Abstract
Overexpression of cyclin D1 is a hallmark feature of mantle cell lymphoma (MCL). Many of the oncogenic effects of cyclin D1 are mediated through cyclin dependent kinases (CDKs). P276-00 is a potent small-molecule inhibitor of CDK4-D1, CDK1-B, and CDK9-T with promising activity in pre-clinical models. In Phase I studies of P276-00 in patients with refractory solid neoplasms, it was well-tolerated with a mild trend of single-agent efficacy. A Phase II study of this agent was conducted in patients with relapsed or refractory MCL at the recommended dose of 185 mg/m2/day from days 1-5 of a 21-day cycle. Thirteen patients were enrolled on this study: 11 patients had disease progression, 1 patient was withdrawn due to an adverse event (AE), and 1 patient died. Eleven patients (84.6%) experienced a treatment-emergent AE deemed related to P276-00. Nine patients (69.2%) received at least 2 cycles of treatment, which was the pre-defined threshold to be evaluable for efficacy; treatment was discontinued early in 2 patients due to AEs (one of which was attributed to P276-00 administration) and in 2 patients due to disease progression. Two patients experienced stable disease for an estimated median duration of 60.5 days (range 58-63 days). The estimated median time to progression for the pre-defined efficacy population was 43 days (range 38-58 days). Given the results observed in this study, if continued evaluation of CDK inhibition in MCL occurs, it should be considered earlier in the disease course or as part of combination strategies for relapsed or refractory disease.
Introduction
Overexpression of cyclin D1 as a result of t(11;14)(q13;q32) translocation is the pathognomic hallmark of mantle cell lymphoma (MCL).1,2 Cyclin D1 plays a central role in the control of the G1 phase of the cell cycle by binding to cyclin-dependent kinase 4 (CDK4) and CDK6. Cyclin D1 complexes with CDK4 and CDK6, phosphorylate the retinoblastoma protein (pRb), leading to the inactivation of its suppressor effect on cell cycle progression. The hyperphosphorylation of pRb by these complexes leads to the release of the E2F family of transcription factors, allowing the transcription of various genes necessary for DNA synthesis, thus facilitating G1/S transition and uncontrolled cell proliferation.3 It is a l s o postulated that cyclin D1 may have an oncogenic role independent of pRb in MCL.4,5 Therefore, inhibition of the cyclin D1-CDK4 complex formation appears to have a potentially promising target in MCL.
P276-00 is a novel, potent, small-molecule, flavone-derived inhibitor of CDK4-D1, CDK1-B, and CDK9-T, with potent cytotoxic effects against chemosensitive as well as chemoresistant tumor cell lines.6 Anti-tumor activity of P276-00 has also been demonstrated in clonogenic assays, murine tumor models, and in human tumor xenograft models in mice.7,8 The safety of P276-00 in humans was previously established in two phase I clinical trials with this agent in patients with advanced refractory neoplasms.9 It was administered as a daily intravenous (IV) infusion. The most common adverse effects reported were Grade 1 hypotension, Grade 1 dizziness, and Grade 2 fatigue; dose-limiting toxicities were infusion reactions, fatigue, and lung infection (all of which were Grade 3). Based on the results of these studies, the recommended phase II dose of P276-00 was 185 mg/m2/day on Days 1-5 of each 21-day cycle. Efficacy was observed in the form of stable disease of duration ranging from 2 to 8 cycles in 14 patients and minor responses in 2 patients.
Based on these favorable pre-clinical and phase I clinical data, we pursued a phase II study of P276-00 as monotherapy at the recommended phase II dose. The primary objective of this study was to evaluate the efficacy of this agent in patients with relapsed or refractory MCL. There is strong rationale for this approach, as there is a growing list of malignancies that reliably respond to agents that target a critical or (in some cases) pathognomonic oncogenic mutation.10-13 This strategy has yet to be fully realized in MCL, marked by cyclin D1 overexpression, where inhibiting the effects of cyclin D1 could have a significant clinical impact.
Patients and Methods
Patients
All patients were at least 18 years of age with a histologically confirmed diagnosis of MCL, measurable disease, and documented progression or relapse of disease after at least 1 line of prior chemotherapy. Patients were included with presence of either nuclear cyclin D1 determined by immunohistochemistry or t(11;14) by fluorescence in situ hybridization (FISH), polymerase chain reaction (PCR), or conventional karyotyping. Additional inclusion criteria included Eastern Cooperative Oncology Group (ECOG) performance status of 2 or more; life expectancy of at least 3 months; ability to understand and the willingness to sign a written informed consent document; and full recovery from all prior treatment toxicities.
Study exclusion criteria were patients who received any other therapy within 4 weeks of study drug administration; prior treatment with monoclonal antibodies or any radio- or toxin-immunoconjugates within 3 months of study drug administration (except a patient who had rituximab treatment within 3 months and had progressive disease after such treatment); prior allogeneic stem cell transplantation within 1 year of study drug administration; current or prior CNS lymphoma; QTc interval greater than 450 msec; unstable angina, myocardial infarction, CHF or stroke within the previous 6 months of study drug administration; presence of active and serious comorbidity and uncontrolled illness other than MCL; history of other prior malignancies except for properly treated basal cell or squamous cell carcinoma of skin, in situ cervical or breast cancer or early stage prostate cancer; inadequate hematopoietic, hepatic, and renal function; patients known to be infected with human immunodeficiency virus (HIV), tuberculosis, or hepatitis B or C viruses; and pregnant or lactating women. All patients provided written informed and verbal consent prior to participation in this study. This trial was registered at ClinicalTrials.gov (NCT00843050).
Plan of Treatment
Patients were administered P276-00 as an IV infusion on days 1 to 5 of each of the 21-day cycles for a minimum of 6 cycles and a maximum of 12 cycles, or until progressive disease (PD) or unacceptable toxicity occurred. Pharmacokinetic (PK) assessments were done on Cycle 1, Day 1 (pre-dose and post-dose time points), and optional biomarker assessments were scheduled pre-dose within 4 weeks of Day 1 and post-dose on Day 4 or 5 in the form of peripheral blood, lymph node, and bone marrow samples. Follow-up visits occurred up to 4 weeks (±1 week window) after the end of the last cycle for final safety assessments.
Response and Toxicity Evaluation and Definitions
Tumor measurements were performed at baseline by complete physical examination along with computed tomography (CT) and/or magnetic resonance imaging (MRI). Positron emission tomography (PET) scans were recommended for eligible patients but were not required. Response evaluation was performed using the International Working Group (IWG) revised response criteria for malignant lymphoma.14 Adverse events (AEs) were graded by the National Cancer Institute's Common Terminology Criteria for Adverse Events (version 4). Safety and efficacy evaluations were performed on Days 1 to 5 and 11 of each cycle, with follow-up imaging studies performed Day 21 of every 2 cycles (i.e. every 6 weeks).
Statistical Considerations
This was an open-label, single-arm, 2-stage trial. The primary efficacy endpoint was the proportion of subjects achieving an objective response (defined as either a complete response [CR] or partial response [PR]). Secondary endpoints included duration of response (defined as the time from when the measurement criteria were met for CR or PR until the first date that recurrent or PD is objectively or clinically documented) and time to progression (defined as the time from day 1 of the study drug administration until the first date of PD). Approximately 35 patients were planned to be enrolled in two stages into the study to obtain a total of 25 efficacy evaluable patients (patients who complete at least 2 cycles of study treatment and have tumor measurements at the end of 2 cycles). A total of 15 efficacy-evaluable patients were planned to be evaluated in Stage I of the study. If there was at least 1 response (CR or PR) of any duration or at least 2 stable disease (SD) cases for 4 or more cycles observed in Stage I, then the study would continue into Stage II, in which additional patients would be treated until there were 10 additional efficacy-evaluable patients. Duration of response and time to progression were estimated using the Kaplan-Meier method.
Results
A total of 14 patients were screened in this trial, of which a total of 13 patients enrolled on this study; their baseline characteristics are described in Table 1. Study participants were generally of relatively advanced age (median of 67 years, maximum of 83 years) with an approximately 2:1 male predominance. The median number of prior treatments was 4 (interquartile range: 3-10), with three patients (23.1%) having disease refractory to the most recent previous treatment. None of the patients had previously undergone hematopoietic cell transplantation (HCT).
Table 1.
Baseline patient characteristics (n = 13).
| Gender, n (%) | |
|---|---|
| Male | 9 (69.2%) |
| Female | 4 (30.8%) |
| Age (years) | |
| Mean (Std. Dev.) | 65.8 (9.99) |
| Median (Range) | 67.0 (45-83) |
| Race, n (%) | |
| Asian | 5 (38.5%) |
| Black or African-American | 1 (7.7%) |
| White or Caucasian | 7 (53.8%) |
| Disease Status at Enrollment, n (%) | |
| Relapsed | 10 (76.9%) |
| Refractory | 3 (23.1%) |
| ECOG Performance Status, n (%) | |
| 0 | 8 (61.5%) |
| 1 | 4 (30.8%) |
| 2 | 1 (7.7%) |
| Prior Therapies, n (%) | |
| Chemotherapy | 13 (100.0%) |
| Radiotherapy | 4 (30.8%) |
| HCT | 0 |
| Prior Lines of Chemotherapy | |
| Median (Interquartile Range) | 4 (3-10) |
Abbreviations: ECOG = Eastern Cooperative Oncology Group; HCT = hematopoietic cell transplantation; Std. Dev. = standard deviation.
The median number of cycles of treatment that were completed was 2: 4 patients (30.8%) received 1 cycle; 7 patients (53.8%) received 2 cycles; and 2 patients (15.4%) received 3 cycles. The 4 patients who received only 1 cycle of therapy were discontinued early due to toxicity in 2 patients (see below for details) and disease progression in 2 patients. This yielded a mean duration of exposure to P276-00 of 11.1 days (range 10-15 days, standard deviation 2.20 days). The mean total cumulative dose was 3835 mg/m2 (range: 2745-5160 mg/m2, standard deviation of 760 mg/m2).
All 13 patients enrolled were evaluable for safety, and Tables 2 and 3 summarize these results. There were no significant changes seen in vital signs or electrocardiographic parameters. Eleven patients (84.6%) experienced an AE that was deemed related to P276-00. Of the 7 serious adverse events (SAEs) observed, 2 patients (15.4%) experienced 1 SAE each that was possibly related to study drug administration: grade 4 thrombocytopenia and grade 3 herpes zoster. The latter of these led to early discontinuation of study drug for the affected patient. The second patient whose study treatment was discontinued early experienced grade 2 elevated creatinine that required hospitalization for monitoring. This event was deemed unrelated to P276-00; nevertheless, in the interest of safety, study treatment was stopped. Two fatal adverse events were observed, both of which were from disease progression.
Table 2.
Summary of adverse events experienced by patients receiving P276-00.
| N (%) | |
|---|---|
| Number of Patients Experiencing AEs | 11 (84.6%) |
| AEs Related to Study Drug | 11 (84.6%) |
| AEs Leading to Discontinuation of Study Drug by the PI | 3 (23.1%) |
| SAEs | 4 (30.8%) |
| SAEs Related to Study Drug | 2 (15.4%) |
| Any SAE with Outcome Other Than Death | 4 (30.8 %) |
| Fatal AEs | 2 (15.4 %) |
| Fatal AEs Related to Study Drug | 0 |
| Subjects with CTCAE Grade 3 and Above | 8 (61.5 %) |
Abbreviations: AE = adverse event; CTCAE = Common Terminology Criteria for Adverse Events; PI = principal investigator; SAE = serious adverse event
Table 3.
Summary of serious adverse events experienced by patients receiving P276-00.
| System Organ Class a | Preferred Term a | Grade a | Relationship to Study Drug | Action Taken with Study Drug | Other Action Taken | Outcome |
|---|---|---|---|---|---|---|
| Investigations | Blood creatinine increased | 2 | None/Unrelated | Discontinued | Hospitalization | Ongoing |
| Respiratory, thoracic and mediastinal disorders | Dyspnea | 3 | None/Unrelated | None | None | Ongoing |
| Cardiac disorders | Atrial fibrillation | 3 | None/Unrelated | None | Hospitalization | Complete Recovery |
| Infections and infestations | Herpes zoster | 3 | Possible | Discontinued | Hospitalization | Complete Recovery |
| Blood and lymphatic system disorders | Thrombocytopenia | 4 | Possible | None | Transfusion | Ongoing |
| General disorders and administration site conditions | Disease progression | 5 | Unlikely | None | Medication Given | Death |
| General disorders and administration site conditions | Disease progression | 5 | None/Unrelated | Discontinued | None | Death |
Terminology and definitions are taken from the National Cancer Institute's Common Terminology Criteria of Adverse Events.
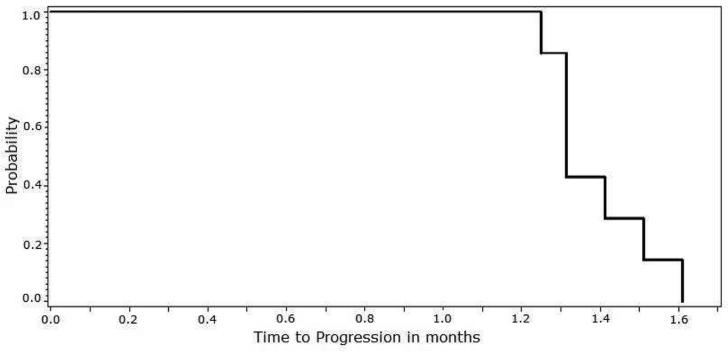
Nine patients (69.2%) received at least 2 cycles of study treatment and were thus evaluable for efficacy. These results are summarized in Table 4. No objective disease responses were observed among these subjects as per IWG revised response criteria. Two patients (22.2%) had SD for an estimated median duration of 60.5 days (range 58-63 days). As mentioned above, 2 patients did not receive 2 cycles of study treatment due to early PD. Thus, all patients ultimately experienced PD. Due to the lack of observed efficacy, enrollment in the study was terminated early. For the pre-defined efficacy population (n = 9), the estimated median time to progression was 43 days (range 38-58 days), as depicted in Figure 1.
Table 4.
Summary of best overall response to P276-00.
| Response a | N (%) |
|---|---|
| Not Evaluable for Responseb | 2 (15.4%) |
| Best Response | 11 (84.6%) |
| Complete Response | 0 |
| Partial Response | 0 |
| Stable Disease | 2 (15.4%) |
| Progressive Disease | 9 (69.2%) |
Responses are according to International Working Group definitions and are further explained in the text.
Two cycles of treatment were required to be evaluable for response. Two patients experienced toxicity requiring study discontinuation after only 1 cycle. See text for further details.
Figure 1.
Kaplan-Meier curve depicting time to progression in patients receiving P276-00, limited to patients included in the pre-defined efficacy population (n = 9).
Discussion
Targeted therapy directed against the downstream mediators of cyclin D1 is a rational line of investigation in the management of MCL. Despite data supporting this approach, inhibition of several CDK isoforms with P276-00 failed to demonstrate any notable anti-tumor effects in patients with relapsed/refractory MCL when administered daily from Days 1 to 5 in a 21-day cycle. Several plausible reasons may be underlying this observation. For example, patients who enrolled on this study were generally high risk, with the majority of them having received at least three prior lines of therapy and approximately one-quarter having chemorefractory disease. Additionally, MCL is known to be a genomically unstable disease.1,15 As a consequence, particularly in patients with more advanced and heavily-pretreated disease, inhibition of select CDK isoforms may not interfere sufficiently with critical proliferative and/or survival signals to lead to clinically-significant anti-tumor effects. Use of agents like P276-00 earlier in the natural history of MCL may yield more promising results.
Another potential avenue for CDK inhibitors such as P276-00 is to explore them in combination with other chemotherapeutic agents. In a recent study, P276-00 combined with either doxorubicin or bortezomib showed synergistic cytotoxic activity in an in vitro MCL model.8 Others have demonstrated similar potentiating activity of P276-00 in combination with doxorubicin in non-small cell lung carcinoma cell lines and xenografts as well as with gemcitabine in pancreas cancer xenografts.16-18 In fact, the latter of these strategies was being investigated in a phase I/II clinical trial in patients with advanced pancreatic cancer, in which P276-00 was well-tolerated in these patients when administered with gemcitabine with mild trends of efficacy.19 Others have shown potential deleterious effects of combining DNA-damaging agents with a CDK4/6 inhibitor (PD0332991; Pfizer, Inc.) in a mouse xenograft model of breast cancer.20 Interestingly, this CDK inhibitor seemed to ameliorate some of the hematologic toxicity observed with cytotoxic agents. The authors of this study postulated that it occurred through a mechanism known as pharmacologic quiescence, whereby inhibition of the CDK enzymes in hematopoietic progenitors protected them from the effects caused by DNA-damaging agents. Consequently, they also speculated that the same effect contributed to the inferior anti-tumor effects when their CDK inhibitor was combined with carboplatin. Thus, perhaps combining CDK inhibitors like P276-00 with other agents that do not directly damage DNA (e.g. bortezomib, lenalidomide, mammalian target of rapamycin [mTOR] inhibitors, etc.) will yield improved results in MCL.
Contrary to our observations, a phase I clinical trial of PD0332991 did yield objective disease responses in patients with relapsed MCL, including 1 CR and 2 PRs from a cohort of 17 patients.21 This agent differs from P276-00 in that it is a more selective inhibitor of CDK4 and CDK6.22 This clinical study included pharmacodynamics evaluations and pathologic correlative studies corroborating the objective clinical responses observed. The investigators noted decreased tumor-cell proliferation as assessed by Ki-67 staining from biopsy specimens pre- and post-treatment and via functional imaging with fluorothymidine positron emission tomography. In general, the patient population in this study was relatively similar to ours: a majority of patients had received at least 2 prior lines of therapy, while 3 of 17 from their study had previously received autologous HCT compared to none in our study. Their observed responses are noteworthy, though a large majority of patients on this study also did not respond to CDK inhibitor monotherapy as we observed. Additionally, these 3 responses were not observed until 4-8 cycles (12-24 weeks) of treatment, suggesting perhaps that these patients had less aggressive disease.
Similarly modest clinical findings have been observed with single-agent treatment with flavopiridol (alvocidib; Sanofi S.A.), another CDK inhibitor in development for MCL.23 More recently, though, flavopiridol has been combined with fludarabine and rituximab in a phase I study of patients with a variety of indolent B-cell non-Hodgkin lymphomas and MCL.24 Interestingly, majority of the patients in this study were previously untreated, including 6 of 10 patients with MCL. Notably, there were 8 objective responses among these 10 patients, including 7 CRs, comparing favorably with historical results of other fludarabine-based regimens. Thus, the authors of this study concluded that this regimen is particularly promising in older patients with MCL and warrants further evaluation. These findings also support using CDK inhibitors earlier in the natural history of MCL or in combination with other agents, as mentioned above.
Conclusions
Single-agent treatment with the CDK inhibitor, P276-00, was generally well-tolerated but failed to produce any objective responses among 13 patients with relapsed/refractory MCL in a phase II study. The role of CDK inhibitors in the treatment of MCL remains uncertain. However, data from other trials with this class of agents suggest that anti-tumor effects can be observed in MCL, though these responses take time to occur. Because of these results and their generally favorable safety profile, further study of these compounds may be warranted, though, future efforts should consider evaluation in patients with less refractory disease or in combination with agents with more established efficacy and safety to explore potential synergistic effects in pre-clinical models. This approach may produce more frequent and durable remissions without significant added toxicity, thus improving the outcomes of patients with this challenging lymphoma.
Clinical Practice Points.
Mantle cell lymphoma (MCL) is a rare subtype of B-cell non-Hodgkin lymphoma that is particularly challenging to treat. For fit patients, aggressive treatment with multi-agent chemoimmunotherapy followed by consolidative autologous hematopoietic cell transplantation can produce durable remissions. However, relapse rates remain high. Furthermore, many patients diagnosed with MCL are ineligible for such an aggressive strategy of treatment.
Targeted approaches with a more favorable toxicity profile are of great interest for treating this disease. One potential avenue is through inhibition of cyclin-dependent kinases (CDKs) by blocking the cyclin D1 complex formation with the CDKs. These enzymes play a key role in cell-cycle regulation and are major downstream mediators of cyclin D1, which is overexpressed in the vast majority of MCL.
We describe the results of a phase II study of P276-00, a novel inhibitor of several CDK isoforms, in patients with relapsed or refractory MCL. This agent was generally well-tolerated, however no objective responses were seen among 13 patients enrolled. Several plausible reasons may underlie this observation, among these are that we included relatively high-risk patients with significant prior treatment of their lymphoma.
Given the targeted nature of this new therapy, perhaps using it earlier in the natural history of MCL or in combination with other effective agents could produce better results with an improved safety profile. Such efforts are being explored by other groups. If these studies are successful, perhaps CDK inhibitors will become a part of the treatment options available to patients with MCL.
Acknowledgements
R.D.C. is supported by NIH K12CA076930. A.K.G. is supported by NIH K24CA184039, the Lymphoma Research Foundation MCL Initiative, and a Clinical Scholar Award by the Leukemia and Lymphoma Society. This work was also supported by gifts from Frank and Betty Vandermeer, Don and Debbie Hunkins, and the Mary Aileen Wright Memorial Fund. We would also like to acknowledge Dr. Martha Glenn, Dr. Craig Reeder, Dr. Vinod Raina, Dr. Cecil Ross, Dr. Alan Hatfield, Dr. Himanshu Parikh, Dr. Mudgal Kothekar, Dr. Ajit Patil and Ms. Poornima Dhobe.
Funding Sources: Piramal Enterprises Limited (PEL) (formerly Piramal Life Sciences Limited)
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of Interest
Drs. Goy, Gopal, and Advani received research support from PEL to participate in this study. Dr. Chawla, Dr. Nachankar and Ms. Gandhi are all employees of PEL and have no stock options in the company. Dr. Cassaday has no conflicts of interest.
Manufacturer Name
P276-00 is a proprietary compound of Piramal Enterprises Limited (PEL) (formerly known as Piramal Life Sciences Limited), which is also manufactured by PEL.
References
- 1.Pileri SA, Falini B. Mantle cell lymphoma. Haematologica. 2009;94:1488–92. doi: 10.3324/haematol.2009.013359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jares P, Campo E. Advances in the understanding of mantle cell lymphoma. Br J Haematol. 2008;142:149–65. doi: 10.1111/j.1365-2141.2008.07124.x. [DOI] [PubMed] [Google Scholar]
- 3.Jares P, Campo E, Pinyol M, et al. Expression of retinoblastoma gene product (pRb) in mantle cell lymphomas. Correlation with cyclin D1 (PRAD1/CCND1) mRNA levels and proliferative activity. Am J Pathol. 1996;148:1591–600. [PMC free article] [PubMed] [Google Scholar]
- 4.Jares P, Colomer D, Campo E. Genetic and molecular pathogenesis of mantle cell lymphoma: perspectives for new targeted therapeutics. Nat Rev Cancer. 2007;7:750–62. doi: 10.1038/nrc2230. [DOI] [PubMed] [Google Scholar]
- 5.Martin P, Leonard JP. Novel therapeutic targets in mantle cell lymphoma. Expert Opin Ther Targets. 2007;11:929–40. doi: 10.1517/14728222.11.7.929. [DOI] [PubMed] [Google Scholar]
- 6.Joshi KS, Rathos MJ, Joshi RD, et al. In vitro antitumor properties of a novel cyclin-dependent kinase inhibitor, P276-00. Mol Cancer Ther. 2007;6:918–25. doi: 10.1158/1535-7163.MCT-06-0613. [DOI] [PubMed] [Google Scholar]
- 7.Joshi KS, Rathos MJ, Mahajan P, et al. P276-00, a novel cyclin-dependent inhibitor induces G1- G2 arrest, shows antitumor activity on cisplatin-resistant cells and significant in vivo efficacy in tumor models. Mol Cancer Ther. 2007;6:926–34. doi: 10.1158/1535-7163.MCT-06-0614. [DOI] [PubMed] [Google Scholar]
- 8.Shirsath NP, Manohar SM, Joshi KS. P276-00, a cyclin-dependent kinase inhibitor, modulates cell cycle and induces apoptosis in vitro and in vivo in mantle cell lymphoma cell lines. Mol Cancer. 2012;11:77. doi: 10.1186/1476-4598-11-77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hirte H, Digumarti R, Baetz T, et al. A phase I study of the selective cyclin dependent kinase inhibitor P276-00 in patients with advanced refractory neoplasms: final report. 100th AACR Annual Meeting. 2009 Abstract #3308. [Google Scholar]
- 10.Druker BJ, Talpaz M, Resta DJ, et al. Efficacy and safety of a specific inhibitor of the BCR-ABL tyrosine kinase in chronic myeloid leukemia. N Engl J Med. 2001;344:1031–7. doi: 10.1056/NEJM200104053441401. [DOI] [PubMed] [Google Scholar]
- 11.Demetri GD, von Mehren M, Blanke CD, et al. Efficacy and safety of imatinib mesylate in advanced gastrointestinal stromal tumors. N Engl J Med. 2002;347:472–80. doi: 10.1056/NEJMoa020461. [DOI] [PubMed] [Google Scholar]
- 12.Thomas DA, Faderl S, Cortes J, et al. Treatment of Philadelphia chromosome-positive acute lymphocytic leukemia with hyper-CVAD and imatinib mesylate. Blood. 2004;103:4396–407. doi: 10.1182/blood-2003-08-2958. [DOI] [PubMed] [Google Scholar]
- 13.Kwak EL, Bang YJ, Camidge DR, et al. Anaplastic lymphoma kinase inhibition in non-small-cell lung cancer. N Engl J Med. 2010;363:1693–703. doi: 10.1056/NEJMoa1006448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cheson BD, Pfistner B, Juweid ME, et al. Revised response criteria for malignant lymphoma. J Clin Oncol. 2007;25:579–86. doi: 10.1200/JCO.2006.09.2403. [DOI] [PubMed] [Google Scholar]
- 15.Camacho E, Hernandez L, Hernandez S, et al. ATM gene inactivation in mantle cell lymphoma mainly occurs by truncating mutations and missense mutations involving the phosphatidylinositol-3 kinase domain and is associated with increasing numbers of chromosomal imbalances. Blood. 2002;99:238–44. doi: 10.1182/blood.v99.1.238. [DOI] [PubMed] [Google Scholar]
- 16.Rathos MJ, Joshi K, Khanwalkar H, et al. Molecular evidence for increased antitumor activity of gemcitabine in combination with a cyclin-dependent kinase inhibitor, P276-00 in pancreatic cancers. J Transl Med. 2012;10:161. doi: 10.1186/1479-5876-10-161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Subramaniam D, Periyasamy G, Ponnurangam S, et al. CDK-4 inhibitor P276 sensitizes pancreatic cancer cells to gemcitabine-induced apoptosis. Mol Cancer Ther. 2012;11:1598–608. doi: 10.1158/1535-7163.MCT-12-0102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rathos MJ, Khanwalkar H, Joshi K, et al. Potentiation of in vitro and in vivo antitumor efficacy of doxorubicin by cyclin-dependent kinase inhibitor P276-00 in human non-small cell lung cancer cells. BMC Cancer. 2013;13:29. doi: 10.1186/1471-2407-13-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Clinicaltrialsgov [Web site] [9/19/2013];A Phase I/II Study to Evaluate Safety and Efficacy of P276-00 in Combination With Gemcitabine in Patients with Cancer of Pancreas (SAVIOR) Available at: http://clinicaltrials.gov/show/NCT00898287.
- 20.Roberts PJ, Bisi JE, Strum JC, et al. Multiple roles of cyclin-dependent kinase 4/6 inhibitors in cancer therapy. J Natl Cancer Inst. 2012;104:476–87. doi: 10.1093/jnci/djs002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Leonard JP, LaCasce AS, Smith MR, et al. Selective CDK4/6 inhibition with tumor responses by PD0332991 in patients with mantle cell lymphoma. Blood. 2012;119:4597–607. doi: 10.1182/blood-2011-10-388298. [DOI] [PubMed] [Google Scholar]
- 22.Marzec M, Kasprzycka M, Lai R, et al. Mantle cell lymphoma cells express predominantly cyclin D1a isoform and are highly sensitive to selective inhibition of CDK4 kinase activity. Blood. 2006;108:1744–50. doi: 10.1182/blood-2006-04-016634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kouroukis CT, Belch A, Crump M, et al. Flavopiridol in untreated or relapsed mantle-cell lymphoma: results of a phase II study of the National Cancer Institute of Canada Clinical Trials Group. J Clin Oncol. 2003;21:1740–5. doi: 10.1200/JCO.2003.09.057. [DOI] [PubMed] [Google Scholar]
- 24.Lin TS, Blum KA, Fischer DB, et al. Flavopiridol, fludarabine, and rituximab in mantle cell lymphoma and indolent B-cell lymphoproliferative disorders. J Clin Oncol. 2010;28:418–23. doi: 10.1200/JCO.2009.24.1570. [DOI] [PMC free article] [PubMed] [Google Scholar]