Abstract
Purpose
Understanding the etiology of cancer-related fatigue (CRF) is critical to identify targets to develop therapies to reduce CRF burden. The goal of this systematic review was to expand on the initial work by the National Cancer Institute CRF Working Group to understand the state of the science of the biology of CRF. Specifically, to evaluate studies that examined the relationships between biomarkers and CRF, and to develop an etiologic model of CRF to guide researchers on pathways to explore or therapeutic targets to investigate.
Methods
This review was completed by the Multinational Association of Supportive Care in Cancer Fatigue Study Group – Biomarker Working Group. The initial search used three terms (biomarkers, fatigue, cancer), which yielded 11,129 articles. After removing duplicates, 7,175 articles remained. Titles were assessed for the keywords, “cancer” and “fatigue” resulting in 3,811 articles. Articles published before 2010 and those with samples <50 were excluded, leaving 75 articles for full-text review. Of the 75 articles, 25 were further excluded for not investigating the associations of biomarkers and CRF.
Results
Of the 47 articles reviewed, 25 were cross-sectional and 22 were longitudinal studies. Less than half (44%) were published recently (2010-2013). Almost half (46%) enrolled breast cancer participants. A majority of studies assessed fatigue using self-report questionnaires, and only two studies used clinical parameters to measure fatigue.
Conclusions
The findings from this review suggest that CRF is linked to immune/inflammatory, metabolic, neuroendocrine, and genetic biomarkers. We also identified gaps in knowledge and made recommendations for future research.
Keywords: cancer-related fatigue, inflammation, metabolic, neuroendocrine, genetic
1. Introduction
Cancer-related fatigue (CRF) is a common, distressing symptom that negatively affects health-related quality of life (QOL) of oncology patients [1-3]. The pathobiology of CRF is also complex and is thought to be caused by a cascade of events resulting in pro-inflammatory cytokine production, hypothalamic-pituitary-adrenal (HPA) activation dysfunction, metabolic and/or endocrine dysregulation, disruption to circadian, rhythm, and neuromuscular function abnormalities [4-7]. As a result, CRF often goes undiagnosed and unmanaged, which negatively impacts treatment adherence, disease control, and patient outcomes. Multiple programs have been initiated by different organizations (e.g., National Cancer Institute [NCI], American Cancer Society, Oncology Nursing Society) to define CRF and to fund research activities to understand the etiological basis of CRF. Moreover, the Canadian Association of Psychosocial Oncology [8], the American Society of Clinical Oncology [9], the Oncology Nursing Society [10], and The National Comprehensive Cancer Network [11] have developed clinical practice guidelines for CRF.
In 2013, the NCI CRF Working Group (a sub-committee of the NCI Symptom Management and QOL Steering Committee) summarized the recommendations from a NCI Clinical Trials Planning Meeting on CRF. One of the major gaps impeding progress in advancing the development of effective treatments for CRF was an inadequate understanding of its underlying biology [1]. Subsequently, the Multinational Association of Supportive Care in Cancer (MASCC) established a Fatigue Study Group – Biomarker Working Group composed of international CRF expert clinicians and researchers.
The goal of this review by the MASCC Fatigue Study Group was to expand on the initial work by the NCI CRF Working Group by conducting a systematic review of the state of the science related solely to the biology of CRF. Specifically, the review plans to evaluate studies that examined the relationship between potential biological markers of CRF with subjective reports of CRF and to develop an etiologic model of CRF that could guide researchers on potential pathways to explore or therapeutic targets to investigate. Although there is no widely accepted definition of biological marker, for the purposes of this review, we defined a biological marker as a molecule whose level is thought to associate with fatigue level.
2. Methods
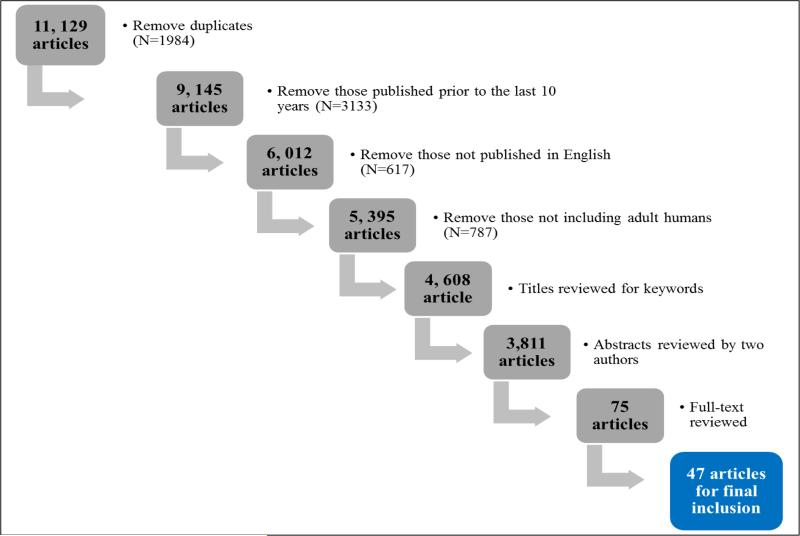
An initial literature query was conducted with the assistance of a medical librarian at the National Institutes of Health. Four reference databases were searched using the strategies summarized in Table 1. The initial search resulted in 11,129 articles. After removing duplicate articles, 9, 145 articles remained. Studies were included if they were published between 2004 and 2013, were written in English, and enrolled human adults. The 4,608 articles remaining articles were assessed for relevance to the area by visually examining their titles for the keywords, “cancer” and “fatigue.” Letters, literature reviews, meeting abstracts, editorials, and dissertations were excluded. Visual review of the titles left 3,811 articles for consideration. The abstracts of these studies were screened by two of the authors (LS and KF) and those with samples <50 were further excluded, which left 75 articles for full-text review. Of the 75 articles, 28 were excluded because they did not investigate the associations of biomarkers and CRF. The literature search strategies are summarized in Figure 1.
Table 1.
Search Terms
| Database | Search Terms | Yield |
|---|---|---|
| PubMed | (biomarkers OR biomarker OR markers OR marker OR inflammatory OR inflammation OR genetics OR genetic OR epigenetics OR epigenetic OR immune OR immunogenomic OR pathophysiology OR etiology) AND fatigue AND (neoplasms OR cancer[tiab]) | N=6921 |
| “cancer related fatigue”[ti] | ||
| Scopus | (TITLE(biomarkers OR biomarker OR markers OR marker OR inflammatory OR inflammation OR genetics OR genetic OR epigenetics OR epigenetic OR immune OR immunogenomic OR pathophysiology OR etiology) AND TITLE(fatigue)) | N=3297 |
| (TITLE(biomarkers OR biomarker OR markers OR marker OR inflammatory OR inflammation OR genetics OR genetic OR epigenetics OR epigenetic OR immune OR immunogenomic OR pathophysiology OR etiology) AND ABS(fatigue)) | ||
| (ABS(biomarkers OR biomarker OR markers OR marker OR inflammatory OR inflammation OR genetics OR genetic OR epigenetics OR epigenetic OR immune OR immunogenomic OR pathophysiology OR etiology) AND TITLE(fatigue)) | ||
| (TITLE-ABS-KEY(biomarkers OR biomarker OR markers OR marker OR inflammatory OR inflammation OR genetics OR genetic OR epigenetics OR epigenetic OR immune OR immunogenomic OR pathophysiology OR etiology) AND TITLE-ABS-KEY(“cancer related fatigue”)) | ||
| Embase | ‘marker’/exp OR ‘inflammation’/exp OR ‘genetic marker’/exp OR ‘epigenetics’/exp OR ‘immunopathology’/exp OR ‘immunity’/exp OR ‘pathophysiology’/exp OR ‘etiology’/exp AND (‘fatigue’/exp/mj OR ‘cancer fatigue’/exp/mj) AND ‘neoplasm’/exp | N=681 |
| CINAHL | (MH “Biological Markers+”) OR “biomarkers” OR biomarker OR markers OR marker OR inflammatory OR inflammation OR genetics OR genetic OR epigenetics OR epigenetic OR immune OR immunogenomic OR pathophysiology OR etiology | N=230 |
| (MH “Cancer Fatigue”) OR “cancer related fatigue” |
Figure 1.
Process of selecting the articles to be included in this review.
3. Results
Of the 47 articles included for full review, 25 were cross-sectional and 22 were longitudinal in design. More than half (34/47, about 70%) were published recently (2010-2013). The predominant cancer population studied was breast cancer. Almost half (21/47, 45%) enrolled solely breast cancer participants; other studies enrolled other patients with mixed cancer diagnosis aside from breast cancer participants. A majority (46/47, 98%) of studies assessed fatigue using single-item and/or multi-item questionnaires; only 1 study used a different form of fatigue assessment, the NCI Common Toxicity Criteria [12]. About half (24/47, 51%) used a cutoff score to define CRF. A total of 16 different multi-item questionnaires were used, with the Functional Assessment of Cancer Therapy-Fatigue questionnaire (FACT-F) being used the most, followed by the Fatigue Questionnaire (FQ). Seven studies used single-item assessments; 4 of which used a single item assessment as their only fatigue measure. Two studies looked at toxicities as criteria for fatigue; two studies used the NCI Common Toxicity criteria to assess for fatigue. One study used a diagnostic clinical interview to diagnose fatigue in addition to self-report questionnaires.
A majority of studies (40/47; 85%) assessed biologic markers only from peripheral blood. The remaining studies used medical record review (2) [13, 14], saliva (3) [15-17], a combination of blood and saliva (1) [18], blood and urine (1) [19], and 2 studies did not state the source of the biological markers [20-21]. Biomarkers with significant associations with CRF were related to immune/inflammatory response, metabolic, neuroendocrine function, and genetics. For ease of presentation, the review is organized into those categories.
3.1 Immune/Inflammation
Overview
The majority (24/47; 51%) of the articles focused on exploring potential immune and inflammatory contributors to CRF (Table 2). Of those 24 articles, 13 were cross-sectional and 11 were longitudinal studies. A majority of the 24 studies (17/24; 71%) were recently published (2010-2013), and the predominant cancer population explored was breast cancer (11/24; 46%). In about 90% (n=21/24) of the studies fatigue was assessed using multi-item self-report questionnaires. In four studies single-item assessments were used; in two studies it was used in combination with other assessment techniques and in two studies only a single-item fatigue assessment was used.
Table 2.
Studies Investigating Biomarkers of Cancer-Related Fatigue.
| Authors | Study Design | Sample Characteristics | Fatigue Measurement | Biomarker Assessed | Sample Source | Association to Fatigue |
|---|---|---|---|---|---|---|
| Inflammation/Immune | ||||||
| Gélinas et al., 2004 | Cross-sectional | N=103 Breast Cancer (remission) | MFI: only 1 subscale was used, general and physical aspects Cut-off score: None |
IL-1P | Blood | No correlation between IL-1β and fatigue. |
| Pusztai et al., 2004 | Longitudinal | N=90 Breast Cancer Controls: N= 15 Healthy Volunteers |
Single-Item Question Brief Fatigue Inventory Daily Toxicity Diary (n=30) Cut-off score: None |
IL-1β, IL-6, IL-8, IL-10, IL-12p70, TNF-α | Blood | No observed correlations between transient fatigue and cytokines. |
| Meyers et al., 2005 | Longitudinal | N=54 AML and MDS | BFI Cut-off scores: Scores ≥4 indicate moderate to severe fatigue |
IL-1, IL-1RA, IL-6, IL-8, and TNF-α Hgb levels |
Blood | IL-6, IL-1RA and TNF-α were significantly related to fatigue at baseline. Not enough individuals had biological data at 1 month for analysis |
| Collado-Hidalgo et al., 2006 | Cross-sectional | N=50 Fatigued Breast Cancer Survivors (n=32) with matched cohort of non-fatigued Breast Cancer Survivors (n=18). | SF-36 vitality scale Cut-off score: > 50 were considered non-fatigued ≤ 50 were considered fatigued |
Leukocyte Subsets Intracellular Cytokines: IL-6, TNF-α Plasma Cytokines: IL-6, sIL-6R, IL-1ra, and TNF-rII In vitro regulation of cytokine receptor expression |
Blood | Alterations in immune and inflammatory markers were found in those with persistent fatigue. |
| Capuano et al., 2008 | Cross-sectional | N=164 Mixed Diagnoses | MFSI-SF Cut-off score: None |
Anemia (Hgb <12) CRP |
Not stated | Only anemia and weight loss influenced fatigue. |
| Booker et al., 2009 | Cross-sectional | N=56 Multiple Myeloma | EORTC-QLQ-C30 fatigue subscale FACT-F Cut-off score: None |
Hgb CRP |
Medical Records | Negative significant correlation between Hb and fatigue, however Hb was not a significant predictor of fatigue when the effect of inflammation (CRP) was removed. CRP was a significant predictor of fatigue; however, CRP is elevated in patients with multiple myeloma. |
| Orre et al., 2009 | Cross-sectional | N= 92 Testicular Cancer Survivors with fatigue Controls: n= 191 Testicular Cancer Survivors without fatigue |
FQ Cut-off score: CF was defined as a score ≥4 on a dichotomized total score and a duration of ≥6 months |
IL-1ra, IL-6, neopterin, sTNF-R1, serum CRP. | Blood | Significantly higher IL-1ra found in patients with chronic fatigue compared to controls. Physical fatigue was correlated with IL-1ra (r=.18, p<0.01) and CRP (r=0.16, p<.05). |
| Steel et al., 2010 | Longitudinal | N= 206 Hepatobiliary Carcinoma | single-item measure of fatigue from the FACT-Hep Cut-off score: None |
Laboratory tests: including total bilirubin, prothrombin time, partial thromboplastin time, albumin, alkaline phosphatase, gamma-glutamyl transpeptidase, hemoglobin (Hgb), hematocrit, alpha-fetoprotein, and creatine Leukocyte counts, including percent of cell types (lymphocyte subsets) |
Blood | Participants with a symptom cluster of high pain, high fatigue, and low emotional well-being had significantly higher levels of eosinophils compared to participants with low levels of symptoms or those with just fatigue. Changes in fatigue variation over time were not statistically associated with the change with immune system parameters over time. |
| Wang et al., 2010 | Longitudinal | N= 62 NSCLC | MDASI Cut-off score: None; symptoms were clustered |
IL-6, IL-8, IL-10, IL-12p40p70, IL1RA, tumor necrosis factor (TNF)α, sTNFRl | Blood | Fatigue reported as part of combined five most severe symptoms. IL-6 was associated with increase in the mean severity of the five most severe symptoms. |
| Bower et al., 2011 | Cross-sectional | N=103 Breast Cancer | FSI Cut-off score: Clinically significant score ≥ 3. |
IL1ra, sTNF-RII, CRP | Blood | sTNF-RII was significantly associated with higher fatigue. When comparing chemotherapy-treated to no chemotherapy groups, the relationship only remained in the chemotherapy-treated patients. |
| Gerber et al. 2011 | Longitudinal | N=223 Breast Cancer | Verbal numerical rating 0-10 Cut-off score: Clinically significant fatigue ≥4. |
Hgb Glucose White Blood Cell Count (WBC) |
Medical Records | Significant correlation between clinically significant fatigue and abnormal WBC count at > 9 months after primary treatment for breast cancer. |
| Kwak et al., 2011 | Cross-sectional | N=90 Mixed Diagnoses | Brief Fatigue Inventory-Korean (BFI-K) Cut-off score: 0-4 mild 4-6 moderate 7-10 severe |
Cytokines: IL-6, TNF-α Laboratory data: WBC, Hgb, BUN, creatinine, albumin, AST, ALT, total bilirubin, and CRP |
Blood | The only inflammatory parameter significantly associated with fatigue score was CRP.Step wise linear regression, higher concentrations of BUN, severe pain, and poor performance status were significant predictors of fatigue. |
| Orre et al., 2011 | Cross-sectional | N=299 Breast Cancer Survivors | FQ Cut-off score: None |
Hgb Leukocyte levels Inflammatory Markers: hsCRP, IL-1ra, IL-6, sTNF-R1, and neopterin, |
Blood | Significant association between fatigue and CRP. Leukocyte count was significant in crude analysis but lost in regression analysis. No significance for IL-1ra, IL-6, sTNF-R1, or neopterin. |
| Alfano et al., 2012 | Longitudinal | N=633 Breast Cancer Survivors | PFS-R SF-36 vitality subscale Cut-off score: > 50 were considered non-fatigued ≤ 50 were considered fatigued |
CRP serum amyloid A (SAA) |
Blood | Significant trend for higher CRP levels with higher fatigue scores. No significant associations for SAA. |
| Clevenger et al., 2012 | Longitudinal | N=136 Ovarian Cancer Follow-up N= 63 women who were Disease-Free at one year post diagnosis |
POMS-SF fatigue subscale Cut-off score: None |
IL-6 | Blood | There was a significant association between increased IL-6 and fatigue prior to surgery; however, significance was lost when sleep disturbance was included. There was no association between IL-6 and fatigue at one year. |
| deRaaf et al., 2012 | Cross-sectional | N= 45 Advanced Cancer N= 47 Cancer Survivors |
MFI: physical fatigue and mental fatigue subscales of the Cut-off score: None |
CRp, neopterin, IL-1-ra, IL-6, and IL-8 | Blood | In advanced cancer patients, physical fatigue was significantly correlated with CRP, IL-6, IL-1-ra. No inflammatory markers were related to mental fatigue. In cancer survivors, Il-1ra was related to both physical fatigue and mental fatigue. |
| Fagundes et al., 2012 | Cross-sectional | N=158 Breast Cancer | Research and Development (RAND)Short Form (SF)-36 vigor/vitality scale Cut-off score: > 50 were considered non-fatigued ≤ 50 were considered fatigued |
Epstein-Barr virus, cytomegalovirus (CMV) C-reactive protein (CRP) | Blood | Higher CMV antibody titers were associated with a greater likelihood of being fatigued. CRP was not associated with fatigue. |
| Liu et al., 2012 | Longitudinal | N=53 Breast Cancer | MFSI-SF Cut-off score: None |
IL-6, IL-1RA CRP |
Blood | Changes in total MFSI-SF scores were significantly associated with IL-6; an increase of 1pg/ml was associated with an increase of 14 points on total MFSI-SF. No significant associations with IL-1RA or CRP. When sleep disturbance was controlled, the association remained. |
| Courtier et al., 2013 | Longitudinal | N= 100 Breast Cancer | FACIT-F Cut-off score: ≤ 34 used to categorize as fatigued; clinically significant change is 3-4 points |
Interleukin (IL)-6sR | Blood | Statistically significant correlation between baseline IL-6sR and fatigue. Vague reference to changes over the course of radiotherapy. |
| Fung et al., 2013 | Longitudinal | N=74 AML | FACT-F Fatigue Visual Analog Scale (VAS) Cut-off scores: Clinically significant changes based upon MCIDs; 3-point change and a 1-point change for FACT-F and VAS respectively |
13 Cytokines: IFN-y, IL-1β, IL-2, IL-4, IL-5, IL-8, IL-10, IL-12 p70, IL-13, TNF-α, IL-6, IP-10, IL-1ra | Blood | Cytokines TNF-α and IP-10 were consistently associated with fatigue. Clinically significant changes were observed between FACT-F and TNFα and IL-6. |
| Hamre et al., 2013 | Cross-sectional | N=232 childhood Lymphoma or Acute Lymphoblastic Leukemia Survivors Controls: cytokine values of Survivors who did not display fatigue |
FQ Cut-off score: Chronic fatigue (CF) is defined as a score ≥4 on a dichotomized total score and a duration of ≥6 months |
27 cytokines, 17 detected: IL-1ra, IL-6, IL-7, IL-8/CXCL8, IL-9, IL-10, IL-12, FGF, eotaxin/CCL11, IP-10/CXCL10, MCP-1 β/CCL2, MIP-1B/CCL4, RANTES/CCL5, PDGF, TNF, VEGF, IFN-y | Blood | No significant difference in cytokine levels between survivors with chronic fatigue compared to those without chronic fatigue. However, when looking at just non-Hodgkin's lymphoma survivors, survivors with chronic fatigue had significantly increased serum levels of FGF, PDGF and eotaxin, and IL-9. |
| Laird, et al., 2013 | Cross-sectional | N=1466 Mixed Diagnoses | EORTC-QLQ-C30 | CRP Cut-off score: None |
Blood | Fatigue was significantly associated with increased CRP. Remained significant when place of care and cancer type were investigated as sub-categories. |
| Paiva et al., 2013 | Cross-sectional | N = 221 or 223 (varies in paper) Mixed Diagnoses | EORTC QLQ-C30 fatigue subscale (EORTC-FS) Edmonton Symptom Assessment (ESAS) Cut-off score: Clinically significant fatigue defined as > 66.67 on EORTC-FS |
CRP Hgb, WBC, platelets, LDH, BUN, and serum albumin |
Blood | Cases (with fatigue) had lower Hb (p=0.015 and higher levels of WBC (p=0.047), LDH (p=0.012), albumin (p=0.0002) and CRP (p=0.0007). Predictive model for fatigue produced from logistic regression included CRP (OR 1.083, 95%CI 1.025-1.143, p=0.004) |
| Pertl et al., 2013 | Longitudinal | N = 61 Breast Cancer | FACT-F Cut-off score: ≤ 35 implies clinically significant fatigue |
CRP, IFN-γ, IL-1β, IL-6, TNF-α, tryptophan (TRP) and kynurenine (KYN), and KYN/TRP ratio | Blood | Pre chemo: fatigue not correlated with IFN-γ, IL-6, or TNF-α, tryptophan, kynurenine, or the KYN/TRP ratio; but was significantly associated with CRP. Without time parameters IL-6 was a significant predictor of fatigue with BMI, age, pain, number of comorbidities, and treatments received as covariates. |
| Metabolic and Neuroendocrine | ||||||
|---|---|---|---|---|---|---|
| Authors | Study Design | Sample Characteristics | Fatigue Measurement | Biomarker Assessed | Sample Source | Association to Fatigue |
| Meyerhardt et al., 2005 | Longitudinal | N=526 Colorectal Cancer | Single Item on the McCorkle & Young Symptom Distress Scale Cut-off score: None |
Insulin growth factor-I (IGF-I), IGF-II, IGF-binding protein 3 (IGFBP), C-peptide, and IGF ratio | Blood | Fatigue was correlated with IGF-II and the IGF-ratio. |
| Thornton et al., 2010 | Cross-sectional | N=104 Breast Cancer | FSI Disruption Index Cut-off score: None |
Cortisol, Adrenocorticotrophic hormone (ACTH), Epinephrine and Norepinephrine | Blood | The neuroendocrine biomarker cluster significantly predicted the pain/ depression/ fatigue symptom cluster after controlling for disease and demographic variables. |
| Weinrib et al., 2010 | Cross-sectional | N=100 Women post-surgery diagnosed with Ovarian Cancer Controls: 77 Women post-surgery diagnosed with Benign Disease N=33 Healthy women |
POMS-SF fatigue subscale Cut-off Score: None |
Cortisol | Saliva | High nocturnal cortisol and less cortisol variability were associated with greater fatigue in those with ovarian cancer. These correlations were not observed in those with benign disease. |
| Fagundes et al., 2011 | Cross-sectional | N= 109 Breast Cancer Survivors | MFSI-SF RAND SF-36 vigor-vitality scale Cut-off score: > 50 were considered non-fatigued ≤ 50 were considered fatigued |
Norepinephrine | Blood | Norepinephrine levels were higher among fatigued women than less fatigued women based on scores from the MFSI. No differences in norepinephrine levels between groups based on the RAND SF-36 |
| Genetic | ||||||
|---|---|---|---|---|---|---|
| Authors | Study Design | Sample Characteristics | Fatigue Measurement | Biomarker Assessed | Sample Source | Association to Fatigue |
| Massacesi et al., 2006 | Longitudinal | N= 56 Colorectal Cancer | NCI Common Toxicity Criteria Cut-off score: None |
Polymorphisms in UGT1A1, MTHFR, and TS genes | Blood | Univariate analysis: UGT1A1 6/6 variation is associated with a decreased incidence of fatigue Multivariate analysis: UGT1A1 (6/6 < 6/7 < 7/7) were observed to have more significance as risk factors for fatigue; TS (2/2 > 2/3 > 3/3) is associated with fatigue (p<0.042) |
| Miaskowski et al., 2010 | Longitudinal | N= 253 n = 168 Mixed Diagnoses n = 85 Family Caregivers |
LFS Cut-off score: Clinically significant Morning Fatigue level ≥ 3.2 Clinically significant Evening Fatigue level ≥5.6 |
IL-6 c.-6101A>T (rs4719714) | Blood | Common allele homozygotes for the gene of interest reported higher morning and evening fatigue compared to minor allele carriers. Genotype |
| Rausch et al., 2010 | Longitudinal | N=1149 Lung Cancer Survivors |
Lung Cancer Symptom Scale (LCSS) fatigue questions Cut-off score: ≥ 10-point change was indicative of clinical significance |
37 SNPS in the following 6 genes: IL-1B, IL-1RN, IL-6, IL-8, IL-10, and TNF-α | Not stated | 2 SNPs for IL-1β at 2 different time points and 1 SNP for IL-1RN at 1 time point were significantly associated with fatigue. |
| Fernandez-de-las-Penas et al., 2012a | Cross-sectional | N=128 Breast Cancer Survivors | PFS Cut-off score: None |
COMT Val158Met polymorphisms | Saliva | Val/Met or Met/Met genotypes were associated with higher levels of fatigue as compared to the Val/Val genotype. |
| Jim et al., 2012 | Longitudinal | N=53 Prostate Cancer | FSI Cut-off score: None |
SNPs in three pro-inflammatory cytokine genes: IL1B, IL6, and TNF-α | Blood | TNFA-308 rs1800629 is associated with fatigue severity. The total sum of variants of each SNP significantly predicted increases in fatigue duration and interference. |
| Bower et al., 2013 | Cross-sectional | N= 171 Breast Cancer | MFSI-SF Cut-off score: Top 1/3 of distribution of scores determined fatigue status |
3 key proinflammatory cytokine gene SNPS: 1.ILB-511 C>T 2.IL6-174 G>C 3.TNF-308 G>A |
Blood | The genetic risk index, sum of high expression alleles was significantly associated with fatigue. Individually, the SNPS for TNF-308 and IL6-174 were significantly associated with fatigue. Additive genetic risk factor was associated with elevated fatigue |
| Reyes-Gibby et al., 2013 | Cross-sectional | N=599 NSCLC | Single Item from the 12-item Short Form Health Survey Cut-off score: Score ≤ 2 indicated severe fatigue; whereas, > 2 indicated non-severe fatigue |
SNPs in 26 immune-response genes | Blood | Among patients with advanced-stage disease, interleukin (IL) genotype IL8-T251A was the most associated with fatigue. Certain variants of this gene were associated with higher risk of sever fatigue. Among those with early-stage NSCLC, women with the Lys_Lys type of IL-10RBLys47Glu and men with the C/C genotype of IL1A C-889T, experienced significant fatigue. These two gene variants also placed the respective groups at higher risk for severe fatigue. |
| Multimodal | ||||||
|---|---|---|---|---|---|---|
| Authors | Study Design | Sample Characteristics | Fatigue Measurement | Biomarker Assessed | Sample Source | Association to Fatigue |
| Wratten, et al., 2004 | Longitudinal | N= 52 Breast Cancer | FACT-F Cut-off score: < 37 defined significant fatigue |
Electrolytes Liver Function Tests Lipid Studies WBC with diff Cytokines Coagulation Factors CRP |
Blood | Baseline fatigue correlated with soluble thrombomodulin, TPA, VWF antigen, monocyte and neutrophil counts. The best baseline predictive factors for development of significant fatigue during RT were lower baseline fatigue scores, and higher neutrophil, hemoglobin, red cell counts, and D-dimer levels. At week 5, those in the fatigue group had lower sodium and higher red cell counts. A significant decrease in Albumin and red cell count for those with fatigue and in increase in eosinophil count and decrease in fibroblast growth factor beta for those with no fatigue differentiated the groups. There were many correlations between fatigue and various biomarkers at each time point. Baseline fatigue score, baseline neutrophil count, and baseline red blood cell count were able to best predict fatigue during RT. |
| Rich et al., 2005 | Cross-sectional | N=80 Colorectal Cancer | EORTC QLQ-C30 Cut-off score: > 33% indicates fatigue |
TGF-α, IL6, TNF-α Cortisol | Blood | Patients with fatigue had higher TGF-α level. TGF-α correlated significantly with higher fatigue scores. |
| Shafqat et al., 2005 | Cross-sectional | N=174 Mixed Diagnoses | BFI FACT-F Cut-off score: BFI score > 4 for clinically significant fatigue |
Hgb, albumin, thyroid stimulating hormone (TSH), dehydroepiandrosterone-sulfate (DHEAS), and testosterone TNF-α |
Blood | Albumin and HB correlated weakly with BFI. In male patients, BFI correlated with testosterone and DHEAS; however, depression scores altered the correlations. |
| Alexander et al., 2009 | Cross-sectional | N=200 Breast Cancer Survivors | FACT-F BFS FCS WAS EORTC QLQ-C30 Diagnostic and Clinical Interview with SCID Cut-off score: None Interview; to determine if participants qualified for CRF Syndrome diagnosis |
Blood: Full blood count; urea and electrolytes; liver function tests; bone profile; thyroid function; glucose and CRP Urine: Cortisol |
Blood Urine |
Fatigued participants had several significantly different biomarkers, most notable of which were white cell count, sodium, some of the liver function tests, and CRP |
| Landmark-Hoyvik et al., 2009 | Longitudinal | N=137 Breast Cancer Survivors | FQ Cut-off score: CF is defined as a score ≥ 4 on a dichotomized total score and a duration of ≥6 months |
White Blood Cell counts Genome-wide expression analyses |
Blood | Evidence for dysfunctional B-cell-mediated inflammation might be present in chronic fatigue |
| Reinertsen et al., 2010 | Longitudinal | N=249 Breast Cancer Survivors | FQ Cut-off score: CF is defined as a score ≥ 4 on a dichotomized total score and a duration of ≥6 months Persistent fatigue (PF): CF at both time points. |
TSH Leukocyte counts Hgb CRP levels |
Blood | Using univariate methods, increasing leucocyte count and CRP were significant predictors of PF. Higher CRP levels were related to CF at the initial assessment but did not remain a significant predictor of persistent fatigue in the multivariate model. |
| Reinersten et al., 2011 | Longitudinal | N=302 Breast Cancer Survivors | FQ Cut-off score: CF is defined as a score ≥ 4 on a dichotomized total score and a duration of ≥6 months Persistent fatigue (PF): CF at both time points. |
SNPs in the IL1b, IL6, IL6R and CRP genes CRP Leukocyte counts |
Blood | Women who were non-depressed but with CF had increased hsCRP levels than those without fatigue. Women with CF at both time points (PF) had higher hsCRP and leukocyte levels than those without fatigue at both time points. Women who were not depressed with PF had significantly different serum hsCRP levels compared to the never-fatigued women |
| Fernandez-de-las-Penas et al., 2012b | Cross-sectional | N=100 Breast Cancer Survivors | POMS-fatigue subscale (Spanish version) Cut-off score: None |
COMT Val158Met genotypes: Val/Val, Val/Met, Met/Met HPA axis, SNS, and immune biomarkers |
Saliva | Val158Met genotype has a significant effect for fatigue domain of POMS Met/Met genotype is significantly associated with higher fatigue scores as compared to Val/Met and Val/Val. There was a significant association between fatigue scores and salivary cortisol concentration in those with Val/Met, but this was not observed with the other genotypes. |
| Kurz et al., 2012 | Cross-sectional | N= 50 NSCLC and SCLC | FACT-F Single-Item Assessment Cut-off score: 0-34 FACT-F score moderate to severe fatigue >34 FACT-F score little to no fatigue |
Tryptophan, kynurenine, IDO Activity (KYN/TRP ratio) Neopterin, CRP Hgb |
Blood | Those with worse fatigue had higher levels of inflammatory markers, more tryptophan breakdown, and lower hemoglobin levels. Antidepressant treatment nullified correlations between fatigue and biomarkers. Hgb and CRP levels as well as antidepressant intake were predictive for fatigue (FACT-F <34). |
| Minton et al., 2012 | Cross-sectional | N= 720 Mixed Diagnoses | EORTC QLQ-C30 fatigue subscale Cut-off score: ≥ 66.67 on the fatigue subscale indicated clinically significant fatigue |
CRP Hgb albumin |
Blood | There were significant differences in fatigued vs non-fatigued participants; Hgb and Albumin levels were lower and CRP levels were higher. Severe fatigue was moderately correlated with Hb. |
| Schrepf et al., 2012 | Longitudinal | N=163 Ovarian Cancer | POMS-SF fatigue subscale Cut-off score: None |
Cortisol, IL-6 | Blood Saliva |
Reductions in IL-6 and nocturnal cortisol were associated with decreased fatigue. |
| Wang et al., 2012 | Longitudinal | N=103 Colorectal and Esophageal Cancers. | MDASI Cut-off score: None |
IL-6, IL-8, IL-10, IL-1RA, VEGF, and sTNF-R1 Hgb Albumin |
Blood | Concentrations of sTNF-R1 were positively associated with fatigue severity. sTNF-R1 and IL-6 were positively related to the component score of a fatigue-centered symptom cluster . |
Functional Assessment of Cancer Therapy (FACT); Functional Assessment of Chronic Illness Therapy-Fatigue subscale (FACIT-F); MD Anderson Symptom Inventory (MDASI); European Organisation for Research and Treatment of Cancer QLQ-C30 (EORTC QLQ-C30); FACT-Fatigue subscale (FACT-F); revised Piper Fatigue Scale (rPFS); Fatigue Symptom Inventory (FSI); Multidimensional Fatigue Symptom Inventory (MFSI); Multidimensional Fatigue Inventory (MFI); chemokine (C-X-C motif) ligand (CXCL); fibroblast growth factor (FGF); chemokine (C-C motif) ligand; monocyte chemoattractant protein (MCP); macrophage inflammatory peptide (MIP); regulated and normal T cell expressed and secreted (RANTES); platelet-derived growth factor (PDGF); vascular endothelial growth factor (VEGF); interferon (IFN); TNF-receptor (TNF-R);Fatigue Questionnaire (FQ); Profile of Mood States-Short Form (POMS-SF); Minimal Clinically Important Difference (MCIDs); blood urea nitrogen (BUN); aspartate aminotransferase (AST); alanine aminotransferase (ALT); high-sensitivity CRP (hsCRP); lactate dehydrogenase (LDH); Lee Fatigue Scale (LFS); National Cancer Institute (NCI); single nucleotide polymorphisms (SNP); small cell lung cancer (SCLC); non-SCLC (NSCLC); transforming growth factor (TGF); Bidimensional Fatigue Scale (BFS); Fatigue Catastrophising Scale (FCS); Work and Social Adjustment Scale (WAS); Structured clinical interview for the diagnostic and statistical manual (SCID); cancer-related fatigue (CRF); hypothalamic-pituitary-adrenal (HPA); sympathetic nervous system (SNS)
The single-item assessments consisted of one question pulled from a multi-item questionnaire [22], a verbal numerical rating (VNR) scale [14], visual analog scale (VAS) [23], and the NCI Common Toxicity Criteria [24]. Two of the single-item assessments, the VNR and VAS, were used with cut-off scores to define clinically significant CRF [14, 23]; in the other two studies using single-item assessments CRF was not defined. In slightly more than half of the 24 articles (13/24, 54%) cut-off scores were used to define CRF: in 6 articles cut-off scores for clinically significant CRF were defined [14, 23, 25-28], in 5 articles cut-off scores were used to dichotomize the study participants into fatigue groups [29-33], and in 2 articles cut-off scores were used to define chronic fatigue [34-35]. Biomarkers were measured predominantly from peripheral blood (n=21/24); in two articles, data obtained from medical records were used and in one study the source of biologic data was not identified [13-14, 20]. Most of the studies (20/24; 83%) looked at a panel of immune and inflammatory biologic markers. However, in four studies there was only one biologic marker investigated; in three studies a sole cytokine was explored [28, 36-37] and in the other study only C-Reactive Protein was explored [38].
Summary of Results
A number of studies explored the associations between concentrations of cytokines (e.g., TNF-α, IL-6) or markers of their activities and levels of CRF. The association of levels of IL6 or its receptors and fatigue severity was the most frequently investigated and had mixed results; in seven studies there was a significant association [25, 28, 32, 35, 37, 39-40], and in two studies there was no significant relationship [24, 33]. Collado-Hidalgo et al. [31] observed ex vivo production of IL-6 and tumor necrosis factor-alpha (TNF-α) following exposure to toll-like receptor 4 (TLR4) ligand lipolysaccharide and low levels of IL-6R on CD14+ cells and higher plasma levels of IL-1ra and sIL-6R. Significant associations of CRF were observed with increased concentrations of IL-1ra, and TNF-α in patients with acute myelogenous leukemia or myelodysplastic syndrome [32]. However, increased IL-1ra levels were not associated with CRF severity in women with early stage breast cancer who recently received primary therapy, but elevations of sTNF-RII were associated with fatigued breast cancer survivors who specifically received chemotherapy [27]. In addition, one investigation of impairment in immune response related to CRF revealed that fatigued breast cancer survivors had relatively lower frequencies of activated T lymphocytes (CD3+/CD69+) and myeloid dendritic cells (HLA-DR+/CD11c+/CD14dim) [31]. The inconsistencies in the results may be related to the data collection procedures, sensitivity of assay used, or treatment of covariates during analyses.
Inconsistent results were also found for the association between levels of C-reactive protein (CRP) and CRF. Higher CRP levels were associated with chronic fatigue in testicular cancer survivors [35] and with fatigue in those with advanced disease [42]. In addition, CRP was found to be a good predictor of CRF in patients with multiple myeloma [13] and was independently associated with CRF among disease-free breast cancer survivors [33, 41]. Investigators of several studies, however, did not find empirical support for the association between CRP and CRF [20, 29, 40].
In two studies, researchers found significant associations between blood cell counts (eosinophil percentage and white blood cell count) and fatigue scores [14, 22]. The association of lower levels of hemoglobin and fatigue were found to be statistically significant [13, 26]; however, this association was no longer significant when the effect of inflammation was removed from the analysis [13]. CRF was also observed to be significantly associated with increased cytomegalovirus antibody titers [29] and several growth factors such as fibroblast growth factor, platelet-derived growth factor, and eotaxin [34].
3.2 Metabolic and Neuroendocrine
Overview
Fewer than 10% (4/47) of the articles obtained for this review explored the association of CRF with metabolic and neuroendocrine etiologies (Table 2) [17, 43-45]. Of those four studies, three were cross-sectional [17, 43-44] and one was longitudinal in design [45]. A majority of the four studies (3/4; 75%) were recently published (2010-2013), and the predominant cancer population explored was breast cancer (2/4; 50%). In most (3/4; 75%) of the studies, fatigue was using multi-item self-report questionnaires; in one study a single-item assessment was used. The single item assessment was one question taken from a multi-item assessment [45]. In only one study, a cut-off score was used to define CRF; scores were used to dichotomize participants [43]. Biomarkers were measured predominantly from peripheral blood (n=3/4); however, in one study, data was obtained from saliva [17]. In half of the studies (2/4), a panel of metabolic or neuroendocrine biologic markers was examined; whereas, in the other 2 studies, only one biologic marker was investigated; cortisol [17] or norepinephrine [43].
Summary of Results
The studies had diverse objectives and results (Table 2); therefore, they are grouped by design, with the cross-sectional studies presented first. In a study by Thornton et al. [44], plasma cortisol, adrenocorticotropic hormone, epinephrine, and norepinephrine were explored in patients who were newly diagnosed with advanced breast cancer. The primary aim was to determine whether clusters of pain, depression, and fatigue were linked to neuroendocrine-immune models. Major findings were that these hormones predicted clustering of pain, depression, and fatigue. One limitation is the one-time, early morning measure of stress hormones that may not be reflective of diurnal or circadian rhythm effects.
Fagundes et al. [43] followed breast cancer survivors to explore relationships between fatigue and the sympathetic nervous system, using the neurotransmitter norepinephrine. Norepinephrine levels were observed to be higher among fatigued than less fatigued women based upon their MFSI questionnaire score, but this relationship was not observed with the RAND SF-36 questionnaire. Furthermore, investigators of the study observed a 20-year difference between fatigued and non-fatigued breast cancer survivors, which led to the proposition that fatigue may be a marker for accelerated aging. Additionally, elevated norepinephrine levels were also associated with other adverse health outcomes, which suggested that fatigue may indicate a need for increased monitoring of these other health issues. A limitation of this study included a lack of investigation of whether the study findings may be a result of patient deconditioning and poor activity levels. In addition, some of the patients were only two months post-cancer treatment and the level of fatigue in this study was much higher than in another comparable trial using the same population and fatigue measure [46].
Weinrib et al. [17] explored whether diurnal cortisol rhythm in 100 ovarian cancer patients scheduled for surgery was associated with fatigue. Salivary cortisol served as the biomarker and 77 controls with benign disease were also followed. Nocturnal cortisol and cortisol variability were associated with significant dysregulation and greater functional disability, fatigue, and vegetative depression in this study, leading the authors to suggest potential hypothalamic-pituitary-adrenal (HPA) involvement in fatigue. Limitations of this study included the influences of stress related to surgery on the cortisol levels, the large number of patients who did not have pre-surgical cortisol levels, the cross-sectional and correlational design that reduced causal interpretations, and the lack of more specific stimulation studies needed to fully confirm dysregulation of HPA feedback mechanisms.
Lastly, in a longitudinal study, Meyerhardt et al. [44] explored the associations of plasma levels of insulin-like growth factor (IGF)-I, IGF-II, IGF binding protein-3, and C-peptide with fatigue in advanced (metastatic) colorectal cancer patients receiving chemotherapy. Major findings were that baseline plasma IGF-I and IGF-II were significantly associated with symptom distress; specifically, fatigue was significantly correlated with IGF-I and IGF-II; however, after adjusting for confounders, only the association with IGF-II remained significant. There results provide evidence for a potential involvement of the IGF pathway in fatigue development.
3.3 Genetics
Overview
In about 15% (7/47) of the articles obtained for this review, genetic markers of CRF were investigated (Table 2) [12, 15, 21, 47-50]. Of those seven studies, three were cross-sectional and four were longitudinal in design. A majority of the studies (6/7; 86%) were recently published (2010-2013), and there was no predominant cancer population enrolled. In most (5/7; 71%) of the studies fatigue was assessed using multi-item, self-report questionnaires [15, 21, 47-48, 50]; in one study a single-item assessment was used [49] and in another study NCI Common Toxicity Criteria were used [12]. The single-item assessment was taken from a multi-item questionnaire [49]. In two studies, a cut-off score was used to define CRF; in one study clinically significant fatigue was defined [47], and in the other a cut-off score was used to dichotomize participants [49]. Biomarkers were measured predominantly from peripheral blood (5/7; 71%); in one study data was obtained from saliva [15] and in another there was no mention of the source of biologic data [21]. In most of the studies (5/7; 71%) a panel of gene markers was investigated; however, in two studies only one gene was explored in each.
Summary of Results
The studies had diverse objectives and findings (Table 2); therefore, they are grouped by design, with the cross-sectional studies presented first. Three of the studies that explored genetic markers underlying CRF were cross-sectional in design [15, 48-49]. Among the cross-sectional studies reviewed, in one study it was observed that GG genotypes of TNF -308 and IL-6 – 174 SNPs were significantly associated with CRF in women with early breast cancer [48]. In another study IL-8-T251A was observed to be a significant predictor of CRF in individuals with advanced cancer; specifically in men with early stage lung cancer with IL-1A C-889T C/C genotype and women with small lung cancer with IL-10RB Lysine_Lysine genotype [49]. In another cross-sectional study, it was observed that breast cancer survivors carrying catechol-O-methyltransferase (COMT) Methionine/Methionine genotypes were significantly correlated with higher fatigue scores [15].
The other four studies were longitudinal in design the authors from each study observed that specific genes encoding inflammatory cytokines appeared to be related to CRF [12, 21, 47, 50]. Jim et al. [50] observed that prostate cancer men with IL-6-174 (rs1800795) G/C or C/C genotype and those with TNFA-308 (rs1800629) genotype showed greater increases in fatigue, six months after initiation of androgen deprivation therapy; however, after controlling for covariates such as age, race, and baseline depressive symptoms, only TNFA genotype remained significantly associated with fatigue severity. Further, Jim et al. [50] observed that a higher number of genetic variants was associated with increases in fatigue duration and interference; however, the addition of covariates weakened the relationship. In another study, common, homozygous (AA) alleles of IL-6 were observed to be associated with higher levels of evening and morning fatigue symptoms among cancer patients before, during, and in those actively receiving radiation therapy, as well as their caregivers [47]. In a third study, it was observed that SNPs of IL-1β (rs1143633, rs2853550) and IL-1RN (rs397211) were associated with persistent fatigue in lung cancer survivors even years after diagnosis [21]. The authors of last longitudinal study investigated the role of genetic markers that are related to metabolism and cancer treatment [12]. Homozygosity for six TA repeats in the promoter region of uridine diphosphate glucuronosyl tranferase (UGT1A1) and two tandem repeats in the thymidylate synthase promoter region were found to be associated with fatigue in colorectal cancer patients treated with irinotecan and raltitrexed [12].
Findings from the reviewed articles showed some inconsistencies in regard to the associations of inflammatory genetic markers and CRF; however, most studies suggest significant associations of specific pro-inflammatory genotypes and metabolic genetic markers with CRF. There are several limitations to the genomic articles reviewed. The phenotyping of CRF is different between studies because of the lack of a uniform measuring tool, and all of the articles used targeted genomic markers to explore, lacking the unbiased, exploratory approach.
3.4 Multimodal
Overview
In about 25% (12/47) of the articles obtained for this review biologic markers of CRF were explored using mixed biologic methods (Table 2). Of those 12 articles, six were cross-sectional [16, 19, 51-54], and six were longitudinal in design [18, 55-59]. A majority of the studies (7/12; 58%) were recently published (2010-2013). In half of the studies (6/12) biologic markers in the breast cancer population were explored; the remaining studies involved diverse cancer populations. In all of the studies fatigue was assessed using multi-item self-report questionnaires; in one study a diagnostic and clinical interview was used in addition to multi-item self-report assessments [19], and in another study a single-item assessment was used in addition to a multi-item assessment [51]. In eight studies cut-off scores were used to define CRF; in two studies cut-off scores were used to define clinically significant CRF [52, 54], in three studies cut-off scores were used to dichotomize participants [51, 53, 59], and in three studies cutoff scores were used to define chronic fatigue [55-57]. In one study a diagnostic and clinical interview with SCID was used to determine if participants qualified for a cancer-related fatigue syndrome (CRFS) diagnosis [19]. In all of the studies biomarkers were measured from peripheral blood; in one study biomarkers from urine were used in addition to blood [19] and in one study saliva was used in addition to blood [18].
Summary of Results
The studies had diverse objectives and findings (Table 2); therefore, they are grouped by design, with the cross-sectional studies presented first. Half of the studies (6/12; 50%) were cross-sectional in design. A study by Shafqat et al. [54] reported a negative association between CRF and albumin, hemoglobin levels, DHEA, and testosterone levels in patients who received cancer therapy within the previous six months. However, in the final multiple linear regression model, CRF was significantly associated only with the biomarker of low hemoglobin level. These same results were observed in a study looking at albumin, hemoglobin, and CRP in a diverse cancer diagnostic population [52]. This study also observed decreased albumin and hemoglobin in those who were fatigued with an increase in CRP. However, similar to the study above, the final model only contained the biomarker hemoglobin as being significant to fatigue.
In addition to hemoglobin, which was a significant biomarker in half of the cross-sectional studies, the other biomarker explored in a majority of the studies was CRP. Higher CRP levels were found to significantly differ between fatigued vs non-fatigued participants [18, 51-52]. CRP was also found to be a significant predictor for the development of fatigue, implicating inflammation in fatigue development. In addition to CRP, several inflammatory cytokines were explored. TGF-α was observed to significantly correlate with fatigue in those with colorectal cancer [53].
Among the longitudinal studies, the underlying mechanisms found to be significantly associated with CRF were immune/inflammatory activation, disruption in blood cell indices, and sympathetic nervous system dysfunction. A longitudinal study by Wratten et al. [59] assessed various blood, coagulation, immune, and biochemical markers during radiation therapy. The authors observed that the most predictive biologic factors for radiation-related fatigue were neutrophil counts and red cell counts, after controlling for various covariates. They also found some weak evidence for the potential role of inflammation in CRF; however, when controlling for various co-factors, many of these relationships lost statistical significance. The authors concluded from the results of this study that radiation-related fatigue may be related to immune activation or HPA axis alterations.
Immune and inflammatory mechanisms were implicated in several studies. Wang et al. [58] observed evidence for the potential role of immune/inflammatory disruption in CRF. The authors observed that CRF was significantly associated with serum sTNF-R1and IL-6 levels after controlling for numerous covariates, in participants with locally advanced colorectal and esophageal cancer who were receiving concurrent chemo radiation therapy. Schrepf et al. [18] found that decreased CRF was significantly associated with the reduction in nocturnal cortisol and IL-6 levels following one year of primary treatment without recurrence in patients with ovarian cancer, which further supports the role for potential immune/inflammatory disruption in CRF. Two separate studies by the same first author [56-57] observed that changes in CRP were related to fatigue. Higher CRP was significantly associated with worse fatigue in breast cancer survivors. Lastly, Landmark-Hoyvik et al. [55] observed that dysfunction B-cell-mediated inflammation may play a role in CRF in breast cancer survivors. Fernández-de-las Peñas et al. [16] observed altered cortisol and α-amylase activity suggesting further evidence for dysfunctional HPA-axis and altered SNS activity in those with CRF.
4. Discussion
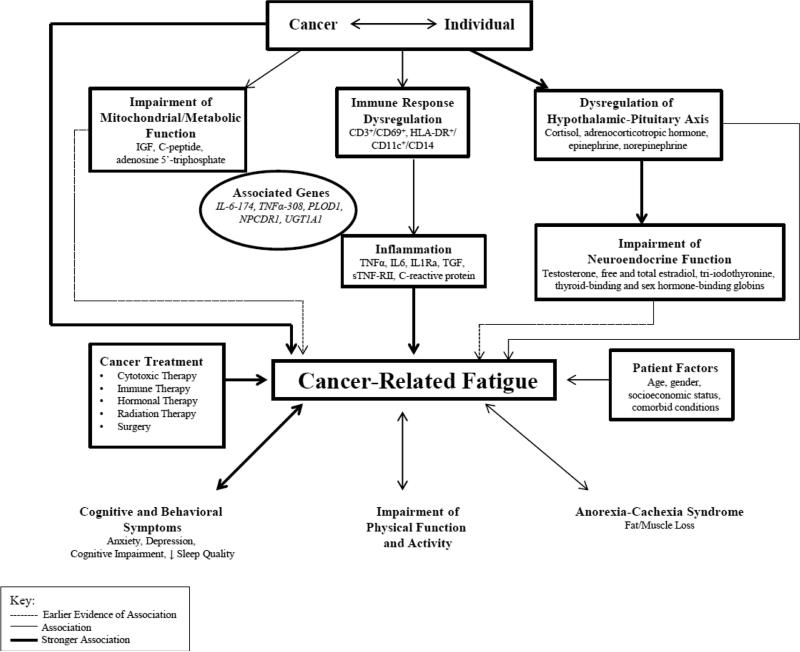
This review illustrates the complexity of studying CRF and possible biomarkers involved in its etiology. Our findings show that the immune response, inflammation, metabolic, neuroendocrine, hypothalamic-pituitary-adrenal axis, and genetics are associated with CRF. We developed a diagrammatic representation of our findings, which is explained in Figure 2.
Figure 2. Biologic Underpinnings of Cancer-Related Fatigue.
The review shows that cancer and/or its treatment induce a cascade of biological changes in an individual contributed by his/her clinical and demographic characteristics. The cascade of genetically-controlled biological events in response to cancer and/or its treatment triggers mitochondrial function impairment and immune dysregulation from an inflammatory response that influence stress response and endocrine function. This cascade of biological events is translated into cancer-related fatigue which is manifested with cognitive and behavioral symptoms, as well as alteration in skeletal muscle function contributing to physical disability.
We hypothesize that fatigue is a result of multiple biologic processes. Cancer and its treatment can lead to immune activation with a release of pro-inflammatory cytokines contributing to peripheral inflammation. Pro-inflammatory cytokine release and immune cell activation trigger a series of events including alterations in endocrine functions, HPA axis dysfunction, as well as mitochondrial impairment in the periphery and in the central nervous system [60-63]. Genetic factors have been reported to exert influence on the biologic processes mentioned [47,64]. These events translate into skeletal muscle dysfunction [65-66], and symptom experiences including fatigue, depression, sleep disturbance, and cognitive impairments [67-71], which can influence physical function and performance. Some of the factors that influence these series of events can include the stage of cancer, type of cancer treatment, comorbidities, concomitant medications, etc.
The reviewed articles reveal that the development of CRF is influenced by immune dysregulation, where specific SNPs and genotypes of IL1b, TNF, IL8, IL6, IL6 receptor, and CRP contributes to worsening or persistent fatigue [21, 47, 50, 57]. Immune dysregulation is known to impact the interactions of the body's cellular components (e.g., cytokines, growth factors), affecting our ability to counter the effect of cancer and/or its therapy [72-73]. In addition, there were also significant associations between levels of growth factors with increasing symptom distress in individuals with advanced cancer on chemotherapy [45]. These latter findings confirm our hypothesis that several cellular components are activated in response to cancer and/or its therapy, which may influence the development or worsening of CRF. The disarray in cellular interactions that trigger immune dysregulation in response to cancer and/or its therapy also influences other mechanisms involving stress response and metabolism. Specific lipid mediators are vital signaling molecules in regulating immune response during inflammation, with a greater role in promoting homeostasis [74]. In addition, adrenal hormone production is thought to be regulated by cytokines [75]. The articles included in our review demonstrated that levels of adrenal hormones were associated with CRF [17, 43-44].
The role of inflammation in the proposed pathobiology of CRF, makes pro-inflammatory markers feasible interventional targets. The use of anti-TNF agents (i.e., infliximab, etarnercept) observed reduction in CRF in some studies [76-77]. Treatment with dexamethasone observed significant short-term improvements in CRF for patients with advanced cancer [78]; however, the use of progestational steroids did not show any effect on CRF [79]. Although, nonpharmacological interventions such as yoga showed reductions in CRF, as well as reductions in NF-κB, an inflammatory regulator [80]. The use of hematopoietic agents generally improved CRF caused by cancer-treatment related anemia [81]; however, most patients with CRF are not anemic, especially post therapy. Additionally, there is a black box label warning issued by the Food and Drug Administration for the use of hematopoietic treatments in patients with cancer [82].
Cancer treated with chemotherapy may accelerate mechanisms associated with stress response. One concept that supports this assertion is allostasis, which refers to the body's adaptation to stress [83]. McEwen & Seeman [83] suggests that excessive stress can cause failure of the body's hormonal stress response and can hasten aging, worsening of psychological distress, and a decline in physical and mental functioning. For cancer patients, the disease and repeated ‘hits’ from its treatment impose overwhelming stress on their allostatic response and can accelerate the aging process, impair their physiologic and behavioral responses, and lead to negative consequences in function, well-being, and symptom experience. Cancer therapy also influences behavioral responses, such as worsening of menopausal symptoms contributing to CRF [84].
4.1 Effect of Age
Cancer treatment is proposed to hasten aging; therefore, there will be a brief mention of studies that sought to describe whether fatigue is influenced by age. Two of the 47 articles included in the review mentioned a possible relationship between fatigue and age [34, 43]. Hamre et al. [34] reported higher levels of fatigue in older individuals; whereas, Fagundes et al. [43] reported no significant differences in fatigue related to age. These conflicting results reflect the current state of the literature of the relationship between CRF and aging. For example, Banthia et al. [85] reported higher fatigue in younger cancer survivors; whereas, Butt et al. [86] reported higher levels of fatigue in older individuals. Krydalen et al. [87], and Luctkar-Flude et al. [88] reported no significant differences in fatigue related to age.
Several studies suggest that perhaps younger patients may have more fatigue because either they receive more aggressive treatments, have greater discrepancies in expected levels of fatigue in relation to their peers, or have expectations of greater health based on their age and higher levels of energy pre-diagnosis [89-90]. Winters-Stone et al. [91] reported that higher levels of fatigue were associated with lower age, lower physical activity, and larger portions of body fat and muscle mass. Interestingly, they reported that older women with leaner body mass had less fatigue compared with older women who had higher body mass. In this study, the sample size was restricted to older women (mean age=68, range=60-89), which limits inferences about physical activity, body fat, and muscle mass in younger women.
In contrast, Storey et al. [92] found no relationship between age and fatigue, but the age range in the sample was restricted to older adults (mean age=78, range=54-95). None of these studies systematically evaluated the reasons for the association between lower age and higher fatigue. More work is needed in this area to determine if there is a relationship between aging processes and the experience of fatigue. If this relationship can be supported, then it can help guide future biological investigations.
4.2 Gaps in knowledge and recommendations for future research
The primary gaps identified in this review that impact the scientific quality of the reviewed studies were mostly the predominant use of cross-sectional designs, the inconsistency in the fatigue measure used, and the inconsistency in collecting study outcomes (e.g., fatigue symptoms and biologic samples) at the same time. These gaps can be readily addressed through longitudinal investigations employing purposeful time points and using consistent outcome measures. Additional gaps identified in this review are related to basic flaws in data collection and analytic approach.
To improve the scientific quality of CRF biomarker investigations, the following factors should be considered: (1) the influence of possible covariates of CRF (e.g., physical activity, age); (2) the use of a statistical approach to address multiple comparisons; (3) the diurnal variations of CRF and biomarker expressions; (4) the use of sensitive assays in the biomarker investigation; (5) the use of adequate sample size; and (6) the use of more appropriate sample (e.g., multiple modes of cancer treatment, various cancer diagnosis). Additionally, the multidimensionality and the lack of a clear definition of CRF also bring inconsistencies with CRF phenotype stratification and complexity to data interpretation, which may produce spurious results and misleading conclusions. Using a single, recommended definition of CRF as proposed by national organizations would be useful in advancing the science of CRF. Future studies of CRF must be designed so that they target the gaps noted above.
While new technologies add power to scientific investigations, the identified gaps in research design and analytic approaches will continue to limit study findings unless they are addressed. Validation studies using careful designs with replication of results from independent groups could address many of the gaps identified. Despite all the limitations mentioned, the reviewed articles collectively indicate that CRF, either due to cancer biology itself or the treatment regimen used, is a common symptom in cancer patients. The severity of fatigue at the time of diagnosis is predictive of the severity of CRF during cancer therapy [59]. However, none of the reviewed studies were able to clearly show the mechanisms linking the biomarkers studied and CRF. Hence, further investigations are warranted.
5.0 Conclusions
In order to develop interventions to alleviate CRF, the mechanistic pathways must be characterized. Translational investigations offer the opportunity to gain new insights into the etiology of CRF. Although the current evidence is limited in proving causality of any biomarker to influence CRF development, there are promising interventional targets that insist some consideration. Research teams will need to have innovative approaches to address the sometimes difficult issues such as non-homogenous sampling, complex study designs, and clustering of variables that influence CRF. Fortunately, these obstacles are not insurmountable. Maintaining an open and collaborative approach between clinicians and researchers to perform thoughtful investigations using inventive strategies may provide new insights into the physiologic mechanisms of CRF and offer opportunities to optimize CRF management.
Acknowledgments
Financial Disclosures:
This research was supported by Multinational Association of Supportive Care in Cancer, and the Division of Intramural Research, National Institute of Nursing Research, National Institutes of Health and Grants NCI K07CA120025, UG1 CA189961 and R01 CA181064.
Footnotes
Conflict of Interest Disclosure
The authors have reported no potential conflicts of interest that exist with any companies/organizations whose products or services may be discussed in this article.
References
- 1.Barsevick AM, Irwin MR, Hinds P, et al. Recommendations for high-priority research on cancer-related fatigue in children and adults. J Natl Cancer Inst. 2013;105:1432–1440. doi: 10.1093/jnci/djt242. doi: 10.1093/jnci/djt242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mustian KM, Peppone LJ, Palesh OG, Janelsins MC, Mohile SG, Purnell JQ, Darling TV. Exercise and Cancer-related Fatigue. US Oncol. 2009;5:20–23. [PMC free article] [PubMed] [Google Scholar]
- 3.Mustian KM, Sprod LK, Janelsins M, Peppone LJ, Mohile S. Exercise recommendations for cancer-related fatigue, cognitive impairment, sleep problems, depression, pain, anxiety, and physical dysfunction: A review. Oncol Hematol Rev. 2012;8:81–88. doi: 10.17925/ohr.2012.08.2.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Berger AM, Gerber LH, Mayer DK. Cancer-related fatigue: Implications for breast cancer survivors. Cancer. 2012;15:2261–9. doi: 10.1002/cncr.27475. doi: 10.1002/cncr.27475. [DOI] [PubMed] [Google Scholar]
- 5.Campos MP, Hassan BJ, Riechelmann R, Del Giglio A. Cancer-related fatigue: A practical review. Ann Oncol. 2011;22:1273–9. doi: 10.1093/annonc/mdq458. doi: 10.1093/annonc/mdq458. [DOI] [PubMed] [Google Scholar]
- 6.Ryan JL, Carroll JK, Ryan EP, Mustian KM, Fiscella K, Morrow GR. Mechanisms of cancer-related fatigue. Oncologist. 2007;12:22–34. doi: 10.1634/theoncologist.12-S1-22. [DOI] [PubMed] [Google Scholar]
- 7.Wang XS, Woodruff JF. Cancer-related and treatment-related fatigue. Gynecol Oncol. 2014 doi: 10.1016/j.ygyno.2014.10.013. doi: 10.1016/j.ygyno.2014.10.013. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Howell D, Keller-Olaman S, Oliver TK, Hack T, Broadfield L, Biggs K, Chung J, Esplen MJ, Gravelle D, Green E, Hamel M, Harth T, Johnston P, McLeod D, Swinton N, Syme A, Olson K on behalf of the Cancer Journey Advisory Group of the Canadian Partnership Against Cancer . A Pan-Canadian Practice Guideline: Screening, Assessment and Care of Cancer- Related Fatigue in Adults with Cancer. Canadian Partnership Against Cancer (Cancer Journey Advisory Group) and the Canadian Association of Psychosocial Oncology; Toronto: Feb, 2011. [Google Scholar]
- 9.Bower JE, Bak K, Berger A, Breitbart W, Escalante CP, Ganz PA, Schnipper HH, Lacchetti C, Ligibel JA, Lyman GH, Ogaily MS, Pirl WF, Jacobsen PB, American Society of Clinical Oncology Screening, assessment, and management of fatigue in adult survivors of cancer: an American Society of Clinical oncology clinical practice guideline adaptation. J Clin Oncol. 2014;32:1840–50. doi: 10.1200/JCO.2013.53.4495. doi: 10.1200/JCO.2013.53.4495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mitchell SA, Hoffman AJ, Clark JC, DeGennaro RM, Poirier P, Robinson CB, Weisbrod BL. Putting evidence into practice: An update of evidence-based interventions for cancer-related fatigue during and following treatment. Clin J Oncol Nurs. 2014;18(Suppl):38–58. doi: 10.1188/14.CJON.S3.38-58. doi:10.1188/14.CJON.S3.38-58. [DOI] [PubMed] [Google Scholar]
- 11.National Comprehensive Cancer Network (NCCN) [27 April 2015];Guidelines Version 2.2015 Panel Members Cancer-Related Fatigue (2015) NCCN clinical practice guidelines in oncology: Cancer-related fatigue. www.nccn.org/professionals/physician_gls/f_guidelines.asp#fatigue.
- 12.Massacesi C, Terrazzino S, Marcucci F, Rocchi MB, Lippe P, Bisonni R, Lombardo M, Pilone A, Mattioli R, Leon A. Uridine diphosphate glucuronosyl transferase 1A1 promoter polymorphism predicts the risk of gastrointestinal toxicity and fatigue induced by irinotecan-based chemotherapy. Cancer. 2006;106:1007–1016. doi: 10.1002/cncr.21722. doi:10.1002/cncr.21722. [DOI] [PubMed] [Google Scholar]
- 13.Booker R, Olson K, Pilarski LM, Noon JP, Bahlis NJ. The relationships among physiologic variables, quality of life, and fatigue in patients with multiple myeloma. Oncol Nurs Forum. 2009;36:209–16. doi: 10.1188/09.ONF.209-216. doi: 10.1188/09.ONF.209-216. [DOI] [PubMed] [Google Scholar]
- 14.Gerber LH, Stout N, McGarvey C, Soballe P, Shieh CY, Diao G, Springer BA, Pfalzer LA. Factors predicting clinically significant fatigue in women following treatment for primary breast cancer. Support Care Cancer. 2011;19:1581–1591. doi: 10.1007/s00520-010-0986-7. doi: 10.1007/s00520-010-0986-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Fernández-de-las-Peñas C, Fernández-Lao C, Cantarero-Villanueva I, Ambite-Quesada S, Rivas-Martínez I, del Moral-Avila R, Arroyo-Morales M. Catechol-O-methyltransferase genotype (Val158met) modulates cancer-related fatigue and pain sensitivity in breast cancer survivors. Breast Cancer Res Treat. 2012;133:405–412. doi: 10.1007/s10549-011-1757-y. doi:10.1007/s10549-011-1757-y. [DOI] [PubMed] [Google Scholar]
- 16.Fernández-de-Las-Peñas C, Cantarero-Villanueva I, Fernández-Lao C, Ambite-Quesada S, Díaz-Rodríguez L, Rivas-Martínez I, del Moral-Avila R, Arroyo-Morales M. Influence of catechol-o-methyltransferase genotype (Val158Met) on endocrine, sympathetic nervous and mucosal immune systems in breast cancer survivors. Breast. 2012;21:199–203. doi: 10.1016/j.breast.2011.09.012. doi:10.1016/j.breast.2011.09.012. [DOI] [PubMed] [Google Scholar]
- 17.Weinrib AZ, Sephton SE, Degeest K, Penedo F, Bender D, Zimmerman B, Kirschbaum C, Sood AK, Lubaroff DM, Lutgendorf SK. Diurnal cortisol dysregulation, functional disability, and depression in women with ovarian cancer. Cancer. 2010;116:4410–4419. doi: 10.1002/cncr.25299. doi:10.1002/cncr.25299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schrepf A, Clevenger L, Christensen D, et al. Cortisol and inflammatory processes in ovarian cancer patients following primary treatment: Relationships with depression, fatigue, and disability. Brain Behav Immun. 2013;30(Suppl):S126–134. doi: 10.1016/j.bbi.2012.07.022. doi:10.1016/j.bbi.2012.07.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Alexander S, Minton O, Andrews P, Stone P. A comparison of the characteristics of disease-free breast cancer survivors with or without cancer-related fatigue syndrome. Eur J Cancer. 2009;45:384–392. doi: 10.1016/j.ejca.2008.09.010. doi:10.1016/j.ejca.2008.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Capauno G, Pavese I, Satta F, Tosti M, Palladino A, Del Grosso A, Di Palma M. Correlation between anemia, unintentional weight loss and inflammatory status on cancer-related fatigue and quality of life before chemo and radiotherapy. e-SPEN, the European e-Journal of Clinical Nutrition and Metabolism. 2008;3:e147–e151. doi:10.1016/j.eclnm.2008.04.008. [Google Scholar]
- 21.Rausch SM, Clark MM, Patten C, Liu H, Felten S, Li Y, Sloan J, Yang P. Relationship between cytokine gene single nucleotide polymorphisms and symptom burden and quality of life in lung cancer survivors. Cancer. 2010;116:4103–4113. doi: 10.1002/cncr.25255. doi:10.1002/cncr.25255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Steel JL, Kim KH, Dew MA, Unruh ML, Antoni MH, Olek MC, Geller DA, Carr BI, Butterfield LH, Gamblin TC. Cancer-related symptom clusters, eosinophils, and survival in hepatobiliary cancer: An exploratory study. J Pain Symptom Manage. 2010;39:859–871. doi: 10.1016/j.jpainsymman.2009.09.019. doi:10.1016/j.jpainsymman.2009.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fung FY, Li M, Breunis H, Timilshina N, Minden MD, Alibhai SM. Correlation between cytokine levels and changes in fatigue and quality of life in patients with acute myeloid leukemia. Leuk Res. 2013;37:274–9. doi: 10.1016/j.leukres.2012.11.013. doi: 10.1016/j.leukres.2012.11.013. [DOI] [PubMed] [Google Scholar]
- 24.Pusztai L, Mendoza TR, Reuben JM, et al. Changes in plasma levels of inflammatory cytokines in response to paclitaxel chemotherapy. Cytokine. 2004;25:94–102. doi: 10.1016/j.cyto.2003.10.004. doi: 10.1016/j.cyto.2003.10.004. [DOI] [PubMed] [Google Scholar]
- 25.Pertl MM, Hevey D, Boyle NT, Hughes MM, Collier S, O'Dwyer AM, Harkin A, Kennedy MJ, Connor TJ. C-reactive protein predicts fatigue independently of depression in breast cancer patients prior to chemotherapy. Brain Behav Immun. 2013;34:108–119. doi: 10.1016/j.bbi.2013.07.177. doi:10.1016/j.bbi.2013.07.177. [DOI] [PubMed] [Google Scholar]
- 26.Paiva CE, Paiva BS. Prevalence, predictors, and prognostic impact of fatigue among Brazilian outpatients with advanced cancers. Support Care in Cancer. 2013;21:1053–1060. doi: 10.1007/s00520-012-1625-2. doi:10.1007/s00520-012-1625-2. [DOI] [PubMed] [Google Scholar]
- 27.Bower JE, Ganz PA, Irwin MR, Kwan L, Breen EC, Cole SW. Inflammation and behavioral symptoms after breast cancer treatment: Do fatigue, depression, and sleep disturbance share a common underlying mechanism? J Clin Oncol. 2011;29:3517–3522. doi: 10.1200/JCO.2011.36.1154. doi: 10.1200/JCO.2011.36.1154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Courtier N, Gambling T, Enright S, Barrett-Lee P, Abraham J, Mason MD. Psychological and immunological characteristics of fatigued women undergoing radiotherapy for early-stage breast cancer. Support Care Cancer. 2013;21:173–181. doi: 10.1007/s00520-012-1508-6. doi: 10.1007/s00520-012-1508-6. [DOI] [PubMed] [Google Scholar]
- 29.Fagundes CP, Glaser R, Alfano CM, et al. Fatigue and herpesvirus latency in women newly diagnosed with breast cancer. Brain Behav Immun. 2012;26:394–400. doi: 10.1016/j.bbi.2011.09.014. doi: 10.1016/j.bbi.2011.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Alfano CM, Imayama I, Neuhouser ML, et al. Fatigue, inflammation, and ω-3 and ω-6 fatty acid intake among breast cancer survivors. J Clin Oncol. 2012;30:1280–1287. doi: 10.1200/JCO.2011.36.4109. doi: 10.1200/JCO.2011.36.4109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Collado-Hidalgo A, Bower JE, Ganz PA, Cole SW, Irwin MR. Inflammatory biomarkers for persistent fatigue in breast cancer survivors. Clin Cancer Res. 2006;12:2759–2766. doi: 10.1158/1078-0432.CCR-05-2398. doi: 10.1158/1078-0432.CCR-05-2398. [DOI] [PubMed] [Google Scholar]
- 32.Meyers CA, Albitar M, Estey E. Cognitive impairment, fatigue, and cytokine levels in patients with acute myelogenous leukemia or myelodysplastic syndrome. Cancer. 2005;104:788–93. doi: 10.1002/cncr.21234. doi: 10.1002/cncr.21234. [DOI] [PubMed] [Google Scholar]
- 33.Kwak SM, Choi YS, Yoon HM, et al. The relationship between interleukin-6, tumor necrosis factor-α, and fatigue in terminally ill cancer patients. Palliat Med. 2012;26:275–82. doi: 10.1177/0269216311406991. doi: 10.1177/0269216311406991. [DOI] [PubMed] [Google Scholar]
- 34.Hamre H, Zeller B, Kanellopoulos A, et al. Serum cytokines and chronic fatigue in adults surviving after childhood leukemia and lymphoma. Brain Behav Immun. 2013;30:80–87. doi: 10.1016/j.bbi.2013.01.006. doi: 10.1016/j.bbi.2013.01.006. [DOI] [PubMed] [Google Scholar]
- 35.Orre IJ, Murison R, Dahl AA, Ueland T, Aukrust P, Fosså SD. Levels of circulating interleukin-1 receptor antagonist and C-reactive protein in long-term survivors of testicular cancer with chronic cancer-related fatigue. Brain Behav Immun. 2009;23:868–74. doi: 10.1016/j.bbi.2009.04.003. doi: 10.1016/j.bbi.2009.04.003. [DOI] [PubMed] [Google Scholar]
- 36.Gélinas C, Fillion L. Factors related to persistent fatigue following completion of breast cancer treatment. Oncol Nurs Forum. 2004;31:269–278. doi: 10.1188/04.ONF.269-278. doi: 10.1188/04.ONF.269-278. [DOI] [PubMed] [Google Scholar]
- 37.Clevenger L, Schrepf A, Christensen D, et al. Sleep disturbance, cytokines, and fatigue in women with ovarian cancer. Brain Behav Immun. 2012;26:1037–1044. doi: 10.1016/j.bbi.2012.04.003. doi: 10.1016/j.bbi.2012.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Laird BJ, McMillan DC, Fayers P, Fearon K, Kaasa S, Fallon MT, Klepstad P. The systemic inflammatory response and its relationship to pain and other symptoms in advanced cancer. Oncologist. 2013;18:1050–5. doi: 10.1634/theoncologist.2013-0120. doi: 10.1634/theoncologist.2013-0120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wang XS, Shi Q, Williams LA, Mao L, Cleeland CS, Komaki RR, Mobley GM, Liao Inflammatory cytokines are associated with the development of symptom burden in patients with NSCLC undergoing concurrent chemoradiation therapy. Brain Behav Immun. 2010;24:968–974. doi: 10.1016/j.bbi.2010.03.009. doi: 10.1016/j.bbi.2010.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Liu L, Mills PJ, Rissling M, Fiorentino L, Natarajan L, Dimsdale JE, Sadler GR, Parker BA, Ancoli-Israel S. Fatigue and sleep quality are associated with changes in inflammatory markers in breast cancer patients undergoing chemotherapy. Brain Behav Immun. 2012;26:706–13. doi: 10.1016/j.bbi.2012.02.001. doi: 10.1016/j.bbi.2012.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Orre IJ, Reinertsen KV, Aukrust P, Dahl AA, Fosså SD, Ueland T, Murison R. Higher levels of fatigue are associated with higher CRP levels in disease-free breast cancer survivors. J Psychosom Res. 2011;71:136–41. doi: 10.1016/j.jpsychores.2011.04.003. doi: 10.1016/j.jpsychores.2011.04.003. [DOI] [PubMed] [Google Scholar]
- 42.de Raaf PJ, Sleijfer S, Lamers CH, Jager A, Gratama JW, van der Rijt CC. Inflammation and fatigue dimensions in advanced cancer patients and cancer survivors: An explorative study. Cancer. 2012;118:6005–11. doi: 10.1002/cncr.27613. doi: 10.1002/cncr.27613. [DOI] [PubMed] [Google Scholar]
- 43.Fagundes CP, Murray DM, Hwang BS, Gouin JP, Thayer JF, Sollers JJ, 3rd, Shapiro CL, Malarkey WB, Kiecolt-Glaser JK. Sympathetic and parasympathetic activity in cancer-related fatigue: more evidence for a physiological substrate in cancer survivors. Psychoneuroendocrinology. 2011;36:1137–1147. doi: 10.1016/j.psyneuen.2011.02.005. doi:10.1016/j.psyneuen.2011.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Thornton LM, Andersen BL, Blakely WP. The pain, depression, and fatigue symptom cluster in advanced breast cancer: Covariation with the hypothalamic-pituitary-adrenal axis and the sympathetic nervous system. Health Psychol. 2010;29:333–337. doi: 10.1037/a0018836. doi:10.1037/a0018836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Meyerhardt JA, Sloan JA, Sargent DJ, et al. Associations between plasma insulin-like growth factor proteins and C-peptide and quality of life in patients with metastatic colorectal cancer. Cancer Epidemiol Biomarkers Prev. 2005;14:1402–1410. doi: 10.1158/1055-9965.EPI-04-0862. doi:10.1158/1055-9965.epi-04-0862. [DOI] [PubMed] [Google Scholar]
- 46.Kiecolt-Glaser JK, Bennett JM, Andridge R, Peng J, Shapiro CL, Malarkey WB, Emery CF, Layman R, Mrozek EE, Glaser R. Yoga's impact on inflammation, mood, and fatigue in breast cancer survivors: a randomized controlled trial. J Clin Oncol. 2014. 2014;32(10):1040–1049. doi: 10.1200/JCO.2013.51.8860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Miaskowski C, Dodd M, Lee K, et al. Preliminary evidence of an association between a functional interleukin-6 polymorphism and fatigue and sleep disturbance in oncology patients and their family caregivers. J Pain Symptom Manage. 2010;40:531–544. doi: 10.1016/j.jpainsymman.2009.12.006. doi:10.1016/j.jpainsymman.2009.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bower JE, Ganz PA, Irwin MR, Castellon S, Arevalo J, Cole SW. Cytokine genetic variations and fatigue among patients with breast cancer. J Clin Oncol. 2013;31:1656–1661. doi: 10.1200/JCO.2012.46.2143. doi:10.1200/jco.2012.46.2143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Reyes-Gibby CC, Wang J, Spitz M, Wu X, Yennurajalingam S, Shete S. Genetic variations in interleukin-8 and interleukin-10 are associated with pain, depressed mood, and fatigue in lung cancer patients. J Pain Symptom Manage. 2013;46:161–172. doi: 10.1016/j.jpainsymman.2012.07.019. doi:10.1016/j.jpainsymman.2012.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Jim HS, Park JY, Permuth-Wey J, Rincon MA, Phillips KM, Small BJ, Jacobsen PB. Genetic predictors of fatigue in prostate cancer patients treated with androgen deprivation therapy: Preliminary findings. Brain Behav Immun. 2012;26:1030–1036. doi: 10.1016/j.bbi.2012.03.001. doi:10.1016/j.bbi.2012.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kurz K, Fiegl M, Holzner B, Giesinger J, Pircher M, Weiss G, Denz HA, Fuchs D. Fatigue in patients with lung cancer is related with accelerated tryptophan breakdown. PLoS One. 2012;7:e36956. doi: 10.1371/journal.pone.0036956. doi:10.1371/journal.pone.0036956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Minton O, Strasser F, Radbruch L, Stone P. Identification of factors associated with fatigue in advanced cancer: a subset analysis of the European palliative care research collaborative computerized symptom assessment data set. J Pain Symptom Manage. 2012;43:226–235. doi: 10.1016/j.jpainsymman.2011.03.025. doi:10.1016/j.jpainsymman.2011.03.025. [DOI] [PubMed] [Google Scholar]
- 53.Rich T, Innominato PF, Boerner J, Mormont MC, Iacobelli S, Baron B, Jasmin C, Levi F. Elevated serum cytokines correlated with altered behavior, serum cortisol rhythm, and dampened 24-hour rest-activity patterns in patients with metastatic colorectal cancer. Clin Cancer Res. 2005;11:1757–1764. doi: 10.1158/1078-0432.CCR-04-2000. doi:10.1158/1078-0432.ccr-04-2000. [DOI] [PubMed] [Google Scholar]
- 54.Shafqat A, Einhorn LH, Hanna N, Sledge GW, Hanna A, Juliar BE, Monahan P, Bhatia S. Screening studies for fatigue and laboratory correlates in cancer patients undergoing treatment. Ann Oncol. 2005;16:1545–1550. doi: 10.1093/annonc/mdi267. doi:10.1093/annonc/mdi267. [DOI] [PubMed] [Google Scholar]
- 55.Landmark-Hoyvik H, Reinertsen KV, Loge JH, Fossa SD, Borresen-Dale AL, Dumeaux V. Alterations of gene expression in blood cells associated with chronic fatigue in breast cancer survivors. Pharmacogenomics J. 2009;9:333–340. doi: 10.1038/tpj.2009.27. doi:10.1038/tpj.2009.27. [DOI] [PubMed] [Google Scholar]
- 56.Reinertsen KV, Cvancarova M, Loge JH, Edvardsen H, Wist E, Fossa SD. Predictors and course of chronic fatigue in long-term breast cancer survivors. J Cancer Surviv. 2010;4:405–414. doi: 10.1007/s11764-010-0145-7. doi:10.1007/s11764-010-0145-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Reinertsen KV, Grenaker Alnaes GI, Landmark-Hoyvik H, Loge JH, Wist E, Kristensen VN, Fossa SD, Edvardsen H. Fatigued breast cancer survivors and gene polymorphisms in the inflammatory pathway. Brain Behav Immun. 2011;25:1376–1383. doi: 10.1016/j.bbi.2011.04.001. doi:10.1016/j.bbi.2011.04.001. [DOI] [PubMed] [Google Scholar]
- 58.Wang XS, Williams LA, Krishnan S, et al. Serum sTNF-R1, IL-6, and the development of fatigue in patients with gastrointestinal cancer undergoing chemoradiation therapy. Brain Behav Immun. 2012;26:699–705. doi: 10.1016/j.bbi.2011.12.007. doi:10.1016/j.bbi.2011.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wratten C, Kilmurray J, Nash S, Seldon M, Hamilton CS, O'Brien PC, Denham JW. Fatigue during breast radiotherapy and its relationship to biological factors. Int J Radiat Oncol Biol Phys. 2004;59:160–167. doi: 10.1016/j.ijrobp.2003.10.008. doi:10.1016/j.ijrobp.2003.10.008. [DOI] [PubMed] [Google Scholar]
- 60.Lee YW, Cho HJ, Lee WH, Sonntag WE. Whole brain radiation-induced cognitive impairment: Pathophysiological mechanisms and therapeutic targets. Biomol Ther (Seoul) 2012;20:357–370. doi: 10.4062/biomolther.2012.20.4.357. doi:10.4062/biomolther.2012.20.4.357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.McDonald B, Conroy S, Ahles T, West J, Saykin A. Gray matter reduction associated with systemic chemotherapy for breast cancer: A prospective MRI study. Breast Cancer Res Treat. 2010;123:819–828. doi: 10.1007/s10549-010-1088-4. doi:10.1007/s10549-010-1088-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Schagen SB, Muller MJ, Boogerd W, Mellenbergh GJ, van Dam FSAM. Change in cognitive function after chemotherapy: A prospective longitudinal study in breast cancer patients. J Natl Cancer Inst. 2006;98:1742–1745. doi: 10.1093/jnci/djj470. [DOI] [PubMed] [Google Scholar]
- 63.Trinchieri G. Cancer and inflammation: An old intuition with rapidly evolving new concepts. Annu Rev Immunol. 2012;30:677–706. doi: 10.1146/annurev-immunol-020711-075008. doi:10.1146/annurev-immunol-020711-075008. [DOI] [PubMed] [Google Scholar]
- 64.Filler K, Lyon D, Bennett J, McCain N, Elswick R, Lukkahatai N, Saligan LN. Association of mitochondrial dysfunction and fatigue: a review of literature. BBA Clin. 2014;1:12–23. doi: 10.1016/j.bbacli.2014.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Christensen JF, Jones LW, Andersen JL, Daugaard G, Rorth M, Hojman P. Muscle dysfunction in cancer patients. Ann Oncol. 2014;25:947–958. doi: 10.1093/annonc/mdt551. doi:10.1093/annonc/mdt551. [DOI] [PubMed] [Google Scholar]
- 66.Reid MB, Moylan JS. Beyond atrophy: Redox mechanisms of muscle dysfunction in chronic inflammatory disease. J Physiol. 2011;589:2171–2179. doi: 10.1113/jphysiol.2010.203356. doi:10.1113/jphysiol.2010.203356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Bower JE, Ganz PA, Irwin MR, Kwan L, Breen EC, Cole SW. Inflammation and behavioral symptoms after breast cancer treatment: Do fatigue, depression, and sleep disturbance share a common underlying mechanism? J Clin Oncol. 2011;29:3517–3522. doi: 10.1200/JCO.2011.36.1154. doi:10.1200/JCO.2011.36.1154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Cumiskey D, Pickering M, O'Connor JJ. Interleukin-18 mediated inhibition of LTP in the rat dentate gyrus is attenuated in the presence of mGluR antagonists. Neurosci Lett. 2007;412:206–210. doi: 10.1016/j.neulet.2006.11.007. [DOI] [PubMed] [Google Scholar]
- 69.Irwin MR, Olmstead RE, Ganz PA, Haque R. Sleep disturbance, inflammation and depression risk in cancer survivors. Brain Behav Immun. 2013;30(Suppl):S58–S67. doi: 10.1016/j.bbi.2012.05.002. doi:10.1016/j.bbi.2012.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Morris G, Berk M, Galecki P, Walder K, Maes M. The neuro-immune pathophysiology of central and peripheral fatigue in systemic immune-inflammatory and neuro-immune diseases. Mol Neurobiol. 2015 doi: 10.1007/s12035-015-9090-9. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 71.Santello M, Bezzi P, Volterra A. NFα controls glutamatergic gliotransmission in the hippocampal dentate gyrus. Neuron. 2011;69:988–1001. doi: 10.1016/j.neuron.2011.02.003. doi:10.1016/j.neuron.2011.02.003. [DOI] [PubMed] [Google Scholar]
- 72.Fowler JA, Mundy GR, Lwin ST, Edwards CM. Bone marrow stromal cells create a permissive microenvironment for myeloma development. Cancer Res. 2012;72:2183–2189. doi: 10.1158/0008-5472.CAN-11-2067. doi:10.1158/0008-5472.CAN-11-2067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Nefedova Y, Landowski TH, Dalton WS. Bone marrow stromal-derived soluble factors and direct cell contact contribute to de novo drug resistance of myeloma cells by distinct mechanisms. Leukemia. 2003;17:1175–1182. doi: 10.1038/sj.leu.2402924. [DOI] [PubMed] [Google Scholar]
- 74.Serhan CN. Novel lipid mediators and resolution mechanisms in acute inflammation: To resolve or not? Am J Pathol. 2010;177:1576–1591. doi: 10.2353/ajpath.2010.100322. doi:10.2353/ajpath.2010.100322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Kanczkowski W, Alexaki VI, Tran N, et al. Hypothalamo-pituitary and immune-dependent adrenal regulation during systemic inflammation. Proc Natl Acad Sci USA. 2013;110:14801–14806. doi: 10.1073/pnas.1313945110. doi:10.1073/pnas.1313945110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Monk JP, Phillips G, Waite R, et al. Assessment of tumour necrosis factor alpha blockade as an intervention to improve tolerability of dose-intensive chemotherapy in cancer patients. J Clin Oncol. 2006;24:1852–1859. doi: 10.1200/JCO.2005.04.2838. [DOI] [PubMed] [Google Scholar]
- 77.Tookman AJ, Jones CL, DeWitte M, Lodge P J. Fatigue in patients with advanced cancer: A pilot study of an intervention with infliximab. Support Care Cancer. 2008;16:1131–1140. doi: 10.1007/s00520-008-0429-x. doi:10.1007/s00520-008-0429-x. [DOI] [PubMed] [Google Scholar]
- 78.Yennurajalingam S, Frisbee-Hume S, Palmer JL, et al. Reduction of cancer-related fatigue with dexamethasone: A double-blind, randomized, placebo-controlled trial in patients with advanced cancer. J Clin Oncol. 2013;31:3076–3082. doi: 10.1200/JCO.2012.44.4661. doi:10.1200/JCO.2012.44.4661. [DOI] [PubMed] [Google Scholar]
- 79.Minton O, Richardson A, Sharpe M, Hotopf M, Stone P. A systematic review and meta-analysis of the pharmacological treatment of cancer-related fatigue. J Natl Cancer Inst. 2008;100:1155–1166. doi: 10.1093/jnci/djn250. doi:10.1093/jnci/djn250. [DOI] [PubMed] [Google Scholar]
- 80.Bower JE, Crosswell AD, Stanton AL, Crespi CM, Winston D, Arevalo J, Ma J, Cole SW, Ganz PA. Mindfulness meditation for younger breast cancer survivors: A randomized controlled trial. Cancer. 2014 doi: 10.1002/cncr.29194. doi: 10.1002/cncr.29194. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Bower JE. Cancer-related fatigue – mechanisms, risk factors, and treatments. Nat Rev Clin Oncol. 2014;11:597–609. doi: 10.1038/nrclinonc.2014.127. doi: 10.1038/nrclinonc.2014.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.NCCN Guidelines Version 2.2015 Panel Members Cancer- and Chemotherapy-Induced Anemia. [27 April 2015];NCCN clinical practice guidelines in oncology: Cancer- and chemotherapy-induced anemia. 2015 www.nccn.org/professionals/physician_gls/pdf/anemia.pdf.
- 83.McEwen BS, Seeman T. Protective and damaging effects of mediators of stress: Elaborating and testing the concepts of allostasis and allostatic load. Ann NY Acad Sci. 1999;896:30–47. doi: 10.1111/j.1749-6632.1999.tb08103.x. [DOI] [PubMed] [Google Scholar]
- 84.Glaus A, Boehme Ch, Thürlimann B, Ruhstaller T, Hsu Schmitz SF, Morant R, Senn HJ, von Moos R. Fatigue and menopausal symptoms in women with breast cancer undergoing hormonal cancer treatment. Ann Oncol. 2006;17:801–6. doi: 10.1093/annonc/mdl030. doi:10.1093/annonc/mdl030. [DOI] [PubMed] [Google Scholar]
- 85.Banthia R, Malcarne VL, Ko CM, Varni JW, Sadler GR. Fatigued breast cancer survivors: The role of sleep quality, depressed mood, stage and age. Psychol Health. 2009;24:965–980. doi: 10.1080/08870440802110831. doi:10.1080/08870440802110831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Butt Z, Rao AV, Lai JS, Abernethy AP, Rosenbloom SK, Cella D. Age-associated differences in fatigue among patients with cancer. J Pain Symptom Manage. 2010;40:217–223. doi: 10.1016/j.jpainsymman.2009.12.016. doi:10.1016/j.jpainsymman.2009.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Kyrdalen AE, Dahl AA, Hernes E, Hem E, Fossa SD. Fatigue in prostate cancer survivors treated with definitive radiotherapy and LHRH analogs. Prostate. 2010;70:1480–1489. doi: 10.1002/pros.21183. doi:10.1002/pros.21183. [DOI] [PubMed] [Google Scholar]
- 88.Luctkar-Flude M, Groll D, Woodend K, Tranmer J. Fatigue and physical activity in older patients with cancer: A six-month follow-up study. Oncol Nurs Forum. 2009;36:194–202. doi: 10.1188/09.ONF.194-202. doi:10.1188/09.onf.194-202. [DOI] [PubMed] [Google Scholar]
- 89.Singer S, Kuhnt S, Zwerenz R, et al. Age- and sex-standardised prevalence rates of fatigue in a large hospital-based sample of cancer patients. Br J Cancer. 2011;105:445–451. doi: 10.1038/bjc.2011.251. doi:10.1038/bjc.2011.251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Soltow D, Given BA, Given CW. Relationship between age and symptoms of pain and fatigue in adults undergoing treatment for cancer. Cancer Nurs. 2010;33:296–303. doi: 10.1097/NCC.0b013e3181ce5a1a. doi:10.1097/NCC.0b013e3181ce5a1a. [DOI] [PubMed] [Google Scholar]
- 91.Winters-Stone KM, Bennett JA, Nail L, Schwartz A. Strength, physical activity, and age predict fatigue in older breast cancer survivors. Oncol Nurs Forum. 2008;35:815–821. doi: 10.1188/08.ONF.815-821. doi:10.1188/08.onf.815-821. [DOI] [PubMed] [Google Scholar]
- 92.Storey DJ, McLaren DB, Atkinson MA, Butcher I, Frew LC, Smyth JF, Sharpe M. Clinically relevant fatigue in men with hormonesensitive prostate cancer on long-term androgen deprivation therapy. Ann Oncol. 2012;23:1542–1549. doi: 10.1093/annonc/mdr447. doi:10.1093/annonc/mdr447. [DOI] [PubMed] [Google Scholar]