Abstract
The relationship between comorbidity and ovarian cancer survival has been controversial so far. Therefore, we conducted a meta-analysis to summarize the existing evidence from prospective studies on this issue. Relevant studies were identified by searching the PubMed, EMBASE, and ISI Web of Science databases through the end of January 2015. Two authors independently performed the eligibility evaluation and data abstraction. Random-effects models were used to estimate summary hazard ratios (HRs) and 95% confidence intervals (CIs) for overall survival. Eight prospective studies involving 12,681 ovarian cancer cases were included in the present study. The summarized HR for presence versus absence of comorbidity was 1.20 (95% CI = 1.11–1.30, n = 8), with moderate heterogeneity (I2 = 31.2%, P = 0.179). In addition, the summarized HR for the highest compared with the lowest category of the Charlson’s comorbidity index was 1.68 (95% CI = 1.50–1.87, n = 2), without heterogeneity (I2 = 0%, P = 0.476). Notably, a significant negative impact of comorbidity on ovarian cancer survival was observed in most subgroup analyses stratified by the study characteristics and whether there was adjustment for potential confounders. In conclusion, the findings of this meta-analysis suggest that underlying comorbidity is consistently associated with decreased survival in patients with ovarian cancer. Comorbidity should be taken into account when managing these patients.
Epithelial ovarian cancer (EOC) is the third most common malignancy worldwide among gynecologic cancers, with almost 0.23 million new cases diagnosed in 20121. Since effective screening programs are still lacking, patients with EOC are always diagnosed at advanced stages. Consequently, this cancer caused more than 0.15 million deaths worldwide in 20121. Notably, EOC is the most lethal gynecological malignancy in developed countries1, with a 5-year survival rate of 44% based on data from the Surveillance, Epidemiology, and End Results (SEER) program registries2. During the past decade, the outcomes of many EOC patients have improved with primary treatments (e.g., surgery and some forms of chemotherapy), but long-term survival of these patients has still not been satisfactory3. Given this, there is an urgent need for identifying modifiable prognostic factors that may improve more targeted therapeutic regimens for this disease.
Age, tumor stage and grade, and residual tumor are well-established prognostic factors for the survival of patients with EOC4. Meanwhile, previous cohort studies have provided conflicting evidence indicating comorbidity, which is defined as the presence of one or more diseases in addition to the primary disease, as a prognostic factor for the survival of EOC patients5. Some studies6,7,8,9,10 have reported an increased risk of mortality with comorbidity, while others11,12,13 have found no association. Additionally, to our knowledge, the aforementioned studies have not been systematically reviewed. Therefore, to summarize results and clarify the association between comorbidity and the survival of EOC patients, we conducted the present meta-analysis based on published prospective studies.
Results
Search results and study characteristics
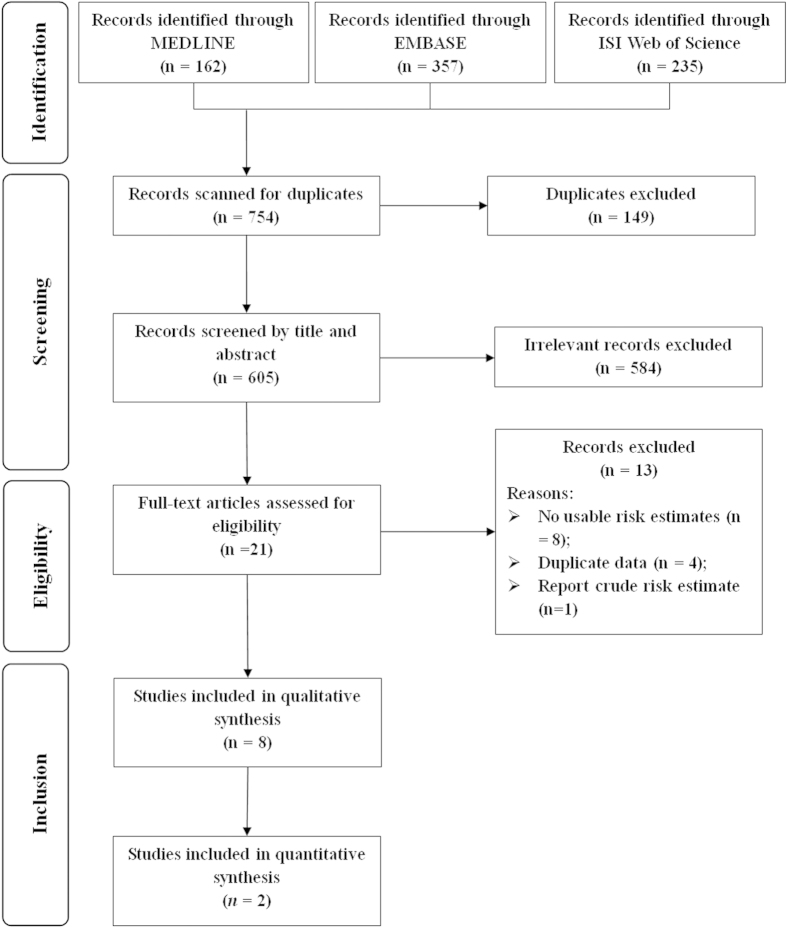
Our systematic literature search in three databases identified 754 articles for eligibility. After initial screening, 149 were excluded as duplicates, 584 by title and abstract scan, and 21 by full-text assessment. Subsequently, thirteen articles were excluded after further evaluation of the full text and for following reasons. Four articles14,15,16,17 were replaced by updated or more informative studies, and eight articles18,19,20,21,22,23,24,25 did not present usable results, while one article26 reported the crude risk estimate without adjustment for any potential confounders or established prognostic factors. In the end, a total of 8 articles6,7,8,9,10,11,12,13 met our inclusion criteria for the final meta-analysis (Fig. 1).
Figure 1. Flow-chart of study selection.
Table 1 provides characteristics of the included studies which were published between 1997 and 2014. Eight studies comprising 12,681 patients with EOC reported an association between comorbidity and overall survival of ovarian cancer. Three studies were carried out in the United States6,10,12, two in Denmark7,8, and one study each in Australia13, Germany9, and the Netherlands11. Sample size of the included studies ranged from 137 to 5213. All included studies adjusted for age and tumor stage. Half of the included studies adjusted for tumor grade (n = 4) and tumor histology (n = 4). However, fewer included studies adjusted for cancer treatment (n = 2), ascites (n = 2), and residual tumor (n = 2).
Table 1. Characteristics of studies of comorbidity and ovarian cancer survival.
| First author, (reference), year, country | Age (Year) | No. of Cases | Study period | CCI assessment | Survival rate (%) | Adjusted factors |
||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Age | Stage | Grade | Histology | Treatment | Ascites | Residual tumor | ||||||
| Anuradha et al.13, 2014, Australia | ≥18 | 1192 | 2005–2012 | √ | 7-year (31%) | √ | √ | √ | √ | — | √ | — |
| Erickson et al.6, 2014, USA | N/A | 367 | 2004–2009 | √ | N/A | √ | √ | √ | — | — | — | √ |
| Sperling et al.7, 2013, Denmark | All | 3129 | 2005–2011 | √ | Overall (46.5%) | √ | √ | — | √ | — | — | √ |
| Tetsche et al.8, 2008, Denmark | >15 | 5213 | 1995–2003 | √ | Overall (32.4%) | √ | √ | — | — | — | — | — |
| Du bois et al.9, 2005, Germany | All | 476 | 2001–2003 | — | Overall (69.1%) | √ | √ | √ | √ | — | √ | — |
| Mass et al.11, 2005, Netherlands | All | 1116 | 1995–2004 | √ | Overall (42%) | √ | √ | — | — | √ | — | — |
| O’Malley et al.10, 2003, USA | All | 1051 | 1994–2001 | √ | Overall (36.1%) | √ | √ | √ | √ | √ | — | — |
| DiSilvestro et al.12, 1997, USA | All | 137 | 1987–1992 | √ | 4-year (51%) | √ | √ | — | — | — | — | — |
CCI, Charlson’s comorbidity index; N/A, not available.
Comorbidity and EOC survival
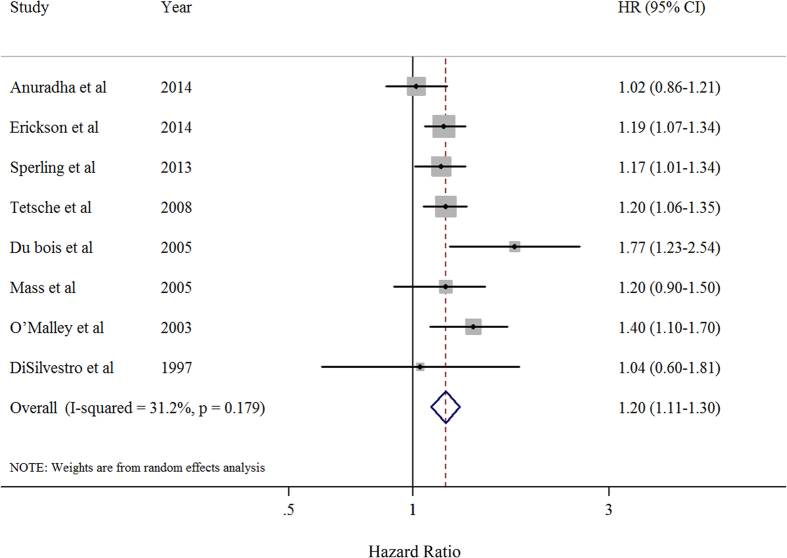
Figure 2 shows the study-specific and summarized hazard ratios (HRs) and 95% confidence intervals (CIs) of EOC survival for the presence versus absence categories of comorbidity. Overall, the summarized analysis showed an HR of 1.20 (95% CI = 1.11–1.30) with moderate heterogeneity (P = 0.179, I2 = 31.2%). There was no evidence of publication bias, both quantitatively (P = 0.448 for Egger’s test and P = 0.902 for Begg’s test) and qualitatively, on visual inspection of the funnel plot (Supplementary Figure S1).
Figure 2. Forest plot (random-effects model) of hazard ratios and 95% confidence intervals of individual studies and pooled estimate for death in patients with ovarian cancer who had comorbidity compared with those without comorbidities.
Squares indicate study-specific hazard ratios (size of the square reflects the study-specific statistical weight); horizontal lines indicate 95% CIs; diamond indicates the summary hazard ratio estimate with its 95% CI. HR: hazard ratio.
In addition, two studies were available for the analysis of the highest versus the lowest categories of CCI8,13. The results showed poorer survival among the highest CCI group compared with the lowest CCI women with EOC (HR = 1.68, 95% CI = 1.50–1.87), without heterogeneity (P = 0.476, I2 = 0%).
Subgroup and sensitivity analysis
To explore the heterogeneity between studies of comorbidity and EOC survival, we performed the stratified and sensitivity analyses. The results of stratified analyses for the association between comorbidity and EOC overall survival are given in Table 2. When the analysis was stratified according to geographic location, the summarized HRs of studies from North America, Europe, and Australia were 1.23 (95% CI = 1.11–1.36), 1.23 (95% CI = 1.10–1.38), and 1.02 (95% CI = 0.86–1.21), respectively. Additionally, similar significant results were observed in the stratified analysis by study period and adjustment for potential confounders (Table 2).
Table 2. Summary risk estimates of the association between comorbidity and ovarian cancer survival.
| No. of studies | Summary HR (95% CIs) | I2 value (%) | Ph* | |
|---|---|---|---|---|
| Overall | 8 | 1.20 (1.11–1.30) | 31.2 | 0.179 |
| Subgroup analyses | ||||
| Geographic location | ||||
| North America | 3 | 1.23 (1.11–1.36) | 1.9 | 0.361 |
| Europe | 4 | 1.23 (1.10–1.38) | 32.7 | 0.216 |
| Australia | 1 | 1.02 (0.86–1.21) | N/A | N/A |
| Study period (years) | ||||
| ≥7 | 4 | 1.19 (1.05–1.34) | 43.0 | 0.154 |
| <7 | 4 | 1.23 (1.08–1.39) | 37.6 | 0.186 |
| Adjustment for potential confounders or prognostic factors | ||||
| Age at diagnosis | ||||
| Yes | 8 | 1.20 (1.11–1.30) | 31.2 | 0.179 |
| No | 0 | N/A | N/A | N/A |
| Tumor stage | ||||
| Yes | 8 | 1.20 (1.11–1.30) | 31.2 | 0.179 |
| No | 0 | N/A | N/A | N/A |
| Tumor grade | ||||
| Yes | 4 | 1.26 (1.05–1.50) | 69.5 | 0.020 |
| No | 4 | 1.19 (1.09–1.29) | 0 | 0.961 |
| Tumor histology | ||||
| Yes | 4 | 1.25 (1.04–1.51) | 69.8 | 0.019 |
| No | 4 | 1.19 (1.10–1.29) | 0 | 0.969 |
| Cancer treatment | ||||
| Yes | 2 | 1.31 (1.12–1.55) | 0 | 0.368 |
| No | 6 | 1.18 (1.08–1.29) | 36.7 | 0.162 |
| Residual tumor | ||||
| Yes | 2 | 1.18 (1.08–1.19) | 0 | 0.854 |
| No | 6 | 1.23 (1.08–1.40) | 50.4 | 0.073 |
| Three aforementioned factors | ||||
| Yes | 6 | 1.21 (1.09–1.35) | 49.7 | 0.077 |
| No | 2 | 1.19 (1.06–1.34) | 0 | 0.620 |
| Four aforementioned factors | ||||
| Yes | 5 | 1.22 (1.08–1.38) | 59.7 | 0.042 |
| No | 3 | 1.19 (1.07–1.33) | 0 | 0.883 |
CI: confidence interval; HR: hazard ratio; N/A: not available.
*P value for heterogeneity within each subgroup.
A sensitivity analysis omitting one study at a time and calculating the summarized HRs for the remainder of the studies showed that the 8 study-specific HRs ranged from a low of 1.18 (95% CI = 1.09–1.27; P = 0.244; I2 = 24.3%) after omitting the study by O’Malley et al.10 to a high of 1.22 (95% CI = 1.14–1.31; P = 0.368; I2 = 7.9%) after omitting the study by Anuradha et al.13.
Discussion
To our knowledge, this is first meta-analysis that addressed the role of comorbidity in predicting survival of EOC. The findings of the present study showed that EOC patients with comorbidity had a worse survival than patients without comorbidity. Additionally, survival in patients with EOC may decrease with higher CCI scores. Clinicians are urged to evaluate and reduce any underlying comorbidity carefully in the management of patients with EOC.
It has been proposed that comorbidity in cancer patients may influence the choice of primary treatment (e.g., surgery and chemotherapy) as well as the tumor stage at diagnosis, which in turn affects cancer prognosis and survival8,21. Compared to EOC patients with comorbidity, patients without comorbidity were more likely to receive standard treatment (primary surgery in combination with chemotherapy)11. Additionally, several studies indicated that patients with comorbidity might have lower tolerance to the adjuvant chemotherapy and that there was a possible interaction between the drugs used to treat comorbid diseases and chemotherapy regimens21,27. Moreover, Tetsche et al.8 found that the presence of severe comorbidity was associated with an advanced stage of EOC and proposed that patients with comorbidities could experience delay in diagnosis, resulting in a more advanced cancer stage8. However, only one study8 carried out a stratified analysis according to FIGO stage, and therefore, whether the impact of comorbidity on ovarian cancer survival varies by tumor stage or grade needs further investigation.
This is the first meta-analysis evaluating the association between comorbidity and survival following EOC diagnosis. By combining the evidence from these published prospective studies, we could detect weaker associations than in the individual studies because of the increased statistical power. Additionally, a number of subgroup and sensitivity analyses were carried out to explore the source of heterogeneity. Of note, the results were robust in the aforementioned analyses (Table 2). However, the impact of comorbidity on survival of EOC patients should be quantified by identifying the proportion of these patients in which comorbid disease was the primary cause of death28, considering the difficulty in determining causality in this volatile clinical setting, we evaluated the association between comorbidity and EOC survival by comparing survival in patients with and without comorbidity28. Thus, several limitations of this study should be acknowledged for the interpretation of our findings.
First, a meta-analysis cannot control for confounders for which there was no adjustment in the primary analysis of individual studies. We excluded the study of Elit et al.26 because they reported the crude risk estimates without adjustment for any potential confounders. In contrast, all included studies had been adjusted for at least two potential confounders in their primary analyses. Furthermore, we cannot exclude the possibility of unmeasured or residual confounding as a potential explanation for the observed associations. Several studies demonstrated that severe comorbidity was associated with older age, higher tumor stage, performance status, and cancer treatment7,8. Although we found that the associations were robust in analyses stratified by adjustment for these aforementioned confounding factors (Table 2), less than half the studies adjusted for tumor grade and histology and only two studies adjusted for cancer treatment or residual tumor. Therefore, further studies stratified by established or additional prognostic factors are warranted to better rule out potential effects of residual confounding.
Second, the use of administrative databases, instead of medical records, has been criticized for lacking the accuracy required for research7. According to the prevalence of the comorbid illness and characteristic of the administrative databases, studies may translate different comorbidity into CCI. For example, because of the treatment progress of ulcer disease and low prevalence of liver disease in the Danish populations7, these two diseases were not registered in their database. On the other hand, CCI has been shown to have a high specificity but relatively low sensitivity29. Applying the CCI to administrative databases could introduce misclassifications of comorbidity. Since comorbidity is often underreported and assumed to be homogenous across outcome groups7, this misclassification could be non-differential and bias the association between comorbidity and EOC survival toward the null30, which implies that our results are likely to be conservative estimates of the true underlying association.
Third, although this meta-analysis summarized the results for the highest versus the lowest categories of CCI, the cogency of the evidence is limited by this comparison being available from only two studies. Notably, it would be important to identify the individual comorbid diseases that have the greatest impact on the survival of EOC patients. A recent study using data from the National Cancer Register of Sweden suggested that thromboembolism, hematologic complications and infections have a pronounced effect on survival in women with EOC19. Hence, future studies should provide more detail of the association between specific comorbid diseases and survival of EOC, as well as measuring and analyzing the comorbidity as a continuous approach.
Finally, we pre-defined the outcome as overall survival, which was evaluated in the analyses, as opposed to EOC-specific survival. However, EOC patients generally died from their cancer disease, and thus, overall survival was a good surrogate for cancer-specific survival. Besides, although we employed three large databases and checked the references of included studies, a limited number of studies were included in this meta-analysis, and thus, some of the stratified analyses were difficult to conduct, possibly being less reliable, which restricted the interpretation of these findings.
In conclusion, the results from this meta-analysis, based on prospective studies, suggest that comorbidity has a negative impact on EOC survival. The status and severity of comorbid diseases should be taken into consideration by clinicians when making decisions on EOC management. Further studies need to figure out which individual comorbid diseases may have the greatest impact on survival of EOC patients.
Materials and Methods
Search strategy
We followed the guidelines developed by the Meta-analysis Of Observational Studies in Epidemiology group (MOOSE) in this meta-analysis31. Two independent investigators systematically searched the MEDLINE (PubMed; http://www.ncbi.nlm.nih.gov/pubmed), EMBASE, and ISI Web of Knowledge databases for eligible prospective studies through the end of January, 2015 without limitations. The following terms were used in the search procedure: (comorbidity) and (ovary or ovarian) and (cancer or neoplasm or tumor or carcinoma) and (survival or mortality or prognosis or recurrence). In addition, the reference lists of retrieved articles were carefully hand-searched for additional publications.
Study selection criteria
Eligibility of studies for inclusion was assessed independently by two investigators. Studies were eligible for inclusion if all the following criteria were fulfilled: (1) the study used a prospective study design; (2) the exposures were defined as comorbidity or Charlson’s comorbidity index (CCI) (Supplementary Table S1); (3) the outcome was defined as overall survival among women with EOC; and (4) there were estimates of the relative risks (RRs) or HRs with 95% Cis, or data was provided for their calculation. If multiple articles were based on the same study population, the most recent report or the report with the most applicable estimates was selected for our analysis. The study reported by Elit et al.26 was excluded because they provided risk estimates without adjustment for any potential confounders.
Data extraction
From each study, the following information was extracted in a standardized manner by the two independent investigators: first author’s last name, publication year, geographic location(s) and age of the patients studied, study sample size, study period, whether using CCI to assess the exposure, survival rate, and factors for which adjustment was made in the primary analysis. From each study, we extracted the risk estimates that reflected the greatest degree of control for potential confounders. Differences in data extraction between investigators were uncommon and were resolved by consensus. Similar to our previous study32,33,34,35, for studies8,13 that did not report the results for the presence versus absence category of comorbidity, we used the effective-count method proposed by Hamling et al.36 to recalculate HRs.
Statistical analysis
In this meta-analysis, we used the random-effects model37 to calculate summary HRs and 95% CIs for the presence versus absence of comorbidity and the highest versus lowest categories of CCI. Statistical heterogeneity between studies was assessed using I2-statistics38. Small study effects, such as publication bias, were evaluated via funnel plots and Egger’s39 and Begg’s40 tests, where potential small-study bias was considered when P < 0.10. Pre-specified sensitivity analysis was performed by deleting each study in turn to determine the influence of each individual data set on the overall estimate. We also conducted pre-planned stratified analyses according to potentially relevant factors to investigate possible sources of heterogeneity between studies. For all tests, a probability level <0.05 was considered statistically significant. All statistical analyses were conducted by using Stata version 12 software (StataCorp, College Station, TX).
Additional Information
How to cite this article: Jiao, Y.-S. et al. Comorbidity and survival among women with ovarian cancer: evidence from prospective studies. Sci. Rep. 5, 11720; doi: 10.1038/srep11720 (2015).
Supplementary Material
Acknowledgments
This work was supported by The National Natural Science Foundation of China (81272873 for Yi-Sheng Jiao); the Younger research fund of Shengjing Hospital (Grant 2014sj09 for Qi-Jun Wu). Qi-Jun Wu was supported by the Fogarty International Clinical Research Scholars and Fellows Support Center at the Vanderbilt Institute for Global Health, funded by the Fogarty International Center, NIH, through an R24 Training Grant (D43 TW008313 to Xiao-Ou Shu).
Footnotes
Author Contributions Y.-S.J. and Q.-J.W. designed research; Y.-S.J., T.-T.G., and Q.-J.W. conducted research; Y.-S.J., T.-T.G. and Q.-J.W. analyzed data; Y.-S.J., T.-T.G., Y.-L.W. and Q.-J.W. wrote the draft; All authors read, reviewed and approved the final manuscript. Q.-J.W. had primary responsibility for final content.
References
- Ferlay J. et al. GLOBOCAN 2012 v1.0, Cancer Incidence and Mortality Worldwide: IARC Cancer Base No. 11 [Internet]. Lyon, France: International Agency for Research on Cancer; 2013. Available from: http://globocan.iarc.fr. (Date of access: 15/February/2015).
- DeSantis C. E. et al. Cancer treatment and survivorship statistics, 2014. CA Cancer J Clin 64, 252–71 (2014). [DOI] [PubMed] [Google Scholar]
- Agarwal R. & Kaye S. B. Prognostic factors in ovarian cancer: how close are we to a complete picture? Ann Oncol 16, 4–6 (2005). [DOI] [PubMed] [Google Scholar]
- Jayson G. C., Kohn E. C., Kitchener H. C. & Ledermann J. A. Ovarian cancer. Lancet 384, 1376–88 (2014). [DOI] [PubMed] [Google Scholar]
- Valderas J. M. et al. Defining comorbidity: implications for understanding health and health services. Ann Fam Med 7, 357–63 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erickson B. et al. The Charlson Comorbidity Index predicts survival in women with epithelial ovarian cancer independent of surgical debulking status. Gynecol Oncol 134, 428–9 (2014). [Google Scholar]
- Sperling C. et al. Comorbidity is an independent prognostic factor for the survival of ovarian cancer: a Danish register-based cohort study from a clinical database. Gynecol Oncol 129, 97–102 (2013). [DOI] [PubMed] [Google Scholar]
- Tetsche M. S. et al. The impact of comorbidity and stage on ovarian cancer mortality: a nationwide Danish cohort study. Bmc Cancer 8, 31 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du Bois A., Rochon J., Lamparter C. & Pfisterer J. Pattern of care and impact of participation in clinical studies on the outcome in ovarian cancer. Int J Gynecol Cancer 15, 183–91 (2005). [DOI] [PubMed] [Google Scholar]
- O’Malley C. D., Cress R. D., Campleman S. L. & Leiserowitz G. S. Survival of Californian women with epithelial ovarian cancer, 1994-1996: a population-based study. Gynecol Oncol 91, 608–15 (2003). [DOI] [PubMed] [Google Scholar]
- Maas H. A. et al. The influence of age and co-morbidity on treatment and prognosis of ovarian cancer: a population-based study. Gynecol Oncol 97, 104–9 (2005). [DOI] [PubMed] [Google Scholar]
- DiSilvestro P., Peipert J. F., Hogan J. W. & Granai C. O. Prognostic value of clinical variables in ovarian cancer. J Clin Epidemiol 50, 501–5 (1997). [DOI] [PubMed] [Google Scholar]
- Anuradha S. et al. Survival of Australian women with invasive epithelial ovarian cancer: a population-based study. Med J Aust 201, 283–8 (2014). [DOI] [PubMed] [Google Scholar]
- Jorgensen T. L., Hallas J., Friis S. & Herrstedt J. Comorbidity in elderly cancer patients in relation to overall and cancer-specific mortality. Br J Cancer 106, 1353–60 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fago-Olsen C. L. et al. Centralized treatment of advanced stages of ovarian cancer improves survival: a nationwide Danish survey. Acta Obstet Gynecol Scand 90, 273–9 (2011). [DOI] [PubMed] [Google Scholar]
- Elit L. M. et al. Surgical outcomes in women with ovarian cancer. Can J Surg 51, 346–54 (2008). [PMC free article] [PubMed] [Google Scholar]
- Tetsche M. S. et al. Comorbidity and ovarian cancer survival in Denmark, 1995-2005: a population-based cohort study. Int J Gynecol Cancer 18, 421–7 (2008). [DOI] [PubMed] [Google Scholar]
- Jorgensen T. L. et al. Significance of age and comorbidity on treatment modality, treatment adherence, and prognosis in elderly ovarian cancer patients. Gynecol Oncol 127, 367–74 (2012). [DOI] [PubMed] [Google Scholar]
- Stalberg K., Svensson T., Lonn S. & Kieler H. The influence of comorbidity on mortality in ovarian cancer patients. Gynecol Oncol 133, 298–303 (2014). [DOI] [PubMed] [Google Scholar]
- Grann A. F. et al. Comorbidity and survival of Danish ovarian cancer patients from 2000-2011: a population-based cohort study. Clin Epidemiol 5, 57–63 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janssen-Heijnen M. L. et al. Prognostic impact of increasing age and co-morbidity in cancer patients: a population-based approach. Crit Rev Oncol Hematol 55, 231–40 (2005). [DOI] [PubMed] [Google Scholar]
- Tingulstad S., Skjeldestad F. E., Halvorsen T. B. & Hagen B. Survival and prognostic factors in patients with ovarian cancer. Obstet Gynecol 101, 885–91 (2003). [DOI] [PubMed] [Google Scholar]
- Robinson K. M., Christensen K. B., Ottesen B. & Krasnik A. Socio-demographic factors, comorbidity and diagnostic delay among women diagnosed with cervical, endometrial or ovarian cancer. Eur J Cancer Care 20, 653–61 (2011). [DOI] [PubMed] [Google Scholar]
- Rannestad T. & Skjeldestad F. E. Co-morbidity and pain sites in long-term gynecological cancer survivors and women in the general population. Gynecol Oncol 127, 168–71 (2012). [DOI] [PubMed] [Google Scholar]
- Baron E. et al. Comorbidity and prognosis in serous and papillary serous ovarian cancer. Gynecol Oncol 112, S116–7 (2009). [Google Scholar]
- Elit L., Bondy S. J., Chen Z. & Paszat L. A tale of two time periods: ovarian cancer trends in Ontario. Curr Oncol 14, 57–60 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monfardini S. Prescribing anti-cancer drugs in elderly cancer patients. Eur J Cancer 38, 2341–6 (2002). [DOI] [PubMed] [Google Scholar]
- Leontiadis G. I., Molloy-Bland M., Moayyedi P. & Howden C. W. Effect of comorbidity on mortality in patients with peptic ulcer bleeding: systematic review and meta-analysis. Am J Gastroenterol 108, 331-45, 346 (2013). [DOI] [PubMed] [Google Scholar]
- de Groot V., Beckerman H., Lankhorst G. J. & Bouter L. M. How to measure comorbidity. a critical review of available methods. J Clin Epidemiol 56, 221–9 (2003). [DOI] [PubMed] [Google Scholar]
- Rothman K. J., Greenland S. & Lash T. Validity in epidemiologic studies. In: Modern Epidemiology. 3rd edn, (eds Rothman K. J. et al. ) 128–147. (Lippincott Williams & Wilkins 2008). [Google Scholar]
- Stroup D. F. et al. Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis Of Observational Studies in Epidemiology (MOOSE) group. JAMA 283, 2008–12 (2000). [DOI] [PubMed] [Google Scholar]
- Luan N. N. et al. Breastfeeding and ovarian cancer risk: a meta-analysis of epidemiologic studies. Am J Clin Nutr 98, 1020–31 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gong T. T. et al. Age at menarche and risk of ovarian cancer: a meta-analysis of epidemiological studies. Int J Cancer 132, 2894–900 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guan H. B., Wu Q. J. & Gong T. T. Parity and kidney cancer risk: evidence from epidemiologic studies. Cancer Epidemiol Biomarkers Prev 22, 2345–53 (2013). [DOI] [PubMed] [Google Scholar]
- Luan N. N. et al. Nonlinear reduction in risk for colorectal cancer by oral contraceptive use: a meta-analysis of epidemiological studies. Cancer Causes Control 26, 65–78 (2015). [DOI] [PubMed] [Google Scholar]
- Hamling J., Lee P., Weitkunat R. & Ambuhl M. Facilitating meta-analyses by deriving relative effect and precision estimates for alternative comparisons from a set of estimates presented by exposure level or disease category. Stat Med 27, 954–70 (2008). [DOI] [PubMed] [Google Scholar]
- DerSimonian R. & Laird N. Meta-analysis in clinical trials. Control Clin Trials 7, 177–88 (1986). [DOI] [PubMed] [Google Scholar]
- Higgins J. P. & Thompson S. G. Quantifying heterogeneity in a meta-analysis. Stat Med 21, 1539–58 (2002). [DOI] [PubMed] [Google Scholar]
- Egger M., Davey S. G., Schneider M. & Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ 315, 629–34 (1997). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Begg C. B. & Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics 50, 1088–101 (1994). [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.