Abstract
Abnormal activity of stress hormone (hypothalamic-pituitary-adrenal [HPA]), and gonadal hormone (hypothalamic-pituitary-gonadal [HPG]) systems is reported following prenatal alcohol exposure (PAE). PAE increases vulnerability of brain regions involved in regulation of these systems to stressors or challenges during sensitive periods of development, such as adolescence. In addition, HPA and HPG functions are linked to higher order functions such as executive function (EF), with dysregulation of either system adversely affecting EF processes, including attention and response inhibition, that influence cognition. However, how HPA and HPG systems interact to influence cognitive performance in individuals with an FASD is not fully understood. To investigate, we used a rat model of moderate PAE. Adolescent female PAE and control offspring were exposed to 10 days of chronic mild stress (CMS) and cognitive function was assessed on the radial arm maze (RAM) in adulthood. On the final test day, animals were sacrificed, with blood collected for hormone analyses, and vaginal smears taken to assess estrus stage at the time of termination. Analyses showed that adolescent CMS significantly increased levels of CORT and RAM errors during proestrus in adult PAE but not control females. Moreover, CORT levels were correlated with estradiol levels and with RAM errors, but only in PAE females, with outcomes dependent on adolescent CMS condition. These results suggest that PAE increases sensitivity to the influences of stress and gonadal hormones on cognition, and thus, in turn, that HPA and HPG dysregulation may underlie some of the deficits in executive function described previously in PAE females.
Keywords: FASD, Prenatal alcohol exposure, adolescent stress, cognition, HPA axis, gonadal hormones, female rat
1.1 Introduction
Prenatal alcohol exposure (PAE) produces a range of cognitive, behavioral, and physiological abnormalities, which fall under the umbrella term of fetal alcohol spectrum disorder (FASD). Many of the cognitive abnormalities in children with FASD are caused by disturbances in executive function (EF), which includes deficits in attention, planning, inhibition and appropriate behaviors that could contribute to impairments in learning and memory (Streissguth and O’Malley, 2000, Sokol et al., 2003). Deficits vary considerably amongst individuals, even if timing and extent of alcohol exposure are taken into account.
In addition to cognitive deficits, results of both human studies and animal models indicate that PAE-related abnormalities in physiological function may also occur, including alterations in hypothalamic-pituitary-adrenal (HPA) and hypothalamic-pituitary-gonadal (HPG) activity and regulation. Dysregulation of the HPA axis following PAE is well documented in both the clinical (Haley et al., 2006, Jacobson et al., 1999, Ramsay et al., 1996) and the animal (Taylor et al., 1982a, Weinberg and Gallo, 1982, Lee et al., 2000, Weinberg et al., 2008b, Hellemans et al., 2010b) literature. Furthermore, children with an FASD are more likely than their non-exposed counterparts to experience adverse conditions in early life (for review see (May and Gossage, 2011). Exposure to stressors during vulnerable periods of development may not only exacerbate PAE-related dysregulation of the HPA axis (Hellemans et al., 2010a, Weinberg et al., 2008b, Rivier et al., 1990), but also result in synergistic effects with PAE, at least during the adolescent period (Henry et al., 2007). As excessive levels of glucocorticoid hormones (cortisol in humans, corticosterone, CORT, in most rodents) are often detrimental to cognition (Diamond et al., 1996, de Quervain et al., 1998), enhanced stress responsiveness could further potentiate PAE-related cognitive deficits. Furthermore, as the HPA axis appears to show increased plasticity during postnatal development, especially during adolescence (Brunson et al., 2003, McCormick et al., 2010, Goldman et al., 1973) adverse experiences during this period may have significant consequences for functioning later in life (Wright et al., 2008, Avital et al., 2006, McCormick et al., 2008).
Sex and gender effects add to the variability in outcome following PAE. Cognitive functions are known to be altered by ovarian hormones, especially estradiol (E2), although whether its influence is positive or negative appears less clear (Chesler and Juraska, 2000, Kimura and Hampson, 1994, Frye, 1995). In rats, estradiol can enhance cognitive performance under certain conditions (Daniel et al., 1997, Frye et al., 2007), but also enhance activity of the HPA axis (Carey et al., 1995), which could lead to impairments in cognition (Diamond et al., 1996). Indeed, the function of the HPA axis is highly influenced by the activity of the HPG system. Estrogens appear to have an overall stimulatory effect on HPA function, as they can disrupt negative feedback and directly stimulate production of glucocorticoids, amplifying hormonal responses to stress (McCormick and Mathews, 2007, Shansky et al., 2003, Weiser and Handa, 2009). In turn, the HPA hormones can modulate activity of the HPG axis. Of importance, PAE is known to alter both HPG and HPA activity and regulation as well as interactions between these systems (Handa et al., 1985, Lee and Rivier, 1996, Weinberg et al., 2008a). For example, we have shown previously that PAE-induced changes in HPG and HPA activity may be estrous phase-specific (Lan et al., 2009). PAE, but not control females had higher basal and stress E2 levels in proestrus (when E2 levels are normally high) compared to other phases of the cycle, and greater variation in LH than control females across the cycle. As well, both basal and stress CORT levels and overall ACTH levels were greater in PAE than control females in proestrus. These data suggest that altered HPA-HPG interactions may differentially affect sensitivity to ovarian steroids in PAE compared to control females.
Despite data showing that HPA and HPG systems appear especially vulnerable to challenges during sensitive periods of development, that HPA and HPG functions are linked to higher order functions such as executive function (EF), and that PAE alters both HPA and HPG systems, how HPA and HPG systems interact to influence EF in individuals with FASD, and particularly in females, is not fully understood. We hypothesized that PAE would produce an increased sensitivity to the physiological and behavioral effects of CORT and E2, which would be further impacted by a period of chronic stress during adolescence; a sensitive period of development, translating into altered cognitive function in adulthood. To investigate, we used our well-established rat model of moderate PAE. Female offspring were exposed to a 10-day period of chronic mild stress (CMS) during adolescence, followed by a battery of behavioral tests, the last of which was the radial arm maze (RAM), a task that assesses aspects of EF such as working memory. Animals were sacrificed on the final RAM test day and we assessed the possibility that PAE females would exhibit altered sensitivity to the influence of stress and sex hormones on cognitive function in adulthood
2.1. Material and methods
2.1.1. Animals and Prenatal Treatments
This study utilized 102 female offspring obtained from 66 litters (24 PAE, 19 Pair-fed, 23 ad libitum-fed control). Sprague Dawley rats were obtained from Charles River Laboratories Inc., St. Constant, Quebec. Animals were pair-housed in standard Plexiglas cages on a ventilated rack with same sex cage mates for a 1–2 week adaptation period, provided with laboratory chow and water ad libitum, and maintained on a 12:12 light-dark cycle. All experimental protocols were carried out in accordance with the Canadian Council on Animal Care guidelines and the Guide for the Care and Use of Laboratory Animals (National Research Council, 2011).
At the start of breeding, females were individually paired with a single male just prior to lights-off. Each morning thereafter, vaginal smears were taken shortly after lights-on to check for the presence of sperm, which confirmed pregnancy and was denoted as gestation day (GD) 1. At this time, the dam was removed from the male, singly housed on a separate ventilated rack and randomly assigned to one of three prenatal treatment groups: Prenatal alcohol exposure (PAE; ad libitum access to a liquid ethanol diet, 36% ethanol-derived calories; Pairfed (PF; yoked to a PAE dam and provided liquid control liquid diet (maltose-dextrin isocalorically substituted for ethanol) in the amount consumed by their PAE partner (g/kg/body weight/gestation day); or Control (Con; pelleted control diet ad libitum). Experimental diets were prepared by Dyets Inc. Bethlehem, PA (Weinberg/Keiver High Protein Experimental Diet #710324, and Control Diet # 710109) and formulated to provide optimal nutrition, regardless of ethanol intake. Food consumption was recorded daily and fresh diet presented approximately 1 hr before lights-off, as studies have shown that under restricted feeding conditions, such as those of the Pair-fed group, circadian rhythms re-entrain to the feeding time rather than the light cycle. Feeding at lights off, toward the peak of the CORT circadian rhythm, results in normal circadian elevations, comparable to those in control animals (Ventura et al., 1984, Gallo and Weinberg, 1981). On GD 21, experimental diets were replaced with laboratory rat chow (19% Protein Extruded Rodent Diet, #2019, Teklad Global). Throughout, dams were maintained in a controlled environment (21°C) on a 12/12 light/dark cycle (lights on 0800 – 2000 h), with ad libitum access to water.
On postnatal day (PND) 1, offspring and dams were weighed and litters culled to ten (five females, five males when possible), and the dam and litter were moved to standard Plexiglas cages with environment controlled filtered lids. Cages were changed weekly thereafter at which time dam and offspring weights were recorded. On PND 22, pups were weaned, ear notched for identification purposes, and housed with same-sex littermates. Only the results from female offspring were assessed in this study. At PND 30, two females from each litter (with a few exceptions in which a single female was taken from a given dam) were pair-housed with same-sex, same prenatal treatment, non-littermate cage partners. Female cage-mates were then assigned together to either the CMS or Non CMS experimental condition, and remained housed together throughout testing until the end of the study. Animals in the CMS and Non CMS conditions were housed in separate colony rooms.
2.1.2. Chronic Mild Stress
Animals in the CMS condition were exposed to mild stressors twice a day for ten consecutive days, on PND 31–41, during the peri-pubertal period, as defined by vaginal opening (Rivest, 1991). Morning stressors took place between 0700 and 1100 hr, and afternoon stressors took place between 1300 and 1830 hr. Animals were assigned to cohorts by age so that their entrance into the CMS condition could be coordinated. All animals received the same number of each stressor over the 10 days of CMS, but the randomized order and timing of stressors varied by cohort. This CMS regimen was designed to be as unpredictable as possible, as research has shown that female rats do not habituate to unpredictable stressors in the same manner as they do predictable ones (Muir and Pfister, 1987, Muir and Pfister, 1986, Weinberg, 1992).
The stressors utilized included: soiled cage (another animal’s soiled bedding), 60 min; cage tilt on a 30° angle, 120 min; elevated platform, 10 min; restraint stress in a PVC tube (tube varied with animal size to ensure a snug fit without physical pressure on the animal), 30 min; cage with novel bedding, 60 min; overnight social isolation, immediately followed by a period of water deprivation in the home cage in the presence of an empty water bottle, 60 min. All stressors, with the exception of the water deprivation, took place in a test room separate from the colony room.
All animals (CMS and Non CMS) were weighed on the first and fifth day of CMS, as well as on the morning following the last day of CMS exposure. In addition, basal blood samples were taken by tail nick on the first day of CMS and again the morning following the last day of the CMS period. Blood collection occurred in an adjacent procedure room within 1 hr of lights-on and prior to stress exposure to obtain measures of basal corticosterone levels at the trough of the circadian rhythm. Samples were collected within 2 min of disturbing the home cage, and care was taken to avoid disturbing animals within the colony room.
2.1.3. Behavior Tests
All animals went through a battery of tests including play behavior, motor activity monitoring, novel odor recognition, a non-match to sample T-maze task and finally, radial arm maze testing. The present study presents only the cognitive measures from a final RAM challenge that occurred after all testing was complete, and also includes estrous stage, as determined by vaginal smears, as well as stress and sex hormone levels from blood samples taken at termination, 30 min after the start of the RAM challenge. This enabled us to assess possible relationships among CORT, E2, stage of the estrous cycle, and cognitive performance during a final RAM challenge.
2.1.3.1. Radial Arm Maze (RAM)
Beginning about PND 110, animals were trained to perform a non-match-to-sample task in an 8-arm radial arm maze. On the 2 days prior to the start of testing, animals were allowed to habituate for a minimum of 5 min in the testing room and 10 min in the maze itself. Animals also had a 5 min habituation period before all test trials. Testing was carried out under low-light conditions with white masking noise (about 40db) in the background. Females were tested at 1200–1700 hr. All animals were food deprived at 95% of their free feeding body weight and allowed to gain 1 – 2% of their weight per week.
Animals ran 1 trial per day. Prior to the start of a trial, a food reward (about 1/3 a Kellogg’s Froot LoopsTM) was placed at the end of each of the 8 arms and 4 of the arms were blocked pseudo-randomly (a random generation of combinations was predetermined and assigned by day of test). Trials were separated into a training phase and a testing phase. During the training phase, animals were placed in the centre area of the maze, with all rats facing the same arm each time. Rats were allowed a maximum of 5 min to enter each of the 4 open arms and retrieve the food reward. The number of entries required for the rat to retrieve all of the rewards was recorded; an animal was considered to have entered an arm when its hind legs crossed the boundary between the arm and the centre of the maze. When an animal did not reach the end of an arm before turning back, or re-entered an arm that it had previously entered, that entry was designated as an error. If the rat completed the task within 5 min, it was removed and placed into a holding cage for a specified delay period before being returned to the maze for the test phase.
During the delay, doors were removed from the previously blocked arms, and the maze wiped with a 20% vinegar and water solution so the animal would not be able to retrace its path using scent. The test phase began when the animal was reintroduced to the maze following the delay and given another 5 min to retrieve the 4 remaining food rewards in the arms that had been blocked during training. Again, the number of entries that it took to retrieve all of the rewards was recorded. To meet the task criteria, the animal had to retrieve all 4 rewards within the 5 min time limit, while making 5 or fewer entries into the arms (i.e., with 1 error or less). Animals ran the task with a 5 min delay until they could successfully meet criteria for 2 consecutive days or after a maximum of 21 days, whichever occurred first. Animals then performed the task with a 20 min delay, and were run until they again met criteria for 2 consecutive days or for a maximum of 7 days.
2.1.3.2. Challenge Task, Termination and Tissue Collection
After completing RAM testing, animals were left undisturbed for 48 hrs before being tested again for a final challenge test with a 20-min delay. As animals were pair-housed, any animal that reached criterion in all versions of the RAM task would be required to wait for its cage-mate to finish as well before both ran the final challenge task. To maintain approximately the same levels of performance, animals that completed the task ahead of their cage-mates were tested every 2–3 days in “sham” trials in which no score was recorded.
Animals were terminated by decapitation at 30 min from the start of the final challenge test. Trunk blood was collected into chilled polystyrene tubes containing 200μL 0.5M EDTA (to prevent coagulation). Brains were extracted and immediately frozen on dry ice, and vaginal smears taken to determine estrous cycle stage. Blood samples were centrifuged, the plasma transferred into 600μL Eppendorf tubes, and tubes stored at −80°C until assays were performed.
2.1.4. Radioimmunoassay for corticosterone and 17β-estradiol
Corticosterone was assayed using a commercial RIA kit from MP Biomedicals, (Solon, OH). Levels of total plasma CORT, bound and free, were measured with [125I] corticosterone as the tracer. The cross-reaction of the antiserum in this kit is 100% for corticosterone. Minimum detectable corticosterone concentration was 0.63 μg/dl and the intra- and inter-assay coefficients of variation were 1.55 % and 4.26 % respectively. To control for batch effects within subjects, CORT assays were run in sets of 56 samples, which were comprised of 4 samples from each of 14 animals, randomized across prenatal treatment and test condition.
Plasma E2 levels were assayed using the Coat-A-Count Estradiol RIA kit (Diagnostic Products Corporation, Los Angeles, CA, USA), with [125I] estradiol as the tracer. The E2 antibody cross-reacts 100% with E2, 10.0% with estrone and 4.4% with d-Equileni but it does not cross-react with aldosterone or corticosterone. The minimum detectable E2 concentration was 8 pg/mL, and the intra- and inter-assay coefficients of variation were 7.0% and 8.1%, respectively.
2.1.5. Estrous Staging
Vaginal smears were collected by lavage using a few drops of physiological saline solution. Slides were dried, stained with a 1% concentration of Toluidine Blue, and examined under a light microscope to determine stage of estrous cycle (as per (McLean et al., 2012) and previous studies from our laboratory (Lan et al., 2009, Sliwowska et al., 2008)). Because we were interested specifically in the relationships among E2, CORT and cognitive function, estrous stage was categorized as “proestrus” (the stage in which E2 levels peak) or “not proestrus” (estrus and diestrus, lower E2 levels).
2.1.6. Statistical Analysis
Analyses were performed using PASW Statistics for Windows (version 18.0. Chicago: SPSS Inc.). ANOVAs were used throughout and pairwise comparisons conducted when F ratios were significant or where a priori comparisons were the focus. Homogeneity of variance was assessed for each test using the Mauchly sphericity test and when violated, the more conservative Huynh-Feldt correction was used to calculate P-values for the F ratios. In the latter case, dfs were rounded for ease of presentation. Significance was set at P ≤ 0.05 and trends at P > 0.05 and ≤ 0.10. Pairwise comparisons are presented in the figure captions to reduce redundancy.
Note: collection of vaginal smears for estrus staging was inadvertently missed for ten females (5 Cons, 4 PF, 1 PAE) so these females are missing from analysis related to stage.
3.1. Results
3.1.1. Vaginal Opening: PAE delays sexual maturation
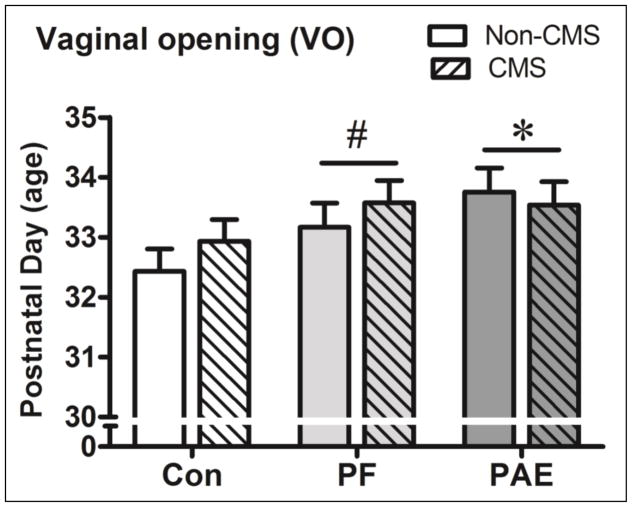
Vaginal opening (VO), as an assessment of reproductive maturation in peri-adolescent females, was systematically assessed in only a portion of the total females (N = 80). Data showed that PAE (N= 25), and to a lesser extent PF (N= 26), females showed delayed VO relative to control (N= 29) females (see Figure 1).
Figure 1.
Vaginal opening was significantly delayed in PAE females and showed a trend towards delay in PF (P = 0.014, Ps = 0.07) relative to Con females. There was no effect of CMS on VO. * significant at (P< 0.05); # trend at (P = 0.07).
An ANOVA with prenatal treatment and adolescent condition as variables revealed a significant main effect of treatment, F(2,74) = 3.49, P = 0.037, but not condition, nor an interaction (Ps > 0.45).
3.1.2. PAE and PF females differentially respond to CMS-related effects on weight gain
3.1.2.1. Adolescent Weight
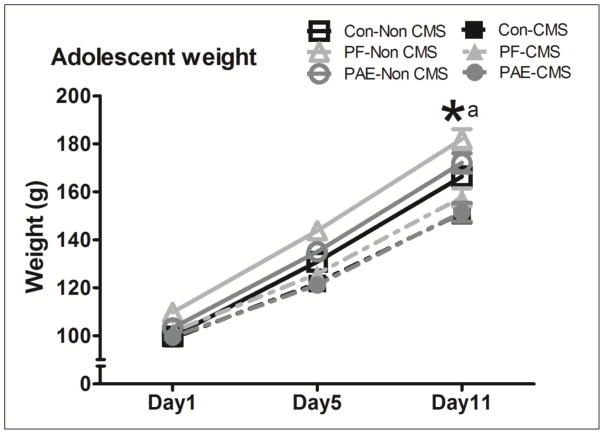
Overall, adolescent CMS inhibited weight gain in females across prenatal groups. Animals in all Non CMS groups gained significantly more weight than their CMS counterparts by PND41, the morning following the 10-day CMS regimen, although PF - Non CMS maintained higher weights relative to Con-Non CMS animals throughout (see Figure 2).
Figure 2.
The pairwise comparison revealed no differences amongst Con and PAE groups to start (Ps > 0.30), although PF females in the Non CMS condition were significantly heavier than their Con-Non CMS (P = 0.012) and PF CMS (P = 0.037) counterparts. Nonetheless, by the end of the CMS period, exposure to CMS significantly inhibited weight gain in all females, irrespective of prenatal treatment group (all Ps < 0.007). (*a) significant at (P< 0.05).
A repeated measures ANOVA with prenatal treatment and adolescent condition as variables showed a main effect of day, F(1.7,164 ) = 4005.24, P < 0.001, a day by condition interaction, F(1.7,164 ) = 66.54, P < 0.001, and a trend toward significant interaction of day by treatment, F(3.5,164 ) = 2.28, P = 0.073.
3.1.2.2. Adult Weight
Overall, there was no long-term effect of CMS on female weight in adulthood (PND110+). However, an analysis of percent gain from adolescence to adulthood showed that PAE and PF females had a greater percent weight gain than Con females (Table 1).
Table 1.
Across CMS condition, PF females showed a significantly greater percent increase in weight (P = 0.033) and PAE females showed a marginally significant increase (P = 0.051) compared with Con females.
| Weight (grams) | PND41 | Adulthood | % Change | N | |
|---|---|---|---|---|---|
| Con | Non CMS | 166.4 (± 3.8) | 301.3 (± 7.2) | 201% (± 7.2) | 18 |
| CMS | 151.1 (± 4.0) | 296.0 (± 7.4) | 196% (± 7.4) | 17 | |
| PF | Non CMS | 180.0 (± 4.1) | 321.9 (± 7.6) | 222% (± 7.6)* | 16 |
| CMS | 157.7 (± 3.8) | 307.1 (± 7.2) | 207% (± 7.2)* | 18 | |
| PAE | Non CMS | 172.1 (± 4.1) | 317.3 (± 7.3) | 217% (± 7.3)† | 16 |
| CMS | 151.5 (± 4.0) | 309.2 (± 7.2) | 209% (± 7.2)† | 17 | |
significant relative to Con females;
trend towards significance relative to Con females when treatment groups were collapsed across CMS condition.
ANOVA of percent change from PND41 to adulthood showed a marginally significant main effect of prenatal treatment, F(2,96) = 2.89, P = 0.060, but no main effect of adolescent condition nor interaction (Ps > 0.12).
3.1.3. Radial Arm Maze: Post-training Challenge Test and Estrus Stage
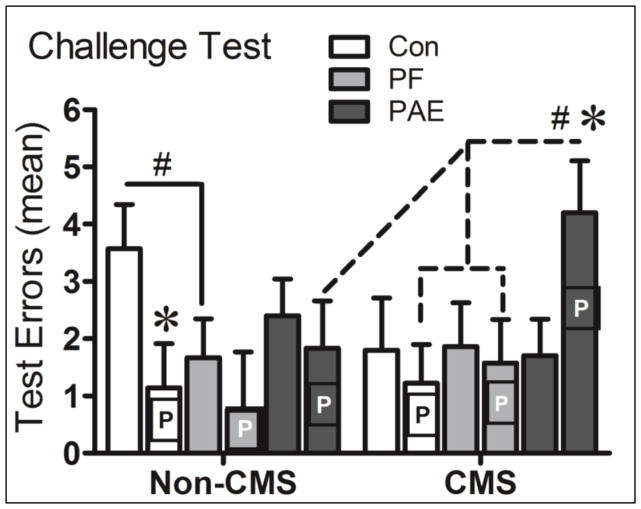
Overall, we found that PAE-CMS females in proestrus made significantly more errors in the Challenge test than Con- and PF-CMS females in proestrus and their non-proestrus PAE–CMS counterparts. In contrast, Con-Non CMS females in proestrus made fewer errors than their non-proestrus counterparts (Figure 3).
Figure 3.
Proestrus (P) PAE-CMS females made significantly more errors than Con- and PF-CMS females in proestrus and their non proestrus (blank bars) PAE-CMS counterparts, as well as more errors (marginally significant) than their PAE-Non CMS counterparts (P = 0.011; P = 0.03; P = 0.028; P = 0.059, respectively). In contrast, proestrus Con-Non CMS females made significantly fewer errors than their non-proestrus Con counterparts (P = 0.029). * significant at P ≤ 0.05; # trend at P ≤ 0.07.
A three-way ANOVA with prenatal treatment, adolescent condition, and estrous stage as variables showed a significant interaction of condition and stage, F(1,74) = 4.12, P = 0.046, and a trend towards an interaction of treatment and stage, F(2,74) = 2.61, P = 0.081.
3.1.4. Effects of adolescent CMS on adolescent basal CORT levels and adult activated CORT levels
3.1.4.1. Adolescent basal CORT levels: Differential effects of CMS in Control and PAE females
The percent change in basal CORT from PND 31 to PND 41 (from pre- to post-CMS) was utilized to assess the effect of adolescent CMS on basal HPA activity. We found that CMS increased basal CORT levels, but only in Con females. Indeed, PAE-CMS females showed a lower percent change in CORT relative to Con-CMS females (see Table 1). By contrast, PAE animals in the Non CMS condition showed a higher percent change in basal CORT relative to Con and PF-Non CMS females. Note, difficulty in obtaining sufficient blood from PND31 females resulted in basal CORT analysis performed for 94, rather than 102 females.
Table 1.
Actual CORT levels are presented, but analysis is based on the calculated percent change of basal CORT from PND 31 to PND 41 (pre- to post-CMS). PAE-Non CMS females had a greater % change in basal CORT from PND31-PND41 relative to both (a) Con and (b) PF - Non CMS females (P = 0.043; P = 0.070, respectively), whereas PAE-CMS females showed a marginally lower % change in CORT levels relative to that of (c)Con-CMS females (P = 0.062). On the other hand, Con-CMS females alone showed a significantly higher % change from pre- to post-CMS relative to their (a) Con-Non CMS counterparts (P = 0.005).
| Basal Corticosterone (μg/dL) | PND31 | PND41 | N | |
|---|---|---|---|---|
| Con | Non CMS | 1.61 (± 0.83) | 0.76 (± 2.03) | 17 |
| CMS | 1.30 (± 0.83) | 10.60 (± 2.03)*a | 17 | |
| PF | Non CMS | 2.49 (± 0.95) | 0.77 (± 2.32)ns | 13 |
| CMS | 3.77 (± 0.85) | 6.54 (± 2.09)ns | 16 | |
| PAE | Non CMS | 1.32 (± 0.88) | 5.80 (± 2.16)*a†b | 15 |
| CMS | 1.79 (± 0.85) | 3.83 (± 2.09)†c | 16 | |
significant at P ≤ 0.05;
trend towards difference.
different from Con-Non CMS,
different from PF-Non CMS,
different from Con-CMS.
An ANOVA on percent change with prenatal treatment and adolescent condition as variables showed a significant interaction of treatment and condition F(2,88) = 4.07, P = 0.020, and a marginally significant effect of condition F(1,88) = 3.50, P = 0.065, but no main effect of treatment (P = 0.717).
3.1.4.2. Adult activated CORT levels: Influence of adolescent CMS and estrous stage
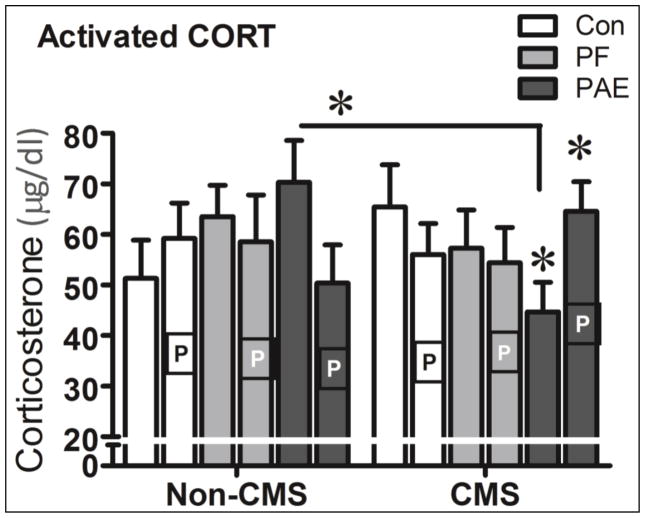
In adulthood, overall, there were no significant effects of prenatal treatment or adolescent CMS condition on activated CORT levels. Importantly, however, both adolescent CMS and estrous stage differentially influenced activated CORT levels among prenatal groups in adulthood. PAE-CMS females in proestrus had higher CORT levels than their non-proestrus PAE-CMS counterparts. In non-proestrus stages, by contrast, PAE-CMS females had lower activated CORT levels than both their Con-CMS and their PAE Non-CMS counterparts (Figure 5).
Figure 5.
Non proestrus (blank bars) PAE-Non CMS females had significantly higher activated CORT than their non proestrus PAE-CMS counterparts (P = 0.018), and these non proestrus PAE-CMS females, in turn had lower CORT levels relative to their Con-CMS counterparts (P = 0.045) as well. In contrast, Proestrus (P bars) PAE-CMS females had significantly higher CORT levels than their non proestrus PAE-CMS female counterparts (P = 0.014). * significant at P < 0.05.
A three-way ANOVA with prenatal treatment, adolescent condition and estrus cycle as variables showed a marginally significant three-way interaction, F(2,72) = 2.91, P = 0.061, but no other interactions or main effects (all Ps > 0.35).
3.1.6. Correlations
Overall, E2 levels did not differ significantly among prenatal treatments or between CMS conditions, irrespective of estrus stage. However, correlations between E2 and CORT and RAM errors and CORT were observed differentially in PAE and control females (with stage as an additional factor, Ns per group ranged from 6 to 10 with three exceptions: Con-CMS Non proestrus, N = 5; PF-Non CMS proestrus, N= 4; PAE–CMS Proestrus, N=5).
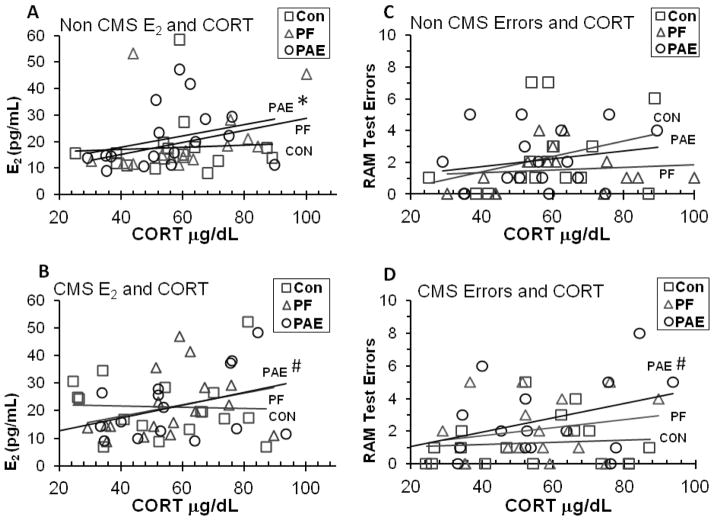
Linear bivariate correlations were conducted to investigate the potential influence of E2 and CORT on cognition. To begin, separate analyses of each prenatal treatment group showed a significant positive linear correlation between E2 and CORT in PAE (r = 0.46, P = 0.008), with only a trend toward a positive correlation in PF (r = 0.31, P = 0.089), and no significant correlation in Con animals (P > 0.20). Further analysis then separated the prenatal treatment groups by adolescent condition, Non CMS and CMS (Figure 6). We found that PAE-Non CMS females showed a marginally significant positive correlation (r = 0.48, P = 0.057) between E2 and CORT, whereas PAE-CMS females showed trends toward positive correlations between E2 and CORT (r = 0.44, P = 0.075), and between RAM Errors and CORT (r = 0.44, P = 0.081). Neither Con nor PF females showed any correlations between E2 and CORT or between Errors and CORT, regardless of CMS condition (all Ps > 0.10).
Figure 6.
(A) PAE-Non CMS females showed a marginally significant positive correlation (P = 0.057) between E2 and CORT, whereas (B) PAE-CMS females showed trends toward positive correlations between E2 and CORT (P = 0.075), and (D) between Errors and CORT (r = 0.44, P = 0.081). Neither Con nor PF females showed any correlations between E2 and CORT or between Errors and CORT, regardless of CMS condition (all Ps > 0.10).
4.1. Discussion
Effects of early adversity, such as prenatal alcohol exposure, can be accentuated by stress, especially during sensitive periods of growth and maturation such as adolescence. In the current study, a 10-day regimen of chronic mild stress simulated a period of stress and unpredictability during adolescence. PAE and CMS are known to act on at least one shared pathway, the HPA axis, but have also been implicated in abnormalities in the HPG axis, again, with particular impact during sensitive periods of development. Alterations in HPA and HPG systems and their interactions can potentially produce a variety of effects on physiological and behavioral development, and on brain regions involved in other functions, including higher-order cognitive processes, such as executive function. Thus, we hypothesized that PAE would produce an increased sensitivity to CORT and E2 that would be further impacted by CMS, and that this sensitivity would translate into a differential influence of these systems on cognitive function in adulthood.
Our study revealed six major novel findings: 1) There was a PAE-related delay in sexual maturation beyond what would be expected from PAE induced nutritional restriction alone; 2) While a 10-day period of CMS in adolescence inhibited weight gain equally across prenatal groups, PAE and PF animals showed a period of enhanced catch-up growth into adulthood; 3) CMS exposure in adolescence differentially altered basal CORT levels in females from the three prenatal treatment groups, with greater effects in PAE than control females, suggesting a dysregulation in HPA function; 4) Activated CORT levels in adulthood differed according to adolescent CMS condition and estrous stage at termination; 5) A relatively short period of CMS in adolescence differentially affected estrous stage-dependent cognitive performance among prenatal groups in adulthood, which was influenced further by a stressful adolescent environment (CMS); and 6) We found a link between HPA, HPG and cognitive function that may help to elucidate the mechanism(s) by which life experience may continue to play a role in the final behavioral phenotype following PAE.
4.1.1. PAE delays sexual maturation
Our finding of a PAE-related delay in sexual maturation is consistent with previous reports of PAE-related delayed sexual development/maturation reported by our laboratory and others in both female and male offspring (Esquifino et al., 1986, Lan et al., 2013, McGivern and Yellon, 1992). Maturational delay occurred irrespective of adolescent CMS condition, indicating a more direct effect of alcohol rather than of stress or nutritional deficiency. Indeed, similar to previous reports (Gavin et al., 1994), PAE females showed an augmented delay in maturation relative to the nutritional control group, indicating that PAE-related nutritional deficiencies alone cannot account for the observed delay.
4.1.2. PAE and PF females differentially respond to CMS-related weight gain
As in adulthood (Uban et al., 2010), adolescent CMS produced attenuated weight gain in all prenatal treatment groups over the CMS period. However, weight gain from adolescence into adulthood was significantly increased in both pair-fed and PAE females. Of interest, all females in this study had undergone a period of restricted food access throughout cognitive testing in adulthood, in which they consumed equal amounts of food per day. Despite this restricted feeding regimen, weight gain in PAE and pair-fed females was significantly greater than that of control females.
Most research investigating weight gain/loss in PAE offspring has focused on early stages of development. Often, though not always, PAE offspring show reduced weight at birth and during early postnatal life (Sigh and Snyder, 1982, Weinberg, 1984, Lopez-Tejero et al., 1986, Weinberg et al., 1995, Gilliam and Kotch, 1996). To our knowledge, this is the first study to assess weight gain or maintenance in adulthood, specifically, following a prolonged period of food restriction. Why PAE and PF females would show increased weight gain is unknown. However, one provocative explanation is related to fetal programming as first discussed by Barker (now referred to as the Barker Hypothesis), linking reduced birth weight with adult cardiovascular disease (Barker, 1995), and metabolic syndrome (Hales and Barker, 2001). Subsequent research argues that under conditions of reduced nutrition prenatally, the fetus is programmed to expect adversity postnatally as well, and is metabolically set up to thrive under such conditions (De Boo and Harding, 2006). Underlying mechanisms of this fetal programming may include changes in gene expression (epigenetic) (De Boo and Harding, 2006) and endocrine function including insulin efficiency, as well as increased sensitivity of the HPA axis (Kajantie et al., 2007, Guilloteau et al., 2009). Incongruence between the pre- and postnatal environment, however, can abolish the proposed benefits of this metabolic reprogramming, and instead produce an increased vulnerability to developmental disorders and disease into adulthood. Thus, it is possible that mild undernutrition during prenatal life may have “benefited” PAE and PF females in their ability to thrive during times of food deprivation in adulthood. Additional support for this comes from a recent study by Fuglestad and colleagues (2014) that found that rates of obesity in children with an FASD was over 40%, which was significantly higher than expected in typically developing individuals. Moreover, the rate of female obesity was even higher, at 50%, which was in significantly greater than the approximate 18% rate in typically developing females. We hypothesize, that the increased weight gain observed in PAE and PF females in our study may be related to endocrine/metabolic alterations produced by nutritional restriction in utero (Fuglestad et al., 2014) that may have altered insulin efficiency and HPA axis function, leading to enhanced fat storage but with possible negative influences on other systems including HPG development and cognitive processes, as well as possible increased risk for later-life disorders such as diabetes or cardiovascular disease.
4.1.3. Altered basal CORT levels following adolescent CMS
Control females showed a significantly greater percent change in basal CORT from pre- to post-CMS compared with their Non CMS counterparts, consistent with the literature linking early stress exposure with heightened basal CORT (Sterlemann et al., 2008). By contrast, the percent change in basal CORT levels of PAE females did not differ between the CMS and Non CMS conditions, suggesting a blunting of the basal HPA response to CMS. On the other hand, under Non-CMS conditions, PAE females had higher basal CORT levels than control females.
While the finding of increased HPA responsiveness to stress appears to be robust across studies (Weinberg et al., 2008a), reports of increased basal CORT in PAE offspring have been inconsistent, and may vary depending on factors including age, type of stressor, time course measured, and postnatal experience (Weinberg et al., 2008a). For instance, Taylor and colleagues (Taylor et al., 1982b) found no change in basal CORT levels in PAE animals raised to adulthood under non-stressed conditions, in contrast to our finding of higher basal CORT in non-stressed PAE females and a trend towards a blunted change in basal CORT levels relative to control females following adolescent CMS. Furthermore, we have shown that even in the face of similar basal CORT levels, central regulation of HPA activity may be altered under both basal and stress conditions (Glavas et al., 2007). Findings similar to ours have been reported in human studies (Halligan et al., 2004) in which basal CORT levels were elevated in adolescents exposed postnatally to maternal depression. Lupien and colleagues (2000) have also reported a relationship between low socioeconomic status (SES) and cortisol, indicating that the stress system may act as the mechanism by which SES negatively influences development, especially in the early years. Although consistent with the literature, our current findings are notable owing to the relatively mild stressors incorporated into the CMS regimen, and provide a longitudinal approach to begin to elucidate the possible relationship between PAE, adolescent experience, and dysregulated HPA function on cognitive performance in adulthood. Of note, these relationships may not be unique to adolescent experiences, as we have shown previously that basal corticosterone levels are increased in control, but not PAE, females following CMS exposure in adulthood, suggesting that PAE may blunt the normal change in basal CORT levels during CMS in adulthood as well (Uban et al., 2013). Moreover, chronic stress may affect males and females differently. In some instances, females may be more sensitive to the effects of chronic stress in terms of hormonal stress response, showing greater changes in hormone secretion after being exposed to the same stressors as males (Galea et al., 1997, Cameron, 2004). Relevant to this point, in contrast to the differences in basal CORT levels observed in PAE compared to control females, Uban and colleagues (Uban et al., 2013) found no differences in basal CORT levels between PAE and control males following CMS, highlighting the importance of sex differences in the investigation of PAE-related responses to early and later life environmental stressors.
4.1.4. Adult activated CORT levels: Influence of adolescent CMS and estrous stage
The finding that activated CORT levels in adulthood differed according to adolescent CMS condition and estrus stage at termination suggests a PAE-related increase in sensitivity to CORT and ovarian hormones. Previous data suggest that females generally show greater CORT activation in response to a stressor during proestrus than at other stages of the cycle (Viau and Meaney, 1991), consistent with the finding that estradiol potentiates HPA activity (Handa et al., 1994). Indeed, we have shown previously (Lan et al., 2009) that under moderate levels of stress, HPA activation did not differ across the estrous cycle in control females, while PAE females showed both increased basal CORT and HPA activation during proestrus. Data from the current study support and significantly extend these results. We did not observe higher activated CORT levels following the RAM challenge test in Control females in proestrus compared to non-proestrus phases, regardless of adolescent CMS experience. By contrast, differential effects of adolescent CMS and estrous phase at termination were observed selectively in PAE females, who showed higher activated CORT following adolescent CMS under proestrus compared to non-proestrus conditions. One possible explanation for the lack of proestrus effects in Control females may relate to the method used to designate estrous stage. In the Viau and Meaney study (1991), females in proestrus were subdivided into late and early phases, with only those rats in early proestrus showing enhanced HPA response to stress, whereas we did not separate animals by early and late proestrus. On the other hand, the finding that CMS PAE females in proestrus showed increased HPA activation following the RAM challenge not only demonstrates an increased sensitivity to stress in PAE animals, as reported previously in our laboratory (Weinberg, 1988, Weinberg et al., 2008a), but extends these findings to suggest the possibility that adolescent stress sensitized the HPA axis in PAE compared to Control females such that subsequent exposure to stress (RAM challenge) unmasked this sensitization selectively under conditions of high estradiol levels (i.e., proestrus).
4.1.5. Estrous stage differentially influences cognitive function of PAE compared to control females
Control females that did not experience CMS during adolescence showed enhanced performance on the RAM during proestrus compared to non-proestrous stages of the cycle, whereas proestrus did not similarly enhance performance of Non CMS PF and Con females. Importantly, however, adolescent CMS differentially influenced the performance of PAE and control females in adulthood, with PAE females showing poorer performance on the RAM during proestrus than control females in the CMS condition, as well as PAE females in the non-CMS condition. On the other hand, under non-proestrus conditions, the performance of PAE-CMS females did not differ from that of their PF and Con counterparts.
As noted, the influence of ovarian hormones on cognitive function can be positive or negative, enhancing performance under certain conditions, (Daniel et al., 1997, Frye et al., 2007), but also enhancing activity of the HPA axis (Carey et al., 1995), which could then impair cognitive performance (Diamond et al., 1996). Our data suggest that differential sensitivity to the effects of both CORT and estradiol played a role in the RAM performance in PAE compared to control females in the present study. While the increased estradiol levels known to occur in proestrus may have enhanced RAM performance in control females, the greater facilitation of HPA activity that likely occurred in PAE compared to control animals during proestrus (Lan et al., 2009) may have interfered with cognitive performance, an effect that was exacerbated in PAE females previously exposed to CMS. We make this suggestion cautiously, however, as both progesterone and estradiol levels peak during proestrus. Although the role of progesterone on cognitive and HPA function is not fully understood, progesterone is known to antagonize the effects estrogen, thereby reducing HPA activity (Viau and Meaney, 1991, Carey et al., 1995) and possibly reducing the more direct influence of estradiol on cognition. Indeed, the findings of the current study are likely a result of a complex interaction of HPA, HPG, and cognitive functioning, and further research is needed to understand how these factors act and interact to influence outcome. Further research is also needed to understand the interactive effects of PAE, stress and ovarian hormones on neuronal structures, such as the prefrontal cortex and hippocampus, which, in addition to subserving cognitive function, play a major role in HPA regulation, and in turn, are influenced by the sex steroids.
The finding that despite exposure to adverse conditions (CMS) during adolescence, PAE animals performed at a level comparable to their control counterparts during the non-proestrus stages of the estrous cycle is not entirely surprising in light of the moderate levels of alcohol in our paradigm combined with the extensive amount of training provided throughout the experiment. These results suggest that cognitive deficits may only become apparent in PAE offspring during or immediately following estrous stage-related changes in estrogen, and possibly also progesterone, as is the case in proestrus (Butcher et al., 1974).
4.1.6. Unique interactions of HPA, HPG and cognitive function in PAE but not control females
In the current study, we found novel correlations between E2 and CORT and RAM errors and CORT in PAE but not control females, and importantly, these correlations depended on whether animals had been exposed to CMS during adolescence. In PAE females that had not been exposed to adolescent stress, we found a positive correlation between E2 and activated CORT levels in response to the RAM challenge. Moreover, following adolescent CMS, PAE females alone not only showed a positive correlation between E2 and activated CORT following the RAM challenge, but also were the only group to show a correlation between RAM errors and CORT levels at the time of testing.
Correlations between E2 levels and CORT are not novel, with E2 known to facilitate the stress response (Viau and Meaney, 1991, Patchev and Almeida, 1996). However, what is novel is that this correlation occurred only in PAE animals. A study by Weiser and Handa (2009) may help to explain this result. In their study, it was shown that E2 works to inhibit the negative feedback of the HPA axis in response to a stressor, thereby amplifying the effects of CORT. As animals in the current study had been exposed to daily testing for an extended time, it would be reasonable to assume that habituation would have occurred, reducing stress responsivity and subsequent increases in CORT levels. As such, a correlation between E2 levels and CORT in PAE females suggests differential hypersensitivity of the HPA axis, HPG axis, or both, in PAE compared to control females, as overall, neither E2 levels nor CORT levels of PAE females were significantly different from levels in control females. This proposed hypersensitivity may also explain the additional correlation of RAM errors and CORT levels during the challenge test that was found only in PAE females from the CMS condition. We have shown previously (Lan et al., 2009) that PAE females exhibit differential changes in both HPA and HPG activity that are estrous-phase specific, with higher basal and stress E2 and CORT levels than controls in proestrus compared to other phases of the cycle, suggesting that HPA activity in PAE females may reflect differential sensitivity to ovarian steroids. The present data support and significantly extend these previous findings to demonstrate differential influences of E2 not only on CORT levels but also on cognitive performance in the radial arm maze, a task that assesses aspects of executive function, in PAE compared to control females. Moreover, we demonstrate that these effects of E2 occur specifically in animals that had been exposed to stress during adolescence, a “second hit” that appears to increase sensitivity to subsequent stressors or challenges, resulting in the observed correlation between RAM errors and CORT levels at the time of testing. This novel finding is critical in providing insight into potential mechanisms underlying long-term effects of PAE on functional outcomes, and in particular, cognitive deficits.
5.1. Summary/Conclusion
The results of the current study help to elucidate the impact of prenatal and postnatal adversity on developmental processes that impact functional outcome in adulthood. The results demonstrate unique metabolic effects of prenatal treatment on postnatal weight gain under restricted feeding conditions that may predispose PAE offspring to later life diseases or disorders. As well, we show novel interactions of HPA, HPG and cognitive function in adulthood in PAE animals alone, that were differently impacted by the adolescent environment, providing important insight into underlying mechanisms of PAE-related deficits. Finally, this study highlights the need to consider sex/gender in expected outcomes, adding to the growing literature of sexually dimorphic outcomes as a consequence of environmental factors.
Figure 4.
Experimental time line for the collection of blood in adolescence and adulthood for corticosterone (CORT) assays.
The results of the current study help to elucidate the impact of prenatal and postnatal adversity on developmental processes that impact functional outcome in adulthood.
unique metabolic effects of prenatal treatment on postnatal weight gain
show novel interactions of HPA, HPG and cognitive function in PAE animals alone
Interactions were differently impacted by the adolescent environment.
illustrates sexually dimorphic outcomes as a consequence of environmental factors.
Acknowledgments
This work was supported by NIH/NIAAA R37 AA007789 and NeuroDevNet, Network of Centers of Excellence of Canada to JW
We would like to thank Wayne Yu for his expertise and technical help with the radioimmunoassays and Parker Holman and Vivian Lam for their time and assistance with the animal work.
Abbreviations
- Con
control
- CMS
chronic mild stress
- CORT
corticosterone (rat); cortisol (human)
- DA
dopamine
- EF
executive function
- FASD
fetal alcohol spectrum disorder
- HPA
hypothalamic-pituitary-adrenal
- HPA
hypothalamic-pituitary-gonadal
- PAE
prenatal alcohol exposure
- PF
pair-fed
- PND
postnatal day
- S
Seconds
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Avital A, Ram E, Maayan R, Weizman A, Richter-Levin G. Effects of early-life stress on behavior and neurosteroid levels in the rat hypothalamus and entorhinal cortex. Brain Res Bull. 2006;68:419–24. doi: 10.1016/j.brainresbull.2005.09.015. [DOI] [PubMed] [Google Scholar]
- Barker DJ. Fetal origins of coronary heart disease. BMJ. 1995;311:171–4. doi: 10.1136/bmj.311.6998.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunson KL, Chen Y, Avishai-Eliner S, Baram TZ. Stress and the developing hippocampus: a double-edged sword? Mol Neurobiol. 2003;27:121–36. doi: 10.1385/MN:27:2:121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butcher RL, Collins WE, Fugo NW. Plasma concentration of LH, FSH, prolactin, progesterone and estradiol-17beta throughout the 4-day estrous cycle of the rat. Endocrinology. 1974;94:1704–8. doi: 10.1210/endo-94-6-1704. [DOI] [PubMed] [Google Scholar]
- Cameron JL. Interrelationships between hormones, behavior, and affect during adolescence: complex relationships exist between reproductive hormones, stress-related hormones, and the activity of neural systems that regulate behavioral affect. Comments on part III. Ann N Y Acad Sci. 2004;1021:134–42. doi: 10.1196/annals.1308.015. [DOI] [PubMed] [Google Scholar]
- Carey MP, Deterd CH, De Koning J, Helmerhorst F, De Kloet ER. The influence of ovarian steroids on hypothalamic-pituitary-adrenal regulation in the female rat. J Endocrinol. 1995;144:311–21. doi: 10.1677/joe.0.1440311. [DOI] [PubMed] [Google Scholar]
- Chesler EJ, Juraska JM. Acute administration of estrogen and progesterone impairs the acquisition of the spatial morris water maze in ovariectomized rats. Hormones and Behavior. 2000;38:234–42. doi: 10.1006/hbeh.2000.1626. [DOI] [PubMed] [Google Scholar]
- Council NR. Guide for the Care and Use of Laboratory Animals. 8. Washington, DC: The National Academies Press; 2011. [PubMed] [Google Scholar]
- Daniel JM, Fader AJ, Spencer AL, Dohanich GP. Estrogen Enhances Performance of Female Rats during Acquisition of a Radial Arm Maze. Hormones and Behavior. 1997;32:217–225. doi: 10.1006/hbeh.1997.1433. [DOI] [PubMed] [Google Scholar]
- De Boo HA, Harding JE. The developmental origins of adult disease (Barker) hypothesis. Australian and New Zealand Journal of Obstetrics and Gynaecology. 2006;46:4–14. doi: 10.1111/j.1479-828X.2006.00506.x. [DOI] [PubMed] [Google Scholar]
- De Quervain DJ, Roozendaal B, Mcgaugh JL. Stress and glucocorticoids impair retrieval of long-term spatial memory. Nature. 1998;394:787–90. doi: 10.1038/29542. [DOI] [PubMed] [Google Scholar]
- Diamond DM, Fleshner M, Ingersoll N, Rose GM. Psychological stress impairs spatial working memory: relevance to electrophysiological studies of hippocampal function. Behav Neurosci. 1996;110:661–72. doi: 10.1037//0735-7044.110.4.661. [DOI] [PubMed] [Google Scholar]
- Esquifino AI, Sanchis R, Guerri C. Effect of prenatal alcohol exposure on sexual maturation of female rat offspring. Neuroendocrinology. 1986;44:483–7. doi: 10.1159/000124690. [DOI] [PubMed] [Google Scholar]
- Frye CA. Estrus-associated decrements in a water maze task are limited to acquisition. Physiol Behav. 1995;57:5–14. doi: 10.1016/0031-9384(94)00197-d. [DOI] [PubMed] [Google Scholar]
- Frye CA, Duffy CK, Walf AA. Estrogens and progestins enhance spatial learning of intact and ovariectomized rats in the object placement task. Neurobiol Learn Mem. 2007;88:208–16. doi: 10.1016/j.nlm.2007.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuglestad AJ, Boys CJ, Chang P-N, Miller BS, Eckerle JK, Deling L, Fink BA, Hoecker HL, Hickey MK, Jimenez-Vega JM, Wozniak JR. Overweight and Obesity Among Children and Adolescents with Fetal Alcohol Spectrum Disorders. Alcoholism: Clinical and Experimental Research. 2014:n/a–n/a. doi: 10.1111/acer.12516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galea LA, Mcewen BS, Tanapat P, Deak T, Spencer RL, Dhabhar FS. Sex differences in dendritic atrophy of CA3 pyramidal neurons in response to chronic restraint stress. Neuroscience. 1997;81:689–97. doi: 10.1016/s0306-4522(97)00233-9. [DOI] [PubMed] [Google Scholar]
- Gallo PV, Weinberg J. Corticosterone rhythmicity in the rat: interactive effects of dietary restriction and schedule of feeding. J Nutr. 1981;111:208–18. doi: 10.1093/jn/111.2.208. [DOI] [PubMed] [Google Scholar]
- Gavin CE, Kates B, Gerken LA, Rodier PM. Patterns of growth deficiency in rats exposed in utero to undernutrition, ethanol, or the neuroteratogen methylazoxymethanol (MAM) Teratology. 1994;49:113–21. doi: 10.1002/tera.1420490207. [DOI] [PubMed] [Google Scholar]
- Gilliam DM, Kotch LE. Dose-related growth deficits in LS but not SS mice prenatally exposed to alcohol. Alcohol. 1996;13:47–51. doi: 10.1016/0741-8329(95)02010-1. [DOI] [PubMed] [Google Scholar]
- Glavas MM, Ellis L, Yu WK, Weinberg J. Effects of prenatal ethanol exposure on basal limbic-hypothalamic-pituitary-adrenal regulation: role of corticosterone. Alcoholism-Clinical and Experimental Research. 2007;31:1598–610. doi: 10.1111/j.1530-0277.2007.00460.x. [DOI] [PubMed] [Google Scholar]
- Goldman L, Winget C, Hollingshead GW, Levine S. Postweaning development of negative feedback in the pituitary-adrenal system of the rat. Neuroendocrinology. 1973;12:199–211. doi: 10.1159/000122169. [DOI] [PubMed] [Google Scholar]
- Guilloteau P, Zabielski R, Hammon HM, Metges CC. Adverse effects of nutritional programming during prenatal and early postnatal life, some aspects of regulation and potential prevention and treatments. J Physiol Pharmacol. 2009;60(Suppl 3):17–35. [PubMed] [Google Scholar]
- Hales CN, Barker DJ. The thrifty phenotype hypothesis. Br Med Bull. 2001;60:5–20. doi: 10.1093/bmb/60.1.5. [DOI] [PubMed] [Google Scholar]
- Haley DW, Handmaker NS, Lowe J. Infant Stress Reactivity and Prenatal Alcohol Exposure. Alcoholism: Clinical and Experimental Research. 2006;30:2055–2064. doi: 10.1111/j.1530-0277.2006.00251.x. [DOI] [PubMed] [Google Scholar]
- Halligan SL, Herbert J, Goodyer IM, Murray L. Exposure to postnatal depression predicts elevated cortisol in adolescent offspring. Biol Psychiatry. 2004;55:376–81. doi: 10.1016/j.biopsych.2003.09.013. [DOI] [PubMed] [Google Scholar]
- Handa RJ, Burgess LH, Kerr JE, O’keefe JA. Gonadal steroid hormone receptors and sex differences in the hypothalamo-pituitary-adrenal axis. Hormones and Behavior. 1994;28:464–76. doi: 10.1006/hbeh.1994.1044. [DOI] [PubMed] [Google Scholar]
- Handa RJ, Mcgivern RF, Noble ES, Gorski RA. Exposure to alcohol in utero alters the adult patterns of luteinizing hormone secretion in male and female rats. Life Sci. 1985;37:1683–90. doi: 10.1016/0024-3205(85)90295-4. [DOI] [PubMed] [Google Scholar]
- Hellemans KG, Sliwowska JH, Verma P, Weinberg J. Prenatal alcohol exposure: fetal programming and later life vulnerability to stress, depression and anxiety disorders. Neurosci Biobehav Rev. 2010a;34:791–807. doi: 10.1016/j.neubiorev.2009.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hellemans KG, Verma P, Yoon E, Yu WK, Young AH, Weinberg J. Prenatal alcohol exposure and chronic mild stress differentially alter depressive- and anxiety-like behaviors in male and female offspring. Alcohol Clin Exp Res. 2010b;34:633–45. doi: 10.1111/j.1530-0277.2009.01132.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henry J, Sloane M, Black-Pond C. Neurobiology and neurodevelopmental impact of childhood traumatic stress and prenatal alcohol exposure. Lang Speech Hear Serv Sch. 2007;38:99–108. doi: 10.1044/0161-1461(2007/010). [DOI] [PubMed] [Google Scholar]
- Jacobson SW, Bihun JT, Chiodo LM. Effects of prenatal alcohol and cocaine exposure on infant cortisol levels. Dev Psychopathol. 1999;11:195–208. doi: 10.1017/s0954579499002011. [DOI] [PubMed] [Google Scholar]
- Kajantie E, Feldt K, Raikkonen K, Phillips DI, Osmond C, Heinonen K, Pesonen AK, Andersson S, Barker DJ, Eriksson JG. Body size at birth predicts hypothalamic-pituitary-adrenal axis response to psychosocial stress at age 60 to 70 years. J Clin Endocrinol Metab. 2007;92:4094–100. doi: 10.1210/jc.2007-1539. [DOI] [PubMed] [Google Scholar]
- Karla SP, Karla PS. Temporal Interrelationships Among Circulating Levels of Estradiol, Progesterone and LH During the Rat Estrous Cycle: Effects of Exogenous Progesterone. Endocrinology. 1974;95:1711–1718. doi: 10.1210/endo-95-6-1711. [DOI] [PubMed] [Google Scholar]
- Kimura D, Hampson E. Cognitive Pattern in Men and Women Is Influenced by Fluctuations in Sex Hormones. Current Directions in Psychological Science. 1994;3:57–61. [Google Scholar]
- Lan N, Vogl AW, Weinberg J. Prenatal ethanol exposure delays the onset of spermatogenesis in the rat. Alcoholism-Clinical and Experimental Research. 2013;37:1074–81. doi: 10.1111/acer.12079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lan N, Yamashita F, Halpert AG, Sliwowska JH, Viau V, Weinberg J. Effects of prenatal ethanol exposure on hypothalamic-pituitary-adrenal function across the estrous cycle. Alcoholism-Clinical and Experimental Research. 2009;33:1075–88. doi: 10.1111/j.1530-0277.2009.00929.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S, Rivier C. Gender differences in the effect of prenatal alcohol exposure on the hypothalamic-pituitary-adrenal axis response to immune signals. Psychoneuroendocrinology. 1996;21:145–55. doi: 10.1016/0306-4530(95)00038-0. [DOI] [PubMed] [Google Scholar]
- Lee S, Schmidt D, Tilders F, Rivier C. Increased activity of the hypothalamic-pituitary-adrenal axis of rats exposed to alcohol in utero: role of altered pituitary and hypothalamic function. Mol Cell Neurosci. 2000;16:515–28. doi: 10.1006/mcne.2000.0890. [DOI] [PubMed] [Google Scholar]
- Lopez-Tejero D, Ferrer I, MLL, Herrera E. Effects of prenatal ethanol exposure on physical growth, sensory reflex maturation and brain development in the rat. Neuropathol Appl Neurobiol. 1986;12:251–60. doi: 10.1111/j.1365-2990.1986.tb00138.x. [DOI] [PubMed] [Google Scholar]
- Lupien SJ, King S, Meaney MJ, Mcewen BS. Child’s stress hormone levels correlate with mother’s socioeconomic status and depressive state. Biol Psychiatry. 2000;48:976–80. doi: 10.1016/s0006-3223(00)00965-3. [DOI] [PubMed] [Google Scholar]
- May PA, Gossage JP. Maternal risk factors for fetal alcohol spectrum disorders: not as simple as it might seem. Alcohol Res Health. 2011;34:15–26. [PMC free article] [PubMed] [Google Scholar]
- Mccormick CM, Mathews IZ. HPA function in adolescence: role of sex hormones in its regulation and the enduring consequences of exposure to stressors. Pharmacol Biochem Behav. 2007;86:220–33. doi: 10.1016/j.pbb.2006.07.012. [DOI] [PubMed] [Google Scholar]
- Mccormick CM, Mathews IZ, Thomas C, Waters P. Investigations of HPA function and the enduring consequences of stressors in adolescence in animal models. Brain Cogn. 2010;72:73–85. doi: 10.1016/j.bandc.2009.06.003. [DOI] [PubMed] [Google Scholar]
- Mccormick CM, Smith C, Mathews IZ. Effects of chronic social stress in adolescence on anxiety and neuroendocrine response to mild stress in male and female rats. Behav Brain Res. 2008;187:228–38. doi: 10.1016/j.bbr.2007.09.005. [DOI] [PubMed] [Google Scholar]
- Mcgivern RF, Yellon SM. Delayed onset of puberty and subtle alterations in GnRH neuronal morphology in female rats exposed prenatally to ethanol. Alcohol. 1992;9:335–40. doi: 10.1016/0741-8329(92)90077-n. [DOI] [PubMed] [Google Scholar]
- Mclean AC, Valenzuela N, Fai S, Bennett SaL. Performing Vaginal Lavage, Crystal Violet Staining, and Vaginal Cytological Evaluation for Mouse Estrous Cycle Staging Identification. Journal of Visualized Experiments. 2012:67. doi: 10.3791/4389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muir JL, Pfister HP. Corticosterone and prolactin responses to predictable and unpredictable novelty stress in rats. Physiol Behav. 1986;37:285–8. doi: 10.1016/0031-9384(86)90234-9. [DOI] [PubMed] [Google Scholar]
- Muir JL, Pfister HP. Time course of the corticosterone and prolactin response following predictable and unpredictable novelty stress in Rattus norvegicus. Physiol Behav. 1987;40:103–7. doi: 10.1016/0031-9384(87)90191-0. [DOI] [PubMed] [Google Scholar]
- Ramsay DS, Bendersky MI, Lewis M. Effect of prenatal alcohol and cigarette exposure on two- and six-month-old infants’ adrenocortical reactivity to stress. J Pediatr Psychol. 1996;21:833–40. doi: 10.1093/jpepsy/21.6.833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rivier C, Imaki T, Vale W. Prolonged exposure to alcohol: effect on CRF mRNA levels, and CRF- and stress-induced ACTH secretion in the rat. Brain Res. 1990;520:1–5. doi: 10.1016/0006-8993(90)91685-a. [DOI] [PubMed] [Google Scholar]
- Shansky RM, Glavis-Bloom C, Lerman D, Mcrae P, Benson C, Miller K, Cosand L, Horvath TL, Arnsten AFT. Estrogen mediates sex differences in stress-induced prefrontal cortex dysfunction. Mol Psychiatry. 2003;9:531–538. doi: 10.1038/sj.mp.4001435. [DOI] [PubMed] [Google Scholar]
- Sigh SP, Snyder AK. Ethanol ingestion during pregnancy: effects on pregnant rats and their offspring. J Nutr. 1982;112:98–103. doi: 10.1093/jn/112.1.98. [DOI] [PubMed] [Google Scholar]
- Sliwowska JH, Lan N, Yamashita F, Halpert AG, Viau V, Weinberg J. Effects of prenatal ethanol exposure on regulation of basal hypothalamic-pituitary-adrenal activity and hippocampal 5-HT1A receptor mRNA levels in female rats across the estrous cycle. Psychoneuroendocrinology. 2008;33:1111–23. doi: 10.1016/j.psyneuen.2008.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sokol RJ, Delaney-Black V, Nordstrom B. Fetal alcohol spectrum disorder. JAMA. 2003;290:2996–9. doi: 10.1001/jama.290.22.2996. [DOI] [PubMed] [Google Scholar]
- Spornitz UM, Socin CD, Dravid AA. Estrous stage determination in rats by means of scanning electron microscopic images of uterine surface epithelium. Anatomical Record. 1999;254:116–26. doi: 10.1002/(SICI)1097-0185(19990101)254:1<116::AID-AR15>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- Sterlemann V, Ganea K, Liebl C, Harbich D, Alam S, Holsboer F, Muller MB, Schmidt MV. Long-term behavioral and neuroendocrine alterations following chronic social stress in mice: implications for stress-related disorders. Hormones and Behavior. 2008;53:386–94. doi: 10.1016/j.yhbeh.2007.11.001. [DOI] [PubMed] [Google Scholar]
- Streissguth AP, O’malley K. Neuropsychiatric implications and long-term consequences of fetal alcohol spectrum disorders. Semin Clin Neuropsychiatry. 2000;5:177–90. doi: 10.1053/scnp.2000.6729. [DOI] [PubMed] [Google Scholar]
- Taylor AN, Branch BJ, Cooley-Matthews B, Poland RE. Effects of maternal ethanol consumption in rats on basal and rhythmic pituitary-adrenal function in neonatal offspring. Psychoneuroendocrinology. 1982a;7:49–58. doi: 10.1016/0306-4530(82)90054-3. [DOI] [PubMed] [Google Scholar]
- Taylor AN, Branch BJ, Liu SH, Kokka N. Long-term effects of fetal ethanol exposure on pituitary-adrenal response to stress. Pharmacol Biochem Behav. 1982b;16:585–9. doi: 10.1016/0091-3057(82)90420-8. [DOI] [PubMed] [Google Scholar]
- Uban KA, Comeau WL, Ellis LA, Galea LA, Weinberg J. Basal regulation of HPA and dopamine systems is altered differentially in males and females by prenatal alcohol exposure and chronic variable stress. Psychoneuroendocrinology. 2013;38:1953–66. doi: 10.1016/j.psyneuen.2013.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uban KA, Sliwowska JH, Lieblich S, Ellis LA, Yu WK, Weinberg J, Galea LA. Prenatal alcohol exposure reduces the proportion of newly produced neurons and glia in the dentate gyrus of the hippocampus in female rats. Hormones and Behavior. 2010;58:835–43. doi: 10.1016/j.yhbeh.2010.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ventura MA, Gardey C, D’athis P. Rapid reset of the corticosterone rhythm by food presentation in rats under acircadian restricted feeding schedule. Chronobiol Int. 1984;1:287–95. doi: 10.3109/07420528409063909. [DOI] [PubMed] [Google Scholar]
- Viau V, Meaney MJ. Variations in the hypothalamic-pituitary-adrenal response to stress during the estrous cycle in the rat. Endocrinology. 1991;129:2503–11. doi: 10.1210/endo-129-5-2503. [DOI] [PubMed] [Google Scholar]
- Weinberg J. Nutritional issues in perinatal alcohol exposure. Neurobehav Toxicol Teratol. 1984;6:261–9. [PubMed] [Google Scholar]
- Weinberg J. Hyperresponsiveness to stress: differential effects of prenatal ethanol on males and females. Alcohol Clin Exp Res. 1988;12:647–52. doi: 10.1111/j.1530-0277.1988.tb00258.x. [DOI] [PubMed] [Google Scholar]
- Weinberg J. Prenatal ethanol exposure alters adrenocortical response to predictable and unpredictable stressors. Alcohol. 1992;9:427–32. doi: 10.1016/0741-8329(92)90043-a. [DOI] [PubMed] [Google Scholar]
- Weinberg J, Gallo PV. Prenatal ethanol exposure: pituitary-adrenal activity in pregnant dams and offspring. Neurobehav Toxicol Teratol. 1982;4:515–20. [PubMed] [Google Scholar]
- Weinberg J, Kim CK, Yu W. Early handling can attenuate adverse effects of fetal ethanol exposure. Alcohol. 1995;12:317–27. doi: 10.1016/0741-8329(95)00005-c. [DOI] [PubMed] [Google Scholar]
- Weinberg J, Sliwowska JH, Lan N, Hellemans KG. Prenatal alcohol exposure: foetal programming, the hypothalamic-pituitary-adrenal axis and sex differences in outcome. Journal of Neuroendocrinology. 2008a;20:470–88. doi: 10.1111/j.1365-2826.2008.01669.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinberg J, Sliwowska JH, Lan N, Hellemans KGC. Prenatal alcohol exposure: Foetal programming, the hypothalamic-pituitary-adrenal axis and sex differences in outcome. Journal of Neuroendocrinology. 2008b;20:470–488. doi: 10.1111/j.1365-2826.2008.01669.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiser MJ, Handa RJ. Estrogen impairs glucocorticoid dependent negative feedback on the hypothalamic–pituitary–adrenal axis via estrogen receptor alpha within the hypothalamus. Neuroscience. 2009;159:883–895. doi: 10.1016/j.neuroscience.2008.12.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright LD, Hebert KE, Perrot-Sinal TS. Periadolescent stress exposure exerts long-term effects on adult stress responding and expression of prefrontal dopamine receptors in male and female rats. Psychoneuroendocrinology. 2008;33:130–42. doi: 10.1016/j.psyneuen.2007.10.009. [DOI] [PubMed] [Google Scholar]