Abstract
Recent research has demonstrated that adaptation to a visuomotor distortion systematically influenced movements to auditory targets in adults and typically developing (TD) children, suggesting that the adaptation of spatial-to-motor transformations for reaching movements is multisensory (i.e., generalizable across sensory modalities). The multisensory characteristics of these transformations in children with developmental coordination disorder (DCD) have not been examined. Given that previous research has demonstrated that children with DCD have deficits in sensorimotor integration, these children may also have impairments in the formation of multisensory spatial-to-motor transformations for target-directed arm movements. To investigate this hypothesis, children with and without DCD executed discrete arm movements to visual and acoustic targets prior to and following exposure to an abrupt visual feedback rotation. Results demonstrated that the magnitudes of the visual aftereffects were equivalent in the TD children and the children with DCD, indicating that both groups of children adapted similarly to the visuomotor perturbation. Moreover, the influence of visuomotor adaptation on auditory-motor performance was similar in the two groups of children. This suggests that the multisensory processes underlying adaptation of spatial-to-motor transformations are similar in children with DCD and TD children.
Keywords: Developmental coordination disorder, Sensorimotor adaptation, Visuomotor, Vision, Audition, Reaching
Introduction
The execution of goal-directed reaching is thought to depend on internal representations that transform sensory information about the spatial localization of the arm and target into the appropriate motor commands (Bullock et al. 1993; Shadmehr and Wise 2005; Wolpert and Kawato 1998). These transformations are typically investigated with visuomotor adaptation tasks during which participants execute discrete reaching movements to visual stimuli under conditions of perturbed feedback (e.g., Kagerer et al. 1997; Krakauer et al. 2000). However, the visual, auditory, and somatosensory systems can all provide information that is critical for the successful execution of goal-directed reaches. Recent research in our laboratory demonstrated that adaptation to an abrupt visuomotor perturbation systematically influenced auditory-motor performance in 5–12-year-old typically developing (TD) children and adults, suggesting that the adaptation of spatial-to-motor transformations is multisensory (Kagerer and Contreras–Vidal 2009; King et al. 2009). Specifically, an updated spatial-to-motor transformation generalizes across sensory modalities and is utilized for reaching movements to both visual and acoustic stimuli. Although visuomotor adaptation in children with movement difficulties such as developmental coordination disorder (DCD) has been investigated (Kagerer et al. 2004, 2006), little is known about the multisensory characteristics of spatial-to-motor transformations in these children. This knowledge gap is the focus of the current research.
Developmental coordination disorder is characterized by marked impairments in the performance of daily living activities that necessitate motor coordination, but cannot be attributed to other general medical conditions such as cerebral palsy, muscular dystrophy, or pervasive developmental disorders (APA 2000). The extant literature is replete with extensive characterizations of behavioral impairments in children with DCD (e.g., Bo et al. 2008; Kagerer et al. 2004; Mackenzie et al. 2008; Oliveira et al. 2006; Schoemaker et al. 2001; Volman and Geuze 1998); however, a unifying, mechanistic explanation is currently lacking. One of the more common explanations posits that children with DCD have behavioral impairments that arise from difficulties in sensorimotor integration (Jongmans et al. 2003; Kagerer et al. 2004, 2006; Piek and Dyck 2004; Schoemaker et al. 2001). More specifically, children with DCD are thought to have deficits in motor tasks that require multisensory (i.e., visuo-proprioceptive) integration (Mon–Williams et al. 1999; Sigmundsson et al. 1997). In the context of goal-directed reaching, information on target location, one of the critical inputs to spatial-motor transformations, can be provided by multiple sensory modalities. Therefore, an investigation of the multisensory characteristics of the transformations for goal-directed reaching may provide insights into the mechanisms underlying the broader behavioral impairments in children with DCD.
The current study sought to replicate and extend previous research in our laboratory investigating sensorimotor adaptation in children with DCD. We employed a cross-modal adaptation paradigm previously used in adults (Kagerer and Contreras–Vidal 2009) and TD children (King et al. 2009) to examine the hypothesis that the multisensory adaptation of spatial-to-motor transformations is impaired in children with DCD. Assuming that the previously reported sensorimotor difficulties in children with DCD represent a “global” multisensory-motor impairment (i.e., not limited to visuo-proprioceptive interactions), it should follow that these children also have difficulties in the multisensory adaptation of spatial-to-motor transformations for goal-directed reaching. The cross-modal (visual-auditory) adaptation paradigm employed in the current study provides a window to examine this hypothesis. Participants performed discrete reaching movements to acoustic stimuli before and after exposure to a visuomotor rotation. Visuomotor distortions are typically used to examine the adaptability of spatial-to-motor transformations (e.g., Kagerer et al. 1997; Krakauer et al. 2000). By having participants execute reaching movements to acoustic stimuli before and after visuomotor adaptation, we can probe the multisensory characteristics of acquired spatial-to-motor transformations. If children with DCD have deficits in the formation of multisensory transformations, then adaptation to the visuomotor distortion will have a smaller impact on auditory-motor performance in the children with DCD compared to the TD children.
Methods
Participants
Thirteen TD children (10 boys) and seven children with DCD (6 boys) between 9 and 11 years of age participated in the study. We elected to restrict our age range to 9–11 years because previous research in our laboratory has demonstrated age-related changes in sensorimotor control of the arm in TD children around 7–8 years of age (Bo et al. 2006; Contreras–Vidal 2006; Contreras–Vidal et al. 2005; King et al. 2010). To avoid potential complications with the interpretation of the data due to these sensorimotor improvements, the current study focused on 9–11-year-old children with and without DCD. Data from the TD children were included in a developmental landscape reported in a previous publication (King et al. 2009). Children with DCD were included in the study if they met the following three criteria. One, performance on the Movement Assessment Battery for Children (MABC; Henderson and Sugden 1992) was below the 5th percentile. Two, a pediatrician independently diagnosed each child with DCD, including a screening for neurological disorders (Physical and Neurological Examination for Subtle Signs; PANESS) (Denckla 1985). Three, cognitive development was considered typical based on the Woodcock–Johnson Psycho-Educational Battery-Revised. Detailed participant characteristics of the children with DCD are included in Table 1. MABC scores for the TD children were above the 20th percentile. All participants were right-handed, based on their preferred hand for everyday activities and confirmed by the MABC criteria. All procedures were approved by the Institutional Review Board at the University of Maryland, College Park. Each child’s parent or legal guardian provided informed consent prior to participation. Children received a toy prize and a modest monetary compensation following completion of the experiment.
Table 1.
Participant information for children diagnosed with DCD
| DCD ID # | Age (years) | Gender | MABC %ile |
|---|---|---|---|
| 28 | 9.5 | M | 1 |
| 33 | 11.4 | M | 3 |
| 36 | 9.2 | M | 5 |
| 40 | 9.6 | F | <1 |
| 45 | 10.7 | M | <1 |
| 68 | 10.4 | M | 2 |
| 69 | 10.3 | M | <1 |
Procedures
Experimental set-up and procedures were identical to those employed in a previous study (see King et al. 2009 for a detailed description). Participants were asked to execute discrete aiming movements with a digitizing pen on a tablet positioned below a horizontally oriented computer monitor. Real-time visual feedback of each participant’s performance as well as the start and target circles was provided on the computer monitor. Vision of the hand was occluded by the experimental apparatus; Cartesian (x/y) coordinates of the computer pen were recorded at a sampling frequency of 99 Hz.
There were two experimental conditions. During the visual condition, participants executed 9 cm aiming movements to one of three visual targets, positioned 25°, 90°, or 155° with respect to the start position. During the auditory condition, participants were blindfolded and instructed to move toward one of two acoustic stimuli. These stimuli were located either 45° or 135° with respect to the start position. Targets were presented in a randomized order with the constraint that each target position appeared the same number of times within each condition (auditory/vision) and experimental phase (see below). During the auditory post-exposure phase, the 135° target appeared one more time than the 45° target due to an odd number of trials. The target sequence remained constant across participants. For both conditions, participants were instructed to move as fast and as accurately as possible. All participants performed the task with their dominant (right) hand.
The experimental protocol contained five phases. Participants first completed a visual baseline phase. During task performance of this phase, accurate visual feedback of the pen trace was displayed on the monitor (24 trials). The participants were then blindfolded in order to complete the auditory baseline phase (24 trials). During the third experimental phase (visual condition), the visual feedback of the participants’ movement paths provided on the computer monitor was rotated 60° clockwise (CW; 126 trials). Following this exposure to the visuomotor distortion, participants repeated the auditory and visual baseline conditions, respectively. Each of these post-exposure phases consisted of nine trials. The purpose of the auditory post-exposure was to determine the effect of visuomotor adaptation on auditory-motor performance (i.e., multisensory effects). The visual post-exposure phase was critical for the assessment of visual aftereffects, which provided a measure of the level of adaptation to the visuomotor distortion. The experimental protocol consisted of a total of 192 trials.
Data analysis
The time series of each trial was dual-pass filtered with a Butterworth filter (8th order; 10 Hz cutoff). Performance during the visual condition was assessed using initial directional error (IDE), variability of IDE (Var IDE), and root mean squared error (RMSE). IDE (measured in degrees) was calculated as the directional error between the participant’s trajectory and an ideal vector connecting the start and target positions 80 ms after movement onset. Since it is computed prior to any feedback-dependent corrective movements, IDE reflects errors in movement planning. Var IDE was computed as the standard deviation of an individual’s IDE scores across the trials within a given experimental phase. RMSE (cm) was a spatial error between the actual trajectory and the straight-line vector between the start and target positions (Contreras–Vidal et al. 2005).
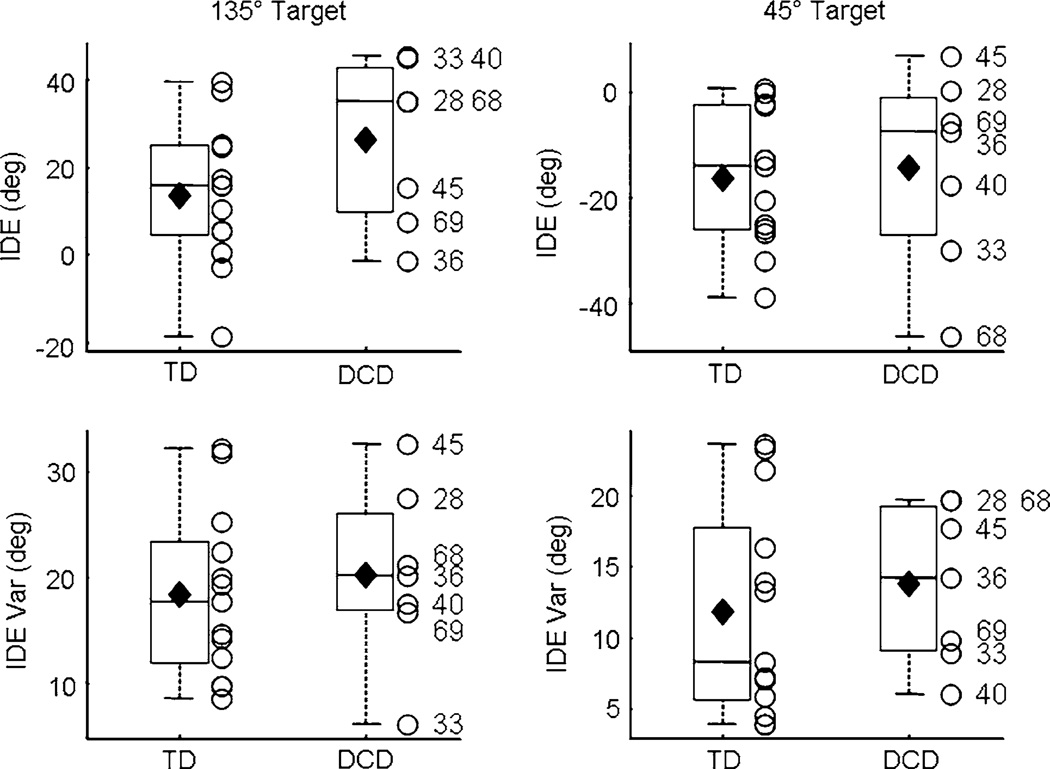
The auditory condition was assessed with IDE and Var IDE. However, consistent with previous research (King et al. 2009), movements during the auditory baseline were directed horizontal with respect to the two acoustic target positions. Since IDE was computed by subtracting the direction of the ideal trajectory from the direction of the actual trajectory, movements to the 135° target consistently resulted in positive IDE values whereas movements to the 45° target were consistently negative (Fig. 2). Thus, analyses for the auditory condition were separated by target position and Bonferroni adjusted. As RMSE is a spatial error between the actual and ideal movement trajectories, it represents errors in the movement plan as well as feedback-dependent corrective movements. When online feedback is not available, as in the auditory condition when participants were blindfolded, there are no feedback-dependent adjustments. Thus, RMSE during the auditory condition can be considered redundant with IDE and was not statistically analyzed.
Fig. 2.
Auditory baseline. IDE (top row) and IDE Var (bottom) during auditory baseline are shown for the two acoustic targets. Horizontal lines depict the lower quartile, median, and upper quartile values; filled diamond = group mean; + = outliers; open circle = individual participants. Outliers are values beyond 1.5 times the interquartile range. Subject ID numbers for the participants with DCD are provided
Baseline data were analyzed with t tests with GROUP as the independent variable. To assess aftereffects and multisensory effects in the visual and auditory post-exposure phases, respectively, repeated measures ANOVAs were conducted with GROUP as the between-subjects factor and BLOCK (pre-/post-exposure) as the within-subjects factor. Given the relatively small sample size and the increased variability evident in children with DCD (e.g., Bo et al. 2008; Mackenzie et al. 2008; Schoemaker et al. 2001), we conducted secondary, non-parametric analyses to provide further support of our main findings. Specifically, the data from the current experiment were randomly resampled, with replacement, to form 3,000 bootstrap data sets for each dependent measure, block and group (Efron and Tibshirani 1994; Zieffler et al. 2011). This approach utilizes the collected data to effectively simulate the results of the experiment thousands of times. Monte Carlo P values, using a correction suggested by Davison and Hinkley (1997), were computed to determine if the mean values from the two groups were significantly different (α = 0.05). Standardized effect sizes (Cohen’s d) and their corresponding nonparametric bootstrap bias-corrected and accelerated 95 percentile intervals using 3,000 bootstrap replicates were computed. The computation of effect sizes and corresponding percentile intervals was suggested by Jones and Tukey (2000) as a preferred method of significance testing. For the current study, the results of the bootstrap test statistics were used to confirm the findings of the t tests and repeated measures ANOVAs employed with the original data. This bootstrap approach is beneficial for several reasons: (1) it is not dependent on the distributional assumption of normality; and, (2) it utilizes the original data to simulate the results of the experiment thousands of times, effectively evaluating the accuracy of parameter estimates computed from a sample (Efron and Tibshirani 1994).
Results
Baseline
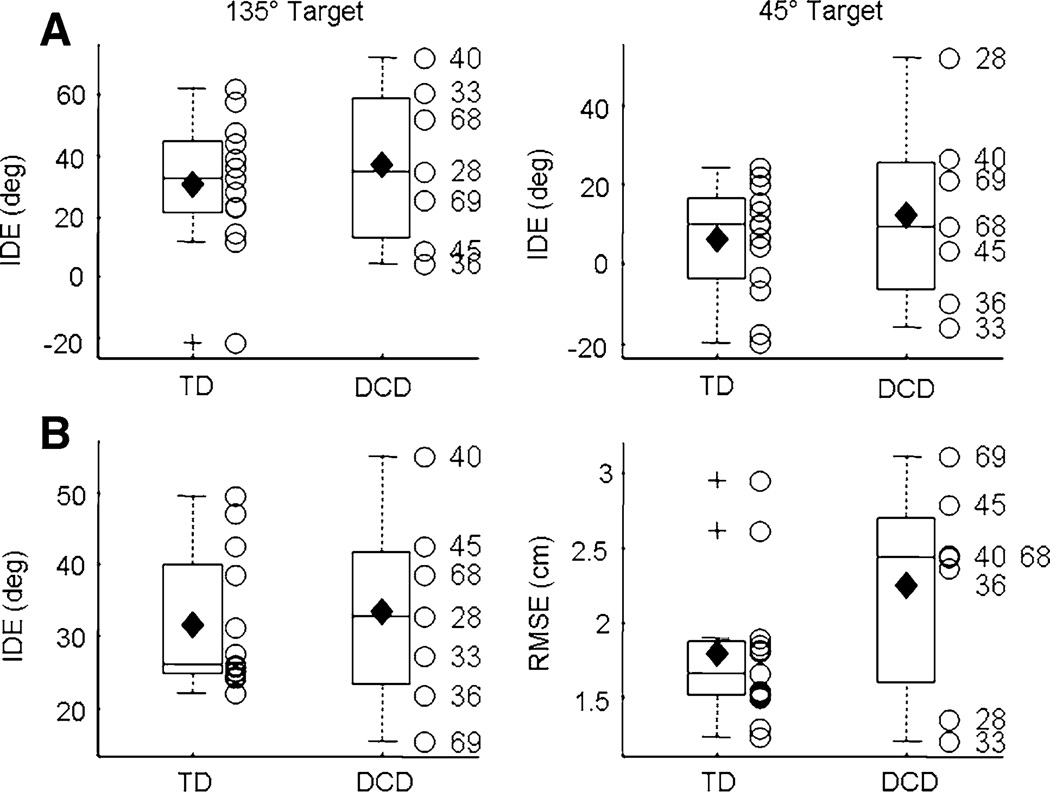
Visual condition
In order to address the heterogeneity of the behavioral deficits evident in DCD, box plots depict group means as well as individual data. There were no significant differences between the two groups for IDE (P > 0.05; Fig. 1). A significant effect of GROUP was revealed for both RMSE (t(18) = 2.61; P = 0.018) and Var IDE (t(18) = 2.50; P = 0.022) during visual baseline (Fig. 1), indicating that the children with DCD exhibited poor spatial control and increased directional variability during the visuomotor task. These results are consistent across the sample of children with DCD, as only one participant (#40) had a RMSE value less than the mean of the TD children and three out of the seven children with DCD exhibited RMSE values greater than the upper quartile of the TD children. Similarly, none of the children with DCD demonstrated Var IDE scores below the TD average.
Fig. 1.
Visual baseline. IDE (top), IDE Var (middle), and RMSE (bottom) for the two groups of participants during the visual baseline phase. Horizontal lines depict the lower quartile, median, and upper quartile values; filled diamond = group mean; + = outliers; open circle = individual participants. Outliers are values beyond 1.5 times the interquartile range. Subject ID numbers for the participants with DCD are provided
To provide additional support for the results presented above, non-parametric bootstrap tests were employed to examine the differences between the means from the two groups of children. There were no group differences in IDE (Monte Carlo P value = 0.85; Cohen’s d = −0.08, 95% interval = [−1.11, 0.09]); but, Var IDE from the children with DCD was significantly larger compared to the TD children (P = 0.019; Cohen’s d = −1.17, 95% interval = [−2.03, −0.13]). Group differences in RMSE were also significant (P = 0.019; Cohen’s d = −1.22, 95% interval = [−2.37, −0.13]). Collectively, these results confirm the findings from the two-sample t tests conducted on the original data.
Auditory condition
Group differences during auditory baseline were investigated by separate t tests on IDE and Var IDE for each target location (Bonferroni adjusted). There were no significant differences between the two groups of children for either of the two targets (Fig. 2). Similar to visual baseline, data from the auditory baseline phase were utilized to form 3,000 bootstrap data sets for each group of children. Results were consistent with those depicted in Fig. 2 (all Monte Carlo P values > 0.1; all Cohen’s d < |0.73|).
Exposure
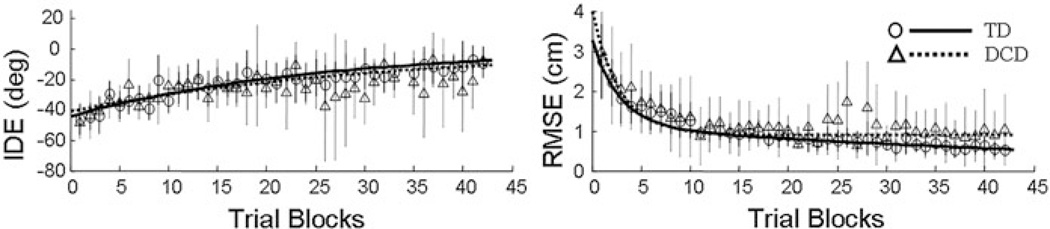
Participants from both groups reduced the magnitude of the movement errors over the course of the exposure to the visuomotor rotation (Fig. 3). These performance improvements, as assessed by IDE and RMSE, were best fit with single- and double-exponential functions, respectively. As the aim of the current study was on the interaction between visuo- and auditory-motor control, our statistical analysis was limited to the pre- and post-exposure phases of the experimental paradigm. A detailed, statistical characterization of the exposure phase for the TD children and children with DCD was included in King et al. (2011).
Fig. 3.
Exposure: Mean Trajectories. Mean IDE (left), and RMSE (right) values are shown for the children with DCD (triangles, dotted lines) and TD children (circles, solid lines) as a function of exposure blocks. Vertical lines depict ± one SD. Trial blocks were computed as a mean of three consecutive trials. Right panel reprinted from Research in Developmental Disabilities, 2011, King BR, Harring JR, Oliveira MA, Clark JE, Statistically characterizing intra- and inter-individual variability in children with developmental coordination disorder, with permission from Elsevier
Post-exposure
Auditory condition
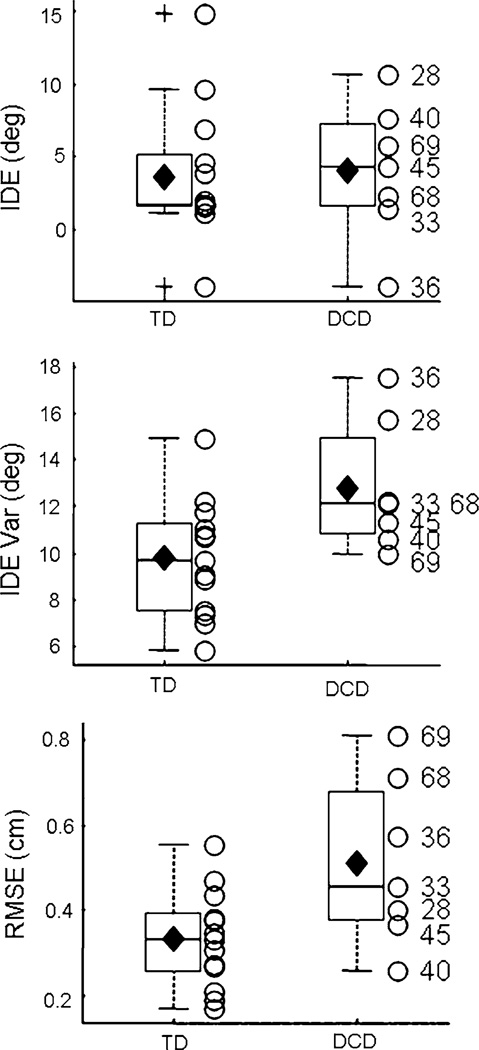
Immediately following the visual exposure phase, participants completed nine trials of the auditory condition. To determine the effects of visuomotor adaptation on auditory-motor performance (i.e., multisensory effects), the auditory post-exposure phase was analyzed with separate 2 (GROUP) × 2 (BLOCK: baseline/post-exposure) ANO-VAs on IDE for each of the two target positions (Bonferroni adjusted). Similar to baseline, results from post-exposure focused on both group differences and individual data. Results revealed a BLOCK effect for both acoustic target positions (45°: F(1,18) = 27.06, P < 0.0001; 135°: F(1,18) = 18.16, P = 0.0005). The magnitude of IDE during auditory post-exposure was significantly larger than during baseline (Fig. 4a), indicating that adaptation to the visuomotor distortion significantly impacted auditory-motor performance. Please note that the data depicted in Fig. 4a are not baseline subtracted. Significant inter-sensory effects are based on differences in IDE between pre- (Fig. 2) and post-exposure (Fig. 4a). The main effect of GROUP and the GROUP × BLOCK interaction were not significant, suggesting that this multisensory effect was similar in both groups of children. Results from the bootstrap tests also revealed no differences between the two groups of children (45° target: P = 0.52; Cohen’s d = −0.26, 95% interval = [−1.32, 0.77]; 135° target: P = 0.45; Cohen’s d = −0.36, 95% interval = [−1.44, 0.78]). An examination of the individual data revealed that two of the participants with DCD (#s 36, 45) demonstrated minimal multisensory effects to both target positions. These data potentially indicate that this subset of children with DCD have impairments in multisensory adaptation; however, this conclusion warrants further investigation as a subset of the TD children demonstrated minimal multisensory effects as well.1
Fig. 4.
Post-exposure. a Multisensory effects for the two target positions during auditory post-exposure. b Visual aftereffects: IDE (left) and RMSE (right). Horizontal lines depict the lower quartile, median, and upper quartile values; filled diamond = group mean; + = outliers; open circle = individual participants. Outliers are values beyond 1.5 times the interquartile range. Subject ID numbers for the participants with DCD are provided
Visual condition
Visual aftereffects (Fig. 4b) were assessed by separate 2 (GROUP) × 2 (BLOCK) ANOVAs for RMSE and IDE. Both IDE and RMSE were larger in visual post-exposure as compared to visual baseline (IDE: F(1,18) = 131.2, P < 0.0001; RMSE: F(1,18) = 157.1, P < 0.0001). A significant GROUP effect for RMSE was also found (F(1,18) = 4.60, P = 0.046), as the children with DCD demonstrated larger RMSE values, collapsed across baseline and post-exposure, than the TD children. This finding is consistent with the differences between groups in visual baseline. The GROUP main effect for IDE and the GROUP × BLOCK interactions for both IDE and RMSE were not significant (P > 0.05). Collectively, results indicate that the participants demonstrated significant visual aftereffects following adaptation to the visuomotor perturbation and there were no differences in the magnitude of the aftereffects between the two groups of children. The lack of differences between the two groups was supported by the non-parametric bootstrap tests (IDE: P = 0.72; Cohen’s d = −0.16, 95% interval = [−1.25, 0.87]; RMSE: p = 0.09; Cohen’s d = −0.80, 95% interval = [−2.25, 0.41]).2 DCD participant #69 demonstrated aftereffects smaller than the lower quartile for the TD children, indicating that this participant did not adapt to the perturbation.
Discussion
The results of our study indicated that children with DCD, as compared to the TD children, demonstrated equivalent multisensory effects and visual aftereffects in the auditory and visual post-exposure phases, respectively. The lack of differences between the two groups of children during the visual post-exposure phase is consistent with previous research examining adaptation to abrupt visual feedback distortions (Kagerer et al. 2006). The similar multisensory effects demonstrated during the auditory post-exposure phase suggest that the processes underlying adaptation of multisensory spatial-to-motor transformations are not impaired in children with DCD.
Multisensory adaptation in children with DCD
The lack of differences between the two groups of children in the magnitude of the multisensory effects is somewhat surprising considering the extant literature has reported children with DCD have deficits in sensorimotor integration (Jongmans et al. 2003; Mon–Williams et al. 1999; Sigmundsson et al. 1997; Wilson and McKenzie 1998). In previous studies, children with DCD were significantly less accurate when localizing visual and/or proprioceptive targets with an unseen hand, a task that requires integrating proprioceptive information about the moving hand with visual and/or proprioceptive information specifying target location (Mon–Williams et al. 1999; Sigmundsson et al. 1997). Based on the results of the current study, these deficits in sensorimotor integration do not extend to multisensory adaptation of acquired spatial-to-motor transformations for goal-directed arm movements. Previous research in our laboratory demonstrated that adaptation of spatial-to-motor transformations is multisensory in 5–12-year-old TD children and adults (Kagerer and Contreras–Vidal 2009; King et al. 2009), indicating that acquired spatial-to-motor transformations are used for goal-directed reaching movements toward both visual and acoustic stimuli. One potential explanation of these previous data is that sensory estimates of target location are mapped to a common reference frame, regardless of target modality. This estimate in a common reference frame serves as an input to the spatial-to-motor transformation that will drive the hand toward the desired target location (Bullock et al. 1993). Additional support for this explanation comes from research in adults demonstrating that visual, auditory, and proprioceptive targets are specified in eye- or gaze-centered reference frames (Pouget et al. 2002). The equivalent multisensory effects in children with DCD and TD children demonstrated in the current study suggests that estimates of target position, independent of target modality, are also mapped to a common reference frame in children with DCD. Moreover, the updated spatial-to-motor transformation acquired during visuomotor adaptation generalizes across sensory modalities in children with DCD.
A potential future direction of this research is to examine age-related differences in multisensory adaptation in children with DCD (e.g., across 6–12-year-olds with DCD). It is possible that multisensory adaptation in 9–11-year-old children with DCD is equivalent to their age-similar TD peers; however, younger children with DCD (i.e., 6–8 years) may have deficits in multisensory adaptation. Previous research has demonstrated a similar age by group interaction in reaching performance with and without prism goggles that horizontally displace the visual field (Zoia et al. 2005).
Visuomotor adaptation in children with DCD
Equivalent visual aftereffects in the two groups of children were not surprising considering the sensorimotor perturbation employed in the current study. Specifically, Kagerer et al. (2006) demonstrated that children with DCD adapted similarly to their TD peers if the perturbation was suddenly introduced (i.e., 60° perturbation for duration of exposure), as in this study. Conversely, if the perturbation was gradually introduced (i.e., 60° perturbation presented in increments of 10°), children with DCD demonstrated significantly smaller aftereffects. Previous literature has suggested that adaptation to gradual and abrupt perturbations may depend on different neural structures. The cerebellum has traditionally been thought to be critical for gradual adaptation (Robertson and Miall 1999) and research on individuals with Parkinson’s disease has indicated that adaptation to abrupt perturbations is dependent on the basal ganglia (Contreras–Vidal et al. 2002; Teulings et al. 2002). Based on these previous studies and the differential effects of gradual and abrupt adaptation in children with DCD, Kagerer et al. (2006) suggested that children with DCD may have functional deficits of the cerebellum. Other research has also suggested the cerebellum as a potential neural substrate underlying the behavioral deficits in children with DCD (Bo et al. 2008; Cantin et al. 2007; Lundy–Ekman et al. 1991; O’Hare and Khalid 2002). However, the suggestion that the cerebellum is specialized for gradual adaptation is not without opposition as a more recent study has demonstrated that cerebellar degeneration impacted adaptation to an abruptly-introduced force field whereas gradual adaptation was relatively spared (Criscimagna–Hemminger et al. 2010). Based on this research on patients with cerebellar damage, the equivalent level of visuomotor adaptation in the children with DCD and the TD children in the current study would speak against compromised cerebellar functioning in DCD. Although identifying the neural substrate(s) underlying DCD is outside the scope of the current paper, it is important to highlight some methodological differences between the two studies investigating the cerebellum in gradual and abrupt adaptation. First, Robertson and Miall (1999) employed a visuomotor rotation paradigm during which visual feedback was rotated up to a magnitude of 15° whereas Criscimagna–Hemminger et al. (2010) utilized a velocity-dependent force field. Second, Robertson and Miall experimentally and reversibly inactivated the dentate nucleus of a monkey. Conversely, the participants in Criscimagna–Hemminger and colleagues were patients with varying severity and types of cerebellar degeneration. It is possible that the type of perturbation and/or the severity and location of cerebellar damage contributed to the equivocal results of these two studies.
It could be argued that the presence of the auditory post-exposure phase in between adaptation and visual post-exposure contaminated the assessment of group differences in the magnitude of visual aftereffects. Specifically, it is possible that the auditory post-exposure phase may have partially “washed out” the visual aftereffects and this washout differentially impacted the two groups of children. There are two pieces of evidence that refute this potential interpretation. First, previous research in adults has demonstrated that kinematic errors (i.e., a visual error signal) are critical for de-adaptation following exposure to a force field (Scheidt et al. 2000). In the absence of such error signals, the persistence of the aftereffects was much greater. In the current experiment, the participants were blindfolded during auditory post-exposure. The lack of a visual error signal during this phase likely prevented any substantial washout. This was confirmed by examining the inter-sensory effects as a function of trials during auditory post-exposure. We conducted linear regressions on the decay of IDE during auditory post-exposure for both groups of children and acoustic target locations. The slopes of these regressions, reflecting changes in IDE as a function of trials, were not significant (P > 0.05), indicating that there was no substantial washout. Second, and as discussed above, a previous study in our laboratory demonstrated similar levels of adaptation between children with DCD and TD children following exposure to an abrupt visual feedback rotation (Kagerer et al. 2006). In this earlier study, there was no auditory condition between the visual exposure and post-exposure phases. The consistency in the results with this previous study suggests that the lack of significant differences in visual aftereffects reported in the current study can not be attributed to any washout in the auditory condition.
Heterogeneity of behavioral performance
Research investigating children with DCD has consistently reported substantial heterogeneity of behavioral deficits (Bo et al. 2008; Hoare 1994; Schoemaker et al. 2001; Volman and Geuze 1998). This between-subject variability can influence the analysis of behavioral data; and, traditional statistical approaches fail to probe data at the individual level. Recent research in our laboratory utilized a flexible, analytic technique to statistically parameterize repeated measures data from children with DCD at the population and individual levels of analysis (King et al. 2011). Our approach in the current experiment also focused on the performance of each individual with DCD; and, we were able to identify children who struggled to perform the sensorimotor task. For example, two of the children with DCD (#s 36 and 45) failed to demonstrate substantial multisensory effects to the two acoustic targets during the post-exposure phase. This potentially suggests that these two children with DCD have difficulties in multisensory adaptation; specifically, the updated spatial-to-motor transformations acquired during the visual feedback rotation do not generalize to other sensory modalities (i.e., audition). One potential interpretation is that the estimates of target location, independent of sensory modality, are not mapped to a common reference frame in these two children, a computation that is thought to be a function of the posterior parietal cortex (Cohen and Andersen 2000). Within this interpretation, estimates of visual target positions in these two children are specified in a gaze-centered reference frame and estimates of acoustic target positions are specified in a head-centered reference frame. With targets specified in distinct reference frames, the acquired spatial-to-motor transformation failed to generalize across modalities. However, this interpretation is purely speculative and additional research is necessary to provide further support. It should also be emphasized that a subset of the TD children did not demonstrate substantial multisensory effects as well. Thus, it is not clear whether the lack of intersensory effects in these two children with DCD (#s 36 and 45) can be directly attributed to DCD-related behavioral deficits.
The visual aftereffect of DCD #69 was less than the lower quartile of the TD children, indicating that this child struggled to update a spatial-to-motor transformation appropriate for the novel sensorimotor environment (Kagerer et al. 1997; Krakauer et al. 2000). This suggests that this individual may have deficits in cerebellar and/or basal ganglia functioning, two neural structures thought to be critical for visuomotor adaptation (Contreras– Vidal et al. 2002; Criscimagna–Hemminger et al. 2010; Robertson and Miall 1999; Teulings et al. 2002).
Spatial-motor control in children with DCD
Children with DCD demonstrated increased RMSE values, as compared to the TD children, during visual baseline. This suggests that these children have poor spatial-motor control during the execution of discrete aiming movements. Previous research in our laboratory showed children with DCD demonstrated increased movement time and movement length during a similar aiming task (Kagerer et al. 2004). The current research extended these previous findings, demonstrating that the movement trajectories of children with DCD are also characterized by increased spatial errors, compared to TD children.
Conclusion
In summary, our results demonstrated that adaptation to a visuomotor perturbation systematically impacted auditory-motor performance in both children with DCD and TD children. Moreover, there were no differences in this multisensory effect between the two groups, indicating that adaptation of spatial-to-motor transformations that facilitate goal-directed reaching is multisensory in children with DCD.
Acknowledgments
This research was funded by National Institutes of Health R01HD42527 (JEC) and R03HD050372 (FAK). We would like to thank the children and their parents for participating in our study and Melissa M. Pangelinan and two anonymous reviewers for feedback during the preparation of this manuscript.
Footnotes
Previous research has standardized (i.e., z-transformed) post-exposure data relative to the baseline phases to account for any inherent group differences in the mean and/or variability of baseline performance (Kagerer et al. 2004, 2006). A two-sample t test on standardized IDE values from auditory post-exposure phase revealed no significant differences between the children with DCD and TD children (P = 0.36). Moreover, non-parametric bootstrap analyses on the standardized data also revealed no significant differences (P = 0.34; Cohen’s d = 0.43, 95% interval = [−0.63, 1.48]). These results are consistent with the findings based on the non-standardized data presented in the main text.
Two-sample t test on standardized IDE and RMSE values from the visual post-exposure phase revealed no significant differences between the children with DCD and TD children (IDE: P = 0.24; RMSE: P = 0.97). Non-parametric bootstrap analyses on the standardized data also revealed no significant differences (IDE: P = 0.22; Cohen’s d = 0.57, 95% interval = [−0.64, 1.78]; RMSE: P = 0.97; Cohen’s d = 0.02, 95% interval = [−1.21, 1.27]). These results are consistent with the findings based on the non-standardized data presented in the main text.
Contributor Information
Bradley R. King, Email: bking7@umd.edu, Cognitive Motor Neuroscience Laboratory, Department of Kinesiology, University of Maryland, 2351 SPH Building, College Park, MD 20742-2611, USA.
Florian A. Kagerer, Department of Kinesiology, Michigan State University, East Lansing, MI, USA Neuroscience Program, Michigan State University, East Lansing, MI, USA.
Jeffrey R. Harring, Department of Measurement, Statistics and Evaluation, University of Maryland, College Park, MD, USA
Jose L Contreras-Vidal, Cognitive Motor Neuroscience Laboratory, Department of Kinesiology, University of Maryland, 2351 SPH Building, College Park, MD 20742-2611, USA; Graduate Program in Neuroscience and Cognitive Science, University of Maryland, College Park, MD, USA; Department of Bioengineering, University of Maryland, College Park, MD, USA.
Jane E. Clark, Cognitive Motor Neuroscience Laboratory, Department of Kinesiology, University of Maryland, 2351 SPH Building, College Park, MD 20742-2611, USA Graduate Program in Neuroscience and Cognitive Science, University of Maryland, College Park, MD, USA.
References
- APA. Diagnostic and statistical manual of mental disorders. Washington: American Psychiatric Association; 2000. [Google Scholar]
- Bo J, Contreras-Vidal JL, Kagerer FA, Clark JE. Effects of increased complexity of visuo-motor transformations on children’s arm movements. Hum Mov Sci. 2006;25:553–567. doi: 10.1016/j.humov.2006.07.003. [DOI] [PubMed] [Google Scholar]
- Bo J, Bastian AJ, Kagerer FA, Contreras-Vidal JL, Clark JE. Temporal variability in continuous versus discontinuous drawing for children with developmental coordination disorder. Neurosci Lett. 2008;431:215–220. doi: 10.1016/j.neulet.2007.11.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bullock D, Grossberg S, Guenther FH. A self-organizing neural model of motor equivalent reaching and tool use by a multijoint arm. J Cogn Neurosci. 1993;5:408–435. doi: 10.1162/jocn.1993.5.4.408. [DOI] [PubMed] [Google Scholar]
- Cantin N, Polatajko HJ, Thach WT, Jaglal S. Developmental coordination disorder: exploration of a cerebellar hypothesis. Hum Mov Sci. 2007;26:491–509. doi: 10.1016/j.humov.2007.03.004. [DOI] [PubMed] [Google Scholar]
- Cohen YE, Andersen RA. Reaches to sounds encoded in an eye-centered reference frame. Neuron. 2000;27:647–652. doi: 10.1016/s0896-6273(00)00073-8. [DOI] [PubMed] [Google Scholar]
- Contreras-Vidal JL. Development of forward models for hand localization and movement control in 6- to 10-year-old children. Hum Mov Sci. 2006;25:634–645. doi: 10.1016/j.humov.2006.07.006. [DOI] [PubMed] [Google Scholar]
- Contreras-Vidal JL, Teulings HL, Stelmach GE, Adler CH. Adaptation to changes in vertical display gain during handwriting in Parkinson’s disease patients, elderly and young controls. Parkinsonism Relat Disord. 2002;9:77–84. doi: 10.1016/s1353-8020(02)00013-5. [DOI] [PubMed] [Google Scholar]
- Contreras-Vidal JL, Bo J, Boudreau JP, Clark JE. Development of visuomotor representations for hand movement in young children. Exp Brain Res. 2005;162:155–164. doi: 10.1007/s00221-004-2123-7. [DOI] [PubMed] [Google Scholar]
- Criscimagna-Hemminger SE, Bastian AJ, Shadmehr R. Size of error affects cerebellar contributions to motor learning. J Neurophysiol. 2010;103:2275–2284. doi: 10.1152/jn.00822.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davison A, Hinkley DV. Bootstrap methods and their application. New York: Cambridge University Press; 1997. [Google Scholar]
- Denckla MB. Revised neurological examination for subtle signs (1985) Psychopharmacol Bull. 1985;21:773–800. [PubMed] [Google Scholar]
- Efron B, Tibshirani RJ. An Introduction to the Bootstrap. Boca Raton: Chapman & Hall/CRC; 1994. [Google Scholar]
- Henderson SE, Sugden DA. Movement assessment battery for children. London: The Psychological Corporation; 1992. [Google Scholar]
- Hoare D. Subtypes of developmental coordination disorder. Adapted Physical Activity Quarterly. 1994;11:158–169. [Google Scholar]
- Jones LV, Tukey JW. A sensible formulation of the significance test. Psychol Methods. 2000;5:411–414. doi: 10.1037/1082-989x.5.4.411. [DOI] [PubMed] [Google Scholar]
- Jongmans MJ, Smits-Engelsman BC, Schoemaker MM. Consequences of comorbidity of developmental coordination disorders and learning disabilities for severity and pattern of perceptual-motor dysfunction. J Learn Disabil. 2003;36:528–537. doi: 10.1177/00222194030360060401. [DOI] [PubMed] [Google Scholar]
- Kagerer FA, Contreras-Vidal JL. Adaptation of sound localization induced by rotated visual feedback in reaching movements. Exp Brain Res. 2009;193:315–321. doi: 10.1007/s00221-008-1630-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kagerer FA, Contreras-Vidal JL, Stelmach GE. Adaptation to gradual as compared with sudden visuo-motor distortions. Exp Brain Res. 1997;115:557–561. doi: 10.1007/pl00005727. [DOI] [PubMed] [Google Scholar]
- Kagerer FA, Bo J, Contreras-Vidal JL, Clark JE. Visuomotor adaptation in children with developmental coordination disorder. Mot Control. 2004;8:450–460. doi: 10.1123/mcj.8.4.450. [DOI] [PubMed] [Google Scholar]
- Kagerer FA, Contreras-Vidal JL, Bo J, Clark JE. Abrupt, but not gradual visuomotor distortion facilitates adaptation in children with developmental coordination disorder. Hum Mov Sci. 2006;25:622–633. doi: 10.1016/j.humov.2006.06.003. [DOI] [PubMed] [Google Scholar]
- King BR, Kagerer FA, Contreras-Vidal JL, Clark JE. Evidence for multisensory spatial-to-motor transformations in aiming movements of children. J Neurophysiol. 2009;101:315–322. doi: 10.1152/jn.90781.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King BR, Pangelinan MM, Kagerer FA, Clark JE. Improvements in proprioceptive functioning influence multisensorymotor integration in 7-to 13-year-old children. Neurosci Lett. 2010;483:36–40. doi: 10.1016/j.neulet.2010.07.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King BR, Harring JR, Oliveira MA, Clark JE. Statistically characterizing intra- and inter-individual variability in children with Developmental Coordination Disorder. Res Dev Disabil. 2011 doi: 10.1016/j.ridd.2010.12.043. doi: 10.1016/j.ridd.2010.12.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krakauer JW, Pine ZM, Ghilardi MF, Ghez C. Learning of visuomotor transformations for vectorial planning of reaching trajectories. J Neurosci. 2000;20:8916–8924. doi: 10.1523/JNEUROSCI.20-23-08916.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lundy-Ekman L, Ivry R, Keele S, Woollacott M. Timing and force control deficits in clumsy children. J Cogn Neurosci. 1991;3:367–376. doi: 10.1162/jocn.1991.3.4.367. [DOI] [PubMed] [Google Scholar]
- Mackenzie SJ, Getchell N, Deutsch K, Wilms-Floet A, Clark JE, Whitall J. Multi-limb coordination and rhythmic variability under varying sensory availability conditions in children with DCD. Hum Mov Sci. 2008;27:256–269. doi: 10.1016/j.humov.2008.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mon-Williams MA, Wann JP, Pascal E. Visual-proprioceptive mapping in children with developmental coordination disorder. Dev Med Child Neurol. 1999;41:247–254. doi: 10.1017/s0012162299000523. [DOI] [PubMed] [Google Scholar]
- O’Hare A, Khalid S. The association of abnormal cerebellar function in children with developmental coordination disorder and reading difficulties. Dyslexia. 2002;8:234–248. doi: 10.1002/dys.230. [DOI] [PubMed] [Google Scholar]
- Oliveira MA, Shim JK, Loss JF, Petersen RDS, Clark JE. Effect of kinetic redundancy on hand digit control in children with DCD. Neurosci Lett. 2006;410:42–46. doi: 10.1016/j.neulet.2006.09.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piek JP, Dyck MJ. Sensory-motor deficits in children with developmental coordination disorder, attention deficit hyperactivity disorder and autistic disorder. Hum Mov Sci. 2004;23:475–488. doi: 10.1016/j.humov.2004.08.019. [DOI] [PubMed] [Google Scholar]
- Pouget A, Ducom JC, Torri J, Bavelier D. Multisensory spatial representations in eye-centered coordinates for reaching. Cognition. 2002;83:B1–B11. doi: 10.1016/s0010-0277(01)00163-9. [DOI] [PubMed] [Google Scholar]
- Robertson EM, Miall RC. Visuomotor adaptation during inactivation of the dentate nucleus. Neuroreport. 1999;10:1029–1034. doi: 10.1097/00001756-199904060-00025. [DOI] [PubMed] [Google Scholar]
- Scheidt RA, Reinkensmeyer DJ, Conditt MA, Rymer WZ, Mussa-Ivaldi FA. Persistence of motor adaptation during constrained, multi-joint, arm movements. J Neurophysiol. 2000;84:853–862. doi: 10.1152/jn.2000.84.2.853. [DOI] [PubMed] [Google Scholar]
- Schoemaker MM, van der Wees M, Flapper B, Verheij-Jansen N, Scholten-Jaegers S, Geuze RH. Perceptual skills of children with developmental coordination disorder. Hum Mov Sci. 2001;20:111–133. doi: 10.1016/s0167-9457(01)00031-8. [DOI] [PubMed] [Google Scholar]
- Shadmehr R, Wise SP. The computational neurobiology of reaching and pointing. Cambridge: The MIT Press; 2005. [Google Scholar]
- Sigmundsson H, Ingvaldsen RP, Whiting HTA. Inter-and intra-sensory modality matching in children with hand-eye co-ordination problems. Exp Brain Res. 1997;114:492–499. doi: 10.1007/pl00005658. [DOI] [PubMed] [Google Scholar]
- Teulings HL, Contreras-Vidal JL, Stelmach GE, Adler CH. Handwriting size adaptation under distorted feedback in Parkinson’s disease, elderly, and young controls. J Neurol Neurosurg Psychiatry. 2002;72:315–324. doi: 10.1136/jnnp.72.3.315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volman JM, Geuze RH. Relative phase stability of bimanual and visuomanual rhythmic coordination patterns in children with a Developmental Coordination Disorder. Hum Mov Sci. 1998;17:541–572. [Google Scholar]
- Wilson PH, McKenzie BE. Information processing deficits associated with developmental coordination disorder: a meta-analysis. J Child Psychol Psychiatry. 1998;39:829–840. [PubMed] [Google Scholar]
- Wolpert DM, Kawato M. Multiple paired forward and inverse models for motor control. Neural Netw. 1998;11:1317–1329. doi: 10.1016/s0893-6080(98)00066-5. [DOI] [PubMed] [Google Scholar]
- Zieffler AS, Harring JR, Long JD. Comparing groups: randomization and bootstrap methods using R. Hoboken: Wiley; 2011. [Google Scholar]
- Zoia S, Castiello U, Blason L, Scabar A. Reaching in children with and without developmental coordination disorder under normal and perturbed vision. Dev Neuropsychol. 2005;27:257–273. doi: 10.1207/s15326942dn2702_4. [DOI] [PubMed] [Google Scholar]