Abstract
Introduction
Sedentary lifestyle is a major risk factor for diabetes, cardiovascular and many other age-related diseases. Heart rate variability (HRV) reflects the function of regulatory systems of internal organs and may sensitively indicate early metabolic disturbances. We hypothesize that quantitative and qualitative changes of HRV in young subjects may reflect early metabolic derangements responsible for further development of clinically significant disease.
Aim
The aim of our study was to determine whether the parameters of carbohydrate metabolism (fasting blood glucose, HBA1c and surrogate insulin sensitivity/resistance indices) correlate with anthropometric data and HRV.
Methods
The study group consisted of 30 healthy sedentary male subjects aged 20–40, nonsmokers, mainly office and research employees, medical staff and students. Athletes, actively training more than one hour per week, severely obese and men of physical work were excluded from the study. HRV parameters were derived from short term ECG records (five minutes intervals) in supine position and during orthostatic test. Anthropometric data included height, weight, body mass index (BMI), age and body composition (estimation by bioelectric impedance method). The fasting blood glucose, insulin and C-peptide, homeostatic model assessment (HOMA-IR) index and glycated hemoglobin (HbA1c) were evaluated. Linear correlation coefficient (r) was calculated using Statistica 10.0 software.
Results and discussion
HOMA-IR index correlated positively with body weight, visceral fat and BMI (p=0.047, 0.027 and 0.017 respectively). In supine position pNN50 positively correlated with glucose/insulin ratio (p=0.011) and heart rate with HOMA-IR (p=0.006). In orthostatic test negative correlations of HBA1c with standard deviation, total and low frequency power were determined (p=0.034, 0.400 and 0.403 respectively), which indicates a gradual worsening of functional capacity of cardiovascular system with low-grade increase (under the conventional threshold) of HBA1c.
Conclusions
In apparently healthy sedentary subjects HRV reduction correlates with the age advancement, subclinical deteriorations of carbohydrate metabolism and excessive fat accumulation.
Keywords: Sedentary lifestyle, Glycated hemoglobin, Heart rate variability, Insulin sensitivity, Correlations
Abbreviations: ANS, autonomous nervous system; BMI, body mass index; CVS, cardiovascular system; ECG, electrocardiogram; FBG, fasting blood glucose; HBA1c, glycated hemoglobin; HF, the power of high frequency oscillations; HOMA, homeostatic model assessment; HR, heart rate; HRV, heart rate variability; HSS, healthy sedentary subjects; IR, insulin resistance; LF, the power of low frequency oscillations; OT, orthostatic test; pNN50, percentage of differences between adjacent normal RR intervals exceeding 50 milliseconds; RMSSD, square root of the mean squared differences of successive RR intervals; SAN, sinoatrial node; SDNN, standard deviation of normal RR intervals; TP, total power of RR-intervals oscillations; VLF, the power of very low frequency oscillations
Graphical abstract
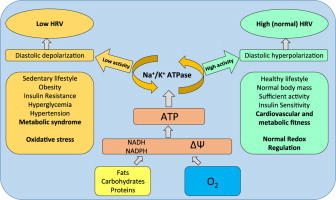
Highlights
-
•
Apparently healthy sedentary young male subjects were enrolled in the study.
-
•
HRV negatively correlates with age, BMI, visceral fat and insulin resistance.
-
•
Glycated hemoglobin negatively correlates with HRV parameters in orthostatic test.
-
•
Changes of HRV may reflect subclinical metabolic deteriorations in sedentary subjects.
1. Introduction
Sedentary lifestyle, obesity, hypertension, dyslipidemia and type 2 diabetes (T2D) are among the most important risk factors of cardiovascular morbidity and mortality [1,2]. These factors contribute to approximately 19.5 million deaths per year, which is more than 1/3 of all fatalities [3]. Due to economic development and gradual elimination of physical work the share of people with physical inactivity (low level of activity) dramatically increases and spreads worldwide from mainly developed countries in the past [4]. It is well known that the development of cardiovascular diseases, in particular atherosclerosis, often starts at young age, sometimes even in childhood and adolescence without the evidence for the presence of these risk factors. Research is mainly focused on already developed disease and little is known about early stages where the main homeostatic parameters (e.g. blood tests) remain within the physiological range and all the changes are on early subclinical stages. Being clinically invisible and generally ignored these conditions gradually progress and it is only a matter of time when they will manifest in the form of the disease. But most of the known risk factors are highly modifiable, and early interventions may be extremely beneficial, cost-effective, and could prevent or at least significantly delay the onset of the disease.
Glycosylated (glycated) hemoglobin (HBA1c) is formed in a non-enzymatic glycation pathway by hemoglobin’s exposure to blood glucose and, since erythrocyte’s lifetime normally is over a hundred days, HBA1c level highly correlates with average blood concentration of glucose for relatively long periods of time (up to 3 months). The level of HBA1c is often used as a stable cumulative index of glycemia reflecting the average level of blood glucose more reliably than commonly used fasting glucose. Therefore it is a major clinical parameter, especially in diabetes. Besides, it may reflect also the general level of posttranslational protein glycation in the whole organism, because the increased rate of glycation takes place in other body compartments proportionally to the glycemia level [5]. There are close relationships of key parameters of carbohydrate metabolism such as fasting blood glucose (FBG), HBA1c, and homeostatic model assessment (HOMA) of insulin resistance (IR), and cardiovascular risk in diabetic patients. However, in non-diabetic subjects where the levels of HBA1c are within normal range (up to 6.0%) despite its potential importance, this parameter has not been extensively studied until recently. In a recent paper of Chang et al. [13] on a large cohort it has been proven that subclinical coronary atherosclerosis is associated with higher levels of HBA1c in non-diabetic subjects without overt cardiovascular disease [6].
Heart rate variability (HRV) reflects oscillations in heart cycle duration over the time and is generally considered as a measure of regulatory influences, mainly activity of the autonomous nervous system (ANS) to regulate function of cardiovascular system (CVS). Classical interpretation of HRV include the activities of parasympathetic and sympathetic branches of ANS, their balance/ratio and a number of other related parameters [7]. However a growing evidence is accumulating since recently concerning metabolic background of HRV [8–10] and the critical dependence of the amplitude of heart rate oscillation on the intracellular energy supply [11]. There are also some indications that the parameters of HRV in orthostatic test (OT) are of particular importance, since the examined subjects are exposed to a mild level of exertion which causes some stress, requiring adaptation. In fact, at rest initial changes may become hidden by adaptive reactions of the human organism, and only mild physiological activation reveals abnormalities [12]. If this assumption is correct, the changes in HRV parameters in apparently healthy subjects should reflect early metabolic shifts and sensitively indicate the initial stages of health problems related with sedentary lifestyle. The aim of our study therefore was to determine whether the parameters of carbohydrate metabolism (FBG, HBA1c and surrogate insulin sensitivity/resistance indices) correlate with anthropometric data and HRV.
2. Materials and methods
2.1. Study group characteristics and anthropometric parameters
In our study 30 apparently healthy sedentary young male subjects (HSS) aged 20–40, nonsmokers, mainly office and research employees, medical staff and students were enrolled. Any athletes, subjects actively training for more than 1 h per week, severely obese (body mass index above 35.0 kg/m2), men physically working and subjects suffering from any chronic diseases were excluded from the study. The questionnaire used in the study was adapted from “The General Practice Physical Activity Questionnaire” (translated into Ukrainian), developed by the London School of Hygiene and Tropical Medicine as a validated short measure of physical activity available online at http://www.patient.co.uk/doctor/general-practice-physical-activity-questionnaire-gppaq. Subjects were considered as “sedentary” if their result was classified qualitatively as “Inactive” or “Moderately Inactive” according to the online calculator. Enrolled subjects never smoked or quit smoking not later than 3 years prior to the participation in the study, no one was vegetarian or had any voluntary qualitative or quantitative food restrictions. All subjects underwent physical examination, routine clinical tests and electrocardiography. Anthropometric data included height, weight, body mass index (BMI), and age. Height was measured with a standardized stadiometer, patients were weighed on electronic scales (OMRON Corporation, Kyoto, Japan), and BMI was calculated. Body composition was estimated by the bioelectric impedance method and the following parameters were determined: fat content (% of body weight), visceral fat (%), muscle mass (%) on the Body Composition Monitor BF500 (OMRON Corporation, Kyoto, Japan). It should be noted that the body composition measurements provide approximate results and are used for rough estimation of body composition. The Ethics Committee of Danylo Halytskyi Lviv National Medical University approved the design and protocol of the study. A written informed consent form was obtained from all the subjects enrolled in the study.
2.2. Clinical laboratory investigations, ELISA and glycated hemoglobin
Routine clinical blood cell count was performed by automatic cell counter ABS-Micros 60-OT (Horiba Medical, Montpellier, France). Blood cell morphology evaluation was performed by an experienced clinical laboratory specialist.
Fasting whole blood glucose was determined by the conventional glucose oxidase method routinely used in clinical laboratories, while HbA1c was assessed using a highly sensitive method of ion-exchange liquid chromatography with a D-10™ System analyzer and BIO-RAD D-10™ reagents (Bio-Rad, Hercules, California, USA). The levels of insulin and C-peptide were determined with respective ELISA-assays (DRG Instruments GmbH, Marburg, Germany).
Surrogate methods for insulin resistance/sensitivity evaluation have also been used in the study. A homeostatic model assessment (HOMA) of insulin resistance (IR) derived from the basal (fasting) levels of glucose and insulin is the most commonly used and highly correlates with the “golden standard” euglycemic clamp method, which is technically much more difficult to perform [13]. HOMA-IR index was calculated by the formula HOMA-IR=FBG×Insulin/22.5. As a measure of insulin sensitivity glucose/insulin ratio was calculated.
2.3. Heart rate variability
HRV parameters were derived from short term electrocardiogram (ECG) records (5 min intervals) in supine position and during orthostatic test. In the morning hours, not less than 24 h after the last significant physical exercise, the short-time records of ECG were performed in a quiet dark room. A computer electrocardiograph “VNS-Micro” (Neurosoft®, Ivanovo, Russia) was used for ECG records. After 20 min of rest, studied subjects were asked to stay supine quietly for 5.0 min for stationary condition HRV recording. Afterwards they were asked to stand up rapidly and remain in the standing position for 6 min – active OT. RR intervals were determined with a sampling frequency of 2.0 kHz and were analyzed with “Poly-Spectrum” (Neurosoft®, Ivanovo, Russia) software designed according to HRV standards [7]. The time-domain parameters – standard deviation of normal RR intervals (SDNN), the square root of the mean squared differences of successive RR interval (RMSSD), and the percentage of differences between adjacent normal RR intervals exceeding 50 m (pNN50) – were determined. The power spectral analysis was performed sequentially with a fast Fourier transformation. The following frequency-domain variables were studied: total power (TP, 0.01–0.40 Hz), high frequency power (HF, 0.15–0.40 Hz), low frequency power (LF, 0.04–0.15 Hz), and very low frequency power (VLF, 0.01–0.04 Hz). Occasional extrasystoles (not more than 1/min) as well as artifacts were detected ad oculus by an experienced physician (A.C.) and removed manually from the analysis. Extrasystoles were excluded from the analysis together with the consecutive compensatory pause, because they cause significant deviation of normal HR with sinus rhythm and may distort the HRV evaluation.
2.4. Statistical analysis
All data were processed using the statistical package Statistica 10.0 (Statsoft, Tulsa, Oklahoma, USA). Normal distribution of the obtained data were confirmed with Shapiro–Wilk's W-test. The descriptive statistics of the data in tables include mean, 95% confidence interval, median, standard deviation, lower and upper quartile, standard error mean. Coefficient of linear correlations r (Pearson) and its significance p was calculated and included in the matrix. If not indicated otherwise, p<0.05 was considered as significant.
3. Results
Since all subjects enrolled in the study were apparently healthy with no documented evidence of any chronic disease, the vast majority of the parameters were found to be within the normal range. The study was intentionally designed and performed to investigate the relationships of the parameters at the stage where no evidence of any changes is present or changes are under the threshold of conventional generally used clinical methods.
The main anthropometric data and body composition parameters are presented in the Table 1. HSS subjects tend to have close to upper normal range limit BMI with mean 24.8 (20.0–24.9). 11 subjects (36.7%) had BMI in the range 25.0–29.9 (overweight), while three of them (10.0%) had between 30.0 and 34.9 indicating first grade of obesity. Total fat content estimation was 21.1% (normal value up to 20.0%) and there was a slightly lower muscle mass of 38.5 (normal value for young male adults above 40%). In six out of 30 HSS the value of visceral fat exceeded 10%, which indicates abdominal type of fat accumulation, typical for metabolic syndrome.
Table 1.
Anthropometric data, body composition parameters, systolic and diastolic blood pressure of healthy sedentary subjects.
| Parameter | Mean (confidence interval 95%) | Median (lower-upper quartile) | Standard deviation | Standard error |
|---|---|---|---|---|
| Age, years | 29.3 (27.3–31.3) | 30.0 (26.0–34.0) | 5.45 | 0.99 |
| Height, cm | 178.4 (176.2–180.5) | 177.5 (175.0–182.0) | 5.77 | 1.05 |
| Weight, kg | 78.8 (73.8–83.8) | 79.0 (70.3–85.5) | 13.3 | 2.43 |
| BMI, kg/m2 | 24.8 (23.3–26.2) | 24.4 (22.7–26.9) | 3.85 | 0.70 |
| Total fat content, % | 21.1 (18.1–24.1) | 22.7 (15.9–26.0) | 8.03 | 1.47 |
| Abdominal fat, % | 7.23 (5.84–8.62) | 7.00 (5.0–10.0) | 3.72 | 0.68 |
| Muscle mass, % | 38.5 (36.76–40.3) | 37.8 (35.0–41.5) | 4.70 | 0.86 |
| Systolic blood pressure, mmHg | 117.3 (115.3–119.3) | 117.0 (113.0–118.0) | 5.28 | 0.96 |
| Diastolic blood pressure, mmHg | 76.2 (74.1–78.4) | 76.0 (72.0–81.0) | 5.79 | 1.06 |
The parameters of carbohydrate metabolism are summarized in Table 2. The mean of FBG was found around the lower normal range limit and HbA1c was slightly elevated to 6.1% only in one subject (normal range is 4.0–6.0, diabetes cut off value is 6.5%). Insulin level was found to be elevated in 3 (10.0%) subjects (reference range 2–25 μlU/mL), while the values of C-peptide were found within the normal range in all HSS (reference range 25–320 pg/ml). HOMA-IR index was above the threshold level of 2.7 in 5 (16.7%) subjects and FBG/insulin ratio level was lower than 0.33 in 14 subjects indicating decreased insulin sensitivity.
Table 2.
Parameters of glucose metabolism and total cholesterol in healthy sedentary subjects.
| Parameter | Mean (confidence interval 95%) | Median (lower-upper quartile) | Standard deviation | Standard error |
|---|---|---|---|---|
| Fasting blood glucose, mM/L | 3.66 (3.42–3.89) | 3.7 (3.2–4.1) | 0.62 | 0.11 |
| Glycated hemoglobin, % | 5.42 (5.31–5.53) | 5.3 (5.2–5.6) | 0.29 | 0.05 |
| Insulin, μlU/mL | 13.27 (8.25–18.28) | 9.11 (5.74–15.02) | 13.44 | 2.45 |
| C-peptide, pg/ml | 135.0 (104.5–165.5) | 134.1 (56.8–208.1) | 81.62 | 14.90 |
| HOMA-IR | 2.09 (1.31–2.86) | 1.37 (0.99–2.24) | 2.09 | 0.38 |
| Glucose/insulin ratio | 0.51 (0.36–0.66) | 0.41 (0.20–0.63) | 0.40 | 0.07 |
| Total cholesterol, mM | 4.54 (4.6-3.5) | 4.6 (4.1–4.9) | 0.55 | 0.10 |
The correlation analysis revealed no significant relationships of age, height and muscular mass with the glucose metabolism parameters. However significant correlations have been found for HOMA-IR with weight, visceral fat and BMI (Table 3). Glucose/insulin ratio, mainly reflecting insulin sensitivity negatively correlated with weight, fat content and visceral fat. No meaningful correlations were observed for C-peptide and anthropometric parameters, while positive correlations were found for insulin with visceral fat and BMI. No significant correlations were found for blood pressure and other studied parameters.
Table 3.
Correlation matrix of the parameters of glucose metabolism and anthropometric data in healthy sedentary subjects (correlations with p<0.05 marked with bold text style).
| Glycated hemoglobin | Fasting glucose | HOMA-IR index | Glucose/insulin ratio | C-peptide | Insulin | |
|---|---|---|---|---|---|---|
| Age | 0.030 p=0.889 | −0.024 p=0.910 | 0.127 p=0.505 | −0.191 p=0.313 | −0.210 p=0.265 | 0.168 p=0.374 |
| Height | −0.282 p = 0.131 | 0.241 p = 0.200 | −0.084 p = 0.661 | 0.074 p=0.696 | −0.148 p = 0.435 | −0.135 p=0.479 |
| Weight | 0.025 p=0.898 | 0.096 p=0.614 | 0.366 p=0.047 | −0.401 p=0.028 | −0.062 p=0.745 | 0.316 p=0.088 |
| Fat content | 0.197 p=0.296 | 0.033 p=0.862 | 0.259 p=0.166 | −0.390 p=0.033 | 0.036 p=0.849 | 0.218 p=0.246 |
| Visceral fat | 0.195 p=0.303 | −0.048 p = 0.803 | 0.407 p = 0.026 | −0.491 p=0.006 | −0.018 p=0.926 | 0.384 p=0.036 |
| Muscles | −0.213 p=0.258 | −0.023 p=0.904 | −0.225 p=0.232 | 0.358 p=0.052 | 0.005 p=0.981 | −0.187 p=0.322 |
| BMI | 0.128 p=0.499 | −0.002 p =0.991 | 0.431 p = 0.017 | −0.475 p=0.008 | −0.015 p = 0.938 | 0.399 p=0.029 |
The correlations of HRV parameters and carbohydrate metabolism are shown in Tables 4 and 5. In supine position (Table 4) the most significant correlations were found for HR, namely negative with FBG, and insulin sensitivity (glucose/insulin ratio) with respective p values 0.013 and <0.001. HOMA-IR index and level of insulin showed positive correlations (p=0.006 and p=0.002 respectively). In contrast pNN50, an important marker of parasympathetic activity, showed negative correlations with HBA1c, insulin and HOMA-IR index, however p values did not reach significance threshold (p>0.05), while the positive correlation with glucose/insulin ratio was significant (p=0.011) (Fig. 1).
Table 4.
Correlation matrix of parameters of glucose metabolism and heart rate variability in healthy sedentary subjects in supine position (correlations with p<0.05 marked with bold text style).
| Glycated hemoglobin | Fasting glucose | HOMA-IR index | Glucose/insulin ratio | C-peptide | Insulin | |
|---|---|---|---|---|---|---|
| HR | 0.238 p=0.206 | −0.449 p=0.013 | 0.489 p=0.006 | −0.599 p<0.001 | 0.040 p=0.832 | 0.545 p=0.002 |
| SDNN | −0.194 p=0.305 | 0.159 p=0.401 | −0.061 p=0.747 | 0.133 p=0.485 | −0.152 p=0.424 | −0.078 p=0.682 |
| pNN50 | −0.308 p =0.097 | 0.273 p=0.144 | −0.276 p=0.140 | 0.456 p=0.011 | −0.075 p=0.694 | −0.307 p=0.099 |
| TP | −0.212 p=0.261 | 0.244 p=0.193 | −0.041 p=0.830 | 0.301 p=0.106 | −0,035 p=0.853 | −0,054 p=0.776 |
| VLF | −0.129 p=0.497 | 0.265 p=0.157 | 0.011 p=0.955 | 0.157 p=0.407 | −0.336 p=0.069 | 0.001 p=0.995 |
| LF | −0.132 p=0.487 | 0.114 p=0.548 | 0.027 p=0.887 | 0.225 p=0.233 | 0.133 p=0.482 | 0.024 p=0.901 |
| HF | −0.232 p=0.217 | 0.241 p=0.200 | −0.101 p=0.596 | 0.315 p=0.090 | −0.005 p =0.977 | −0.118 p=0.534 |
| LF/HF | 0.338 p=0.068 | −0.170 p=0.368 | 0.109 p=0.567 | −0.199 p=0.292 | 0.275 p=0.142 | 0.115 p=0.546 |
Table 5.
Correlation matrix of parameters of glucose metabolism and heart rate variability in healthy sedentary subjects during orthostatic test (correlations with p<0.05 marked with bold text style).
| Glycated hemoglobin | Fasting glucose | HOMA-IR index | Glucose/insulin ratio | C-peptide | Insulin | |
|---|---|---|---|---|---|---|
| HR | −0.045 p=0.814 | −0.271 p=0.148 | 0.265 p=0.158 | −0.231 p=0.220 | 0.0100 p=0.601 | 0.317 p=0.088 |
| SDNN | −0.387 p=0.034 | 0.148 p=0.434 | −0.338 p=0.068 | 0.464 p=0.010 | −0.266 p=0.155 | −0.355 p=0.054 |
| pNN50 | −0.256 p=0.173 | 0.182 p=0.335 | −0.166 p=0.379 | 0.278 p=0.137 | −0.249 p=0.184 | −0.193 p=0.306 |
| TP | −0.400 p = 0.028 | 0.081 p=0.669 | −0.295 p=0.114 | 0.470 p=0.009 | −0.262 p=0.162 | −0.298 p=0.109 |
| VLF | −0.267 p=0.153 | 0.222 p=0.238 | −0.245 p=0.192 | 0.520 p=0.003 | −0.336 p=0.069 | −0.268 p=0.153 |
| LF | −0.403 p=0.027 | −0.202 p=0.285 | −0.234 p=0.214 | 0.145 p=0.444 | 0.056 p=0.768 | −0.205 p=0.277 |
| HF | −0.146 p=0.440 | 0.105 p=0.580 | −0.054 p=0.775 | 0.191 p=0.312 | −0.356 p=0.054 | −0.060 p=0.752 |
| LF/HF | −0.287 p=0.124 | −0.108 p=0.568 | −0.054 p=0.777 | −0.042 p=0.827 | 0.354 p=0.055 | −0.043 p=0.823 |
Fig. 1.
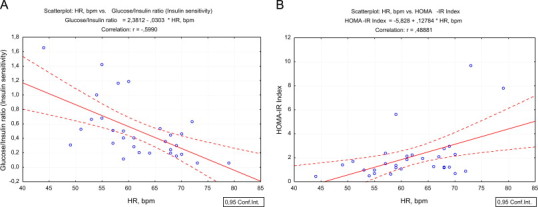
A scatterplot of glucose/insulin ratio and heart rate in supine position (A); HOMA-IR index and heart rate in supine position (B) in healthy sedentary subjects.
A negative correlations of HRV with age were demonstrated, in particular pNN50 is clearly decreasing with age (r=0.527, p=0.003, Fig. 2B) and HF power (r=0.421, p=0.020) in supine position. At the same time no significant correlations of age were confirmed in OT.
Fig. 2.
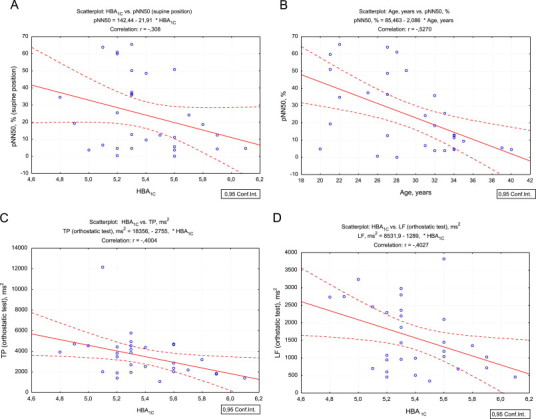
A scatterplot of glycated hemoglobin (HbA1c) and pNN50 in supine position (A); pNN50 in supine position and age (B); glycated hemoglobin (HbA1c) and total spectral power in orthostatic test (C); glycated hemoglobin (HbA1c) and LF-power in orthostatic test in healthy sedentary subjects (D).
During OT the pattern of correlations was different. HBA1c negatively correlated with all HRV parameters and significant values were shown for SDNN (p=0.034), TP (p=0.028) and LF (p=0.027). For glucose/insulin ratio reflecting insulin sensitivity significant positive correlations with SDNN (p=0.010), TP (p=0.009) and VLF (p=0.003) were observed. FBG, HOMA-IR index, insulin and C-peptide did not reveal any significant correlations with HRV parameters in OT.
4. Discussion
There is growing evidence for the importance of sedentary lifestyle in cardiovascular diseases and T2D, in particular due to increase of its rate in developing countries [4]. Together with wider food availability inactivity contributes to weight gain and obesity. Physically active subjects with overweight or even mild forms of obesity often are healthier and have lower risk of cardiovascular events and other related diseases than, for example, lean smokers with sedentary lifestyle. These patients are referred to as “metabolically healthy but obese” and there is no surprise that when they are sufficiently active their obesity remains relatively benign and IR, dyslipidemia, endothelial dysfunction and other related conditions do not develop [14,15].
Most age-related diseases have a long period of subclinical changes and usually remain undetected. The patients gradually approach to the disease onset often being unaware of serious consequences of long-lasting latent period. Existing recommendations for the treatment of hypertension or T2D advise “lifestyle modification” or “moderate physical activity”, however, the intervention could be started far earlier, and potentially could effectively prevent even the development of pathological conditions such as pre-diabetes and pre-hypertension.
It is well known that decreased HRV is associated with numerous health problems including increased all-cause mortality, ventricular arrhythmia mortality, heart failure, myocardial infarction, T2D risks even without evidence of autonomic neuropathy, liver damage, hypertension and many other problems [16]. Recently we reported substantial decreases of HRV in patients with Marfan syndrome which is often associated with worsening of the prognosis and serious cardiovascular complications. There is growing evidence that a decrease in HRV eventually may appear not only as a consequence of decreased activities of sympathetic and parasympathetic branches of ANS, but also as independent metabolic phenomenon, related to possible impairment of cardiomyocytes, especially cells of the sinoatrial node (SAN) that may be less responsive to neural and humoral impacts [11]. Basically, according to this hypothesis, lower ATP supply by mitochondria of the cells of SAN provide lower membrane potential created by Na+/K+ ATPase, which lowers excitation threshold of pacemaker cells. In other words energy (ATP) deficit has the same (or similar) effect on cardiac cells as sympathetic nervous output. Low excitation threshold reduces the impact of regulatory mechanisms and amplitude of HR oscillations remain low. In contrast, sufficient supply of energy provides high threshold for SAN cell excitation, decrease in HR and duration of heart cycle may be more readily manipulated, for example by cardiovascular reflexes, increasing amplitude of HR oscillations (Fig. 3).
Fig. 3.
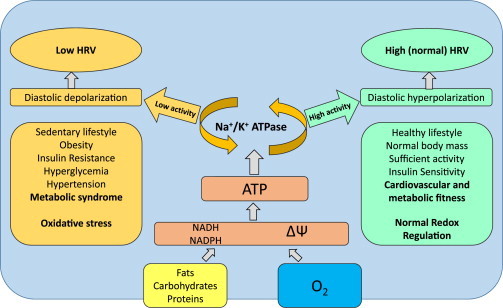
The relationships of heart rate variability and redox state in the pacemaker cells of sinoatrial node. The heart rate regulation is very complex and many regulatory mechanisms are involved on different levels. Sympathetic and parasympathetic branches of autonomic nervous system play a major role in modulation of heart automaticity, however intrinsic metabolic and electrophysiological properties of sinoatrial node cardiocytes may be crucial for the amplitude of heart rhythm oscillations. Higher activity of Na+/K+ ATPase powered by sufficient ATP supply provide diastolic hyperpolarization of the membrane of cardiocyte, decreasing excitability and promoting vagal brake effect (providing heart rate decelerations). Energetic deficit, in contrast, provides depolarization of membrane, increased excitability and lower amplitude of heart rhythm oscillations.
HR fluctuations provide better adaptability of the organism compared with the relatively stable heart rhythm [12] and this principle may be applied to redox biology as well. Recently the redox theory of aging was suggested and, interestingly, among the arguments the author also points out that a greater regularity of the metabolism is associated with poor health [17]. The regulation of the metabolism is organized on multiple hierarchic levels and numerous mechanism are involved. Many of them have feedback loop organization therefore causing physiological oscillations of the levels of substrates, activities of enzymes and periodical functional activities of the cells. The higher variability of metabolism provides wider availability of different adaptive reactions which may become critical for proper functioning or even survival in certain situations. However, possible relationships of metabolic variability and HRV are still not quite clear.
There is a growing evidence of the relationship of carbohydrate metabolism derangements and vascular, in particular, endothelial dysfunction. It was shown that hyperglycemia, glucose fluctuations and carbonyl stress can enhance brain microvascular endothelial barrier dysfunction [18]. Oxidative stress, lipid peroxidation and accumulation of reactive aldehydes, in particular 4-hydroxynonenal is widely involved in the progression of carbohydrate dysregulation as well as other aging related conditions [19–22]. In addition, it was shown that HRV was affected by age, hyperglycemia and accumulation of body fat [23]; however, all the reports concern mainly patients with already developed disease. Our study is an effort to establish relationships far before disease onset.
Worsening functional conditions of CVS in apparently healthy subjects was associated with gradual decrease of time and frequency domain parameters of HRV, and there are negative correlations of a number of HRV parameters with age, BMI, visceral fat and other parameters. These findings were in accordance with the data from positive IR correlations with anthropometric parameters (body mass, visceral fat and BMI, Table 1). Interestingly, we were unable to detect significant correlations of HBA1c with HRV in supine position, despite the evidence of an association of sub-threshold levels of HBA1c with signs of arteriosclerosis and calcification of the vessels in non-diabetic men and women [6]. However, with low-grade functional activation of CVS (active OT) we observed negative correlations of HBA1c with SDNN, TP and LF, which indicates the gradual worsening of functional capacity of CVS with low-grade increase (under the conventional threshold) of HBA1c.
From the physiological point of view HRV is very sensitive and may reflect even mild changes in functional conditions of CVS. For example, there are quite significant variations of HRV depending on the time of day, physical activity, condition of physical exhaustion and recovery. This restricts wide and straightforward use of the method. On the other hand – HRV reflects current conditions of SAN and the integrated regulatory input which is focused specifically on the pacemaker cell [11,12]. This requires a very careful methodological approach to minimize possible artifacts that can significantly distort the results of the HRV records. In our case the studies were carefully standardized, the criteria of inclusion and exclusion allowing to avoid any significant unnatural impacts, and the HR monitoring and recording was performed in similar conditions for all the subjects in the study providing a quasi-stationary condition.
There are several important limitations of this pilot study. Significant degrees of heterogeneity in the group and relatively low number of subjects recruited reduces statistical power and reliability of obtained data in terms of extrapolation to larger populations. The changes caused by sedentary lifestyle in studied subjects in most cases are not clinically significant and are reversible. It would be of great importance to study correlations of HRV with markers of inflammation, oxidative stress and a number of hormones, which we plan for the future. The mid- and long term follow up of these subjects could be a valuable continuation of the study.
In summary, the progression of age-related diseases is gradual and early interventions are the most effective in reduction of the risk of morbidity and development of complications. Conventional criteria of diseases often leave a large number of subclinical cases under the diagnostic threshold leaving those subjects without sufficient attention and adequate recommendations. In apparently healthy sedentary subjects HRV reduction correlates with the age advancement, subclinical deteriorations of carbohydrate metabolism and excessive fat accumulation. The mechanistic relationships of HRV and metabolic parameters remain to be poorly understood and require further studies.
Conflicts of interest
Authors declare no conflict of interest.
Acknowledgements
The work was supported by the State Agency of Science, Innovations and Informatization of Ukraine Contracts nos. M512-2011, and M473-2012, OEAD Project UA 03/2011, and by COST Action CM1001 “Chemistry of non-enzymatic protein modification − modulation of protein structure and function”.
References
- 1.Macniven R., Bauman A., Abouzeid M. A review of population-based prevalence studies of physical activity in adults in the Asia–Pacific region. B.M.C. Public Health. 2012;12(1):41. doi: 10.1186/1471-2458-12-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.León-Latre M., Moreno-Franco B., Andrés-Esteban E.M. Sedentary lifestyle and its relation to cardiovascular risk factors, insulin resistance and inflammatory profile. Rev. Esp. Cardiol. (Engl. Ed.) 2014;67(6):449–455. doi: 10.1016/j.rec.2013.10.015. 24863593 [DOI] [PubMed] [Google Scholar]
- 3.WHO . Global Health Risks: Mortality and Burden of Disease Attributable to Selected Major Risks. 2009. 〈http://www.who.int/healthinfo/global_burden_disease/GlobalHealthRisks_report_full.pdf〉. [Google Scholar]
- 4.Bauman A., Bull F., Chey T. The International Prevalence Study on Physical Activity: results from 20 countries. Int. J. Behav. Nutr. Phys. Act. 2009;6(1):21. doi: 10.1186/1479-5868-6-21. 19335883 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kishabongo A.S., Katchunga P., Van Aken E.H. Glycation of nail proteins: from basic biochemical findings to a representative marker for diabetic glycation-associated target organ damage. PLOS One. 2015;10(3) doi: 10.1371/journal.pone.0120112. 25781337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chang Y., Yun K.E., Jung H.- A1C and coronary artery calcification in nondiabetic men and women. Arterioscler. Thromb. Vasc. Biol. 2013;33(8):2026–2031. doi: 10.1161/ATVBAHA.113.301587. [DOI] [PubMed] [Google Scholar]
- 7.Malik M., Bigger J.T., Camm A.J. Heart rate variability. Standards of measurement, physiological interpretation, and clinical use. Task Force of the European Society of Cardiology and the North American Society of Pacing and Electrophysiology. Eur. Heart J. 1996;17(3):354–381. 8737210 [PubMed] [Google Scholar]
- 8.Yelisyeyeva O., Cherkas A., Semen K., Kaminskyy D., Lutsyk A. Study of aerobic metabolism parameters and heart rate variability and their correlations in elite athletes: a modulatory effect of amaranth oil. Clin. Exp. Med. J. 2009;3(2):293–307. [Google Scholar]
- 9.Yelisyeyeva O., Semen K., Zarkovic N., Kaminskyy D., Lutsyk O., Rybalchenko V. Activation of aerobic metabolism by amaranth oil improves heart rate variability both in athletes and patients with type 2 diabetes mellitus. Arch. Physiol. Biochem. 2012;118(2):47–57. doi: 10.3109/13813455.2012.659259. 22393897 [DOI] [PubMed] [Google Scholar]
- 10.K.O. Semen, O.P. Yelisyeyeva, D.V. Kaminskyy, et al. Interval hypoxic training in complex treatment of Helicobacter pylori-associated peptic ulcer disease. Acta Biochim. Pol. 57 (2) (2010) 199–208 〈http://www.ncbi.nlm.nih.gov/pubmed/20532252〉 (accessed 10.01.15, Pubmed: 20532252) [PubMed]
- 11.Cherkas A., Yatskevych O. The amplitude of heart rate oscillations is dependent on metabolic status of sinoatrial node cells. OA Med. Hypothesis. 2014;2(1):1–8. 〈http://www.oapublishinglondon.com/images/article/pdf/1415968823.pdf〉. [Google Scholar]
- 12.Abrahamovych O., Cherkas A., Abrahamovych U., Abrahamovych M., Serhiyenko V. Heart Rate Variability: Physiological Bases, Clinical Importance and Peculiarities in Patients with Peptic Ulcer and after Resection of Stomach. Danylo Halytskyi Lviv National Medical University; Lviv: 2014. [Google Scholar]
- 13.Wallace T.M., Levy J.C., Matthews D.R. Use and abuse of HOMA modeling. Diabetes Care. 2004;27(6) doi: 10.2337/diacare.27.6.1487. [DOI] [PubMed] [Google Scholar]
- 14.Soriguer F., Gutiérrez-Repiso C., Rubio-Martín E. Metabolically healthy but obese, a matter of time? Findings from the prospective Pizarra study. J. Clin. Endocrinol. Metab. 2013;98(6):2318–2325. doi: 10.1210/jc.2012-4253. 23559087 [DOI] [PubMed] [Google Scholar]
- 15.Martínez-Larrad M.T., Corbatón Anchuelo A., Del Prado N., Ibarra Rueda J.M., Gabriel R., Serrano-Ríos M. Profile of individuals who are metabolically healthy obese using different definition criteria. A population-based analysis in the Spanish population. PLOS One. 2014;9(9):e106641. doi: 10.1371/journal.pone.0106641. 25198070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Xhyheri B., Manfrini O., Mazzolini M., Pizzi C., Bugiardini R. Heart rate variability today. Prog. Cardiovasc. Dis. 2012;55(3):321–331. doi: 10.1016/j.pcad.2012.09.001. 23217437 [DOI] [PubMed] [Google Scholar]
- 17.Jones D.P. Redox theory of aging. Redox Biol. 2015;5:71–79. doi: 10.1016/j.redox.2015.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Li W., Maloney R.E., Aw T.Y. High glucose, glucose fluctuation and carbonyl stress enhance brain microvascular endothelial barrier dysfunction: implications for diabetic cerebral microvasculature. Redox Biol. 2015;5:80–90. doi: 10.1016/j.redox.2015.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zarkovic K., Larroque-Cardoso P., Pucelle M. Elastin aging and lipid oxidation products in human aorta. Redox Biol. 2015;4:109–117. doi: 10.1016/j.redox.2014.12.008. 25553420 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jaganjac M., Tirosh O., Cohen G., Sasson S., Zarkovic N. Reactive aldehydes − second messengers of free radicals in diabetes mellitus. Free Radic. Res. 2013;47(Suppl. 1):S39–S48. doi: 10.3109/10715762.2013.789136. 23521622 [DOI] [PubMed] [Google Scholar]
- 21.Jørgensen P., Milkovic L., Zarkovic N., Waeg G., Rattan S.I. Lipid peroxidation-derived 4-hydroxynonenal-modified proteins accumulate in human facial skin fibroblasts during ageing in vitro. Biogerontology. 2014;15(1):105–110. doi: 10.1007/s10522-013-9482-z. 24264997 [DOI] [PubMed] [Google Scholar]
- 22.Wildburger R., Mrakovcic L., Stroser M. Lipid peroxidation and Age-associated diseases-cause or consequence?: review. Turkiye Klin. J Med. Sci. 2009;29(1):189–193. 〈http://www.turkiyeklinikleri.com/article/en-lipid-peroxidation-and-age-associated-diseases-cause-or-consequence-review-53453.html〉. [Google Scholar]
- 23.Poliakova N., Després J.P., Bergeron J., Alméras N., Tremblay A., Poirier P. Influence of obesity indices, metabolic parameters and age on cardiac autonomic function in abdominally obese men. Metabolism. 2012;61(9):1270–1279. doi: 10.1016/j.metabol.2012.02.006. 22444779 [DOI] [PubMed] [Google Scholar]


