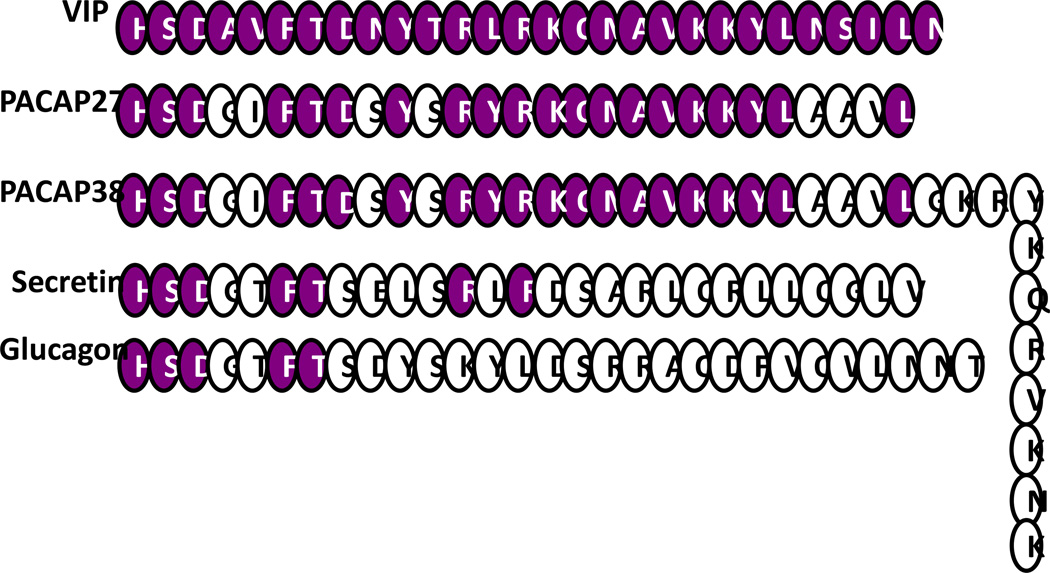Abstract
Neuropeptides represent an important category of endogenous contributors to the establishment and maintenance of immune deviation in immune privileged organs such as the CNS, and in the control of acute inflammation in the peripheral immune organs. Vasoactive intestinal peptide (VIP) is a major immunoregulatory neuropeptide widely distributed in the central and peripheral nervous system. In addition to neurons, VIP is synthesized by immune cells which also express VIP receptors. Here we review the current information on VIP production and VIP receptor mediated effects in the immune system, the role of endogenous and exogenous VIP in inflammatory and autoimmune disorders, and present and future VIP therapeutic approaches.
Keywords: vasoactive intestinal peptide, neuropeptides, innate immunity, T cell differentiation, tolerogenic dendritic cells, autoimmune diseases
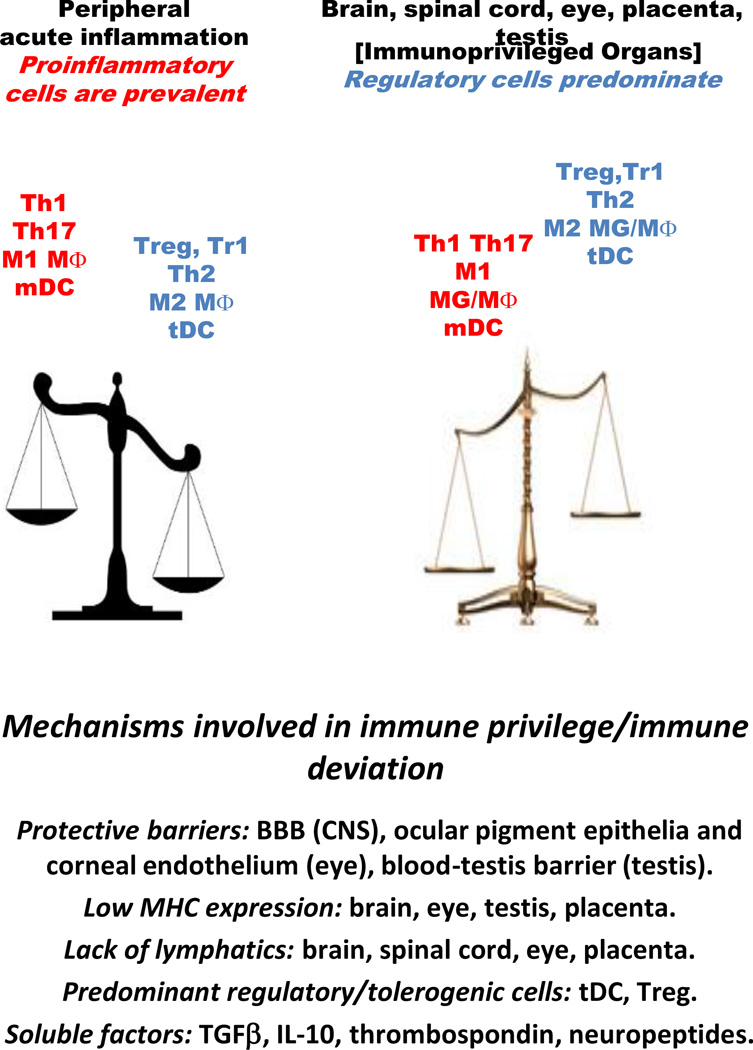
Immune privileged organs such as the CNS, eyes, testis and the feto-maternal interface are protected from potentially damaging robust inflammation that could lead to cell death and loss of function (Kanellopoulos-Langevin et al., 2003, Lampron et al., 2013, Stein-Streilein and Caspi, 2014, Zhao et al., 2014). In the CNS, although perivascular macrophages (MΦ) and meningeal dendritic cells (DC) are located at the blood/brain interface and small numbers of activated T cells recirculate, the parenchyma is guarded primarily through innate immune responses mediated by resident microglia (MG) in the absence of proinflammatory adaptive immune responses [reviewed in (Lampron et al., 2013, Ousman and Kubes, 2012, Ransohoff and Brown, 2012]. Several mechanisms, including the existence of the blood-brain barrier (BBB), low expression of MHC molecules, lack of draining lymphatics, predominant presence of anti-inflammatory, repair-prone M2 type MG and MΦ, presence of regulatory T cells (Treg) and tolerogenic dendritic cells (tDC), and prevalence of IL-10, TGFβ, thrombospondin, and neuropeptides, all contribute to the maintenance of a relative anti-inflammatory environment in the immune privileged organs (Fig. 1).
Fig. 1.
Mechanisms involved in immune privilege/immune deviation
The immunoregulatory activity of neuropeptides occurs not only in the immune privileged organs, but also in peripheral lymphoid organs upon release from the innervation and from the immune cells themselves. Both immune and non-immune cells express neuropeptide receptors which initiate specific signaling pathways leading to regulatory effects on gene expression. An expanding number of studies characterized the effects of neuropeptides on various parameters of the innate and adaptive immune response [reviewed in (Ganea, 2013]. With few exceptions, neuropeptides suppress the production of proinflammatory cytokines and chemokines from innate immune cells and inhibit differentiation of T cells into the proinflammatory Th1 subset, while promoting Treg differentiation and/or activity. In this review we will focus on the vasoactive intestinal peptide (VIP), a neuropeptide found in abundance in the CNS and lymphoid organs, synthesized and released by both the parasympathetic innervation and by activated T cells. We will address its role in MΦ, DC and MG activation and T cell differentiation, its effects in models of inflammatory and autoimmune diseases, and discuss its potential for therapeutic development.
General characteristics of endogenous VIP
The twenty eight aminoacid VIP is structurally related to the secretin/glucagon family of peptide hormones, sharing 70% sequence identity with the neuropeptide pituitary adenylate cyclase-activating polypeptide (PACAP) (Fig. 2). The superfamily is thought to have been generated through an initial duplication of a common ancestral gene followed by extensive divergence. The VIP secondary structure consists of two N-terminal β-turns followed by two helical segments connected by a region of undefined structure conferring mobility (Fry et al., 1989). The precursor molecule preproVIP is processed to mature VIP and the related peptide PHM (peptide with N-terminal histidine and C-terminal methionine amide) in humans or PHI (peptide with N-terminal histidine and C-terminal isoleucine amide), in other mammalian species. There is not consistent expression of both VIP and PHI/PHM in the same cell, suggesting differential protein processing.
Fig. 2.
Aminoacid structure of the VIP/secretin peptide family
VIP is produced by neurons, endocrine and immune cells, and is present in most organs including the CNS, heart, lung, thyroid, kidney, urinary and gastrointestinal tracts, genital organs and the immune system (Henning and Sawmiller, 2001). The widespread VIP distribution supports its biological functions, including increased cardiac output, bronchodilation, smooth muscle relaxation, regulation of secretory processes and motility in the GI tract.As a secretagogue, VIP promotes the release of prolactin, luteinizing hormone, and growth hormone from the pituitary gland, and regulates insulin and glucagon release. VIP also promotes analgesia, hyperthermia, learning and behavior, has neurotrophic effects and regulates bone metabolism, circadian rhythms and embryonic development.
VIP and the immune system
Lymphoid organs exhibit two VIP sources, the nerve endings and immune cells themselves. VIPergic nerve fibers are present in thymus, spleen, lymph nodes and mucosal-associated lymphoid tissue, functioning as the CNS-immune system anatomical link (Bellinger et al., 1996). The fact that autonomic denervation of thymus and spleen did not affect their VIP content (Bellinger et al., 1997), strongly suggested a second VIP source in the immune organs. Both CD4 and CD8 T cells were shown to express VIP mRNA, to process preproVIP, and to secrete VIP during inflammation or antigenic stimulation (Leceta et al., 1994). Moreover, we reported that Th2 CD4 and T2 CD8 T cells produce considerable amounts of VIP following antigen stimulation (Delgado and Ganea, 2001a).
Endogenous immune VIP plays a critical immunoregulatory role. Analysis of Th1/Th2 cell differentiation in cultures depleted of VIP through use of VIPase determined that VIP generated by T cells promoted the development of Th2 cells and inhibited Th1 differentiation (Voice et al., 2004). The in vivo immunoregulatory role of endogenous VIP generated by hematopoietic cells was confirmed by the higher numbers of anti-viral CD8 T cells observed in wild-type (wt) chimeras receiving bone marrow cells from VIP-deficient mice. Since the bone marrow chimeras produced normal levels of neuronal VIP, the increased anti-viral response was attributed to the lack of hematopoietic VIP (Li et al., 2011). The immunosuppressive role of endogenous VIP is supported by the fact that patients with autoimmune diseases such as lupus, autoimmune thyroiditis, MS and RA have low levels of VIP in serum, associated in some cases with high levels of VIPase autoantibodies (Bangale et al., 2003, Andersen et al., 1984, Martinez et al., 2014, Sharpless et al., 1984).
VIP receptors in immune cells
The pleiotropic effects of VIP are mediated through receptors widely distributed in CNS and peripheral tissues [reviewed in (Dickson and Finlayson, 2009). Three types of G protein-coupled VIP/PACAP receptors have been cloned and classified as VPAC1, VPAC2 and PAC1. VIP binds with high affinity (Kd ≈ 1 nM) to VPAC1 and VPAC2, and with low affinity (Kd > 500 nM) to PAC1. In immune cells, VPAC1 is constitutively expressed in lymphocytes, macrophages, monocytes, dendritic cells, microglia and mast cells, whereas VPAC2 is induced following stimulation, especially in T cells [reviewed in (Delgado et al., 2004b]. Studies using receptor agonists and antagonists initially identified VPAC1 as the major mediator for the immunoregulatory role of VIP (Delgado et al., 2004b, Gonzalez-Rey and Delgado, 2007). However, later studies using VPAC2- and PAC1-deficient mice showed increased susceptibility to inflammatory disorders, suggesting the participation of VPAC2 and PAC1 in immune regulation (Goetzl et al., 2001, Martinez et al., 2002, Lauenstein et al., 2010, Samarasinghe et al., 2011, Lauenstein et al., 2011). In comparison to healthy controls, immune cells from patients with ankylosing spondylitis, rheumatoid arthritis and osteoarthritis express less VPAC1 and respond poorly to VIP (Delgado et al., 2008a, Juarranz et al., 2008, Paladini et al., 2008, Sun et al., 2006). Also, a polymorphism in the 3’UTR region of the VPAC1 gene was identified in RA (Delgado et al., 2008a, Paladini et al., 2008). In Sjogren syndrome patients, monocytes exhibit increased VPAC2 expression and are deficient in phagocytosis of apoptotic cells (Hauk et al., 2014). In contrast, reduced VPAC2 expression and a distinct DNA footprinting pattern in the VPAC2 promoter were observed in Th1 cells of MS patients (Sun et al., 2006). These findings suggest that defects in the VIP receptor/signaling system could predispose to autoimmunity.
In immune cells, VPAC1, VPAC2 and PAC1 activate adenylate cyclase and protein kinase A (PKA). In addition, PAC1 also activates phospholipase C and protein kinase C (PKC) in monocytes (Mo) and MΦ [reviewed in (Delgado et al., 2004b]. The cAMP/PKA pathway acts as the major anti-inflammatory signaling pathway in MΦ, Mo, DC and MG, and in T cell differentiation. For the time being the involvement of PAC1 and PKC has been limited to VIP stimulation of IL-6 expression in resting macrophages (Martinez et al., 1998).
VIP: a regulator of innate and adaptive immunity
VIP inhibits macrophage and microglia activation
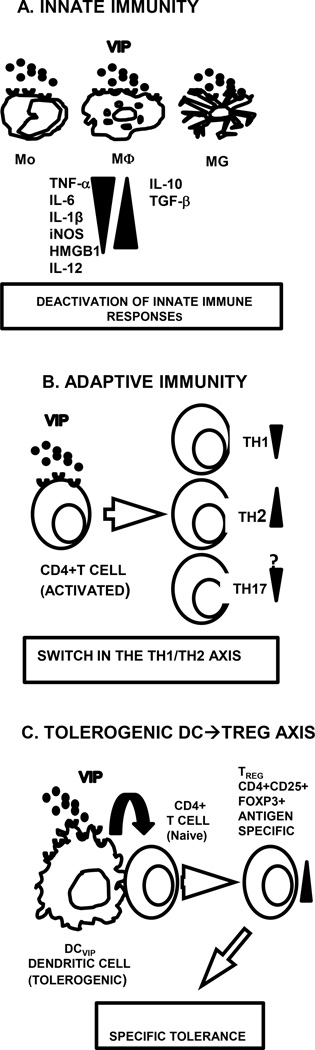
Following stimulation through Toll-like receptors MΦ, Mo and MG release proinflammatory cytokines and chemokines. Although acute inflammation is a requirement for the elimination of pathogens, an exacerbated and/or prolonged inflammatory response leads to tissue damage, organ failure, and death. Endogenous molecules such as anti-inflammatory cytokines, glucocorticoid hormones, pro-resolving lipid mediators, and neuropeptides such as VIP dampen inflammation acting on innate immune cells. VIP was shown to inhibit production of proinflammatory cytokines such as TNFα, IL-6, IL-12, iNOS induction, and to promote IL-10 expression in LPS-stimulated MΦ, Mo and MG. These effects are mediated primarily through VPAC1 (Fig. 3A) [reviewed in (Delgado et al., 2004b]. Recently VIP was reported to also downregulate high mobility group box-1 (HMGB1), a late-occurring cytokine involved in lethal endotoxemia and sepsis, and to suppress the inflammatory response in MG treated with beta-amyloid fibrils or neurotoxins (Chorny and Delgado, 2008, Delgado and Ganea, 2003a, Delgado et al., 2008c). In vivo, VIP prevented neurodegeneration and MG activation in models of neuroinflammation (Delgado and Ganea, 2003c, Kim et al., 2000). The VIP anti-inflammatory activity is also exerted through inhibition of chemokine release from MΦ and MG (Delgado and Ganea, 2001b, Delgado and Ganea, 2003b, Delgado et al., 2002a), and reduced recruitment of neutrophils, MΦ, and lymphocytes in models of acute peritonitis (Delgado and Ganea, 2001b).
Fig. 3. VIP effects on innate and adaptive immunity.
A. VIP inhibits production of pro-inflammatory and promotes production of anti-inflammatory factors in activated innate immune cells; B. VIP inhibits Th1 and promotes Th2 differentiation. Inhibitory effects on Th17 were observed in vivo but not in vitro. C. Bone marrow DC generated in the presence of VIP (DCVIP) are tolerogenic and induce Ag-specific Treg.
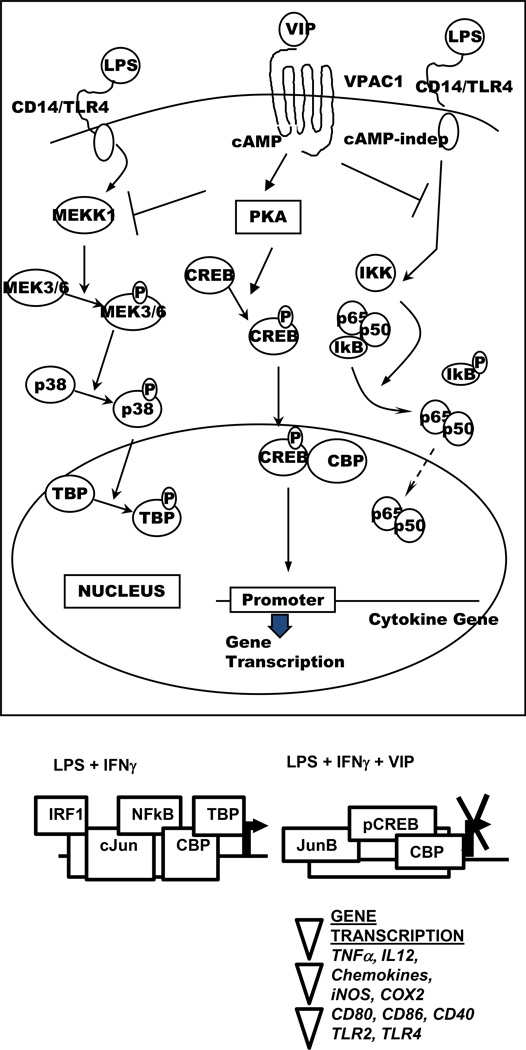
In terms of signaling pathways, VIP affects the expression of proinflammatory factors in LPS− and LPS+IFNγ-stimulated MΦ and MG by affecting the transcription factors (TF) AP-1, NFκB, CREB, and IRF-1 (Fig. 4) [reviewed in (Delgado et al., 2004b]. Effects on NFkB, an ubiquitous TF involved in the expression of proinflammatory genes, explains the inhibitory effect of VIP on a wide range of cytokines and chemokines. VIP affects NFkB through sveral signaling signaling pathways. A cAMP-independent pathway inhibits IKK, stabilizing IkB and maintaining the p65/p50/IkB complex in the cytoplasm. A cAMP-dependent pathway leads to PKA-mediated activation of CREB, its nuclear translocation and subsequent sequestration of the co-activator CBP. As a result, the transcriptional activity of NFkB is severely impaired. cAMP-dependent signaling which inhibits JAK1/STAT1 phosphorylation is also involved in the reduction of IRF-1, a TF required for optimal expression of iNOS and IL-12p40. In addition, VIP acts through a cAMP-dependent MEKK1/MEK4/JNK pathway to replace c-Jun with JunB in AP-1 complexes leading to a reduction in AP-1 binding to the TNFα promoter. Moreover, VIP reduces TATA-box binding protein (TBP) phosphorylation through inhibition of the MEKK1/MEK3/6/p38 pathway (Delgado, 2002, Delgado and Ganea, 2001c, Delgado and Ganea, 2000, Delgado et al., 1998). In a model of experimental colitis, VIP was reported to also inhibit TLR-2 and TLR-4 expression in MΦ, DC and lymphocytes, and to reduce TLR-4 upregulation in human rheumatoid synovial fibroblasts (Arranz et al., 2008, Foster et al., 2007, Gomariz et al., 2005, Gutierrez-Canas et al., 2006).
Fig. 4. Effects of VIP on transcription factors in innate immune cells.
VIP inhibits NFkB transactivating activity in innate immune cells through: a. cAMP-independent inhibition of IkB phosphorylation and subsequent IkB stabilization; b. cAMP-dependent phosphorylation of CREB and subsequent sequestration of the coactivator CBP; c. c-AMP dependent inhibition of the p38 MAPK pathway and subsequent reduced phosphorylation of the TATA-box binding protein (TBP). In addition VIP suppresses IFNγ-induced IRF-1 expression through inhibition of JAK1/STAT1 and alters the composition of AP-1 complexes through JunB replacement of cJun.
Following exposure to pathogens, innate immune cells, DC in particular, have the capacity to act as antigen-presenting cells (APCs), inducing the proliferation and differentiation of antigen-specific CD4 T cells. In agreement with its anti-inflammatory effects, VIP reduces the capacity of DC and MΦ to activate CD4 T cells, primarily by preventing the upregulation of the costimulatory molecules CD40, CD80 and CD86, leading to reduced CD4 T cell proliferation in vivo and in vitro (Delgado et al., 2004c, Delgado et al., 1999).
VIP shifts the Th1/Th2 balance during CD4 T cell differentiation in favor of Th2 cells
Following activation, CD4 T cells differentiate into effector cell subsets, i.e. Th1, Th2, Th17 and Treg with different functions based primarily on their cytokine profile. VIP inhibits Th1 and favors Th2 differentiation both in vivo and in vitro (Fig. 3B) [reviewed in (Ganea et al., 2003]. Th2 preferential differentiation was also observed in vivo in transgenic mice overexpressing VPAC2. In contrast, VPAC2-deficient mice exhibited a predominant Th1 response (Goetzl et al., 2001, Voice et al., 2003). This confirmed that VIP affected the Th1/Th2 balance in vivo primarily through VPAC2. Several non-excluding mechanisms contribute to the VIP-induced Th2 bias:
IL-12 is an essential factor for Th1 differentiation. VIP inhibits IL-12 production in activated APCs, and blocks IL-12 signaling in T cells by inhibiting JAK2/STAT4 phosphorylation and by inducing c-Maf and JunB (Liu et al., 2007, Voice et al., 2004).
VIP supports Th2 survival in vivo and in vitro through inhibition of FasL and granzyme B expression in Th2 cells (Delgado et al., 2002b, Sharma et al., 2006).
VIP promotes Th2 while inhibiting Th1 migration, through the induction of DC-derived CCL22, a Th2-attracting chemokine, and inhibition of the Th1-attracting chemokine CXCL10. Accordingly, in vivo administration of VIP-treated DC results in preferential accumulation of Th2 effectors (Delgado et al., 2004a, Jiang et al., 2002).
VIP and Th17 differentiation: inhibition or stimulation?
Th17 cells play a major role in autoimmunity, dominating the inflammatory response in RA, MS, psoriasis, and Crohn’s disease (Bovenschen et al., 2011, Ferraccioli and Zizzo, 2011, Fujino et al., 2003, Kebir et al., 2007). The effect of VIP on Th17 differentiation and function is controversial. In experimental models of type I diabetes and collagen-induced arthritis, VIP administration resulted in delayed disease onset, and reduced expression of IL-17, RORγt and IL-22, suggesting an inhibitory effect on Th17 differentiation or function (Deng et al., 2010, Jimeno et al., 2010). In contrast to the in vivo data, increased numbers of IL-17+ T cells were observed in vitro in the presence of TGFβ and VIP, following exposure to VIP-treated Langerhans cells, or during differentiation of human Th17 cells (Ding et al., 2012, Yadav et al., 2008, Jimeno et al., 2014). Whether VIP induction of Th17 also occurs in vivo, and whether VIP-induced Th17 cells express the recently described pathogenic signature (Lee et al., 2012), remains to be determined.
VIP induces tolerogenic DC (tDC) and regulatory T cells (Treg)
Regulatory T cells (Treg), including natural and induced Treg, play an essential role in maintaining tolerance. Deficiencies in Treg were documented in autoimmune diseases, and various experimental models. The majority of the anti-inflammatory neuropeptides have been reported to induce Treg (Ganea, 2013). However, VIP is presently the only neuropeptide reported to induce antigen-specific Treg through the generation of tolerogenic DC (tDC) pulsed with specific antigens. Biological and pharmacological agents can induce tDC which can be then manipulated to present specific autoantigens. Representative tDC-inducing biological agents include galectin 1, vitamin D3, IL-10 and TNFα, and more recently VIP (Maldonado and von Andrian, 2010). Exposure to VIP during differentiation of bone marrow- or monocyte-derived DC leads to the development of tolerogenic VIP-generated DC (DCVIP), which further induce CD4+Foxp3+ Treg (Fig. 3C). Treg induced by antigen-pulsed DCVIP inhibit the proliferation of antigen-specific T cells and transfer tolerance to naïve recipients [reviewed in (Gonzalez-Rey et al., 2010].
Induction of Treg in vivo by VIP has been demonstrated in several experimental systems. Inoculation of VIP and antigen (low dose) increased the numbers of CD4+CD25+Foxp3+ Treg which were capable of inhibiting effector T cell proliferation, transferring suppression, and inhibiting in vivo Th1 responses (Delgado et al., 2005a, Delgado et al., 2005b). VIP administration generated Treg and suppressed Th17 in collagen-induced arthritis, murine type I diabetes and EAE (Chen et al., 2008, Delgado et al., 2005a, Delgado et al., 2005b, Deng et al., 2010, Fernandez-Martin et al., 2006, Jimeno et al., 2010). When Treg from VIP-treated arthritic mice were transferred to mice with established disease they ameliorated clinical symptoms and prevented disease progression (Gonzalez-Rey et al., 2006a). Disease amelioration, reduced inflammation and induction of CD4+CD25+Foxp3+ Treg occurred upon delivery of a VIP-expressing viral vector to arthritic mice (Delgado et al., 2008b). In humans, administration of nebulized VIP to patients with sarcoidosis led to increased numbers of CD4+ CD25+ Foxp3+ Treg in the bronchoalveolar lavage (Prasse et al., 2010).
Recently, an apparent contradiction was reported regarding the effects of VIP in EAE. Exogenous VIP administration in active EAE models attenuated disease and induced CD4+CD25+Foxp3+ Treg which inhibited the proliferation of encephalitogenic Th1/Th17 cells (Fernandez-Martin et al., 2006, Gonzalez-Rey et al., 2006b). Similar effects were observed for the related neuropeptide PACAP. The PACAP protective effect was confirmed in PACAP-deficient mice which developed more severe EAE. These mice had decreased Foxp3 expression in spinal cord and lower numbers of Treg in draining lymph nodes (Tan et al., 2009). In contrast, VIP-deficient mice were EAE resistant, although they developed a robust Th1/Th17 response as expected. The explanation for their resistance to EAE resides in the fact that although CD4+ T cells migrated to the meninges, they failed to enter brain parenchyma (Abad et al., 2010). This suggests that VIP may play a dual role, reducing Th1/Th17 differentiation as already reported, but also promoting immune cell infiltration in the CNS, presumably through effects on endothelial cells. This issue has significant impact in terms of potential therapeutic use of VIP in MS, and clarification of the mechanisms involved requires the development of conditional, tissue-specific VIP receptor knock-outs.
Cellular therapy using DCVIP
The goal to generate long-term Ag-specific Treg in vivo through use of Ag-pulsed DCVIP is supported by several encouraging developments. For example, in vivo administration of DCVIP led to the generation of Ag-specific Treg capable of transferring specific tolerance to naïve recipients (Delgado et al., 2005b). Cellular therapy with DCVIP pulsed with collagen II stopped disease progression and reduced T cell proliferation in the CIA arthritis model in an Ag-specific manner (Chorny et al., 2005). DCVIP generated Treg and prevented GVHD while maintaining the graft-versus tumor response (Chorny et al., 2006). In a more recent development, we transduced immature DC with lentiviral vectors expressing preproVIP and generated lentiVIP- DC which secreted VIP, had a tolerogenic phenotype, and were therapeutic in EAE and sepsis models (Toscano et al., 2010). Future developments include the generation and use of tolerogenic VIP-expressing human monocyte-derived DC loaded with relevant autoantigens for the treatment of chronic autoimmune diseases.
VIP related genetic models
VIP transgenics and deficient mice
VIP overexpression
Selective overexpression of the human VIP gene in pancreatic β-cells enhanced glucose-induced insulin secretion and abolished glucose intolerance in mice with reduced functional pancreatic islet cell mass (Kato et al., 1994), suggesting possible therapeutic use of VIP in type I diabetes.
VIP deficiency
Partial (20% reduction in brain VIP) caused learning impairment especially in memory acquisition, and reduced sexual activity in males (Gozes et al., 1993).
Global VIP KO are fertile and generally healthy but exhibit disrupted circadian rhythm, a higher risk of death from gut stenosis, and enhanced susceptibility for pulmonary hypertension and asthma (Aton et al., 2005, Colwell et al., 2003, Hamidi et al., 2006, Lelievre et al., 2007). The role of VIP in the generation of Treg is supported by the fact that VIP KOs have reduced numbers of Foxp3+Treg in thymus and spleen (Szema et al., 2011). Whether VIP KO exhibit an altered susceptibility to endotoxemia is still debatable, with reports of both increased and decreased susceptibility (Abad et al., 2012, Hamidi et al., 2006). The difficulty of interpreting some of the findings from global VIP deficient mice is illustrated by their resistance to EAE (Abad et al., 2010). The opposite was expected based on the beneficial effect of VIP administration to wild-type (wt) mice. A careful analysis showed that encephalitogenic Th1/Th17 develop and migrate to the perivascular and meningeal space in both wt and VIP KO mice. However, in contrast to wt mice, T cells do not enter the spinal cord parenchyma in VIP KO, which is most probably the reason for their resistance to develop EAE. Indeed, in an EAE transfer model, wt encephalitogenic Th1/Th17 cells transferred to VIP KO mice also lose the ability to migrate into the parenchyma and to cause clinical symptoms (Abad et al., 2010). This suggests that VIP acts on cells other than T cells to promote migration into the CNS and emphasizes the need for future studies using cell-specific, conditional VIP deficient mice.
VPAC2 transgenics and KO
Mice overexpressing human VPAC2 showed changes in circadian rhythm, supporting the role of VPAC2 in controlling circadian cycles (Shen et al., 2000). Transgenic mice overexpressing hVPAC2 in T cells exhibited an increased Th2/Th1 ratio (Voice et al., 2001). In contrast, VPAC2 global KO developed a predominant Th1 response, and more severe DSS colitis with increased expression of proinflammatory factors such as IL-1β, IL-6, MMP9, and MPO (Goetzl et al., 2001, Yadav et al., 2011). These results confirmed previous conclusions regarding the anti-inflammatory effect of VIP and the role of VPAC2 receptors.
Studies related to the role of VPAC1 have been limited to the use of specific antagonists, since global homozygous VPAC1 KO mice exhibit prenatal lethality associated with severe neonatal growth failure, enlarged cecum, intestinal hemorrhage, and enterocyte hyperproliferation in addition to disorganized islets and impaired glucose homeostasis in surviving mice (Jackson Labs, Mouse Genome Informatics; http://www.informatics.jax.org/marker/MGI:109272). Future studies in cell specific, conditional VPAC1 and VPAC2 KO are required to dissect their roles in the immune system.
VIP: Therapeutic perspective
To date, VIP (Aviptadil in the clinic) has been successfully used in pulmonary hypertension and sarcoidosis. However, VIP is a potential therapeutic agent for a wider range of inflammatory/autoimmune diseases. VIP has the advantage of targeting both innate and adaptive immune responses and of affecting a large number of pro-inflammatory cytokines and chemokines through the inhibition of TF such as AP-1 and NFkB. Both innate immunity and proinflammatory Th1/Th17 subsets play a role in autoimmune diseases such as MS, RA, and IBD (inflammatory bowel disease), and exogenous VIP administration showed efficacy in the corresponding experimental mouse models of EAE, collagen-induced arthritis, and hapten (TNBS)-induced colitis. Taking into account the multiple VIP cellular and molecular targets leading to immunosuppressive effects, direct VIP administration in inflammatory/autoimmune disorders is a reasonable therapeutic approach. However, the issue of drug delivery remains a significant obstacle in developing a safe and efficacious VIP therapeutic approach. In plasma, VIP is degraded quickly through enzymatic and spontaneous hydrolysis, including catalytic antibodies, having a half-life on only 1–2 min (Chu and Orlowski, 1985). In addition, systemic administration of VIP causes cardiovascular and gastrointestinal side effects including a marked decrease in blood pressure, tachycardia, cutaneous flushing, and watery-diarrhea syndrome (Bloom et al., 1973, Henning and Sawmiller, 2001, Morice et al., 1983). Therefore, therapeutic use of VIP requires targeted delivery combined with protection against degradation. Several options are presently being investigated, such as VIP stabilization through aminoacid substitutions, use of liposomes or nanoparticles to deliver VIP, and combined treatments using peptidase inhibitors or serum neuropeptide-binding proteins. Stable VIP derivatives have already been used and as inhalable formulations in an asthma/COPD model, liposome encapsulated VIP was used in a model of autoimmune uveoretinitis, and silver-protected VIP nanoparticles inhibited microglia activation [reviewed in (Gonzalez-Rey et al., 2010]. VIP inhalations are used in chronic sarcoidosis and idiopathic pulmonary arterial hypertension (Leuchte et al., 2008, Prasse et al., 2010). VIP gene therapy using lentiviral vectors has been used successfully in collagen-induced arthritis (Delgado et al., 2008b), and is being currently tested in experimental models of type 1 diabetes (Sanlioglu, 2014). However, the disadvantage of this therapeutic approach is its lack of tissue- and cell-specificity. Cellular therapies represent another viable alternative, especially since DC generated in the presence of VIP (DCVIP) develop into tolerogenic DC capable of inducing antigen-specific Treg. The potential use of DCVIP is aimed at the in vivo generation of Ag-specific Treg and could be developed in humans by generating DCVIP in vitro from blood monocytes, loading them with specific antigens and re-injecting them into patients. Although the identification of specific antigens for various autoimmune conditions is a challenge, the potential in vivo generation of antigen-specific, long-lived Treg would represent an enormous advantage. Cell-based gene therapy offers a good alternative in terms of targeted VIP delivery. As an example, differentiating DC transduced with a recently developed lentiviral VIP vector proved to be therapeutic in EAE and sepsis models (Toscano et al., 2010). VIP-producing tolerogenic DC serve a double purpose, i.e. to suppress innate immune responses through local delivery of VIP and to induce Treg specific for antigens acquired at the inflammation site.
Acknowledgements
This work was supported by the NIH/NIAID RO1AI47325 (DG) grant.
Footnotes
Conflict of interest
The authors declare that they have no conflict of interest.
References
- Abad C, Tan YV, Cheung-Lau G, Nobuta H, Waschek JA. VIP deficient mice exhibit resistance to lipopolysaccharide induced endotoxemia with an intrinsic defect in proinflammatory cellular responses. PLoS One. 2012;7:e36922. doi: 10.1371/journal.pone.0036922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abad C, Tan YV, Lopez R, Nobuta H, Dong H, Phan P, Feng JM, Campagnoni AT, Waschek JA. Vasoactive intestinal peptide loss leads to impaired CNS parenchymal T-cell infiltration and resistance to experimental autoimmune encephalomyelitis. Proc Natl Acad Sci U S A. 2010;107:19555–19560. doi: 10.1073/pnas.1007622107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersen O, Fahrenkrug J, Wikkelso C, Johansson BB. VIP in cerebrospinal fluid of patients with multiple sclerosis. Peptides. 1984;5:435–437. doi: 10.1016/0196-9781(84)90249-3. [DOI] [PubMed] [Google Scholar]
- Arranz A, Juarranz Y, Leceta J, Gomariz RP, Martinez C. VIP balances innate and adaptive immune responses induced by specific stimulation of TLR2 and TLR4. Peptides. 2008;29:948–956. doi: 10.1016/j.peptides.2008.01.019. [DOI] [PubMed] [Google Scholar]
- Aton SJ, Colwell CS, Harmar AJ, Waschek J, Herzog ED. Vasoactive intestinal polypeptide mediates circadian rhythmicity and synchrony in mammalian clock neurons. Nat Neurosci. 2005;8:476–483. doi: 10.1038/nn1419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bangale Y, Karle S, Planque S, Zhou YX, Taguchi H, Nishiyama Y, Li L, Kalaga R, Paul S. VIPase autoantibodies in Fas-defective mice and patients with autoimmune disease. FASEB J. 2003;17:628–635. doi: 10.1096/fj.02-0475com. [DOI] [PubMed] [Google Scholar]
- Bellinger DL, Lorton D, Brouxhon S, Felten S, Felten DL. The significance of vasoactive intestinal polypeptide (VIP) in immunomodulation. Adv Neuroimmunol. 1996;6:5–27. doi: 10.1016/s0960-5428(96)00008-3. [DOI] [PubMed] [Google Scholar]
- Bellinger DL, Lorton D, Horn L, Brouxhon S, Felten SY, Felten DL. Vasoactive intestinal polypeptide (VIP) innervation of rat spleen, thymus, and lymph nodes. Peptides. 1997;18:1139–1149. doi: 10.1016/s0196-9781(97)00075-2. [DOI] [PubMed] [Google Scholar]
- Bloom SR, Polak JM, Pearse AG. Vasoactive intestinal peptide and watery-diarrhoea syndrome. Lancet. 1973;2:14–16. doi: 10.1016/s0140-6736(73)91947-8. [DOI] [PubMed] [Google Scholar]
- Bovenschen HJ, van de Kerkhof PC, van Erp PE, Woestenenk R, Joosten I, Koenen HJ. Foxp3+ regulatory T cells of psoriasis patients easily differentiate into IL-17A-producing cells and are found in lesional skin. J Invest Dermatol. 2011;131:1853–1860. doi: 10.1038/jid.2011.139. [DOI] [PubMed] [Google Scholar]
- Chen G, Hao J, Xi Y, Wang W, Wang Z, Li N, Li W. The therapeutic effect of vasoactive intestinal peptide on experimental arthritis is associated with CD4+CD25+ T regulatory cells. Scand J Immunol. 2008;68:572–578. doi: 10.1111/j.1365-3083.2008.02178.x. [DOI] [PubMed] [Google Scholar]
- Chorny A, Delgado M. Neuropeptides rescue mice from lethal sepsis by down-regulating secretion of the late-acting inflammatory mediator high mobility group box 1. Am J Pathol. 2008;172:1297–1307. doi: 10.2353/ajpath.2008.070969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chorny A, Gonzalez-Rey E, Fernandez-Martin A, Ganea D, Delgado M. Vasoactive intestinal peptide induces regulatory dendritic cells that prevent acute graft-versus-host disease while maintaining the graft-versus-tumor response. Blood. 2006;107:3787–3794. doi: 10.1182/blood-2005-11-4495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chorny A, Gonzalez-Rey E, Fernandez-Martin A, Pozo D, Ganea D, Delgado M. Vasoactive intestinal peptide induces regulatory dendritic cells with therapeutic effects on autoimmune disorders. Proc Natl Acad Sci U S A. 2005;102:13562–13567. doi: 10.1073/pnas.0504484102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu TG, Orlowski M. Soluble metalloendopeptidase from rat brain: action on enkephalin-containing peptides and other bioactive peptides. Endocrinology. 1985;116:1418–1425. doi: 10.1210/endo-116-4-1418. [DOI] [PubMed] [Google Scholar]
- Colwell CS, Michel S, Itri J, Rodriguez W, Tam J, Lelievre V, Hu Z, Liu X, Waschek JA. Disrupted circadian rhythms in VIP- and PHI-deficient mice. Am J Physiol Regul Integr Comp Physiol. 2003;285:R939–R949. doi: 10.1152/ajpregu.00200.2003. [DOI] [PubMed] [Google Scholar]
- Delgado M. Vasoactive intestinal peptide and pituitary adenylate cyclase-activating polypeptide inhibit the MEKK1/MEK4/JNK signaling pathway in endotoxin-activated microglia. Biochem Biophys Res Commun. 2002;293:771–776. doi: 10.1016/S0006-291X(02)00283-8. [DOI] [PubMed] [Google Scholar]
- Delgado M, Chorny A, Gonzalez-Rey E, Ganea D. Vasoactive intestinal peptide generates CD4+CD25+ regulatory T cells in vivo. J Leukoc Biol. 2005a;78:1327–1338. doi: 10.1189/jlb.0605299. [DOI] [PubMed] [Google Scholar]
- Delgado M, Ganea D. Inhibition of IFN-gamma-induced janus kinase-1-STAT1 activation in macrophages by vasoactive intestinal peptide and pituitary adenylate cyclase-activating polypeptide. J Immunol. 2000;165:3051–3057. doi: 10.4049/jimmunol.165.6.3051. [DOI] [PubMed] [Google Scholar]
- Delgado M, Ganea D. Cutting edge: is vasoactive intestinal peptide a type 2 cytokine? J Immunol. 2001a;166:2907–2912. doi: 10.4049/jimmunol.166.5.2907. [DOI] [PubMed] [Google Scholar]
- Delgado M, Ganea D. Inhibition of endotoxin-induced macrophage chemokine production by vasoactive intestinal peptide and pituitary adenylate cyclase-activating polypeptide in vitro and in vivo. J Immunol. 2001b;167:966–975. doi: 10.4049/jimmunol.167.2.966. [DOI] [PubMed] [Google Scholar]
- Delgado M, Ganea D. Vasoactive intestinal peptide and pituitary adenylate cyclase-activating polypeptide inhibit nuclear factor-kappa B-dependent gene activation at multiple levels in the human monocytic cell line THP-1. J Biol Chem. 2001c;276:369–380. doi: 10.1074/jbc.M006923200. [DOI] [PubMed] [Google Scholar]
- Delgado M, Ganea D. Neuroprotective effect of vasoactive intestinal peptide (VIP) in a mouse model of Parkinson's disease by blocking microglial activation. Faseb J. 2003a;17:944–946. doi: 10.1096/fj.02-0799fje. [DOI] [PubMed] [Google Scholar]
- Delgado M, Ganea D. Vasoactive intestinal peptide inhibits IL-8 production in human monocytes. Biochem Biophys Res Commun. 2003b;301:825–832. doi: 10.1016/s0006-291x(03)00059-7. [DOI] [PubMed] [Google Scholar]
- Delgado M, Ganea D. Vasoactive intestinal peptide prevents activated microglia-induced neurodegeneration under inflammatory conditions: potential therapeutic role in brain trauma. Faseb J. 2003c;17:1922–1924. doi: 10.1096/fj.02-1029fje. [DOI] [PubMed] [Google Scholar]
- Delgado M, Gonzalez-Rey E, Ganea D. VIP/PACAP preferentially attract Th2 effectors through differential regulation of chemokine production by dendritic cells. Faseb J. 2004a;18:1453–1455. doi: 10.1096/fj.04-1548fje. [DOI] [PubMed] [Google Scholar]
- Delgado M, Gonzalez-Rey E, Ganea D. The neuropeptide vasoactive intestinal peptide generates tolerogenic dendritic cells. J Immunol. 2005b;175:7311–7324. doi: 10.4049/jimmunol.175.11.7311. [DOI] [PubMed] [Google Scholar]
- Delgado M, Jonakait GM, Ganea D. Vasoactive intestinal peptide and pituitary adenylate cyclase-activating polypeptide inhibit chemokine production in activated microglia. Glia. 2002a;39:148–161. doi: 10.1002/glia.10098. [DOI] [PubMed] [Google Scholar]
- Delgado M, Leceta J, Ganea D. Vasoactive intestinal peptide and pituitary adenylate cyclase-activating polypeptide promote in vivo generation of memory Th2 cells. Faseb J. 2002b;16:1844–1846. doi: 10.1096/fj.02-0248fje. [DOI] [PubMed] [Google Scholar]
- Delgado M, Munoz-Elias EJ, Kan Y, Gozes I, Fridkin M, Brenneman DE, Gomariz RP, Ganea D. Vasoactive intestinal peptide and pituitary adenylate cyclase-activating polypeptide inhibit tumor necrosis factor alpha transcriptional activation by regulating nuclear factor-kB and cAMP response element-binding protein/c-Jun. J Biol Chem. 1998;273:31427–31436. doi: 10.1074/jbc.273.47.31427. [DOI] [PubMed] [Google Scholar]
- Delgado M, Pozo D, Ganea D. The significance of vasoactive intestinal peptide in immunomodulation. Pharmacol Rev. 2004b;56:249–290. doi: 10.1124/pr.56.2.7. [DOI] [PubMed] [Google Scholar]
- Delgado M, Reduta A, Sharma V, Ganea D. VIP/PACAP oppositely affects immature and mature dendritic cell expression of CD80/CD86 and the stimulatory activity for CD4(+) T cells. J Leukoc Biol. 2004c;75:1122–1130. doi: 10.1189/jlb.1203626. [DOI] [PubMed] [Google Scholar]
- Delgado M, Robledo G, Rueda B, Varela N, O'Valle F, Hernandez-Cortes P, Caro M, Orozco G, Gonzalez-Rey E, Martin J. Genetic association of vasoactive intestinal peptide receptor with rheumatoid arthritis: altered expression and signal in immune cells. Arthritis Rheum. 2008a;58:1010–1019. doi: 10.1002/art.23482. [DOI] [PubMed] [Google Scholar]
- Delgado M, Sun W, Leceta J, Ganea D. VIP and PACAP differentially regulate the costimulatory activity of resting and activated macrophages through the modulation of B7.1 and B7.2 expression. J Immunol. 1999;163:4213–4223. [PubMed] [Google Scholar]
- Delgado M, Toscano MG, Benabdellah K, Cobo M, O'Valle F, Gonzalez-Rey E, Martin F. In vivo delivery of lentiviral vectors expressing vasoactive intestinal peptide complementary DNA as gene therapy for collagen-induced arthritis. Arthritis Rheum. 2008b;58:1026–1037. doi: 10.1002/art.23283. [DOI] [PubMed] [Google Scholar]
- Delgado M, Varela N, Gonzalez-Rey E. Vasoactive intestinal peptide protects against beta-amyloid-induced neurodegeneration by inhibiting microglia activation at multiple levels. Glia. 2008c;56:1091–1103. doi: 10.1002/glia.20681. [DOI] [PubMed] [Google Scholar]
- Deng S, Xi Y, Wang H, Hao J, Niu X, Li W, Tao Y, Chen G. Regulatory effect of vasoactive intestinal peptide on the balance of Treg and Th17 in collagen-induced arthritis. Cell Immunol. 2010;265:105–110. doi: 10.1016/j.cellimm.2010.07.010. [DOI] [PubMed] [Google Scholar]
- Dickson L, Finlayson K. VPAC and PAC receptors: From ligands to function. Pharmacol Ther. 2009;121:294–316. doi: 10.1016/j.pharmthera.2008.11.006. [DOI] [PubMed] [Google Scholar]
- Ding W, Manni M, Stohl LL, Zhou XK, Wagner JA, Granstein RD. Pituitary adenylate cyclase-activating peptide and vasoactive intestinal polypeptide bias Langerhans cell Ag presentation toward Th17 cells. Eur J Immunol. 2012;42:901–911. doi: 10.1002/eji.201141958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez-Martin A, Gonzalez-Rey E, Chorny A, Martin J, Pozo D, Ganea D, Delgado M. VIP prevents experimental multiple sclerosis by downregulating both inflammatory and autoimmune components of the disease. Ann N Y Acad Sci. 2006;1070:276–281. doi: 10.1196/annals.1317.026. [DOI] [PubMed] [Google Scholar]
- Ferraccioli G, Zizzo G. The potential role of Th17 in mediating the transition from acute to chronic autoimmune inflammation: rheumatoid arthritis as a model. Discov Med. 2011;11:413–424. [PubMed] [Google Scholar]
- Foster N, Lea SR, Preshaw PM, Taylor JJ. Pivotal advance: vasoactive intestinal peptide inhibits up-regulation of human monocyte TLR2 and TLR4 by LPS and differentiation of monocytes to macrophages. J Leukoc Biol. 2007;81:893–903. doi: 10.1189/jlb.0206086. [DOI] [PubMed] [Google Scholar]
- Fry DC, Madison VS, Bolin DR, Greeley DN, Toome V, Wegrzynski BB. Solution structure of an analogue of vasoactive intestinal peptide as determined by two-dimensional NMR and circular dichroism spectroscopies and constrained molecular dynamics. Biochemistry. 1989;28:2399–2409. doi: 10.1021/bi00432a010. [DOI] [PubMed] [Google Scholar]
- Fujino S, Andoh A, Bamba S, Ogawa A, Hata K, Araki Y, Bamba T, Fujiyama Y. Increased expression of interleukin 17 in inflammatory bowel disease. Gut. 2003;52:65–70. doi: 10.1136/gut.52.1.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ganea D, Rodriguez R, Delgado M. Vasoactive intestinal peptide and pituitary adenylate cyclase-activating polypeptide: players in innate and adaptive immunity. Cell Mol Biol (Noisy-le-grand) 2003;49:127–142. [PubMed] [Google Scholar]
- Ganea DaMS. Immunoregulatory Neuropeptides. In: Alexander W, Kusnecov HA, editors. The Wiley-Blackwell Handbook of Psychoneuroimmunology. London: Wiley-Blackwell; 2013. [Google Scholar]
- Goetzl EJ, Voice JK, Shen S, Dorsam G, Kong Y, West KM, Morrison CF, Harmar AJ. Enhanced delayed-type hypersensitivity and diminished immediate-type hypersensitivity in mice lacking the inducible VPAC(2) receptor for vasoactive intestinal peptide. Proc Natl Acad Sci U S A. 2001;98:13854–13859. doi: 10.1073/pnas.241503798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomariz RP, Arranz A, Abad C, Torroba M, Martinez C, Rosignoli F, Garcia-Gomez M, Leceta J, Juarranz Y. Time-course expression of Toll-like receptors 2 and 4 in inflammatory bowel disease and homeostatic effect of VIP. J Leukoc Biol. 2005;78:491–502. doi: 10.1189/jlb.1004564. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Rey E, Delgado M. Anti-inflammatory neuropeptide receptors: new therapeutic targets for immune disorders? Trends Pharmacol Sci. 2007;28:482–491. doi: 10.1016/j.tips.2007.07.001. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Rey E, Fernandez-Martin A, Chorny A, Delgado M. Vasoactive intestinal peptide induces CD4+,CD25+ T regulatory cells with therapeutic effect in collagen-induced arthritis. Arthritis Rheum. 2006a;54:864–876. doi: 10.1002/art.21652. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Rey E, Fernandez-Martin A, Chorny A, Martin J, Pozo D, Ganea D, Delgado M. Therapeutic effect of vasoactive intestinal peptide on experimental autoimmune encephalomyelitis: down-regulation of inflammatory and autoimmune responses. Am J Pathol. 2006b;168:1179–1188. doi: 10.2353/ajpath.2006.051081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez-Rey E, Ganea D, Delgado M. Neuropeptides: keeping the balance between pathogen immunity and immune tolerance. Curr Opin Pharmacol. 2010;10:473–481. doi: 10.1016/j.coph.2010.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gozes I, Glowa J, Brenneman DE, McCune SK, Lee E, Westphal H. Learning and sexual deficiencies in transgenic mice carrying a chimeric vasoactive intestinal peptide gene. J Mol Neurosci. 1993;4:185–193. doi: 10.1007/BF02782501. [DOI] [PubMed] [Google Scholar]
- Gutierrez-Canas I, Juarranz Y, Santiago B, Arranz A, Martinez C, Galindo M, Paya M, Gomariz RP, Pablos JL. VIP down-regulates TLR4 expression and TLR4-mediated chemokine production in human rheumatoid synovial fibroblasts. Rheumatology (Oxford) 2006;45:527–532. doi: 10.1093/rheumatology/kei219. [DOI] [PubMed] [Google Scholar]
- Hamidi SA, Szema AM, Lyubsky S, Dickman KG, Degene A, Mathew SM, Waschek JA, Said SI. Clues to VIP function from knockout mice. Ann N Y Acad Sci. 2006;1070:5–9. doi: 10.1196/annals.1317.035. [DOI] [PubMed] [Google Scholar]
- Hauk V, Fraccaroli L, Grasso E, Eimon A, Ramhorst R, Hubscher O, Perez Leiros C. Monocytes from Sjogren's syndrome patients display increased vasoactive intestinal peptide receptor 2 expression and impaired apoptotic cell phagocytosis. Clin Exp Immunol. 2014;177:662–670. doi: 10.1111/cei.12378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henning RJ, Sawmiller DR. Vasoactive intestinal peptide: cardiovascular effects. Cardiovasc Res. 2001;49:27–37. doi: 10.1016/s0008-6363(00)00229-7. [DOI] [PubMed] [Google Scholar]
- Jiang X, Jing H, Ganea D. VIP and PACAP down-regulate CXCL10 (IP-10) and up-regulate CCL22 (MDC) in spleen cells. J Neuroimmunol. 2002;133:81–94. doi: 10.1016/s0165-5728(02)00365-x. [DOI] [PubMed] [Google Scholar]
- Jimeno R, Gomariz RP, Gutierrez-Canas I, Martinez C, Juarranz Y, Leceta J. New insights into the role of VIP on the ratio of T-cell subsets during the development of autoimmune diabetes. Immunol Cell Biol. 2010;88:734–745. doi: 10.1038/icb.2010.29. [DOI] [PubMed] [Google Scholar]
- Jimeno R, Leceta J, Martinez C, Gutierrez-Canas I, Carrion M, Perez-Garcia S, Garin M, Mellado M, Gomariz RP, Juarranz Y. Vasoactive Intestinal Peptide Maintains the Nonpathogenic Profile of Human Th17-Polarized Cells. J Mol Neurosci. 2014 doi: 10.1007/s12031-014-0318-3. [DOI] [PubMed] [Google Scholar]
- Juarranz Y, Gutierrez-Canas I, Santiago B, Carrion M, Pablos JL, Gomariz RP. Differential expression of vasoactive intestinal peptide and its functional receptors in human osteoarthritic and rheumatoid synovial fibroblasts. Arthritis Rheum. 2008;58:1086–1095. doi: 10.1002/art.23403. [DOI] [PubMed] [Google Scholar]
- Kanellopoulos-Langevin C, Caucheteux SM, Verbeke P, Ojcius DM. Tolerance of the fetus by the maternal immune system: role of inflammatory mediators at the feto-maternal interface. Reprod Biol Endocrinol. 2003;1:121. doi: 10.1186/1477-7827-1-121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kato I, Suzuki Y, Akabane A, Yonekura H, Tanaka O, Kondo H, Takasawa S, Yoshimoto T, Okamoto H. Transgenic mice overexpressing human vasoactive intestinal peptide (VIP) gene in pancreatic beta cells. Evidence for improved glucose tolerance and enhanced insulin secretion by VIP and PHM-27 in vivo. J Biol Chem. 1994;269:21223–21228. [PubMed] [Google Scholar]
- Kebir H, Kreymborg K, Ifergan I, Dodelet-Devillers A, Cayrol R, Bernard M, Giuliani F, Arbour N, Becher B, Prat A. Human TH17 lymphocytes promote blood-brain barrier disruption and central nervous system inflammation. Nat Med. 2007;13:1173–1175. doi: 10.1038/nm1651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim WK, Kan Y, Ganea D, Hart RP, Gozes I, Jonakait GM. Vasoactive intestinal peptide and pituitary adenylyl cyclase-activating polypeptide inhibit tumor necrosis factor-alpha production in injured spinal cord and in activated microglia via a cAMP-dependent pathway. J Neurosci. 2000;20:3622–3630. doi: 10.1523/JNEUROSCI.20-10-03622.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lampron A, Elali A, Rivest S. Innate immunity in the CNS: redefining the relationship between the CNS and Its environment. Neuron. 2013;78:214–232. doi: 10.1016/j.neuron.2013.04.005. [DOI] [PubMed] [Google Scholar]
- Lauenstein HD, Quarcoo D, Plappert L, Schleh C, Nassimi M, Pilzner C, Rochlitzer S, Brabet P, Welte T, Hoymann HG, Krug N, Muller M, Lerner EA, Braun A, Groneberg DA. Pituitary adenylate cyclase-activating peptide receptor 1 mediates anti-inflammatory effects in allergic airway inflammation in mice. Clin Exp Allergy. 2010;41:592–601. doi: 10.1111/j.1365-2222.2010.03636.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lauenstein HD, Quarcoo D, Plappert L, Schleh C, Nassimi M, Pilzner C, Rochlitzer S, Brabet P, Welte T, Hoymann HG, Krug N, Muller M, Lerner EA, Braun A, Groneberg DA. Pituitary adenylate cyclase-activating peptide receptor 1 mediates anti-inflammatory effects in allergic airway inflammation in mice. Clin Exp Allergy. 2011;41:592–601. doi: 10.1111/j.1365-2222.2010.03636.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leceta J, Martinez MC, Delgado M, Garrido E, Gomariz RP. Lymphoid cell subpopulations containing vasoactive intestinal peptide in the rat. Peptides. 1994;15:791–797. doi: 10.1016/0196-9781(94)90031-0. [DOI] [PubMed] [Google Scholar]
- Lee Y, Awasthi A, Yosef N, Quintana FJ, Xiao S, Peters A, Wu C, Kleinewietfeld M, Kunder S, Hafler DA, Sobel RA, Regev A, Kuchroo VK. Induction and molecular signature of pathogenic TH17 cells. Nat Immunol. 2012;13:991–999. doi: 10.1038/ni.2416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lelievre V, Favrais G, Abad C, Adle-Biassette H, Lu Y, Germano PM, Cheung-Lau G, Pisegna JR, Gressens P, Lawson G, Waschek JA. Gastrointestinal dysfunction in mice with a targeted mutation in the gene encoding vasoactive intestinal polypeptide: a model for the study of intestinal ileus and Hirschsprung's disease. Peptides. 2007;28:1688–1699. doi: 10.1016/j.peptides.2007.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leuchte HH, Baezner C, Baumgartner RA, Bevec D, Bacher G, Neurohr C, Behr J. Inhalation of vasoactive intestinal peptide in pulmonary hypertension. Eur Respir J. 2008;32:1289–1294. doi: 10.1183/09031936.00050008. [DOI] [PubMed] [Google Scholar]
- Li JM, Southerland L, Hossain MS, Giver CR, Wang Y, Darlak K, Harris W, Waschek J, Waller EK. Absence of vasoactive intestinal peptide expression in hematopoietic cells enhances Th1 polarization and antiviral immunity in mice. J Immunol. 2011;187:1057–1065. doi: 10.4049/jimmunol.1100686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu L, Yen JH, Ganea D. A novel VIP signaling pathway in T cells cAMP-->protein tyrosine phosphatase (SHP-2?)-->JAK2/STAT4-->Th1 differentiation. Peptides. 2007;28:1814–1824. doi: 10.1016/j.peptides.2007.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maldonado RA, von Andrian UH. How tolerogenic dendritic cells induce regulatory T cells. Adv Immunol. 2010;108:111–165. doi: 10.1016/B978-0-12-380995-7.00004-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez C, Abad C, Delgado M, Arranz A, Juarranz MG, Rodriguez-Henche N, Brabet P, Leceta J, Gomariz RP. Anti-inflammatory role in septic shock of pituitary adenylate cyclase-activating polypeptide receptor. Proc Natl Acad Sci U S A. 2002;99:1053–1058. doi: 10.1073/pnas.012367999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez C, Delgado M, Pozo D, Leceta J, Calvo JR, Ganea D, Gomariz RP. VIP and PACAP enhance IL-6 release and mRNA levels in resting peritoneal macrophages: in vitro and in vivo studies. J Neuroimmunol. 1998;85:155–167. doi: 10.1016/s0165-5728(98)00018-6. [DOI] [PubMed] [Google Scholar]
- Martinez C, Ortiz AM, Juarranz Y, Lamana A, Seoane IV, Leceta J, Garcia-Vicuna R, Gomariz RP, Gonzalez-Alvaro I. Serum levels of vasoactive intestinal peptide as a prognostic marker in early arthritis. PLoS One. 2014;9:e85248. doi: 10.1371/journal.pone.0085248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morice A, Unwin RJ, Sever PS. Vasoactive intestinal peptide causes bronchodilatation and protects against histamine-induced bronchoconstriction in asthmatic subjects. Lancet. 1983;2:1225–1227. doi: 10.1016/s0140-6736(83)91272-2. [DOI] [PubMed] [Google Scholar]
- Ousman SS, Kubes P. Immune surveillance in the central nervous system. Nat Neurosci. 2012;15:1096–1101. doi: 10.1038/nn.3161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paladini F, Cocco E, Cauli A, Cascino I, Vacca A, Belfiore F, Fiorillo MT, Mathieu A, Sorrentino R. A functional polymorphism of the vasoactive intestinal peptide receptor 1 gene correlates with the presence of HLA-B*2705 in Sardinia. Genes Immun. 2008;9:659–667. doi: 10.1038/gene.2008.60. [DOI] [PubMed] [Google Scholar]
- Prasse A, Zissel G, Lutzen N, Schupp J, Schmiedlin R, Gonzalez-Rey E, Rensing-Ehl A, Bacher G, Cavalli V, Bevec D, Delgado M, Muller-Quernheim J. Inhaled vasoactive intestinal peptide exerts immunoregulatory effects in sarcoidosis. Am J Respir Crit Care Med. 2010;182:540–548. doi: 10.1164/rccm.200909-1451OC. [DOI] [PubMed] [Google Scholar]
- Ransohoff RM, Brown MA. Innate immunity in the central nervous system. J Clin Invest. 2012;122:1164–1171. doi: 10.1172/JCI58644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samarasinghe AE, Hoselton SA, Schuh JM. The absence of VPAC2 leads to aberrant antibody production in Aspergillus fumigatus sensitized and challenged mice. Peptides. 2011;32:131–137. doi: 10.1016/j.peptides.2010.09.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanlioglu S. Vasoactive Intestinal Peptide-mediated Gene Therapy for Diabetes. JOP. 2014;15:2783. [Google Scholar]
- Sharma V, Delgado M, Ganea D. Granzyme B, a new player in activation-induced cell death, is down-regulated by vasoactive intestinal peptide in Th2 but not Th1 effectors. J Immunol. 2006;176:97–110. doi: 10.4049/jimmunol.176.1.97. [DOI] [PubMed] [Google Scholar]
- Sharpless NS, Thal LJ, Perlow MJ, Tabaddor K, Waltz JM, Shapiro KN, Amin IM, Engel J, Jr, Crandall PH. Vasoactive intestinal peptide in cerebrospinal fluid. Peptides. 1984;5:429–433. doi: 10.1016/0196-9781(84)90248-1. [DOI] [PubMed] [Google Scholar]
- Shen S, Spratt C, Sheward WJ, Kallo I, West K, Morrison CF, Coen CW, Marston HM, Harmar AJ. Overexpression of the human VPAC2 receptor in the suprachiasmatic nucleus alters the circadian phenotype of mice. Proc Natl Acad Sci U S A. 2000;97:11575–11580. doi: 10.1073/pnas.97.21.11575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein-Streilein J, Caspi RR. Immune privilege and the philosophy of immunology. Front Immunol. 2014;5:110. doi: 10.3389/fimmu.2014.00110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun W, Hong J, Zang YC, Liu X, Zhang JZ. Altered expression of vasoactive intestinal peptide receptors in T lymphocytes and aberrant Th1 immunity in multiple sclerosis. Int Immunol. 2006;18:1691–1700. doi: 10.1093/intimm/dxl103. [DOI] [PubMed] [Google Scholar]
- Szema AM, Hamidi SA, Golightly MG, Rueb TP, Chen JJ. VIP Regulates the Development & Proliferation of Treg in vivo in spleen. Allergy Asthma Clin Immunol. 2011;7:19. doi: 10.1186/1710-1492-7-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan YV, Abad C, Lopez R, Dong H, Liu S, Lee A, Gomariz RP, Leceta J, Waschek JA. Pituitary adenylyl cyclase-activating polypeptide is an intrinsic regulator of Treg abundance and protects against experimental autoimmune encephalomyelitis. Proc Natl Acad Sci U S A. 2009;106:2012–2017. doi: 10.1073/pnas.0812257106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toscano MG, Delgado M, Kong W, Martin F, Skarica M, Ganea D. Dendritic cells transduced with lentiviral vectors expressing VIP differentiate into VIP-secreting tolerogenic-like DCs. Mol Ther. 2010;18:1035–1045. doi: 10.1038/mt.2009.293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voice J, Donnelly S, Dorsam G, Dolganov G, Paul S, Goetzl EJ. c-Maf and JunB mediation of Th2 differentiation induced by the type 2 G protein-coupled receptor (VPAC2) for vasoactive intestinal peptide. J Immunol. 2004;172:7289–7296. doi: 10.4049/jimmunol.172.12.7289. [DOI] [PubMed] [Google Scholar]
- Voice JK, Dorsam G, Lee H, Kong Y, Goetzl EJ. Allergic diathesis in transgenic mice with constitutive T cell expression of inducible vasoactive intestinal peptide receptor. FASEB J. 2001;15:2489–2496. doi: 10.1096/fj.01-0671com. [DOI] [PubMed] [Google Scholar]
- Voice JK, Grinninger C, Kong Y, Bangale Y, Paul S, Goetzl EJ. Roles of vasoactive intestinal peptide (VIP) in the expression of different immune phenotypes by wild-type mice and T cell-targeted type II VIP receptor transgenic mice. J Immunol. 2003;170:308–314. doi: 10.4049/jimmunol.170.1.308. [DOI] [PubMed] [Google Scholar]
- Yadav M, Huang MC, Goetzl EJ. VPAC1 (vasoactive intestinal peptide (VIP) receptor type 1) G protein-coupled receptor mediation of VIP enhancement of murine experimental colitis. Cell Immunol. 2011;267:124–132. doi: 10.1016/j.cellimm.2011.01.001. [DOI] [PubMed] [Google Scholar]
- Yadav M, Rosenbaum J, Goetzl EJ. Cutting edge: vasoactive intestinal peptide (VIP) induces differentiation of Th17 cells with a distinctive cytokine profile. J Immunol. 2008;180:2772–2776. doi: 10.4049/jimmunol.180.5.2772. [DOI] [PubMed] [Google Scholar]
- Zhao S, Zhu W, Xue S, Han D. Testicular defense systems: immune privilege and innate immunity. Cell Mol Immunol. 2014;11:428–437. doi: 10.1038/cmi.2014.38. [DOI] [PMC free article] [PubMed] [Google Scholar]