Abstract
We recently showed that visuomotor adaptation acquired under attentional distraction is better recalled under a similar level of distraction compared to no distraction. This paradoxical effect suggests that attentional state (e.g., divided or undivided) is encoded as an internal context during visuomotor learning and should be reinstated for successful recall (Song & Bédard, 2015). To investigate if there is a critical temporal window for encoding attentional state in visuomotor memory, we manipulated whether participants performed the secondary attention-demanding task concurrently in the early or late phase of visuomotor learning. Recall performance was enhanced when the attentional states between recall and the early phase of visuomotor learning were consistent. However, it reverted to untrained levels when tested under the attentional state of the late-phase learning. This suggests that attentional state is primarily encoded during the early phase of learning before motor errors decrease and reach an asymptote. Furthermore, we demonstrate that when divided and undivided attentional states were mixed during visuomotor adaptation, only divided attention was encoded as an internal cue for memory retrieval. Therefore, a single attentional state appears to be primarily integrated with visuomotor memory while motor error reduction is in progress during learning.
Keywords: visuomotor learning, attentional states, attentional-state dependent motor memory
Introduction
To behave appropriately in constantly changing environments, new sensory-motor relationships must be learned and remembered for future use (e.g., Hegele & Heuer, 2010; Krakauer, Ghez, & Ghilardi, 2005; Redding & Wallace, 1996). This adaptive learning often occurs while our attention is divided across several tasks. For instance, when playing tennis, we maneuver the direction of the racquet while simultaneously attending to the opponent's action.
Because attention has been considered to be a necessary resource that facilitates various cognitive functions (Blaser, Pylyshyn, & Holcombe, 2000; Carrasco & McElree, 2001; Culham et al., 1998; Morgan, Ward, & Castet, 1998; Spitzer, Desimone, & Moran, 1988), it is not surprising that dividing attentional resources in a dual task is very costly (Gardiner & Richardson-Klavehn, 2000; Pashler, 1994). Indeed, previous studies have shown that performing a concurrent attention-demanding task impairs somatic motor adaptation and motor sequence learning (Curran & Keele, 1993; Frensch, Lin, & Buchner, 1998; Nissen & Bullemer, 1987; Taylor & Thoroughman, 2007, 2008). Dual-task costs are larger when the complexity of the motor sequence increases (Cohen, Ivry, & Keele, 1990; Rowland & Shanks, 2006) and when explicit rather than implicit learning is involved (Cleeremans, 1993; Hazeltine, Grafton, & Ivry, 1997; Jiménez & Vázques, 2005). These studies have primarily focused on the effect of divided attention on immediate motor performance. Thus its effect on motor memory formation or retrieval has not been examined until recently.
Yet, using a dual-task paradigm that pairs a visuomotor adaptation task and an attention-demanding secondary task, such as a rapid visual serial presentation (RSVP) task, we recently showed that attentional distraction by a secondary task did not always impair the learning performance. Intriguingly, a motor skill learned under distraction by a secondary task was only remembered when a similar distraction was present. When tested without the distracting task, motor performance reverted to untrained levels (Song & Bédard, 2015). This counterintuitive result, in which performance decreases when more attentional resources are available, suggests that diverting attention to a secondary task can be encoded as an internal context to act as a vital context for memory retrieval. Specifically, the internal state of either divided or undivided attention itself is encoded and integrated with visuomotor memory during learning. To achieve similar performance at recall, this attentional state has to be reinstated.
So far, such an attentional state has not been fully investigated as the content that serves as an internal cue for memory formation and retrieval. How and when is attentional state encoded and associated with visuomotor memory to modulate retrieval? To some extent, this newly reported attentional state–dependent visuomotor memory is similar to context-dependent memory (e.g., Baddeley, Eysenck, & Anderson, 2009), in which recall of specific episodes or information is facilitated when encoding and retrieval take place in the same environmental context. However, in contrast to episodic memory (e.g., Eich, 1980), in which congruent external context has a priority over consistent internal states for memory retrieval, Song and Bédard (2015) repeatedly demonstrated that consistent divided attentional states can form an internal cue that overrides the same external environmental cue. Moreover, they reported that attentional state–dependent visuomotor memory could be formed between different types of attentional tasks or sensory modalities as long as attentional state remained the same (e.g., whether divided or undivided). Therefore, attentional state–dependent memory seems to be distinct from context-dependent memory, and attentional state as an internal cue seems to have a priority over external environmental context during visuomotor adaptation. The properties of the mechanism that supports the attentional state–dependent memory need to be investigated.
In order to investigate the nature of attentional state–dependent memory formation, we first examined when, during visuomotor adaptation, attentional state is encoded as an internal cue to be reinstated at recall. Understanding the time course of attentional state–dependent memory formation would tell us how attentional state–dependent memory and visuomotor adaptation interplay with each other.
It has been suggested that different processes operate during visuomotor adaptation. During visuomotor adaptation, rapid motor error reduction occurs during the early phase of visuomotor motor adaptation (i.e., preasymptote) whereas motor performance has attained a plateau during the late phase (i.e., postasymptote). Thus, in the preasymptotic phase, the acquisition of a simple motor skill involves a rapid improvement of task performance (Censor, Sagi, & Cohen, 2012). This preasymptotic phase is suggested to be the cognitive state (Fitts, 1964; Fitts & Posner, 1967) in which motor error reduction requires considerable cognitive activity for adjustment of motor commands according to sensory cues in the environment (Atkeson, 1989; Shadmehr & Mussa-Ivaldi, 1994). In contrast, during the postasymptotic phase, motor performance becomes more efficient and automatic, depending less on error signals and attentional processes (Preilowski, 1977).
However, the previous study (Song & Bédard, 2015) could not characterize the relationship between attentional context encoding and progress in motor error reduction because the secondary task was constantly presented during the entire duration of visuomotor adaptation. To address this question, in Experiment 1, we asked participants to perform the secondary RSVP task during either the preasymptote or postasymptote period of visuomotor adaptation. Then, we manipulated whether or not they performed the RSVP at recall to match the attentional state (divided vs. undivided) to the preasymptote or postasymptote period during learning. If encoding attentional state is independent of motor error signals, the attentional state could be encoded at any time during the learning phase. On the other hand, if encoding attentional states critically depends on the progress of visuomotor adaptation, we would predict that the success of memory retrieval should be affected by whether the attentional state during the early or late learning period is reinstated at recall. For instance, if attentional state is critically encoded while motor error is still decreasing, the attentional state in the preasymptote period should be reinstated at recall.
In Experiment 2, we further examined the flexibility of attentional state–dependent memory by asking whether one can simultaneously associate both divided and undivided attentional states with visuomotor memory. In daily life, we often have to acquire new visuomotor skills while the demand of divided and undivided attention fluctuates moment by moment. It is unknown whether the random mixture of two attentional states can be encoded because in Song and Bédard (2015), either the divided or undivided state was exclusively formed during adaptation. To address this question, in Experiment 2, participants were exposed to both divided and undivided attentional states with randomly intermixed sequence of these two types of trials during learning. Then, we examined whether visuomotor memory could be retrieved in either state at recall. For instance, if both attentional states could be simultaneously encoded, recall performance should be improved regardless of attentional state at recall. Alternatively, if only one attentional state (e.g., divided) is encoded dominantly over the other (e.g., undivided), the groups that reinstated the dominant attentional state at recall could better retrieve the visuomotor memory.
Experiment 1: Is there a critical temporal window for encoding attentional states?
In Experiment 1, we examined whether encoding a diverted attentional state into memory during visuomotor learning is limited to a critical temporal window: the early phase or the late phase of visuomotor learning.
Methods
Similar methods including apparatus, tasks, and data analysis procedures have been implemented in prior work (Song & Bédard, 2013, 2015).
Participants
A total of 110 right-handed participants with normal color vision and normal or corrected-to-normal vision participated in our experiments (18–32 years old). All the experimental protocols were approved by the Institutional Review Board at Brown University. Participants received small monetary compensation or a course credit.
Apparatus
The experiments took place in a dark room. Participants sat on a chair in front of a 21-in. Macintosh iMac computer (60 Hz refresh rate, 1920 × 1080 pixels) viewed from a distance of approximately 57 cm. They used their dominant right arm to perform a goal-directed reaching task with a stylus pen on a table touch screen (Magic Touch, Keytec) located on the table. The stylus pen controlled a cursor (white dot with diameter of 0.5°) presented on the monitor. We presented visual stimuli against a black background on the monitor and recorded cursor displacement using Matlab (R2009b; MathWorks Inc., Natick, MA) and functions from the PsychToolbox (Brainard 1997; Pelli 1997).
Tasks
We adapted a dual-task paradigm combining a visuomotor adaptation task and an RSVP task as in the previous studies (Bédard & Song, 2013; Song & Bédard, 2013, 2015).
Visuomotor adaptation task:
Participants had to reach from a central starting base (annulus with diameter of 1°, corresponding to 1 cm) toward a visual target (white dot with diameter of 1°) located 5.5 cm away from the starting base in the 3, 6, 9, or 12 o'clock direction (Figure 1A). A trial started after the participant positioned the cursor in the starting base for at least 500 ms, after which a visual target appeared. Each visual target appeared in a pseudorandom sequence, such that all the target locations were selected once before they were repeated. Participants were instructed to make ballistic, uncorrected, straight movements toward the visual target and come back to the starting position. Reaching occurred in one of two types of trials: In the null trials, the cursor followed stylus motion normally whereas in the rotation trials the cursor direction was rotated 45° counterclockwise (CCW) to force movement learning. After 40 null practice trials with no perturbation, each participant performed the four sequential experimental phases: baseline (40 null trials), learning (160 rotation trials), washout (80 null trials), and recall (80 rotation trials).
Figure 1.
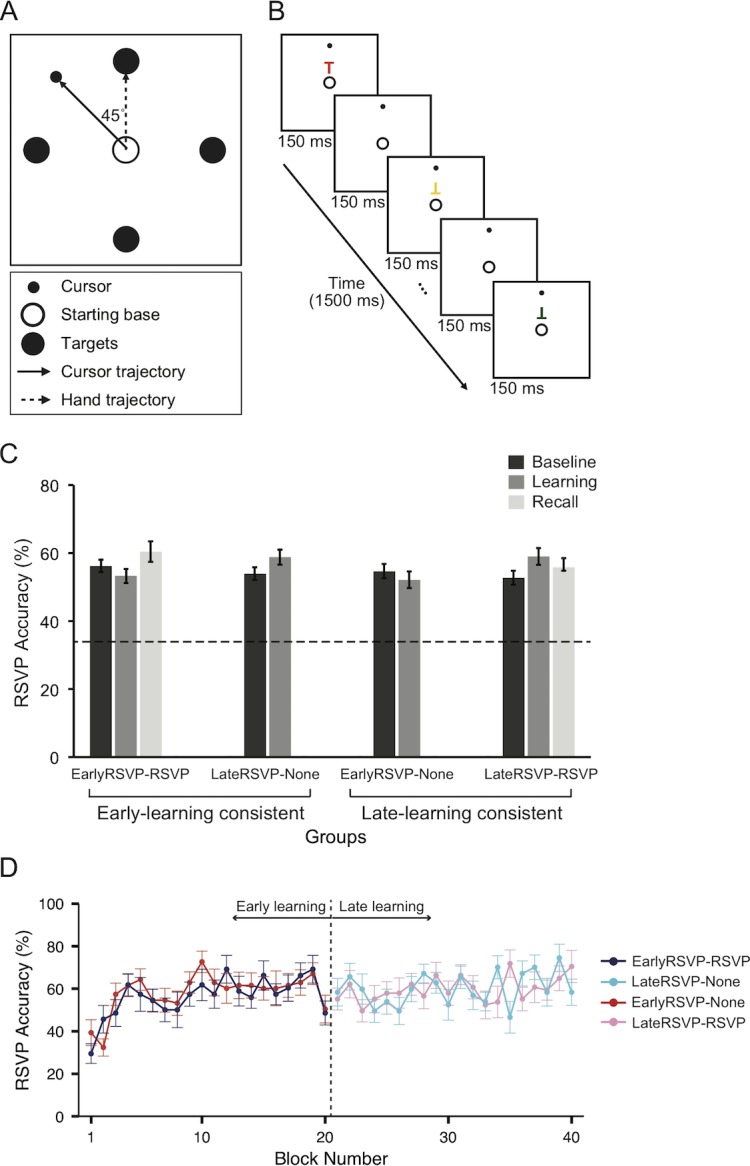
Task schematics and performance in the secondary RSVP task. (A) Reaching task. Targets appeared one at a time and remained visible for the whole trial. During the baseline and washout phases, the cursor followed the mouse normally. However, during the learning and recall phases, the cursor direction was rotated by 45° CCW from the hand trajectory. (B) The secondary RSVP task. A sequence of five Ts were presented for 150 ms, each either upright or inverted in five different colors. Participants had to report how many target Ts (red upright Ts and green inverted Ts) they detected by pressing a keyboard at the end of each trial with their left hand. (C) Average accuracy for the RSVP tasks of each group (EarlyRSVP–RSVP, LateRSVP–None, EarlyRSVP–None, and LateRSVP–RSVP) during baseline, learning, and recall phases. The dotted line indicates the 33% chance level. (D) Average RSVP accuracy of each of the four groups (EarlyRSVP–RSVP, LateRSVP–None, EarlyRSVP–None, and LateRSVP–RSVP) over the blocks (collapsed from four successive trials) during the learning phase. The error bars indicate the standard error of the means (SEM).
RSVP task:
In every trial, upright or inverted Ts (0.5 × 1 cm) of various colors (red, white, green, purple, or yellow) appeared 0.5 cm above the starting base in sequence while participants performed the visuomotor adaptation task (Figure 1B). In each trial, a total of five Ts were sequentially presented in 300-ms intervals, but each remained visible for only 150 ms (for a total duration of 1500 ms). The task was to detect a conjunction target (e.g., red upright Ts and green inverted Ts) from the RSVP stream. The number of relevant Ts varied randomly among 1, 2, and 3 with equal probability, resulting in a 33% chance level. Participants reported the number of targets observed (1, 2, or 3) at the end of each trial by pressing a keyboard with their left hand. In trials in which the RSVP task was not performed, participants were also instructed to press a keyboard in response to a visual cue at the end of each trial (i.e., “Press button 1”) to equate the extra response and time delay. Importantly, Ts appeared in every trial of all experimental phases, so visual stimuli remained the same across all participants.
Procedures
Participants performed the visuomotor adaptation task (Figure 1A) with or without the RSVP task (Figure 1B), depending on the requirements for the groups and experimental phases, as indicated in Table 1. The labels in Table 1 indicate whether participants performed the RSVP during the early (first 80 trials) or late (last 80 trials) learning phase (Early vs. Late) and whether they performed the RSVP task during the entire 80-trial recall phase (None vs. RSVP): EarlyRSVP–RSVP (N = 12), LateRSVP–None (N = 11), EarlyRSVP–None (N = 11), LateRSVP–RSVP (N = 12) groups. For example, the EarlyRSVP–RSVP group performed the RSVP task during the first half of the learning phase (i.e., EarlyRSVP) and during the whole recall phase (i.e., RSVP) whereas the LateRSVP–None group performed the RSVP task during only the second half of the learning phase (i.e., LateRSVP) but not during the recall phase (i.e., None).
Table 1.
RSVP task performed in each group throughout each experimental phase.
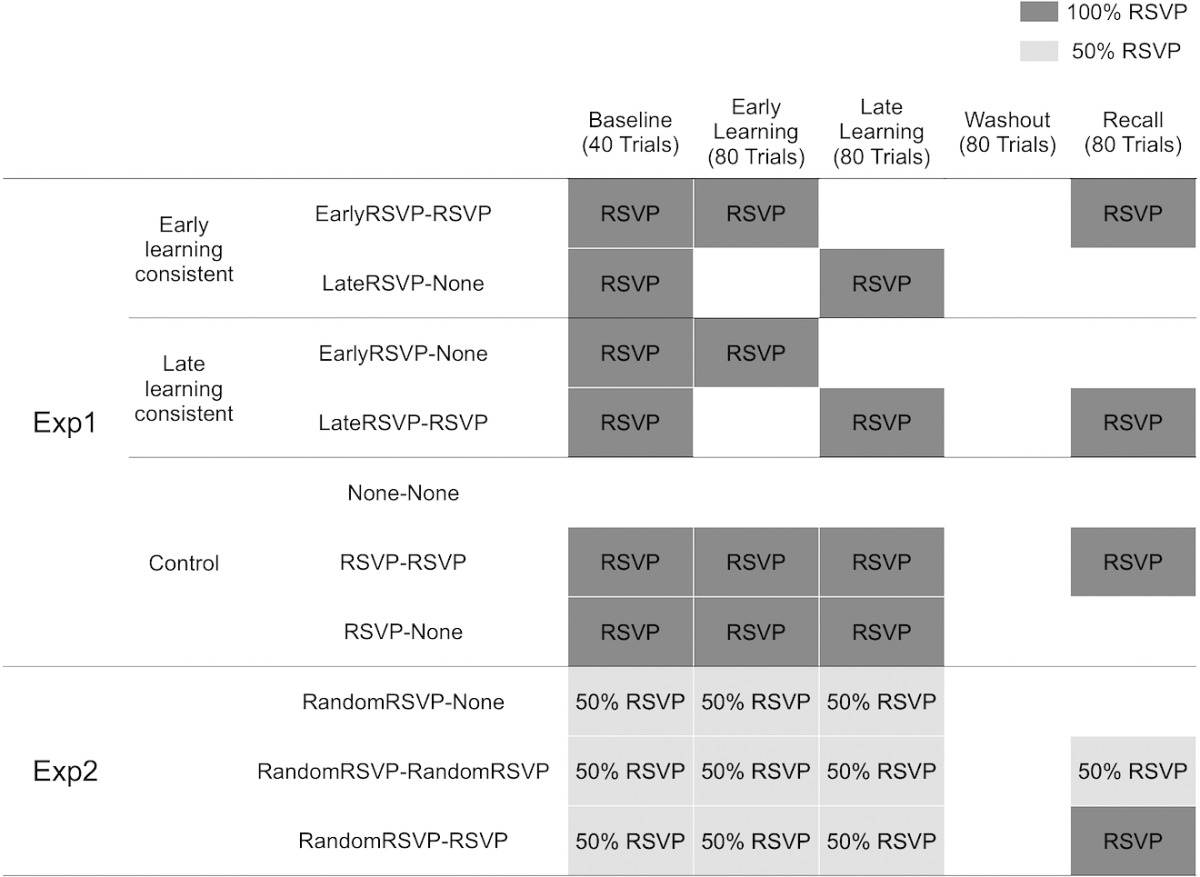
The EarlyRSVP–RSVP and LateRSVP–None groups were categorized as the early-learning consistent groups because their attentional states at recall were consistent with the early phase of learning (divided and undivided, respectively). On the other hand, the LateRSVP–RSVP and EarlyRSVP–None groups were categorized as the late-learning consistent groups because their attentional states at recall were consistent with the late phase of learning (divided and undivided, respectively).
To evaluate the strength of attentional state formed during the early or late learning phase in comparison to that formed during the entire learning phase, we formed three additional control groups: None–None (N = 20), RSVP–RSVP (N = 23), RSVP–None (N = 21) groups. The characteristics of these three groups were closely examined in Song and Bédard (2015). These groups performed the RSVP task during the entire learning phase or had no secondary task. For instance, the RSVP–RSVP group performed the dual task during the entire learning and recall phases whereas the None–None group did not perform the RSVP task at all.
Data analysis
We filtered the x and y coordinates of stylus displacements with a low-pass Butterworth filter using a 10 Hz cutoff and then calculated the cursor trajectory by taking the square root of the sum of squared x and y coordinates at each time point. We differentiated the position of the cursor to yield tangential velocity and determined the onset and end of movement when the cursor reached 5% of peak velocity. We measured reaction time (RT) as the time elapsed from target appearance to movement onset and movement time (MT) as the time elapsed between movement onset and the end of the movement. Reaching error was calculated as the angle between the direct line from the starting base to the target and the line defined by the position of the cursor at movement onset to the cursor position at peak velocity. Clockwise (CW) errors were deemed positive and CCW errors negative. To minimize potential random error variance led by four reach target locations in the visuomotor adaptation task, we averaged RT, MT, and reaching error across the four successive trials, in which each target location was presented once, into blocks.
We measured savings, a metric of memory formation, as the difference between the averages of blocks 2 to 7 of the learning and recall phases in accord with similar work (Krakauer et al., 2005; Song & Bédard, 2015). We did not use the first block in the learning and recall phases because of task-switching costs and the initial difficulty of performing the RSVP task and the reaching task simultaneously. This would have artificially and inappropriately amplified their savings.
We used mixed-effects ANOVAs with Groups as a between-subjects factor and Blocks and Phases as repeated measures. When multiple post hoc comparisons were made, Bonferroni correction was applied.
Results
No disruption of RSVP performance by visuomotor learning
We first confirmed that participants allocated their attention to the secondary RSVP task (Figure 1B). Figure 1C shows average accuracy for RSVP detection of each group (EarlyRSVP–RSVP, LateRSVP–None, EarlyRSVP–None, and LateRSVP–RSVP). All the groups performed the RSVP task above the 33% chance level (dotted line). A two-way ANOVA with Groups (EarlyRSVP–RSVP, LateRSVP–None, EarlyRSVP–None, and LateRSVP–RSVP) and Phases (baseline and learning) showed no significant main effect of Group, F(3, 42) = 0.23, p = 0.87, and Phase, F(1, 84) = 0.26, p = 0.61, as well as no interaction, F(3, 84) = 0.24, p = 0.89. Also, in the LateRSVP–RSVP and EarlyRSVP–RSVP groups, accuracy in the recall phase did not differ from the other phases: LateRSVP–RSVP, F(2, 33) = 0.96, p = 0.39; EarlyRSVP–RSVP, F(2, 36) = 0.91, p = 0.41. This result is consistent with our two previous studies, showing that performing the visuomotor adaptation task does not interfere with performance of the RSVP task (Bédard & Song, 2013; Song & Bédard, 2015).
We also examined whether the RSVP accuracy was relatively stable while visuomotor adaptation was in progress and compatible across the groups during the learning phase. As shown in Figure 1D, except for the first two blocks in the EarlyRSVP groups (blue for EarlyRSVP–RSVP and red for EarlyRSVP–None, respectively), the RSVP accuracy was consistent overall throughout the trials and across the groups. Therefore, the visuomotor learning had only the minimal impact on the RSVP performance during the first few blocks in which large motor error reduction occurs. This result also indicates that the success on the RSVP task and reach error reduction are independent of each other.
To statistically confirm these observations, we directly compared the 20 blocks of the RSVP task performance in each group (e.g., blocks 1–20 for the EarlyRSVP–RSVP and EarlyRSVP–None groups and blocks 21–40 for the LateRSVP–None and LateRSVP–RSVP groups) by running a two-way ANOVA with factors of Groups and Blocks. We found a significant effect by Groups, F(3, 38) = 1.42, p < 0.01, and by Blocks, F(19, 760) = 2.41, p < 0.01, but no significant interaction, F(57, 760) = 1.30, p = 0.07. The significant difference across the groups was mainly due to the impaired RSVP accuracy only for the two EarlyRSVP groups. When we exclude the first two blocks of the learning phase, the main effect of Blocks, F(17, 684) = 1.32, p = 0.17; the main effect of Groups, F(3, 38) = 0.35, p = 0.79; and the interaction, F(51, 684) = 0.92, p = 0.64, were not significant. To sum up, we confirmed that visuomotor learning had only a minimal impact on the RSVP performance. Moreover, we also showed that success on the RSVP task is independent of motor error reduction.
No disruption of immediate motor performance by divided attention
Figure 2A shows reaching trajectories of representative participants in the EarlyRSVP–RSVP, LateRSVP–None, EarlyRSVP–None, and LateRSVP–RSVP groups during the early (red, green, and blue lines for the second, fifth, and ninth trials, respectively) and late learning (gray lines) phases. All the trajectories were realigned to the 12 o'clock direction. Participants gradually reduced reaching errors during the early learning phase independent of whether they performed the RSVP task during the early or the late phases. By the end of the learning phase (i.e., gray lines for the last six trials), movements were straight and directed accurately to the target.
Figure 2.
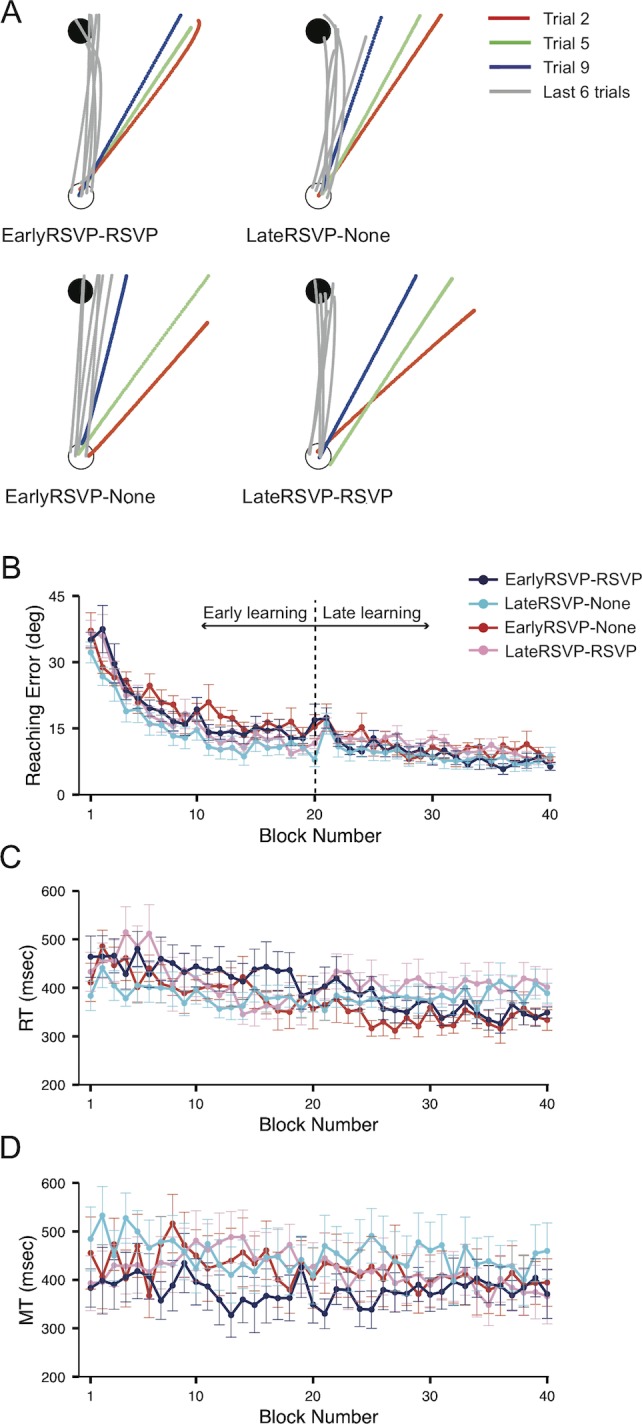
Results of the reaching task in Experiment 1. (A) Reaching trajectories of four representative participants of different groups (EarlyRSVP–RSVP, LateRSVP–None, EarlyRSVP–None, and LateRSVP–RSVP) during the learning phase. All the trajectories are realigned to the 12 o'clock direction. The trajectories of the first three trials are color-coded (red, green, and blue for the second, fifth, and ninth trials, respectively), and the gray lines indicate the trajectories of the last six trials. The trajectories in the beginning of the learning phase deviated from the target (i.e., red, green, blue lines). However, by the end of the learning phase (gray lines), the trajectories became more aligned and straight toward the target, indicating visuomotor learning. (B) Mean reaching errors of the four groups during the learning phase (averaged over blocks of four trials ± SEM: EarlyRSVP–RSVP, N = 12; LateRSVP–None, N = 11; EarlyRSVP–None, N = 11; LateRSVP–RSVP, N = 12). Trial blocks 1–20 are defined as the early-learning phase, and trial blocks 21–40 are defined as the late-learning phase. All groups performed similarly regardless of attentional load. (C) RT (means ± SEM) of the reaching. Regardless of whether they performed the secondary RSVP task during the learning phase or not, the RT of the reaching did not differ across the groups. (D) Mean MT (means ± SEM). Just as with RT, MT did not differ across the groups regardless of whether they performed the secondary RSVP task during the learning phase.
Equivalent learning performance across the four groups can be seen in the fully superimposed reaching error curves shown in Figure 2B. All the groups did not show any systematic difference in the rate of visuomotor learning regardless of when they performed the RSVP task. A two-way ANOVA with Groups (EarlyRSVP–RSVP, LateRSVP–None, EarlyRSVP–None, and LateRSVP–RSVP) and Blocks (all 40 blocks) also confirmed our observation: no main effect of the Groups, F(3, 42) = 1.86, p = 0.14; a significant main effect of the Blocks, F(39, 1680) = 30.62, p < 0.01, indicating motor learning; and no significant interaction, F(117, 1680) = 1.01, p = 0.44. This result demonstrates that visuomotor learning occurred in all the groups and divided attention did not impair immediate motor performance, consistent with previous observations (Bédard & Song, 2013; Song & Bédard, 2015).
It is worth noticing that there seems to be a small peak at block 21 in Figure 2B, which is the first block in which participants had to switch the attentional state during the learning phase (e.g., from divided to undivided for EarlyRSVP groups and from undivided to divided for LateRSVP groups). The sudden peak in the learning curves on this block seems to reflect the cost in reaching error for switching the attentional states in the middle of the learning phase. However, participants seem to have been able to handle such a task switch efficiently and rapidly.
To assess whether our manipulation of early (preasymptotic) and late (postasymptotic) phases of learning was reasonable, we estimated the time to asymptote at which the learning curve attained the low asymptotic level of the reaching error during the learning phase. We fitted the decreasing reaching error curve of each individual participant by an exponential function:
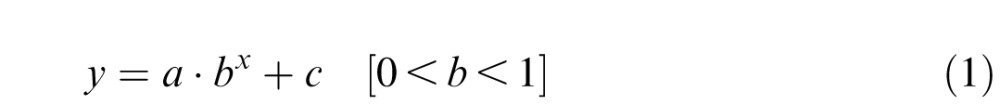 |
where a is the y-intercept, b is the rate of learning, and c is the level of the low asymptote. From the fitted parameter c, reflecting the low asymptote of the learning curve, we obtained the elbow point at which the decreasing reaching error attained the asymptote. We then averaged the estimates of time to asymptote of the learning curves across participants for each of the four groups. On average, the error curves reached the asymptote at about the 18th block on average (i.e., time to asymptote), indicating that our splitting into two learning phases (early vs. late) was reasonable: The reaching errors decreased up to the the 18th block and remained stable near the low asymptote afterward. Also, we found that the estimates of the time to asymptote were not significantly different across the groups, F(3, 42) = 0.30, p = 0.83; mean ± SEM: EarlyRSVP–RSVP: 19.18 ± 1.15, LateRSVP–None: 17.91 ± 1.30, EarlyRSVP–None: 18.0 ± 1.10, LateRSVP–RSVP: 18.10 ± 0.58). This result suggests that these groups learned the visuomotor task equivalently despite the different attentional demand by the secondary task.
Finally, we examined RT and MT during the learning phase. There were no differences in RT (Figure 2C) and MT (Figure 2D) across the groups during the learning phase. A two-way ANOVA with Groups and Blocks (all 40 blocks) confirmed our observations: For RT, no main effect of Groups, F(3, 42) = 1.74, p = 0.16; a significant effect of Blocks, F(39, 1680) = 2.60, p < 0.01; and no interaction, F(117, 1680) = 1.11, p = 0.20; and for MT, no main effect of Groups, F(3, 42) = 0.18, p = 0.91, and Blocks, F(39, 1680) = 0.56, p = 0.99, as well as no interaction, F(117, 1680) = 1.10, p = 0.23. These results suggest that all the groups used similar kinematic strategies regardless of when they performed the RSVP task during learning and that different attentional demand did not affect visuomotor learning.
Reinstatement of the early learning attentional state for memory retrieval
The main question of interest was whether there is a critical time window for association of attentional state and visuomotor memory. In order to examine whether attentional state encoded during the early phase of learning or during the late phase of learning, we compared savings across the groups. If attentional state is critically encoded during the early learning phase, the two early-learning consistent groups would show higher savings than the other two late-learning consistent groups. Alternatively, if attentional state is encoded during the late learning phase, we would expect the opposite pattern.
Figure 3A through D shows visuomotor performance during the learning (open square) and recall (solid square) phases of EarlyRSVP–RSVP (Figure 3A), LateRSVP–None (Figure 3B), EarlyRSVP–None (Figure 3C), and LateRSVP–RSVP (Figure 3D) groups. Again, savings is defined as the difference in reaching error between the early adaptation and early recall phases (gray areas in Figure 3A through D). Interestingly, although performing the RSVP task during learning did not disrupt the process of decreasing reaching error (Figure 2B), the two early-learning consistent groups (Figure 3A, B: EarlyRSVP–RSVP and LateRSVP–None) show much larger savings than the late-learning consistent groups (Figure 3C, D: EarlyRSVP–None and LateRSVP–RSVP). This observation of different savings across the groups is summarized in Figure 3E and also confirmed by a one-way ANOVA, F(3, 42) = 5.59, p < 0.01. The EarlyRSVP–RSVP (dark blue bar) and LateRSVP–None (light blue bar) groups showed significantly larger savings (all ps < 0.05) than both the EarlyRSVP–None and LateRSVP–RSVP groups, who did not differ from each other. This result suggests that the early phase of learning is the critical period for encoding of attentional states into visuomotor memory that facilitate performance when reinstated at recall.
Figure 3.
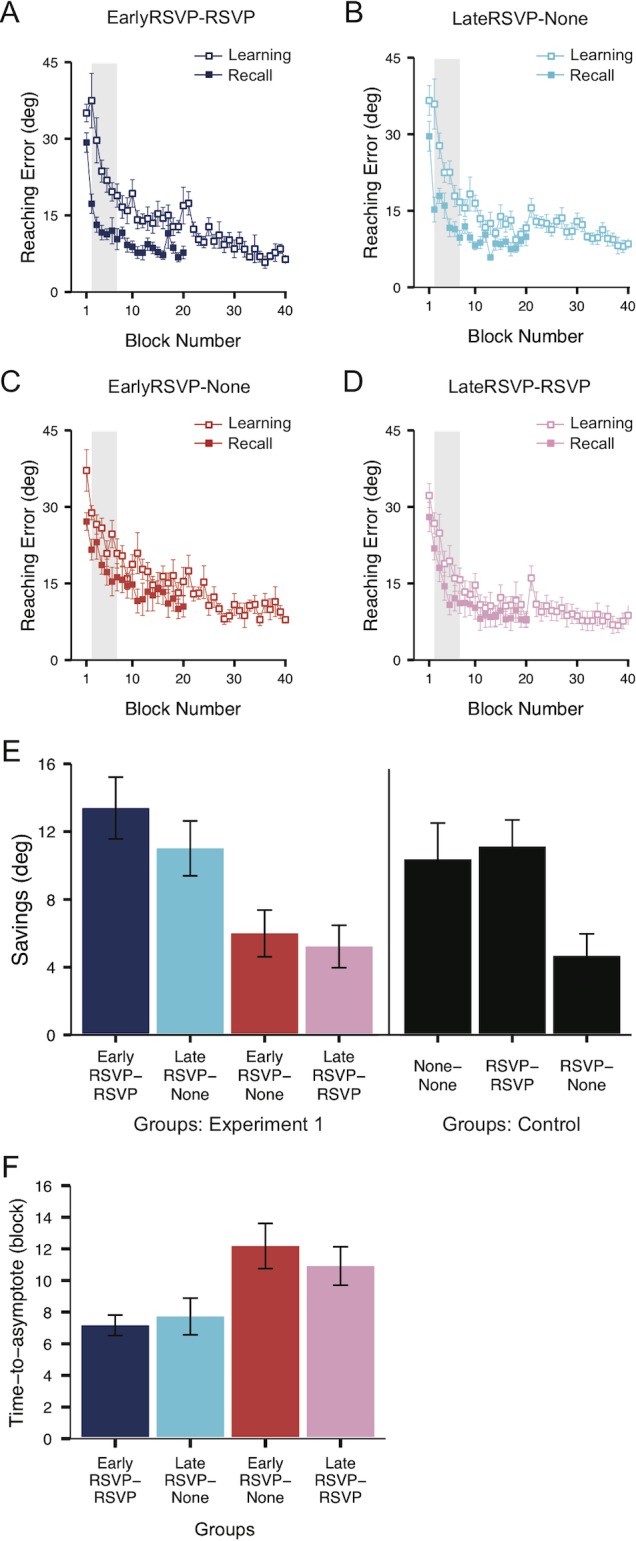
Reaching error and savings of the four groups in Experiment 1. (A–D) Reaching error (averaged over blocks of four trials ± SEM) plotted for each group to highlight savings differences. Gray areas in each figure indicate which blocks were used to calculate savings. (E) Savings (means ± SEM) for EarlyRSVP–RSVP, LateRSVP–None, EarlyRSVP–None, and LateRSVP–RSVP groups and savings for the three control groups (None–None, RSVP–RSVP, and RSVP–None, three rightmost black bars). The two early-learning consistent groups (EarlyRSVP–RSVP and LateRSVP–None) showed higher savings that the two late-learning consistent groups (EarlyRSVP–None and LateRSVP–RSVP). The two consistent groups (None–None and RSVP–RSVP) showed significant savings, replicating previous findings (e.g., Song & Bédard, 2015). The amount of savings of the consistent groups (None–None and RSVP–RSVP) was equivalent to that of the two early-learning consistent groups (EarlyRSVP–RSVP and LateRSVP–None). On the other hand, the amount of savings of the inconsistent group (RSVP–None) was smaller than the two early-learning consistent groups (EarlyRSVP–RSVP and LateRSVP–None) but equivalent to those of the two late-learning consistent groups (EarlyRSVP–None and LateRSVP–RSVP). (F) Time to asymptote (means ± SEM) for the four experimental groups: EarlyRSVP–RSVP, LateRSVP–None, EarlyRSVP–None, and LateRSVP–RSVP. The two early-learning consistent groups (EarlyRSVP–RSVP and LateRSVP–None) reached the low asymptote more rapidly than the two late-learning consistent groups (EarlyRSVP–None and LateRSVP–RSVP), suggesting faster, more efficient recall.
To obtain the converging evidence, we also estimated the time to asymptote by fitting the exponential function (Equation 1) to the recall curve. Again, the estimate of the time to asymptote indicates when reaching error of each participant started to attain the level of low asymptote. The more efficiently memory is retained and transferred from learning, the more quickly performance would have reached asymptote of the recall curve. Thus, smaller values indicate more rapid, efficient learning in which transferred memory from the learning phase facilitated the reaching error reduction. Figure 3F shows the average time to asymptote of the recall curves for each of the four groups (EarlyRSVP–RSVP, LateRSVP–None, EarlyRSVP–None, and LateRSVP–RSVP) in terms of block number. We can observe the significant difference in the time to asymptote of the recall curves across the groups, F(3, 42) = 3.94, p < 0.05. Following contrast analyses further revealed that both the early-learning consistent groups (EarlyRSVP–RSVP and LateRSVP–None) reached the asymptote quicker than the late-learning consistent groups (EarlyRSVP–None and LateRSVP–RSVP) during recall (all ps < 0.05). Thus, in accord with savings, the estimates of time to asymptote also indicate that the consistent attentional state encoded during the early phase of learning indeed facilitates retrieval of visuomotor memory.
Robustness of encoded attentional states in the early learning phase
We also assessed whether attentional states formed during the early learning phase modulate improvement at memory retrieval (i.e., savings) as robustly as those formed during the entire learning phase. We created three control groups: None–None, RSVP–RSVP, and RSVP–None, that either did or did not perform the RSVP task during the entire learning phase instead of only early or late in the learning phase. In accord with the findings of Song and Bédard (2015), the three black bars in Figure 3E show that the None–None (left black bar) and RSVP–RSVP (middle black bar) groups, who maintained consistent attentional states across the learning and recall phases (RSVP task or no task), showed equivalently higher savings than the RSVP–None group (right black bar), who performed the RSVP during the learning but not the recall phase. A one-way ANOVA confirmed that the main effect of Groups (None–None, RSVP–RSVP, and RSVP–None) was significant, F(2, 63) = 4.63, p < 0.05. Further post hoc analyses demonstrated that the None–None and RSVP–RSVP groups had an equivalent savings, t(41) = 0.29, p = 0.77, whereas they showed significantly larger savings than the RSVP–None group: None–None versus RSVP–None, t(39) = −2.30, p < 0.05; RSVP–RSVP versus RSVP–None, t(42) = 3.14, p < 0.01.
Of interest is whether savings of the two early-learning consistent groups (i.e., EarlyRSVP–RSVP, LateRSVP–None) is similar to that of the RSVP–RSVP and None–None groups. We observed that the EarlyRSVP–RSVP group (Figure 3E, dark blue bar) showed equivalent savings compared to the RSVP–RSVP group (Figure 3E, middle black bar), t(33) = 0.89, p = 0.38. In addition, the LateRSVP–None group (Figure 3E, light blue bar) showed equivalent savings to the None–None group (Figure 3E, left black bar), t(29) = 0.21, p = 0.84. Moreover, both EarlyRSVP–RSVP, t(31) = 3.95, p < 0.01, and LateRSVP–None groups, t(30) = 2.95, p < 0.01, showed higher savings than the RSVP–None group (Figure 3E, right black bar).
In contrast, the two late-learning consistent groups (EarlyRSVP–None and LateRSVP–RSVP groups) yielded significantly smaller savings than the None–None and RSVP–RSVP groups: EarlyRSVP–None versus RSVP–RSVP, t(32) = 2.10, p < 0.05; LateRSVP–RSVP versus RSVP–RSVP, t(33) = 2.51, p < 0.05. Rather, these late-learning consistent groups yielded comparable savings to that of the RSVP–None group, t(30) = 0.64, p = 0.52 and t(31) = 0.28, p = 0.78 for the EarlyRSVP–None and LateRSVP–RSVP, respectively. These results suggest that memory retrieval is equally strengthened independent of whether attentional states are integrated with visuomotor memory during the early learning phase or the entire learning phase. Thus, this suggests that attentional state integration must be established before motor performance reaches the plateau to provide a benefit during recall.
Together, we demonstrate that attentional states must be bound to visuomotor memory during the early learning (preasymptotic) phase if they are to serve as an internal context cue to enhance later recall. In daily activities, however, attentional resources are often more dynamically divided during learning of a new visuomotor skill, such that the same attentional state is not maintained throughout the entire learning process. In Experiment 2, therefore, we examined whether two different attentional states (e.g., divided and undivided) can be encoded simultaneously and associated with visuomotor memory during learning so that visuomotor memory can be flexibly retrieved later under both attentional states.
Experiment 2: Can multiple attentional states be simultaneously encoded during learning?
Methods
Participants
A total of 31 new right-handed participants with normal color vision and normal or corrected-to-normal vision participated in the experiments (19–22 years old). All the experimental protocols were approved by the Institutional Review Board at Brown University. Participants received monetary compensation or a course credit.
Apparatus and task
All aspects were identical to those of Experiment 1 except for the following. Before the beginning of each trial, the start base turned green or white to instruct the participants as to whether they had to perform or not perform the RSVP task, respectively.
Procedures
As listed in Table 1, we created three groups: RandomRSVP–None (N = 11), RandomRSVP–RandomRSVP (N = 10), RandomRSVP–RSVP (N = 10). During the baseline and learning phases, all three groups performed the RSVP task in a randomly selected 50% of trials as precued by a green starting base (RandomRSVP). During the recall phase, the RandomRSVP–None group did not perform the RSVP task at all whereas the RandomRSVP–RandomRSVP group continued to perform the RSVP task randomly in 50% of the trials and the RandomRSVP–RSVP group always performed the RSVP task (100%).
Results
No disruption of RSVP performance by visuomotor learning
Figure 4A shows the average RSVP accuracy of each group (RandomRSVP–None, RandomRSVP–RandomRSVP, and RandomRSVP–RSVP). All groups performed the RSVP task at better than 33% chance level (a dotted line). A two-way ANOVA with Groups (RandomRSVP–None, RandomRSVP–RandomRSVP, and RandomRSVP–RSVP) and Phases (baseline and learning) revealed no significant main effect of Group, F(2, 28) = 0.23, p = 0.79, and Phase, F(1, 56) = 0.53, p = 0.47, as well as no interaction, F(2, 56) = 1.21, p = 0.31. In addition, in the RandomRSVP–RandomRSVP and the RandomRSVP–RSVP groups, accuracy in the recall phase did not differ from the other phases: RandomRSVP–RandomRSVP, F(2, 27) = 0.04, p = 0.96; RandomRSVP–RSVP, F(2, 27) = 0.03, p = 0.26. As in Experiment 1, this result confirms that the RSVP performance was equivalent during the baseline, learning, and recall phases, indicating that performing the visuomotor adaptation task did not interfere with performance of the RSVP task.
Figure 4.
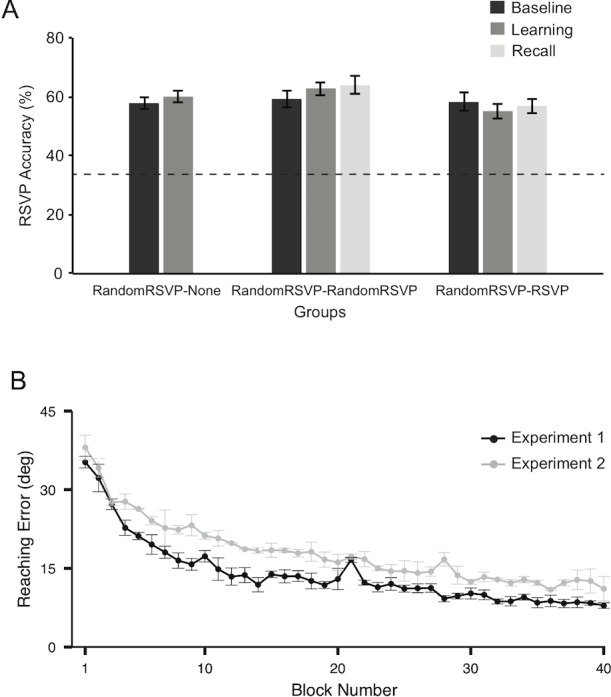
RSVP accuracy and learning curves from Experiment 2. (A) Average accuracy for the RSVP tasks of each group (RandomRSVP–None, RandomRSVP–RandomRSVP, and RandomRSVP–RSVP) during baseline, learning, and recall phases. The dotted line indicates the 33% chance level (33%). (B) The grand mean of the learning curves from Experiments 1 and 2 for a direct comparison. The black line indicates the grand mean of the reaching errors during the learning phase, averaged across the four experimental groups in Experiment 1 (EarlyRSVP–RSVP, LateRSVP–None, EarlyRSVP–None, and LateRSVP–RSVP) and the gray line indicates the grand mean of the reaching errors during the learning phase, averaged across the three experimental groups in Experiment 2 (RandomRSVP–None, RandomRSVP–RandomRSVP, and RandomRSVP–RSVP).
Immediate motor performance under intermixed attentional states
All three groups (RandomRSVP–None, RandomRSVP–RandomRSVP, and RandomRSVP–RSVP) performed the same mixed single and dual tasks during the learning phase. Reaching error decreased throughout the learning phase and reached an asymptote for all three groups. Not surprisingly, there was no systematic difference in the rate of visuomotor learning across the groups. A two-way ANOVA with Groups (RandomRSVP–None, RandomRSVP–RandomRSVP, and RandomRSVP–RSVP) and Blocks (all 40 blocks) confirmed our observation: no main effect of the Groups, F(2, 28) = 1.85, p = 0.19; a significant main effect of the Blocks, F(39, 1120) = 26.25, p < 0.01, indicating motor learning; and no significant interaction, F(78, 1120) = 0.76, p = 0.94. The time to asymptote in their learning curves also showed no difference across these groups, F(2, 28) = 0.51, p = 0.61; mean ± SEM: RandomRSVP–None: 19.45 ± 1.76, RandomRSVP–RandomRSVP: 18.91 ± 1.91, RandomRSVP–RSVP: 21.3 ± 1.42).
We then examined whether this mixed block of single and dual task trials during learning, in which attentional states frequently switched, affected the rate of adaptation. We directly compared learning performance from Experiment 2 with that from Experiment 1. In Figure 4B, we plotted the average learning curves across the groups in Experiment 1 (black line) and Experiment 2 (gray line). Overall, the three groups who performed the mixed single and dual tasks (RandomRSVP groups) in Experiment 2 yielded the shallower learning curves overall compared to the four groups in Experiment 1, who maintained the consistent attentional states. A two-way ANOVA with Experiments (Experiment 1 and Experiment 2) and Blocks (all 40 blocks) confirmed this. We found that the main effect by Experiments, F(1, 75) = 205.1, p < 0.01, and the main effect by Blocks, F(39, 3000) = 47.4, p < 0.01, were significant, but the interaction was not significant, F(39, 3000) = 0.76, p = 0.84. Together, this result suggests that the randomly intermixed sequence of the two different attentional states impaired the rate of adaptation during the learning phase.
Divided attentional state as a dominant internal cue
We next examined whether both divided and undivided attentional states can be simultaneously encoded during learning and later determine the success of recall. Figure 5A through C shows visuomotor performance during the learning (open square) and recall (solid square) phases of the RandomRSVP–None (Figure 5A), RandomRSVP–RandomRSVP (Figure 5B), and RandomRSVP–RSVP (Figure 5C) groups. We compared savings across the three groups (Figure 5A through C, gray area). If the random mixture of attentional states is encoded as an internal cue during learning, only the RandomRSVP–RandomRSVP group would show significant savings. Alternatively, if both attentional states could be simultaneously encoded, all three groups would show equivalent and significant savings. Finally, if only one attentional state (e.g., divided) is encoded dominantly over the other (e.g., undivided), the groups who reinstated the dominant attentional state at recall (i.e., RandomRSVP–RandomRSVP and RandomRSVP–RSVP) would show higher savings than the other (i.e., RandomRSVP–None).
Figure 5.
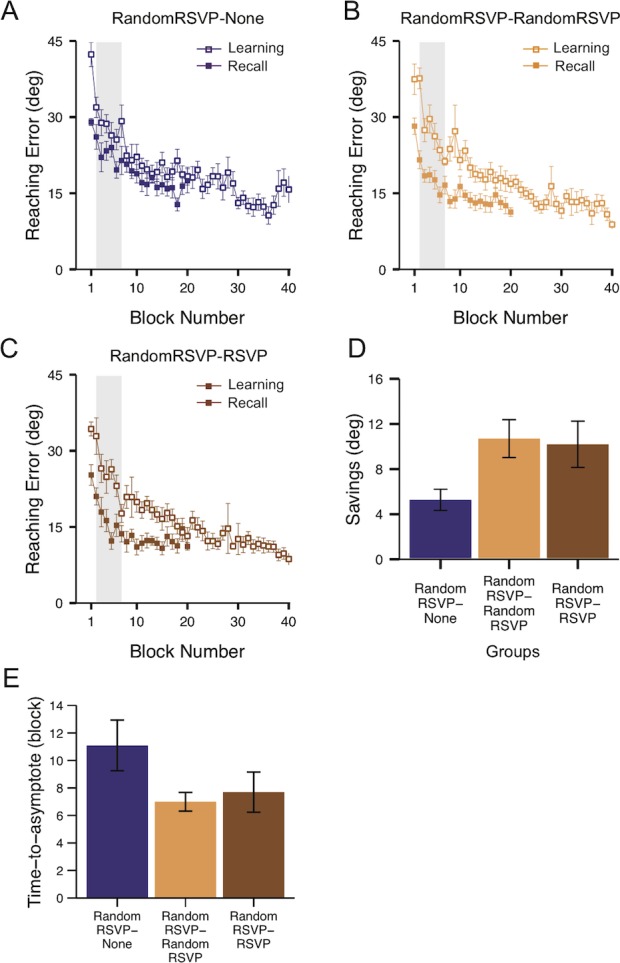
Results of Experiment 2. (A–C) Reaching error plotted for each group separately (averaged over blocks of four trials ± SEM: RandomRSVP–None, N = 11; RandomRSVP–RandomRSVP, N = 10; RandomRSVP–RSVP, N = 10). Gray areas in each figure indicate which blocks were used to calculate savings. (D) RandomRSVP–None, RandomRSVP–RandomRSVP, and RandomRSVP–RSVP groups (means ± SEM). Both the RandomRSVP–RandomRSVP and RandomRSVP–RSVP groups showed significant savings, but the RandomRSVP–None group did not. (E) Time to asymptote (means ± SEM) for the three experimental groups: RandomRSVP–None, RandomRSVP–RandomRSVP, and RandomRSVP–RSVP. Both the RandomRSVP–RandomRSVP and RandomRSVP–RSVP groups reached the low asymptote during the recall phase more rapidly than the RandomRSVP–None group, suggesting faster, more efficient recall.
Savings were significantly smaller for the RandomRSVP–None group (Figure 5A) than the RandomRSVP–RandomRSVP (Figure 5B) and the RandomRSVP–RSVP (Figure 5C) groups. Figure 5D summarizes this observation. A main effect of savings was observed across the groups, F(2, 28) = 3.68, p < 0.05. Post hoc tests confirmed that the RandomRSVP–RandomRSVP group and the RandomRSVP–RSVP group yielded significantly larger savings than the RandomRSVP–None group (all ps < 0.05). However, savings from the RandomRSVP–RandomRSVP group and the RandomRSVP–RSVP group did not statistically differ, t(18) = 0.19, p = 0.85. We also observed that the RandomRSVP–RandomRSVP, t(31) = 0.16, p = 0.87, and RandomRSVP–RSVP groups, t(31) = 0.34, p = 0.74, yielded comparable savings to those of the control RSVP–RSVP group (Figure 3E). Therefore, the RandomRSVP sequence did not reduce the magnitude of memory transfer at recall. This result is also consistent with the notion that divided attentional state is dominantly encoded during learning in the RandomRSVP–RandomRSVP and RandomRSVP–RSVP groups, such that reinstatement of the same divided attentional state at recall facilitates recall performance. In contrast, the RandomRSVP–None group yielded significantly smaller savings than the RSVP–RSVP group, t(32) = 2.74, p < 0.05, but comparable to the savings from the RSVP–None group, t(30) = 0.32, p = 0.75.
As in Experiment 1, to obtain the converging evidence, we also compared the time to asymptote for the three groups (Figure 5E). We found that there was a significant difference in the time to asymptote, F(2, 28) = 3.29, p < 0.05. Further contrast analyses revealed that both RandomRSVP–RandomRSVP and RandomRSVP–RSVP groups reached the asymptote quicker than the RandomRSVP–None group (all ps < 0.05). However, the RandomRSVP–RandomRSVP and RandomRSVP–RSVP groups reached the asymptote with a comparable rate during recall, t(28) = 0.25, p = 0.81.
These results together suggest that although participants experienced both divided and undivided attentional states in an intermixed sequence, the divided attentional state was dominantly associated with visuomotor memory.
Discussion
Although it is generally thought that more attentional resources help when learning a new motor task (e.g., Nissen & Bullemer, 1987), we showed that the rate of visuomotor adaptation is the same even when attention is distracted to a secondary task. In accord with our previous work (Song & Bédard, 2015), we confirmed that an internal context is formed by whether or not participants divide their attention to the secondary task during visuomotor adaptation (divided vs. undivided). When the internal context about attentional states formed during learning is reinstated at recall, memory retrieval is facilitated.
Furthermore, Song and Bédard (2015) have shown that even when participants were instructed to perform two different secondary tasks during learning and recall, visuomotor memory retrieval was facilitated as long as they were under the consistent attentional state (e.g., divided or undivided). Therefore, what is coded during visuomotor learning seems to be the attentional state itself rather than external contexts, such as the task setting. Furthermore, attentional state–dependent memory appears to be invariant across external environmental changes.
In the current study, we extended our prior results and showed that the preasymptote period of visuomotor adaptation is the critical temporal window in which attentional states must be encoded into visuomotor memory to provide a later benefit. We also showed when participants are exposed to intermixed divided and undivided attentional states, the divided attentional state is dominantly encoded as an internal cue.
Temporal window for encoding attentional states
During the early phase of motor learning, movements are unskilled, highly feedback-dependent, and require strong attentional demands to reduce error (Atkeson, 1989; Petersen, Corbetta, Miezin, & Shulman, 1994) whereas during the late phase, accuracy and velocity of actions increase with practice, and feedback becomes less important (Preilowski, 1977). Accordingly, previous studies have suggested different functional roles and neural bases of preasymptotic and postasymptotic phases during motor learning (Ajemian, D'Ausilio, Moorman, & Bizzi, 2013; Halsband & Lange, 2006). For instance, a recent model drew a distinction between preasymptotic and postasymptotic periods, suggesting that once the motor error reaches the asymptote, the postasymptotic phase of learning requires altered network dynamics for memory permanence (Ajemian et al., 2013). Furthermore, Della-Maggiore, Malfait, Ostry, and Paus (2004) showed that transcranial magnetic stimulation, directed to the posterior parietal cortex did not disrupt visuomotor learning when motor error was still decreasing in the early learning phase but impaired learning once performance had attained a plateau. Thus, it appears that motor error reduction primarily occurs during the preasymptote phase whereas motor memory formation occurs during the postasymptote phase. Neuroimaging studies using fMRI have also demonstrated that separate brain areas, such as the striatum and cerebellum, are involved in the early phase, and those such as the motor cortex are involved in the late phase of motor learning (Ungerleider, Doyon, & Karni, 2002).
Here, we showed that the preasymptotic and postasymptote phases of visuomotor adaptation interact differently with attentional states. We demonstrated that the attentional state in the early phase of learning should be reinstated at recall for the successful retrieval of a newly acquired visuomotor skill. Furthermore, attentional states formed only during the early learning phase can facilitate memory recall to the same extent as those formed during the entire learning phase. These results suggest that the preasymptotic period is a critical temporal window to integrating attentional states with visuomotor memory to achieve a later benefit during recall.
Rapid reduction of motor error occurs during the preasymptotic phase. In order to reduce motor error, motor commands should be adjusted in real time according to visual feedback. Thus, the preasymptotic phase relies heavily on the attentional processes for novel association between the visual input and motor execution (Atkeson, 1989; Shadmehr & Mussa-Ivaldi, 1994). Consistent with this notion, previous imaging studies have found the involvement of the dorsolateral prefrontal cortex in the preasymptotic phase of learning (Deiber et al., 1997; Shadmehr & Holcomb, 1997), which is presumably involved in working memory and in establishing a novel association between visual cues and motor commands (Halsband & Lange, 2006; Hazeltine et al., 1997).
We propose that the divided attentional state manifests primarily during the early phase of learning in which both the visuomotor learning and the RSVP task interact closely with attentional processes. During the late phase of learning, however, visuomotor skill has been learned and is more automatic, so that a divided attentional state becomes less prevalent. Although performing the dual task during the early or late learning phases did not impair the visuomotor adaptation task or the RSVP accuracy, it is possible that the divided attentional state was more explicit during the early phase of learning before the visuomotor learning became a skilled, automatic process. Thus, the divided attentional state is encoded as an internal context and associated with visuomotor memory concurrently to the motor error reduction during the preasymptotic phase and before visuomotor memory representation is fully developed.
Difficulty of encoding multiple attentional states
The ability to flexibly generalize the learned skills is crucial for the survival of biological systems (Bédard & Song, 2013). In daily activities, attentional resources are often divided but not always to the same extent. We examined whether both divided and undivided attentional states could be associated with visuomotor memory when participants performed visuomotor adaptation under two intermixed attentional states during the learning phase. Our results indicate that multiple attentional states cannot be simultaneously associated with visuomotor memory even if participants were equally exposed to intermixed divided and undivided attentional states. Rather, it appears that the divided attentional state is primarily encoded with visuomotor memory.
Specifically, we demonstrated that recall performance was equally enhanced when participants performed visuomotor adaptation under the randomly intermixed attentional states as in the learning phase (RandomRSVP–RandomRSVP) or fully divided attentional states (RandomRSVP–RSVP). This finding indicates that participants encoded the sustained, divided attentional state during learning rather than switching attentional states across trials.
Encoding two different internal cues at the same time could have allowed participants to maximize their learning performance by switching flexibly attentional states back and forth to remain consistent during learning and recall. However, it may be too costly to encode both associations and retrieve one of them selectively. Because switching attentional set across trials itself is already very costly (for review, see Monsell, 2003), always maintaining the divided attentional state during the learning phase rather than switching back and forth may also have been a more reasonable strategy.
It may be also possible that our experimental setting may have provided only a limited answer to the question of whether multiple attentional states could be encoded and stored separately for the following reason. The specific setting of our experiments might have led participants to maintain the divided attentional state during the entire learning phase rather than switching back and forth because the trial types were switched frequently in an unpredictable manner. When the two different trial types are switched frequently in a random sequence, it is expected that a better strategy for the participants to maximize the accuracy and reduce the effort and the running time would be to always pay attention to the RSVP stream.
In many real-world situations, however, transition between two different attentional states more likely happens in a gradual and predictable manner. Perhaps, when changes in attentional states are not frequent or predictable, participants might also be able to encode and store both attentional states separately by flexibly allocating attentional resources. For example, what if visuomotor learning in different attentional states were temporally separated by a washout period? Future studies are required to test these various settings in order to investigate in what circumstances multiple states can be encoded and stored.
Choking under pressure
In daily activities, we often face distractions from various sources while we learn to interact with our environment. Here we demonstrate that this is not always disadvantageous. Instead, we demonstrated a paradoxical result that divided attention could be sometimes more beneficial for visuomotor learning than undivided attention if both learning and recall happen in the divided attentional state.
Another example of paradoxical interference led by allocating more attentional resources is “choking under pressure,” defined as unexpected performance impairment during competition (Addou, Krouchev, & Kalaska, 2011; Jordet & Hartman, 2008). One possible explanation for choking is that focusing attention on movements reverts an automatic skill back to an earlier conscious form of control. Accordingly, previous studies have shown that participants with highly practiced skills performed better when attention was allocated to the external events instead of their own movements (Beilock, Bertenthal, McCoy, & Carr, 2004).
Both attentional state–dependent memory and choking under pressure suggest that allocating more attentional resources does not always facilitate performance. Sometimes, allocating more attentional resources can interfere with performance. Despite the similarity, however, we believe that attentional state–dependent memory and choking under pressure are different phenomena. Unlike the notion of choking under pressure, the current study showed that the demand of attentional resources did not affect immediate motor performance. Furthermore, the attentional demand during recall also did not directly determine the recall performance. We observed a failure of memory retrieval when the consistency of attentional states between the early phase learning and recall was disrupted (e.g., EarlyRSVP–None or LateRSVP–RSVP groups). In contrast, as long as attention was continuously divided (e.g., EarlyRSVP–RSVP) or undivided (e.g., LateRSVP–None) during the early phase of learning, there was no difference in savings. Therefore, the observed attentional state–dependent retrieval is different from the choking under pressure phenomenon.
Mechanisms underlying attentional state–dependent visuomotor learning
To date, it has been hard for currently existing models on visuomotor learning to fully explain the current finding on attentional state–dependent visuomotor learning. Thus, a more generalizable model is needed to accommodate the interaction between attentional states and visuomotor learning. One of the representative motor learning models, for example, is a two-state model, proposed by Smith, Ghazizadeh, and Shadmehr (2006). In this model, a fast process enables fast initial learning, and a slow process contributes to long-term retention of motor memory. The two-state model posits that motor adaptation depends on at least two distinct systems that have different sensitivity to motor errors. The fast process is sensitive to motor error reduction during the early phase of learning but has poor retention. On the other hand, the slow process responds to motor error weakly but retains motor memory representation longer, presumably being more dominant during the late phase of learning. The two-state model can successfully account for various phenomena of motor adaptation, including savings and anterograde interference in which learning a second task interferes with the retrieval of the first task (Miall, Jenkinson, & Kulkarni, 2004).
However, it cannot fully account for the current results because we found that the early phase of learning was the critical time window for attentional state–dependent visuomotor memory. Because the fast process which is dominant for motor error reduction during the early phase of learning cannot retain the formed memory representation long enough, the two-state model cannot explain how the association between attentional state and visuomotor memory during the early phase of learning can be retained and reinstated at recall.
More recently, Lee and Schweighofer (2009) proposed a model in which the fast process contains a single state whereas the slow process contains multiple states that can be switched flexibly via a contextual cue. In their model, contexts are mainly defined by the task factors, such as rotational angles across visuomotor adaptation blocks (e.g., 20°–35°–20°). In order for this model to accommodate our results, for instance, it might need to implement attentional states into individual states for the slow process as in task contexts. Moreover, further investigation is needed to systematically describe how multiple slow states can be associated to facilitate the visuomotor learning exclusively for the early-learning consistent groups.
Together, the current finding challenges the existing computational models on visuomotor learning and requires a new, more generalizable model to understand the mechanism underlying the modulation by attentional states on visuomotor learning.
Conclusions
In summary, we find that attentional state can be integrated with visuomotor memory during encoding and function as an internal cue for retrieval of visuomotor memory. Importantly, the integration of attentional state and visuomotor memory is an exclusive and sustained process that occurs during the early phase of learning. We believe this new phenomenon of attentional state–dependent memory will provide important practical implications for various areas in which one pursues learning programs that are more efficient and generalizable to dynamic real-world settings (e.g., training drivers, pilots, and athletes, etc.).
Supplementary Material
Acknowledgments
This project is supported by the Brown University Salomon faculty research award and NIGMS-NIH IDeA P20GM103645 to J. H. S.
Commercial relationships: none.
Corresponding author: Joo-Hyun Song.
Email: joo-hyun_song@brown.edu.
Address: Department of Cognitive, Linguistic & Psychological Sciences, Brown University, Providence, RI, USA.
Contributor Information
Hee Yeon Im, Email: him3@mgh.harvard.edu.
Patrick Bédard, Email: patrick_bedard@brown.edu.
Joo-Hyun Song, joo-hyun_song@brown.edu, http://research.clps.brown.edu/songlab/.
References
- Addou, T.,, Krouchev N.,, Kalaska J. F. (2011). Colored context cues can facilitate the ability to learn and to switch between multiple dynamical force fields. Journal of Neurophysiology, 106, 163–183. [DOI] [PubMed] [Google Scholar]
- Ajemian R.,, D'Ausilio A.,, Moorman H.,, Bizzi E. (2013). A theory for how sensorimotor skills are learned and retained in noisy and nonstationary neural circuits. Proceedings of the National Academy of Sciences, USA, 110, E5078–E5087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atkeson C. G. (1989). Learning arm kinematics and dynamics. Annual Reviews of Neuroscience, 12, 157–183. [DOI] [PubMed] [Google Scholar]
- Baddeley A.,, Eysenck M. W.,, Anderson M. C. (2009). Memory. London: Psychology Press. [Google Scholar]
- Bédard P.,, Song J.-H. (2013). Attention modulates generalization of visuomotor adaptation. Journal of Vision, 13 (12): 20 1–10, doi:10.1167/13.12.12. [PubMed][Article] [DOI] [PubMed] [Google Scholar]
- Beilock S. L.,, Bertenthal B. I.,, McCoy A. M.,, Carr T. H. (2004). Haste does not always make waste: Expertise, direction of attention and speed versus accuracy in performing sensorimotor skills. Psychonomic Bulletin & Review, 11, 373–379. [DOI] [PubMed] [Google Scholar]
- Blaser E.,, Pylyshyn Z. W.,, Holcombe A. O. (2000). Tracking an object through feature space. Nature, 408, 196–199. [DOI] [PubMed] [Google Scholar]
- Brainard D. H. (1997). The Psychophysics Toolbox. Spatial Vision, 10, 433–436. [PubMed] [Google Scholar]
- Carrasco M.,, McElree B. (2001). Covert attention accelerates the rate of visual information processing. Proceedings of the National Academy of Sciences, USA, 98, 5363–5367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Censor N.,, Sagi D.,, Cohen L. G. (2012). Common mechanisms of human perceptual and motor learning. Nature Reviews Neuroscience, 13, 658–664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cleeremans A. (1993). Attention and awareness in sequence learning. In Proceedings of the 15th Annual Conference of the Cognitive Science Society. Hillsdale, NJ: Erlbaum. [Google Scholar]
- Cohen A.,, Ivry R.,, Keele S. W. (1990). Attention and structure in sequence learning. Journal of Experimental Psychology: Learning, Memory, and Cognition, 16, 17–30. [Google Scholar]
- Culham J. C.,, Brandt S.,, Cavanagh P.,, Kanwisher N. G.,, Dale A.,, Tootell R. B. H. (1998). Cortical fMRI activation produced by attentive tracking of moving targets. Journal of Neurophysiology, 80, 2657–2670. [DOI] [PubMed] [Google Scholar]
- Curran T.,, Keele S. W. (1993). Attentional and nonattentional forms of sequence learning. Journal of Experimental Psychology: Learning, Memory, & Cognition, 16, 17–30. [Google Scholar]
- Deiber M. P.,, Wise S. P.,, Honda M.,, Catalan M. J.,, Grafman J.,, Hallett M. (1997). Frontal and parietal networks for conditional motor learning: A positron emission tomography study. Journal of Neurophysiology, 78, 977–991. [DOI] [PubMed] [Google Scholar]
- Della-Maggiore V.,, Malfait N.,, Ostry D. J.,, Paus T. (2004). Stimulation of the posterior parietal cortex interferes with arm trajectory adjustments during the learning of new dynamics. The Journal of Neuroscience, 24, 9971–9976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eich J. E. (1980). The cue-dependent nature of state-dependent retrieval. Memory & Cognition, 8, 157–173. [DOI] [PubMed] [Google Scholar]
- Fitts P. M. (1964). Perceptual-motor skill learning. Melton A. W. (Ed.) Categories of human learning (pp 243–285). New York: Academic Press. [Google Scholar]
- Fitts, P. M.,, Posner M. I. (1967). Learning and skilled performance in human performance. Belmont, CA: Brock-Cole. [Google Scholar]
- Frensch P. A.,, Lin A.,, Buchner A. (1998). Learning versus behavioral expression of the learned: The effects of a secondary tone-counting task on implicit learning in the serial reaction task. Psychological Research, 61, 83–98. [Google Scholar]
- Gardiner J. M.,, Richardson-Klavehn A. (2000). Remembering and knowing. Tulving E., Craik F. I. M. (Eds.) The Oxford handbook of memory (2nd ed.) ( 229–244). New York: Oxford University Press. [Google Scholar]
- Halsband, U.,, Lange R. K. (2006). Motor learning in man: A review of functional and clinical studies. Journal of Physiology, 99, 414–424. [DOI] [PubMed] [Google Scholar]
- Hazeltine E.,, Grafton S.,, Ivry R. (1997). Attention and stimulus characteristics determine the locus of motor-sequence encoding. Brain, 120, 123–140. [DOI] [PubMed] [Google Scholar]
- Hegele M.,, Heuer H. (2010). Implicit and explicit components of dual adaptation to visuomotor rotations. Conscious Cognition, 19, 906–917. [DOI] [PubMed] [Google Scholar]
- Jiménez L.,, Vázquez G. A. (2005). Sequence learning under dual-task conditions: Alternatives to a resource-based account. Psychological Research, 69, 352–368. [DOI] [PubMed] [Google Scholar]
- Jordet G.,, Hartman E. (2008). Avoidance motivation and choking under pressure in soccer penalty shootouts. Journal of Sport and Exercise Psychology, 30, 450–457. [DOI] [PubMed] [Google Scholar]
- Krakauer J. W.,, Ghez C.,, Ghilardi M. F. (2005). Adaptation to visuomotor transformations: Consolidation, interference, and forgetting. The Journal of Neuroscience, 25, 473–478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J.-Y.,, Schweighofer N. (2009). Dual adaptation supports a parallel architecture of motor memory. The Journal of Neuroscience, 19, 10396–10404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miall R. C.,, Jenkinson N.,, Kulkarni K. (2004). Adaptation to rotated visual feedback: A re-examination of motor interference. Experimental Brain Research, 154, 201–210. [DOI] [PubMed] [Google Scholar]
- Monsell S. (2003). Task switching. Trends in Cognitive Sciences, 7, 134–140. [DOI] [PubMed] [Google Scholar]
- Morgan M. J.,, Ward R. M.,, Castet E. (1998). Visual search for a tilted target: Tests of spatial uncertainty models. The Quarterly Journal of Experimental Psychology, 51, 347–370. [DOI] [PubMed] [Google Scholar]
- Nissen M. J.,, Bullemer P. (1987). Attentional requirements of learning: Evidence from performance measures. Cognitive Psychology, 19, 1–32. [Google Scholar]
- Pashler H. (1994). Dual-task interference in simple tasks: Data and theory. Psychological Bulletin, 116, 220–244. [DOI] [PubMed] [Google Scholar]
- Pelli D. G. (1997). The VideoToolbox software for visual psychophysics: Transforming numbers into movies. Spatial Vision, 10, 437–442. [PubMed] [Google Scholar]
- Petersen S. E.,, Corbetta M.,, Miezin F. M.,, Shulman G. L. (1994). PET study of parietal involvement in spatial attention: Comparison of different task types. Canadian Journal of Experimental Psychology, 48, 319–338. [DOI] [PubMed] [Google Scholar]
- Preilowski B. (1977). Phases of motor skills acquisition: A neuropsychological approach. Journal of Human Movement Studies, 3, 169–181. [Google Scholar]
- Redding G. M.,, Wallace B. (1996). Adaptive spatial alignment and strategic perceptual-motor control. Journal of Experimental Psychology: Human Perception and Performance, 22, 379–394. [DOI] [PubMed] [Google Scholar]
- Rowland L. A.,, Shanks D. R. (2006). Attention modulates the learning of multiple contingencies. Psychonomic Bulletin & Review, 13, 643–648. [DOI] [PubMed] [Google Scholar]
- Shadmehr R.,, Holcomb H. H. (1997). Neural correlates of motor memory consolidation. Science, 277, 821–825. [DOI] [PubMed] [Google Scholar]
- Shadmehr R.,, Mussa-Ivaldi F. A. (1994). Adaptive representation of dynamics during learning of a motor task. The Journal of Neuroscience, 14, 3208–3224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith M. A.,, Ghazizadeh A.,, Shadmehr R. (2006). Interacting adaptive processes with different timescales underlie short-term motor learning. PLoS Biology, 4, e179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song J.-H.,, Bédard P. (2013). Allocation of attention for dissociated visual and motor goals. Experimental Brain Research, 226, 209–219. [DOI] [PubMed] [Google Scholar]
- Song J.-H.,, Bédard P. (2015). Paradoxical benefits of dual-task contexts for visuomotor memory. Psychological Science, 26, 148–158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spitzer H.,, Desimone R.,, Moran J. (1988). Increased attention enhances both behavioral and neuronal performance. Science, 240, 338–340. [DOI] [PubMed] [Google Scholar]
- Taylor J. A.,, Thoroughman K. A. (2007). Divided attention impairs human motor adaptation but not feedback control. Journal of Neurophysiology, 98, 317–326. [DOI] [PubMed] [Google Scholar]
- Taylor J. A.,, Thoroughman K. A. (2008). Motor adaptation scaled by the difficulty of a secondary cognitive task. PLoS ONE, 3, e2485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ungerleider L. G.,, Doyon J.,, Karni A. (2002). Imaging brain plasticity during motor skill learning. Neurobiology of Learning and Memory, 78, 553–564. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


