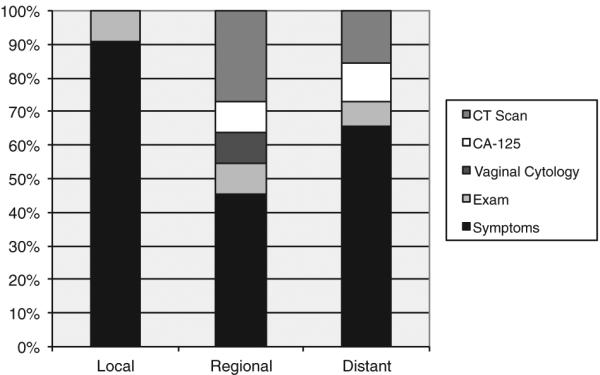
Surveillance method leading to detection of recurrence by site.
To determine whether site of disease recurrence was associated with outcome, PFS and OS were compared based on local, regional or distant first recurrence site. The PFS and OS were significantly different amongst the initial sites of recurrence: PFS was 16.1 months for those with local recurrences, 17.4 months for regional recurrences and 11 months for distant recurrences (P = 0.04); OS was 48.4 months versus 31.6 months vs 23.2 months respectively (P= 0.005).
The optimal surveillance technique would identify a salvageable locoregional recurrence. Therefore, we sought to ascertain whether a particular surveillance modality could detect salvageable recurrences based on the stage of disease (Fig. 2). For Stage I, a total of 76% of recurrences were detected by symptoms or physical exam. The remaining recurrences were asymptomatic and detected by CT scan (19%) or vaginal cytology (5%). Stage II recurrences were primarily detected by symptoms and physical exam findings (80%) and 2 recurrences were detected by CT scan (20%). Combining Stage III and Stage IV recurrences, 72% of recurrences were detected by symptoms or physical exam. Fifteen percent of recurrences were detected by CA-125 and 13% detected by CT scan in the absence of symptoms. There were no recurrences with a distant component detected by vaginal cytology in the advanced stage patients. The PFS and OS for advanced stage patients with recurrences detected by symptoms was no different than that for recurrences detected by CT scan: PFS was 9.9 months compared to 16.4 months (P=0.59); OS was 18 months vs 31.9 months, respectively (P= 0.07).
Fig. 2.
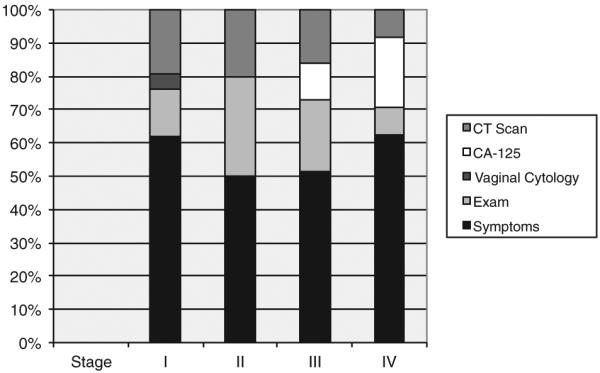
Surveillance method leading to detection of recurrence by stage.
Discussion
The role of surveillance is based on the concept that the detection of earlier asymptomatic recurrences results in earlier intervention, better therapeutic options and overall outcomes [19]. This multi-institutional study demonstrates that recurrence occurs in greater than 30% of patients with high grade endometrial cancer. The majority of patients, even with early stage disease, will have a recurrence that presents with a distant component. Over 70% of initial recurrences were detected by routine physical exam or a workup based on symptoms. The OS for patients with a local or regional recurrence was better than for those with a distant initial recurrence. The predictors of the patients destined to develop salvageable locoregional recurrences who may be longer term survivors of high grade endometrial cancer would be beneficial when assessing the role and cost effectiveness of regular routine surveillance modalities. To date, however, such predictors have not been well characterized.
Certainly, physical examination and assessment of symptoms are routine components of good clinical care and provide the opportunity to counsel the patients on lifestyle modification and implementation of other preventative health measures. In this study, 74% of initial recurrences were detected through symptom assessment and physical exam. In another study, 86% of recurrences were symptomatic [20].
Vaginal cytology has routinely been performed as part of the physical exam. The patients have come to associate the pelvic examination with getting a Pap smear and they frequently expect a Pap smear to be done. Yet, the rate of recurrence detected by vaginal vault cytology ranges from 0 to 6.8% in asymptomatic patients and the majority of these patients have low grade, early stage disease [19]. In our high risk patient population where one would expect the recurrence rate to be higher, a Pap smear detected only 1% of recurrences in the absence of symptoms or visible exam findings. For those patients with distant recurrences, no recurrence was detected solely by cytologic evaluation. Several cost effectiveness analyses have been performed to analyze the cost associated with vaginal cytology in routine surveillance and found vaginal cytology to not be a cost effective surveillance modality [10–14]. Despite the detection of a single local recurrence, the findings of the present study support the recommendations of the NCCN panel and the Society of Gynecologic Oncology (SGO) that vaginal vault cytology should not be used for surveillance in endometrial cancer patients [15,19].
The majority of the patients with high grade endometrial cancer in this study who recurred (76%) had a distant component to their initial recurrence. In this situation, the use of routine CT surveillance in detecting asymptomatic recurrences could have a rationale. Routine CT scan with no accompanying symptoms or physical exam findings found 15% of recurrences. Other studies have shown that 5–21% of asymptomatic recurrences are detected by CT imaging [5]. Despite this, earlier detection has not been shown to improve survival over detection of recurrence when the patients develop symptoms or recurrence detected by physical exam [10,21]. Otsuka et al. reviewed 51 patients with all grades and all stages of endometrial cancer who developed recurrence [22]. Their multivariate analysis showed that the independent predictors of prolonged survival after recurrence were site of recurrence and time to recurrence. There was no statistically significant survival advantage for symptomatic patients compared to asymptomatic patients. This analysis concluded that detecting recurrence by imaging studies and CA-125 does not improve prognosis, even if recurrence is detected before symptoms develop. Studies involving cancers outside of gynecologic malignancies have also found no survival advantage to routine CT scan surveillance [23–28]. The present study found that CT scan did detect 15% of recurrences. However, survival in these advanced stage patients where recurrence was detected by CT was not statistically different than the survival in the patients where recurrence was detected by exam (31.9 months CT versus 18 months exam, P = 0.07). For now, CT scan should play a role when a recurrence is suspected to best outline the appropriate treatment modality.
Lastly, in the present study, CA-125 detected 1 of 11 (9%) regional recurrences and 8 of 70 (11%) distant recurrences in asymptomatic women. Seven of these 9 patients had papillary serous histology. The number of the patients with asymptomatic distant recurrences identified by CA-125 was small, but was associated with an OS of 31 months, which was not statistically different from other methods of detection for distant recurrences. The CA-125 tumor marker is not routinely used in the followup for low grade, early stage endometrial cancer [5]. One study reported the detection of recurrence by CA-125 in asymptomatic patients to be 15% [29]. A large (n = 236) retrospective study by Rose et al. included the evaluation of all endometrial cancer patients and assessed pre-operative CA-125 levels and followup levels [30]. They stratified the patients by grade and stage of disease and classified the patients as low risk, medium risk or high risk. The median followup time was 39 months and 29 recurrences were detected. There were no recurrences detected by CA-125 in the low risk group (n=97), two in the medium risk group (n=42) and 27 (n=97) in the high risk group. Fifteen of the 27 women in the high risk group had elevated CA-125 levels at recurrence, but whether they were symptomatic prior to CA-125 elevation or not was not clear from the study. No medium-risk recurrent patients had an elevated CA-125. The results of the MRC OV05/EORTC 55955 study where advanced stage ovarian cancer patients in remission were randomized to immediate treatment based on rising CA-125 versus treatment based on symptoms reported no survival advantage with earlier detection of recurrence [31]. The uniformity in these results may be even more applicable in high grade endometrial cancers where there are far fewer effective treatment regimens in the recurrent setting than in ovarian cancer.
A retrospective analysis published by Carrara et al. in 2012 evaluated the followup modalities in the diagnosis of endometrial cancer relapses. In particular, they identified the patients as symptomatic or asymptomatic at the time of recurrence. The patients with an asymptomatic recurrence had a longer median survival time from relapse when compared to the patients who experienced a symptomatic recurrence: 35 months versus 13 months (P = 0.0001) [32]. Because of the retrospective nature of this the study and the possibility of lead time bias, the value of detecting asymptomatic recurrences remains inconclusive but the results are provocative. To explore the role of surveillance in detecting endometrial cancer recurrences and the subsequent effect on survival, there is an ongoing prospective Italian trial (ClinicalTrials.gov identifier NCT00916708) that randomizes endometrial cancer patients at low and high risks of recurrences to a minimalist regimen of followup versus an intensive regimen of followup. The results of this study should serve to determine if the intensity of followup is associated with differences in overall survival. This trial opened in September, 2008 and is due to be completed in August, 2015.
The strengths of our study include the multi-institutional nature of a comprehensively surgically staged sample population to elucidate the association between surveillance practices and the detection of recurrences. Our large study corroborates the findings and trends of a paper by Zakhour et al. evaluating the surveillance practices and methods of detection of recurrence for Type II endometrial cancer patients at a single institution [33]. Furthermore, this study validates the recurrence rates for high grade endometrial cancer from previously reported series as well as recommendations of the NCCN and the SGO for routine surveillance [15,19]. However, the main weaknesses of this study are its retrospective nature with relatively small number of recurrences, the lack of a uniform patient reported system for assessing symptoms, and inability to associate post-recurrence treatment with outcome based on surveillance technique.
Conclusion
The optimal surveillance strategy for high grade endometrial cancer patients that balances the detection of salvageable recurrences, cost, and patient reassurance remains uncertain. From our study, it is clear that symptom assessment and physical examination will detect the majority of recurrences and that use of vaginal cytology is not warranted. CT imaging and CA-125 do pick up asymptomatic recurrences, but the definitive role of these modalities in routine surveillance is yet to be determined.
HIGHLIGHTS.
The majority of recurrences, amongst all stages, had a distant component
CT scan detected 15% of locoregional recurrences in the absence of symptoms or exam findings
The majority of locoregional and distant recurrences are detected by symptoms and physical exam
Footnotes
Confliict of interest statement
No conflict of interest.
References
- [1].Querleu D, Planchamp F, Narducci F, Morice P, Joly F, Genestie C, et al. Clinical practice guidelines for the management of patients with endometrial cancer in France: recommendations of the Institut National du Cancer and the Societe Francaise d'Oncologie Gynecologique. Int J Gynecol Cancer. 2011 Jul;21(5):945–50. doi: 10.1097/IGC.0b013e31821bd473. [DOI] [PubMed] [Google Scholar]
- [2].Morrow CP, Bundy BN, Kurman RJ, Creasman WT, Heller P, Homesley HD, et al. Relationship between surgical–pathological risk factors and outcome in clinical Stage I and II carcinoma of the endometrium: a Gynecologic Oncology Group study. Gynecol Oncol. 1991 Jan;40(1):55–65. doi: 10.1016/0090-8258(91)90086-k. [DOI] [PubMed] [Google Scholar]
- [3].Tjalma WA, van Dam PA, Makar AP, Cruickshank DJ. The clinical value and the cost-effectiveness of follow-up in endometrial cancer patients. Int J Gynecol Cancer. 2004 Sep-Oct;14(5):931–7. doi: 10.1111/j.1048-891X.2004.014532.x. [DOI] [PubMed] [Google Scholar]
- [4].Hamilton CA, Cheung MK, Osann K, Chen L, Teng NN, Longacre TA, et al. Uterine papillary serous and clear cell carcinomas predict for poorer survival compared to grade 3 endometrioid corpus cancers. Br J Cancer. 2006 Mar 13;94(5):642–6. doi: 10.1038/sj.bjc.6603012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Fung-Kee-Fung M, Dodge J, Elit L, Lukka H, Chambers A, Oliver T. Follow-up after primary therapy for endometrial cancer: a systematic review. Gynecol Oncol. 2006 Jun;101(3):520–9. doi: 10.1016/j.ygyno.2006.02.011. [DOI] [PubMed] [Google Scholar]
- [6].Keys HM, Roberts JA, Brunetto VL, Zaino RJ, Spirtos NM, Bloss JD, et al. A phase III trial of surgery with or without adjunctive external pelvic radiation therapy in intermediate risk endometrial adenocarcinoma: a Gynecologic Oncology Group study. Gynecol Oncol. 2004 Mar;92(3):744–51. doi: 10.1016/j.ygyno.2003.11.048. [DOI] [PubMed] [Google Scholar]
- [7].Creutzberg CL, van Putten WL, Koper PC, Lybeert ML, Jobsen JJ, Warlam-Rodenhuis CC, et al. Surgery and postoperative radiotherapy versus surgery alone for patients with Stage-1 endometrial carcinoma: multicentre randomised trial. PORTEC Study Group. Post operative radiation therapy in endometrial carcinoma. Lancet. 2000 Apr 22;355(9213):1404–11. doi: 10.1016/s0140-6736(00)02139-5. [DOI] [PubMed] [Google Scholar]
- [8].Aalders J, Abeler V, Kolstad P, Onsrud M. Postoperative external irradiation and prognostic parameters in Stage I endometrial carcinoma: clinical and histopathologic study of 540 patients. Obstet Gynecol. 1980 Oct;56(4):419–27. [PubMed] [Google Scholar]
- [9].Piver MS, Yazigi R, Blumenson L, Tsukada Y. A prospective trail comparing hysterectomy, hysterectomy plus vaginal radium, and uterine radium plus hysterectomy in Stage I endometrial carcinoma. Obstet Gynecol. 1979 Jul;54(1):85–9. doi: 10.1097/00006250-197907000-00020. [DOI] [PubMed] [Google Scholar]
- [10].Morice P, Levy-Piedbois C, Ajaj S, Pautier P, Haie-Meder C, Lhomme C, et al. Value and cost evaluation of routine follow-up for patients with clinical Stage I/II endometrial cancer. Eur J Cancer. 2001 May;37(8):985–90. doi: 10.1016/s0959-8049(01)00066-1. [DOI] [PubMed] [Google Scholar]
- [11].Cooper AL, Dornfeld-Finke JM, Banks HW, Davey DD, Modesitt SC. Is cytologic screening an effective surveillance method for detection of vaginal recurrence of uterine cancer? Obstet Gynecol. 2006 Jan;107(1):71–6. doi: 10.1097/01.AOG.0000194206.38105.c8. [DOI] [PubMed] [Google Scholar]
- [12].Agboola OO, Grunfeld E, Coyle D, Perry GA. Costs and benefits of routine follow-up after curative treatment for endometrial cancer. CMAJ. 1997 Oct 1;157(7):879–86. [PMC free article] [PubMed] [Google Scholar]
- [13].Salani R, Nagel CI, Drennen E, Bristow RE. Recurrence patterns and surveillance for patients with early stage endometrial cancer. Gynecol Oncol. 2011 Nov;123(2):205–7. doi: 10.1016/j.ygyno.2011.07.014. [DOI] [PubMed] [Google Scholar]
- [14].Bristow RE, Purinton SC, Santillan A, Diaz-Montes TP, Gardner GJ, Giuntoli RL., II Cost-effectiveness of routine vaginal cytology for endometrial cancer surveillance. Gynecol Oncol. 2006 Nov;103(2):709–13. doi: 10.1016/j.ygyno.2006.05.013. [DOI] [PubMed] [Google Scholar]
- [15].NCCN Guidelines Version 2.2015 Endometrial carcinoma. Accessed at http://www.nccn.org/professionals/physician_gls/pdf/uterine/pdf.
- [16].Tergas AI, Buell-Gutbrod R, Gwin K, Kocherginsky M, Temkin SM, Fefferman A, et al. Clinico-pathologic comparison of type II endometrial cancers based on tamoxifen exposure. Gynecol Oncol. 2012 Nov;127(2):316–20. doi: 10.1016/j.ygyno.2012.07.105. [DOI] [PubMed] [Google Scholar]
- [17].Voss MA, Ganesan R, Ludeman L, McCarthy K, Gornall R, Schaller G, et al. Should grade 3 endometrioid endometrial carcinoma be considered a type 2 cancer—a clinical and pathological evaluation. Gynecol Oncol. 2012 Jan;124(1):15–20. doi: 10.1016/j.ygyno.2011.07.030. [DOI] [PubMed] [Google Scholar]
- [18].Ayeni TA, Bakkum-Gamez JN, Mariani A, McGree ME, Weaver AL, Haddock MG, et al. Comparative outcomes assessment of uterine grade 3 endometrioid, serous, and clear cell carcinomas. Gynecol Oncol. 2013 Jun;129(3):478–85. doi: 10.1016/j.ygyno.2013.03.011. [DOI] [PubMed] [Google Scholar]
- [19].Salani R, Backes FJ, Fung MF, Holschneider CH, Parker LP, Bristow RE, et al. Posttreatment surveillance and diagnosis of recurrence in women with gynecologic malignancies: Society of Gynecologic Oncologists recommendations. Am J Obstet Gynecol. 2011;204(6):466–78. doi: 10.1016/j.ajog.2011.03.008. [DOI] [PubMed] [Google Scholar]
- [20].Ng TY, Ngan HY, Cheng DK, Wong LC. Vaginal vault cytology in the routine follow-up of patients treated for endometrial carcinoma: is it useful? Aust N Z J Obstet Gynaecol. 1997 Feb;37(1):104–6. doi: 10.1111/j.1479-828x.1997.tb02229.x. [DOI] [PubMed] [Google Scholar]
- [21].Connor JP, Andrews JI, Anderson B, Buller RE. Computed tomography in endometrial carcinoma. Obstet Gynecol. 2000 May;95(5):692–6. doi: 10.1016/s0029-7844(99)00626-2. [DOI] [PubMed] [Google Scholar]
- [22].Otsuka I, Uno M, Wakabayashi A, Kameda S, Udagawa H, Kubota T. Predictive factors for prolonged survival in recurrent endometrial carcinoma: implications for follow-up protocol. Gynecol Oncol. 2010 Dec;119(3):506–10. doi: 10.1016/j.ygyno.2010.08.013. [DOI] [PubMed] [Google Scholar]
- [23].Armitage JO. Who benefits from surveillance imaging? J Clin Oncol. 2012 Jul 20;30(21):2579–80. doi: 10.1200/JCO.2012.42.6189. [DOI] [PubMed] [Google Scholar]
- [24].Voss SD, Chen L, Constine LS, Chauvenet A, Fitzgerald TJ, Kaste SC, et al. Surveillance computed tomography imaging and detection of relapse in intermediate- and advanced-stage pediatric Hodgkin's lymphoma: a report from the Children's Oncology Group. J Clin Oncol. 2012 Jul 20;30(21):2635–40. doi: 10.1200/JCO.2011.40.7841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Radford JA, Eardley A, Woodman C, Crowther D. Follow-up policy after treatment for Hodgkin's disease: too many clinic visits and routine tests? A review of hospital records. BMJ. 1997 Feb 1;314(7077):343–6. doi: 10.1136/bmj.314.7077.343a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Wagner-Johnston ND, Bartlett NL. Role of routine imaging in lymphoma. J Natl Compr Cancer Netw. 2011 May;9(5):575–84. doi: 10.6004/jnccn.2011.0048. quiz 85. [DOI] [PubMed] [Google Scholar]
- [27].Liedtke M, Hamlin PA, Moskowitz CH, Zelenetz AD. Surveillance imaging during remission identifies a group of patients with more favorable aggressive NHL at time of relapse: a retrospective analysis of a uniformly-treated patient population. Ann Oncol. 2006 Jun;17(6):909–13. doi: 10.1093/annonc/mdl049. [DOI] [PubMed] [Google Scholar]
- [28].Gerlinger M, Rohatiner AZ, Matthews J, Davies A, Lister TA, Montoto S. Surveillance investigations after high-dose therapy with stem cell rescue for recurrent follicular lymphoma have no impact on management. Haematologica. 2010 Jul;95(7):1130–5. doi: 10.3324/haematol.2009.020115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Reddoch JM, Burke TW, Morris M, Tornos C, Levenback C, Gershenson DM. Surveillance for recurrent endometrial carcinoma: development of a follow-up scheme. Gynecol Oncol. 1995 Nov;59(2):221–5. doi: 10.1006/gyno.1995.0012. [DOI] [PubMed] [Google Scholar]
- [30].Rose PG, Sommers RM, Reale FR, Hunter RE, Fournier L, Nelson BE. Serial serum CA 125 measurements for evaluation of recurrence in patients with endometrial carcinoma. Obstet Gynecol. 1994 Jul;84(1):12–6. [PubMed] [Google Scholar]
- [31].Rustin GJ, van der Burg ME, Griffin CL, Guthrie D, Lamont A, Jayson GC, et al. Early versus delayed treatment of relapsed ovarian cancer (MRC OV05/EORTC 55955): a randomised trial. Lancet. 2010 Oct 2;376(9747):1155–63. doi: 10.1016/S0140-6736(10)61268-8. [DOI] [PubMed] [Google Scholar]
- [32].Carrara L, Gadducci A, Landoni F, Maggino T, Scambia G, Galletto L, et al. Could different follow-up modalities play a role in the diagnosis of asymptomatic endometrial cancer relapses?: an Italian multicentric retrospective analysis. Int J Gynecol Cancer. 2012 Jul;22(6):1013–9. doi: 10.1097/IGC.0b013e31825ad3ee. [DOI] [PubMed] [Google Scholar]
- [33].Zakhour M, Li AJ, Walsh CS, Cass I, Karlan BY, Rimel BJ. Post treatment surveillance of type II endometrial cancer patients. Gynecol Oncol. 2013 Dec;131(3):609–12. doi: 10.1016/j.ygyno.2013.09.008. [DOI] [PubMed] [Google Scholar]


