Abstract
Objective
Prompt carotid endarterectomy (CEA) in clinically significant carotid stenosis is important in the prevention of neurologic sequelae. The greatest benefit from surgery is obtained by prompt revascularization upon diagnosis. It has been demonstrated that black patients both receive CEA less frequently than white patients and experience worse postoperative outcomes. We sought to test our hypothesis that black race is an independent risk factor for a prolonged time from sonographic diagnosis of carotid stenosis warranting surgery to the day of operation (TDO).
Methods
166 CEA patients from 1998-2013 at a single institution were retrospectively reviewed using Synthetic Derivative, a de-identified electronic medical record. Factors potentially affecting TDO, including demographics, preoperative cardiac stress testing (CST), degree of stenosis, smoking status and comorbidities, were noted. Multivariate analysis was performed on variables that trended with prolonged TDO on univariate analysis (P<.10) to determine independent (P<.05) predictors of TDO. Subgroup analyses were further performed on the symptomatic and asymptomatic stenosis cohorts.
Results
32 black patients and 134 white patients and were studied; the mean TDO was 78±17 days vs. 33±3 days, respectively (P<.001). In addition to the need for preoperative CST, black race was the only variable that demonstrated a trend with (P<.10), or was an independent risk factor (P<.05) for prolonged TDO among all patients (B=42 days; P<.001) and within the symptomatic (B=35 days; P=.08) and asymptomatic cohorts (B=35 days; P=.003). On Kaplan-Meier analysis, black patients in each strata of symptomatology (all, symptomatic and asymptomatic patients) experienced prolonged TDO (log-rank P<.03 for all three groups).
Conclusions
Black race is a risk factor for a temporal delay in CEA for carotid stenosis. Awareness of this disparity may help surgeons avoid undesirable delays in operation for their black patients.
INTRODUCTION
The carotid endarterectomy (CEA) is the primary operative intervention in the prevention of stroke due to carotid artery stenosis.1 CEA is a valuable tool in preventing cerebrovascular accident, currently the fourth leading cause of death in the United States.2 The North American Symptomatic Carotid Endarterectomy Trial (NASCET) and Asymptomatic Carotid Artery Stenosis (ACAS) trials of the 1990s provided high-level evidence that operative removal of carotid plaque in the presence of carotid stenosis offered a relative risk reduction in 5-year stroke and death rate.3, 4 As these trials had a relatively small proportion of black enrollees (~3%), a number of ensuing studies have shown that black patients underwent CEA with less frequency than white patients, received fewer carotid imaging studies, and had more severe neurological disease and comorbidities preoperatively.2, 5-13
The impact of these racial disparities has been realized in outcomes studies, leading some to question the external validity of NASCET and ACAS. In 2000, Dardik et al. reported that black patients had poorer outcomes in the immediate postoperative period. Black patients had a greater postoperative stroke rate, longer length of stay and more costly hospitalization. Black race was identified as an independent risk factor for postoperative stroke.6 However, these findings remained equivocal throughout the decade; Schneider et al., in 2012, found that while black patients have increased risk of stroke postoperatively, it may not be race that explains the disparity.14
Race has been a risk factor in postoperative death with greater consistency than postoperative stroke. CEA operations performed from 2005-2010 in the National Surgical Quality Improvement Program (NSQIP) were examined in 2013, in which black race was identified as an independent risk factor for both 30-day postoperative and in-hospital mortality.2 Explanations for these outcomes disparities have included increased comorbidities preoperatively, poor patient selection, confounding by socioeconomic status and other non-medical factors, and increased proportion of CEA operations on black patients performed at low volume institutions, by less experienced surgeons.2, 6, 9, 11, 15
Prompt CEA in the presence of symptoms with carotid stenosis in candidates with appropriate surgical risk is unequivocally advocated and represents the standard of care in modern vascular surgery.4 The present study aims to evaluate a gap in knowledge that may contribute to the disparity in postoperative CEA outcomes between white and black patients, but also may illustrate a disparity in care. While it is the judgment of the vascular surgeon as to when an operation is indicated, there is often a sonographic or radiographic imaging study that prompts the decision to operate, often coincident with workup of neurologic symptoms (symptomatic patients) or as part of a routine screen or workup for other reasons (asymptomatic patients). It is our hypothesis that a racial disparity will emerge in the duration from sonographic determination that an operation is warranted, to the day of operation (Time from sonographic Diagnosis to Operation, TDO), and that race may be independently associated with prolonged TDO.
METHODS
The Vanderbilt University Synthetic Derivative (SD) is a database that captures all patient data representing interactions within the Vanderbilt University medical system, and contains records for > 2.1 million patients available with a wide array of search methods.16 The standard electronic medical record at Vanderbilt University, StarPanel, is scrubbed of personal identifiers, and uploaded via one-way hash to the SD. As no identifying information is available, this study was approved as non-human research by the Vanderbilt University Institutional Review Board, and informed consent was waived. In SD, a search was performed for only patients who identified as either white or black race, who were diagnosed with carotid stenosis sonographically (CPT code 93880 or 93882), and who subsequently underwent CEA (CPT code 35301). Patients who identified as other races, such as Hispanic or Asian, were not assessed due to prohibitively low sample size.
All black patients identified (n=39), and a cohort of white patients (n=169) randomly generated by the SD algorithm were chosen for chart review. Date of sonographic diagnosis of carotid stenosis deemed to warrant operation by the operating surgeon (no less than 50-79%), and date of surgery were noted. Variables potentially influencing TDO, including demographics, degree of stenosis (defined as the average of the range given on sonographic assessment), preoperative smoking status and symptomatology were recorded. Major comorbidities with prevalence over 10% in the cohort were examined individually as well, and ICD-9 code query with individual chart confirmation was used for attribution. These comorbidities were hypertension (ICD-9 401,405; HTN), coronary artery disease (ICD-9 410-414; CAD), congestive heart failure (ICD-9 428.0; CHF), chronic pulmonary disease (ICD-9 490-493; CPD), cancer in the last 5 years (ICD-9 140-149, 201-208, 238.4) peripheral vascular disease including aortic aneurysm (ICD-9 443, 443.89, 443.9, 441; PVD) and diabetes mellitus (ICD-9 250; DM).2, 8 The Charlson Comorbidity Index (CCI) was also calculated for all patients, as a validated measure of comorbidity burden.17 Anticipating that patients with cardiac risk factors may require preoperative workup, the presence of cardiac stress testing (CST) was also noted. Patients were excluded if the sonogram and CEA were both performed within the same hospitalization (22 patients), if there was insufficient information in the patient record (19 patients), or if acuity increased during TDO (1 symptomatic patient had another stroke, 1 asymptomatic patient became symptomatic; both white female patients). For the final analysis of this retrospective cohort study, 166 patients were included; 134 white patients and 32 black patients, a ratio of approximately 4 to 1.
For analysis, patients were primarily stratified in two ways: symptomatology (all cases, symptomatic carotid stenosis, and asymptomatic carotid stenosis) and race (white and black).9 Comparison between groups was performed using Fisher's exact test for categorical variables and unpaired Student's t-test for continuous variables. Measures of central tendency and variance for continuous variables were described by mean and standard error of the mean. Within each of the three strata of symptomatology, univariate linear regression analysis was performed using each baseline characteristic as an independent variable and TDO as the dependent variable. Factors on univariate analysis that trended toward an association with TDO (P<.10; defined a priori) were entered into a stepwise multivariate linear regression analysis. Collinearity was assessed via variance inflation factor (VIF); in all cases, VIF was <1.4, and therefore there was minimal collinearity and no modifications were made to analysis. Finally, Kaplan-Meier curves for each symptomatology stratum were generated to model TDO by race, and these curves were compared via the Mantel-Cox Log-rank test.18 Statistical analysis was performed with IBM SPSS Statistical Data Editor 21 (IBM, Somers, NY) and GraphPad Prism (La Jolla, CA). Unless stated otherwise, for all tests, P<.05 was used for statistical significance.
RESULTS
Baseline demographics and clinical characteristics
From 1998-2013, 47 black patients and 810 white patients underwent CEA at Vanderbilt University. 166 operations from 1998-2013 were studied, representing a random sample of white patients and all black patients who met inclusion criteria. There was no difference in mean age at surgery, gender, degree of stenosis or tobacco use between races (P>.05). Black patients more frequently received preoperative CST than white patients (P<.001). Proportion of symptomatic patients did not vary with race (P=1.0). No other comorbidities were significantly different between the two groups (P>.05; Table I).
Table I.
Baseline characteristic for all patients
| Variable | White (n=134) | Black (n=32) | P |
|---|---|---|---|
| Age (y) | 67±0.8a | 68±1a | 0.14 |
| Female gender | 48 (36%) | 16 (50%) | 0.16 |
| Preop CST | 24 (18%) | 17 (53%) | <0.001 |
| Symptomatic | 44 (33%) | 10 (31%) | 1.0 |
| % Stenosis | 82±1%a | 83±2%a | 0.88 |
| Tobacco User | 52 (39%) | 10 (31%) | 0.54 |
| Comorbidities | |||
| HTN | 113 (84%) | 25 (78%) | 0.43 |
| CAD | 78 (58%) | 24 (75%) | 0.11 |
| CHF | 28 (21%) | 10 (31%) | 0.24 |
| CPD | 13 (10%) | 7 (22%) | 0.07 |
| DM | 64 (48%) | 16 (50%) | 0.85 |
| PVD | 64 (48%) | 17 (53%) | 0.70 |
| Cancer | 18 (13%) | 4 (13%) | 1.0 |
| CCI | 2.5±0.2a | 2.7±0.3a | 0.59 |
Mean ± standard error of the mean
There were 54 symptomatic patients and 112 asymptomatic patients. Within each subgroup, there was no difference between races with respect to age at surgery, gender, degree of stenosis or tobacco use (P>.05). Frequency of preoperative CST remained significantly greater in the black cohort (70% vs. 18% for symptomatic patients; 46% vs. 18% for asymptomatic patients; P≤.01). Among symptomatic patients, black patients had a significantly greater prevalence of CAD (90% vs. 52%; P=.04), CHF (50% vs. 14%; P=.02) and CPD (50% vs. 11%; P=.01). Differences in other comorbidities assessed, as well as CCI, were not significant (P>.05). Among asymptomatic patients, there were no differences in any specific comorbidity (P>.05; Table II).
Table II.
Baseline characteristics for symptomatic and asymptomatic subgroups
| Symptomatic (n=54) | Asymptomatic (n=112) | |||||
|---|---|---|---|---|---|---|
| Variable | White (n=44) | Black (n=10) | P | White (n=90) | Black (n=22) | P |
| Age (y) | 69±1a | 68±3a | 0.74 | 66±1a | 67±2a | 0.15 |
| Female gender | 14 (32%) | 5 (50%) | 0.30 | 56 (62%) | 11 (50%) | 0.34 |
| Preop CST | 8 (18%) | 7 (70%) | 0.003 | 16 (18%) | 10 (46%) | 0.01 |
| % Stenosis | 80±2%a | 81±4%a | 0.85 | 83±1%a | 83±3%a | 0.98 |
| Tobacco User | 19 (43%) | 5 (50%) | 0.74 | 33 (37%) | 5 (23%) | 0.32 |
| Comorbidities | ||||||
| HTN | 40 (91%) | 10 (100%) | 1.0 | 73 (81%) | 15 (68%) | 0.25 |
| CAD | 23 (52%) | 9 (90%) | 0.04 | 55 (61%) | 15 (68%) | 0.34 |
| CHF | 6 (14%) | 5 (50%) | 0.02 | 22 (24%) | 5 (23%) | 1.0 |
| CPD | 5 (12%) | 5 (50%) | 0.01 | 8 (9%) | 2 (9%) | 1.0 |
| DM | 14 (32%) | 5 (50%) | 0.30 | 50 (56%) | 11 (50%) | 0.64 |
| PVD | 21 (48%) | 7 (70%) | 0.30 | 43 (48%) | 10 (46%) | 1.0 |
| Cancer | 8 (18%) | 2 (20%) | 1.0 | 10 (11%) | 2 (9%) | 1.0 |
| CCI | 2.6±0.3a | 3.5±0.7a | 0.23 | 2.4±0.2a | 2.3±0.4a | 0.838 |
Mean ± standard error of the mean
Factors independently associated with TDO
For all patients, baseline factors that trended toward an association with TDO upon univariate analysis (P<.10) were female gender, black race, preoperative CST, degree of stenosis, HTN, CAD, CHF, CPD, PVD and CCI. These were entered into a stepwise multivariate linear regression. Factors which were independently associated with TDO for all patients were female gender (B=−16 days, 95% CI −34, −4 days; P=.01), black race (B=42 days, 95% CI 23, 62 days; P<.001), preoperative CST (B=36 days, 95% CI 3, 39 days; P=.02) and CHF (B=26 days, 95% CI 9, 44 days, P=.004; Table III).
Table III.
Baseline characteristics for symptomatic and asymptomatic subgroups
| Univariate | Multivariate | |||||
|---|---|---|---|---|---|---|
| Variable | B | (95% CI) | P | B | (95% CI) | P |
| Age (y) | 0 | (−1,0.9) | 0.96 | - | - | - |
| Female gender | −16 | (−32,0.8) | 0.06 | −19 | (−34,−4.4) | 0.01 |
| Black Race | 46 | (26,65) | <0.001 | 42 | (23,62) | <0.001 |
| Preop CST | 36 | (18,54) | <0.001 | 21 | (2.9,39) | 0.02 |
| % Stenosis | −0.7 | (−1.4, −0.06) | 0.03 | * | * | * |
| Symptomatic | −10 | (−27,7) | 0.26 | - | - | - |
| Tobacco User | 3.6 | (−13.3,20) | 0.68 | - | - | - |
| Comorbidities | ||||||
| HTN | 18 | (−3.3,40) | 0.10 | * | * | * |
| CAD | 20 | (3.9,37) | 0.02 | * | * | * |
| CHF | 27 | (8.3,46) | 0.005 | 26 | (8.7,44) | 0.004 |
| CPD | 22 | (−2.7,47) | 0.08 | * | * | * |
| DM | −1.2 | (−18,15) | 0.89 | - | - | - |
| PVD | 20 | (3.8,36) | 0.02 | * | * | * |
| Cancer | −17 | (−40,6.6) | 0.16 | * | * | * |
| CCI | 4.2 | (0.1,8.3) | 0.05 | * | * | * |
For symptomatic patients, black race, preoperative CST, CHF, CPD, PVD and CCI trended with TDO on univariate analysis (P<.10). These were entered into stepwise multivariate linear regression. Preoperative CST (B=34 days, 95% CI 1, 67 days; P=.05) and CPD (B=57 days, 95% CI 19, 95 days; P=.004) were significant independent predictors of prolonged TDO while black race demonstrated a trend (B=35, 95% CI −4, 74 days; P=.08; Table IV).
Table IV.
Regression analysis of independent predictors of TDO, symptomatic patients
| Univariate | Multivariate | |||||
|---|---|---|---|---|---|---|
| Variable | B | (95% CI) | P | B | 95% CI | P |
| Age at surgery | −0.3 | (−2,1.5) | 0.75 | - | - | - |
| Female gender | −6.2 | (−39,27) | 0.71 | - | - | - |
| Black Race | 63 | (26,100) | 0.001 | 35 | (−3.9,74) | 0.08 |
| Preop CST | 46 | (13,79) | 0.007 | 34 | (0.7,67) | 0.05 |
| % Stenosis | −0.7 | (−2.0,0.5) | 0.25 | - | - | - |
| Tobacco User | −4.4 | (−38,29) | 0.80 | - | - | - |
| Comorbidities | ||||||
| HTN | 21 | (−23,65) | 0.35 | - | - | - |
| CAD | 11 | (−21,43) | 0.49 | - | - | - |
| CHF | 36 | (−5.5,77) | 0.09 | * | * | * |
| CPD | 64 | (23,105) | 0.003 | 57 | (19,95) | 0.004 |
| DM | 16 | (−18,49) | 0.36 | - | - | - |
| PVD | 31 | (−1.4,63) | 0.06 | * | * | * |
| Cancer | −5.2 | (−48,37) | 0.81 | - | - | - |
| CCI | 8.8 | (1.5,16) | 0.02 | * | * | * |
For asymptomatic patients, female gender, black race, preoperative CST, degree of stenosis, CAD and CHF trended with TDO in univariate analysis (P<.10). Upon multivariate stepwise regression, independent predictors of TDO were female gender (B=−20 days, 95% CI −38, −2 days; P=.03), black race (B=35 days, 95% CI 12, 57 days; P=0.003), preoperative CST (B=21 days, 95% CI 0.2, 42 days; P=0.05) and degree of stenosis (B=−0.8 days, 95% CI −1.6, −0.1 days; P=0.04), while CAD trended toward significance (B=16 days, 95% CI −2, 35 days; P=.09; Table V). Overall, black race was independently associated with prolonged TDO by an average of 42 days among all patients and 35 days among asymptomatic patients, longer than any other variable.
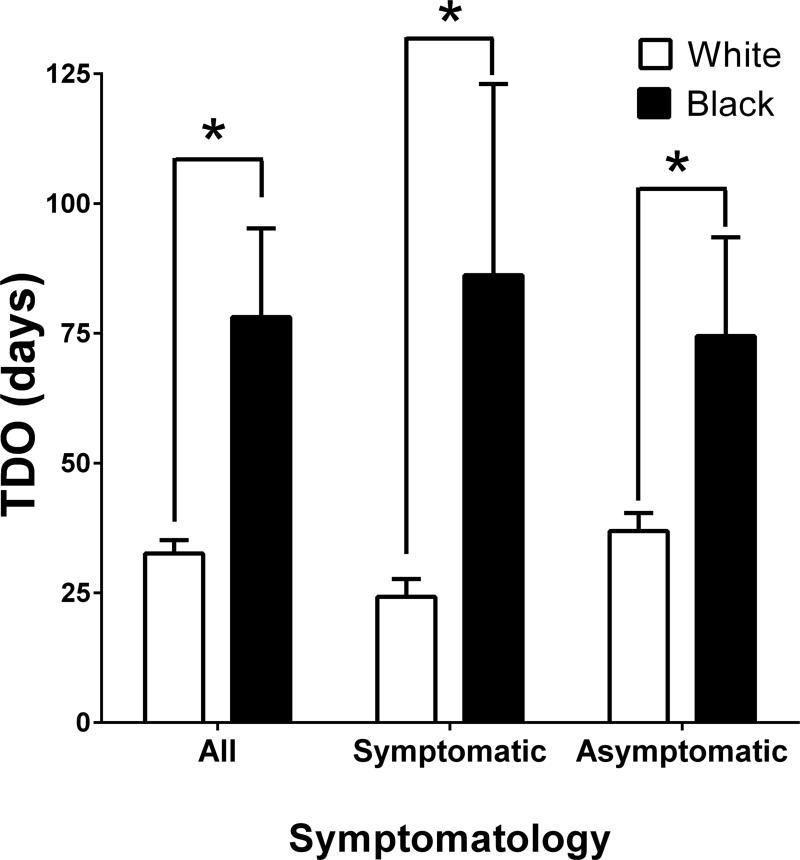
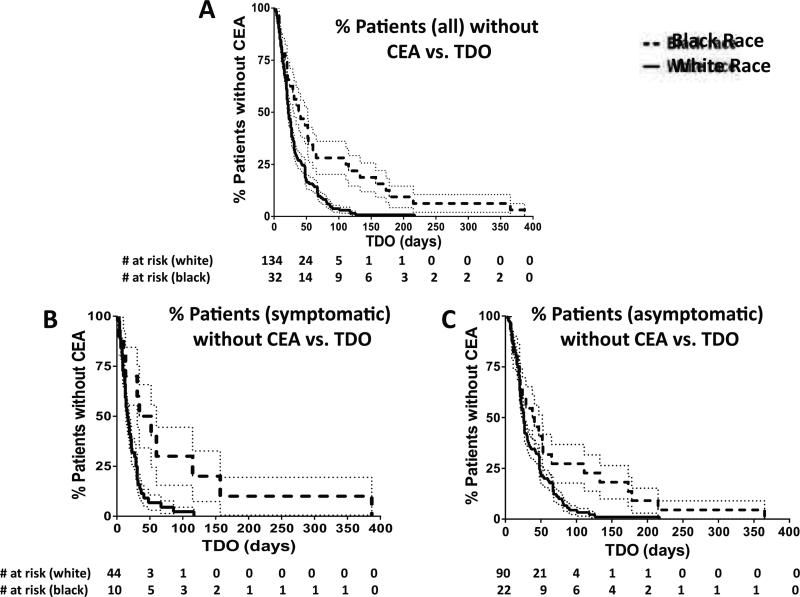
Figure 1 depicts mean TDO differences by race. Among all patients, blacks experienced significantly prolonged TDO (78±17 days vs. 33±3 days; P<.001). Within symptomatic and asymptomatic patient subgroups, the black patients still experienced prolonged TDO (86±37 days vs. 23±3 days, P=.001 for symptomatic patients; 75±19 days vs. 37±3 days, P=.002 for asymptomatic patients). Kaplan-Meier curves demonstrate the proportion of each race that had yet to undergo CEA over time (Figure 2). For the three groups (all patients, symptomatic subgroup, and asymptomatic subgroup), the Mantel-Cox P values were P=.001, P=.007 and P=.03, respectively.19
Figure 1.
TDO by race, for each stratum of symptomatology (*P<.002). Error bars represent standard error of the mean (SEM)
Figure 2.
Kaplan-Meier Curve for TDO, for all patients (A; χ2=11.8; P<.001), for symptomatic patients (B; χ2=7.2, P=.007) and asymptomatic patients (C; χ2=5.0, P=.03). Dotted lines represent standard error.
DISCUSSION
Many level II and level III studies have concluded that black race is independently associated with postoperative sequelae including stroke, reoperation and death; furthermore, black patients tend to experience a more prolonged and costly hospital course.2, 5-13, 20 In Brown et al.'s 2013 NSQIP chart review of >30,000 patients, black race was associated with increased risk of postoperative stroke (P=.009), 30-day mortality (P=.002), operative time (P<.001), length of stay (P<.001), unplanned intubation and ventilation (P<.001), cardiac arrest (P<.001) and return to operating room (P=.02). Black race was also an independent risk factor in postoperative death, just the same as COPD, dialysis, age>80 years and angina were.2 To the authors’ knowledge, this is the first study that examines the role of race in the duration from diagnosis to operation, an outcome measure important to ensure equitable treatment for all patients.
Clinical judgment guides the timing of CEA. Patients who are symptomatic with high-grade stenosis represent the least ambiguous cohort with respect to their risk-benefit ratio for surgery.4
Conversely, asymptomatic patients with known carotid disease may be routinely screened until a critical sonogram at which point the surgeon elects to operate. TDO was standardized using the most temporally proximal sonogram to the day of surgery even if it was unchanged from a previous one, to eliminate bias attributable to being an established patient in the Vanderbilt University system. To optimize uniformity of TDO among all patients, patients who became symptomatic, or had recurrent stroke, during this interval and thus developed enhanced acuity were excluded.
Our analysis of 166 CEAs at a single institution demonstrated that black patients experience prolonged TDO, when scheduled on an outpatient basis. Our analysis included demographics, but also considered other factors potentially influencing TDO including degree of stenosis, need for preoperative CST and whether the patient was a tobacco user. Based on prior reports, it was anticipated that at the time of diagnosis, blacks would have a greater comorbidity burden.2, 9, 14 For this reason, the Charlson Comorbidity Index was included in the analysis to capture the impact of conditions with low prevalence in the cohort, such as end-stage renal disease.17 In addition, major comorbidities were examined individually. A multivariate stepwise analysis was employed to isolate those factors that independently predicted an operative delay. Confirming our hypothesis, black race was the largest contributor to prolonged TDO in all patients (B=42 days) and within the asymptomatic cohort (B=35 days); ostensibly due to sample size, its significance as an independent risk factor for prolonged TDO was not observed in the symptomatic cohort (P=.08).
This study has several limitations affecting the applicability and validity of our findings. As data was gathered retrospectively, we were constrained to the information provided in the medical record.21 To this effect, we aimed to gather only easily definable variables and used a clear-cut endpoint (TDO). Furthermore, as this was a single-institution study, the sample size was affected by the relatively small number of black patients who received CEA, and disproportionately low ratio of black patients to white patients relative to the hospital admission demographics for all diagnoses (0.06:1 vs. 0.17:1). This ratio may reflect a regional bias in site of vascular surgical care, but may also be partially attributable to the lower prevalence of clinically significant carotid stenosis in the black population.22, 23
This study did not capture non-medical variables that may influence TDO. Insurance status and access to Medicaid are among multiple socioeconomic factors that may necessitate postponement of surgery. This concern was proposed as an explanation as to why black patients received CEAs less frequently than white patients and had inferior outcomes postoperatively, but the trend persisted even after accounting for these variables in other studies.5, 24-27 To date, it has been reported using the Nationwide Inpatient Sample database that asymptomatic self-pay/Medicaid patients are less likely to receive carotid revascularization, often waiting until becoming symptomatic.28 One additional confounder might be an increased aversion to surgery among black patients, manifesting in a delay to surgery attributable to operative hesitancy.29-31 These patients were cared for by the same group of surgeons, and received preoperative medical optimization similarly, supported by similar rates of treatment with at least one anti-platelet agent (78% vs. 88% for white and black patients, respectively; P=.45), in accordance with recommended management guildelines32, 33, and a statin (48% vs. 41% for white and black patients, respectively; P=.56). However, it is plausible that a surgeon with one set of practice guidelines primarily managed a disproportionate number of black patients; the de-identified SD database prevented identification of the surgeon.
Finally, this study did not correlate length of stay to postoperative endpoints such as mortality, stroke or reoperation. Nevertheless, the primary focus of this study was to test the hypothesis that there are racial disparities in CEA management, and we have demonstrated that such disparities exist.
CONCLUSION
Understanding the limitations, we conclude there is a delay from sonographic diagnosis of carotid stenosis warranting an operation to actual performance of operation in black patients. This delay is independent of confounding medical factors and represents a disparity in care. Awareness of this disparity may help vascular surgeons ensure that timing of surgery is carefully considered for all patients.
Table V.
Regression analysis of independent predictors of TDO, asymptomatic patients
| Univariate | Multivariate | |||||
|---|---|---|---|---|---|---|
| Variable | B | (95% CI) | P | B | (95% CI) | P |
| Age at surgery | 0.2 | (−0.9,1.4) | 0.69 | - | - | - |
| Female gender | −21 | (−40,1.8) | 0.03 | −20 | (−38,−2.1) | 0.03 |
| Black Race | 37 | (14,60) | 0.002 | 35 | (12,57) | 0.003 |
| Preop CST | 32 | (9.9,54) | 0.005 | 21 | (0.2,42) | 0.05 |
| % Stenosis | −0.9 | (−1.7,0.0) | 0.04 | −0.8 | (−1.6,−0.1) | 0.04 |
| Tobacco User | 6.3 | (−13,26) | 0.52 | - | - | - |
| Comorbidities | ||||||
| HTN | 18 | (−6.8,43) | 0.15 | - | - | - |
| CAD | 24 | (4.1,44) | 0.02 | 16 | (−2.4,35) | 0.09 |
| CHF | 23 | (1.7,44) | 0.04 | * | * | * |
| CPD | −2.8 | (−34,28) | 0.86 | - | - | - |
| DM | −10 | (−29,9.1) | 0.30 | - | - | - |
| PVD | 14 | (−5.1,33) | 0.15 | - | - | - |
| Cancer | −23 | (−51,5.7) | 0.12 | - | - | - |
| CCI | 2.0 | (−3.1,7.0) | 0.44 | - | - | - |
Acknowledgments
Grant Support Acknowledgement: Vanderbilt RedCAP: CTSA Award UL1 TR000445 from NCATS/NIH
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Easton JD. History of carotid endarterectomy then and now: personal perspective. Stroke; a journal of cerebral circulation. 2014;45(6):e101–3. doi: 10.1161/STROKEAHA.113.003501. [DOI] [PubMed] [Google Scholar]
- 2.Brown HA, Sullivan MC, Gusberg RG, Dardik A, Sosa JA, Indes JE. Race as a predictor of morbidity, mortality, and neurologic events after carotid endarterectomy. Journal of vascular surgery. 2013;57(5):1325–30. doi: 10.1016/j.jvs.2012.10.131. [DOI] [PubMed] [Google Scholar]
- 3.Endarterectomy for asymptomatic carotid artery stenosis. Executive Committee for the Asymptomatic Carotid Atherosclerosis Study. JAMA : the journal of the American Medical Association. 1995;273(18):1421–8. [PubMed] [Google Scholar]
- 4.North American Symptomatic Carotid Endarterectomy Trial C. Beneficial effect of carotid endarterectomy in symptomatic patients with high-grade carotid stenosis. The New England journal of medicine. 1991;325(7):445–53. doi: 10.1056/NEJM199108153250701. [DOI] [PubMed] [Google Scholar]
- 5.Oddone EZ, Horner RD, Sloane R, McIntyre L, Ward A, Whittle J, et al. Race, presenting signs and symptoms, use of carotid artery imaging, and appropriateness of carotid endarterectomy. Stroke; a journal of cerebral circulation. 1999;30(7):1350–6. doi: 10.1161/01.str.30.7.1350. [DOI] [PubMed] [Google Scholar]
- 6.Dardik A, Bowman HM, Gordon TA, Hsieh G, Perler BA. Impact of race on the outcome of carotid endarterectomy: a population-based analysis of 9,842 recent elective procedures. Annals of surgery. 2000;232(5):704–9. doi: 10.1097/00000658-200011000-00013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Conrad MF, Shepard AD, Pandurangi K, Parikshak M, Nypaver TJ, Reddy DJ, et al. Outcome of carotid endarterectomy in African Americans: is race a factor? Journal of vascular surgery. 2003;38(1):129–37. doi: 10.1016/s0741-5214(02)75455-4. [DOI] [PubMed] [Google Scholar]
- 8.Kennedy BS, Fortmann SP, Stafford RS. Elective and isolated carotid endarterectomy: health disparities in utilization and outcomes, but not readmission. Journal of the National Medical Association. 2007;99(5):480–8. [PMC free article] [PubMed] [Google Scholar]
- 9.Halm EA, Tuhrim S, Wang JJ, Rojas M, Rockman C, Riles TS, et al. Racial and ethnic disparities in outcomes and appropriateness of carotid endarterectomy: impact of patient and provider factors. Stroke; a journal of cerebral circulation. 2009;40(7):2493–501. doi: 10.1161/STROKEAHA.108.544866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Martin KD, Naert L, Goldstein LB, Kasl S, Molinaro AM, Lichtman JH. Comparing the Use of Diagnostic Imaging and Receipt of Carotid Endarterectomy in Elderly Black and White Stroke Patients. J Stroke Cerebrovasc. 2012;21(7):600–6. doi: 10.1016/j.jstrokecerebrovasdis.2011.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chaturvedi S, Madhavan R, Santhakumar S, Mehri-Basha M, Raje N. Higher risk factor burden and worse outcomes in urban carotid endarterectomy patients. Stroke; a journal of cerebral circulation. 2008;39(11):2966–8. doi: 10.1161/STROKEAHA.108.516062. [DOI] [PubMed] [Google Scholar]
- 12.Lucas FL, Stukel TA, Morris AM, Siewers AE, Birkmeyer JD. Race and surgical mortality in the United States. Annals of surgery. 2006;243(2):281–6. doi: 10.1097/01.sla.0000197560.92456.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kennedy BS. Does race predict short-term mortality after carotid surgery? The results of a meta-analysis. Journal of the National Medical Association. 2002;94(1):25–30. [PMC free article] [PubMed] [Google Scholar]
- 14.Schneider EB, Black JH, 3rd, Hambridge HL, Lum YW, Freischlag JA, Perler BA, et al. The impact of race and ethnicity on the outcome of carotid interventions in the United States. The Journal of surgical research. 2012;177(1):172–7. doi: 10.1016/j.jss.2012.02.050. [DOI] [PubMed] [Google Scholar]
- 15.Nguyen LL, Henry AJ. Disparities in vascular surgery: is it biology or environment? Journal of vascular surgery. 2010;51(4 Suppl):36S–41S. doi: 10.1016/j.jvs.2010.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Roden DM, Pulley JM, Basford MA, Bernard GR, Clayton EW, Balser JR, et al. Development of a large-scale de-identified DNA biobank to enable personalized medicine. Clinical pharmacology and therapeutics. 2008;84(3):362–9. doi: 10.1038/clpt.2008.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. Journal of chronic diseases. 1987;40(5):373–83. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 18.Sedgwick P, Joekes K. Kaplan-Meier survival curves: interpretation and communication of risk. Bmj-Brit Med J. 2013:347. [Google Scholar]
- 19.Mantel N. Evaluation of survival data and two new rank order statistics arising in its consideration. Cancer chemotherapy reports Part 1. 1966;50(3):163–70. [PubMed] [Google Scholar]
- 20.Oddone E. Sydenham Society: racial variations in carotid endarterectomy. Journal of clinical epidemiology. 2007;60(2):208–11. doi: 10.1016/j.jclinepi.2006.07.008. [DOI] [PubMed] [Google Scholar]
- 21.Doll R. Cohort studies: history of the method. II. Retrospective cohort studies. Sozialund Praventivmedizin. 2001;46(3):152–60. doi: 10.1007/BF01324251. [DOI] [PubMed] [Google Scholar]
- 22.Rockman CB, Hoang H, Guo Y, Maldonado TS, Jacobowitz GR, Talishinskiy T, et al. The prevalence of carotid artery stenosis varies significantly by race. Journal of vascular surgery. 2013;57(2):327–37. doi: 10.1016/j.jvs.2012.08.118. [DOI] [PubMed] [Google Scholar]
- 23.Mackinnon AD, Jerrard-Dunne P, Porteous L, Markus HS. Carotid intima-media thickness is greater but carotid plaque prevalence is lower in black compared with white subjects. AJNR American journal of neuroradiology. 2010;31(10):1951–5. doi: 10.3174/ajnr.A2214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Modan B, Wagener DK. Some epidemiological aspects of stroke: mortality/morbidity trends, age, sex, race, socioeconomic status. Stroke; a journal of cerebral circulation. 1992;23(9):1230–6. doi: 10.1161/01.str.23.9.1230. [DOI] [PubMed] [Google Scholar]
- 25.Murray CJ, Lopez AD. Mortality by cause for eight regions of the world: Global Burden of Disease Study. Lancet. 1997;349(9061):1269–76. doi: 10.1016/S0140-6736(96)07493-4. [DOI] [PubMed] [Google Scholar]
- 26.Sacco RL, Boden-Albala B, Gan R, Chen X, Kargman DE, Shea S, et al. Stroke incidence among white, black, and Hispanic residents of an urban community: the Northern Manhattan Stroke Study. American journal of epidemiology. 1998;147(3):259–68. doi: 10.1093/oxfordjournals.aje.a009445. [DOI] [PubMed] [Google Scholar]
- 27.Mitchell JB, Ballard DJ, Matchar DB, Whisnant JP, Samsa GP. Racial variation in treatment for transient ischemic attacks: impact of participation by neurologists. Health services research. 2000;34(7):1413–28. [PMC free article] [PubMed] [Google Scholar]
- 28.Brinjikji W, El-Sayed AM, Kallmes DF, Lanzino G, Cloft HJ. Racial and insurance based disparities in the treatment of carotid artery stenosis: a study of the Nationwide Inpatient Sample. Journal of neurointerventional surgery. 2014 doi: 10.1136/neurintsurg-2014-011294. [DOI] [PubMed] [Google Scholar]
- 29.Oddone EZ, Horner RD, Diers T, Lipscomb J, McIntyre L, Cauffman C, et al. Understanding racial variation in the use of carotid endarterectomy: the role of aversion to surgery. Journal of the National Medical Association. 1998;90(1):25–33. [PMC free article] [PubMed] [Google Scholar]
- 30.Oddone EZ, Horner RD, Johnston DC, Stechuchak K, McIntyre L, Ward A, et al. Carotid endarterectomy and race: do clinical indications and patient preferences account for differences? Stroke; a journal of cerebral circulation. 2002;33(12):2936–43. doi: 10.1161/01.str.0000043672.42831.eb. [DOI] [PubMed] [Google Scholar]
- 31.Horner RD, Oddone EZ, Stechuchak KM, Johnston DC, Grambow SC. Who doesn't receive carotid endarterectomy when appropriate? Journal of vascular surgery. 2004;39(1):162–8. doi: 10.1016/j.jvs.2003.08.004. [DOI] [PubMed] [Google Scholar]
- 32.Chaturvedi S, Bruno A, Feasby T, Holloway R, Benavente O, Cohen SN, et al. Carotid endarterectomy--an evidence-based review: report of the Therapeutics and Technology Assessment Subcommittee of the American Academy of Neurology. Neurology. 2005;65(6):794–801. doi: 10.1212/01.wnl.0000176036.07558.82. [DOI] [PubMed] [Google Scholar]
- 33.Alonso-Coello P, Bellmunt S, McGorrian C, Anand SS, Guzman R, Criqui MH, et al. Antithrombotic therapy in peripheral artery disease: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. 2012;141(2 Suppl):e669S–90S. doi: 10.1378/chest.11-2307. [DOI] [PMC free article] [PubMed] [Google Scholar]