Abstract
Mucormycoses are serious infections caused by filamentous fungi of the order Mucorales. They occur most often in immunocompromised patients. We report five cases of mucormycosis in patients hospitalized in the Infectious Diseases Department in Sousse – Tunisia between 2000 and 2013. They were 4 males and one female, mean age 60 years. Three patients were diabetic and one patient had acute leukemia. The locations of mucormycosis were rhinocerebral, rhino-orbital, auricular, pulmonary and cutaneous. The Mucorales isolated were Rhizopus arrhizus in 3 cases and Lichteimia in 2 cases. All patients were treated with amphotericin B and 2 patients had, in addition, surgical debridement. Two patients died and 2 kept peripheral facial paralysis.
Keywords: Amphotericin B, diabetes, Lichteimia corymbifera, Mucormycosis, Rhizopus arrhizus
Introduction
Mucormycosis is a rare but serious infection due to filamentous fungi of the division of Mucorales, the class Zygomycetes. They occur most often in immunocompromised patients with diabeties or haematological malignancies with prolonged neutropenia they represent the third cause of fungal infection in these patients after invasive candidiasis and aspergillosis [1,2]. We report five cases of confirmed mucormycosis in patients hospitalized in the Infectious Diseases department of Sousse, Tunisia, between 2000 and 2013.
Result
The main data are summarized in Table 1.
Table 1.
Main clinical, therapeutic and outcome data in 5 patients with mucormycosis
| Patient 1 | Patient 2 | Patient 3 | Patient 4 | Patient 5 | |
|---|---|---|---|---|---|
| Year of diagnosis | 2007 | 2011 | 2012 | 2012 | 2013 |
| Gender, age (years) | F, 72 | M, 77 | M, 25 | M, 54 | M, 76 |
| Comorbiditie (s) | Type 2 diabetes | None | Acute leukemia | Type 2 diabetes | Type 2 diabetes |
| Infection localisation | Rhino-orbital pansinusitis | Chronic otitis media | Necrotizing fasciitis | Pneumonia | Rhino-cerebral + pansinusitis |
| Clinical signs | Hemiface inflammation | Otalgia, otorrhea, hypoacusis; PFP | Leg necrotizing inflammation | Cough, fever, dyspnea | Front subcutaneous abscess; confusion |
| Disease duration (days) | 2 | 30 | 15 | 4 | 4 |
| CT scan | Pansinusitis; infraorbital abscess | Filling the mastoid cells | — | Bilateral alveolo-interstitial syndrome | Pansinusitis |
| Direct examination Culture |
Hyphae Lichtemia |
Hyphae Lichtemia |
Negative Lichtemia |
Hyphae Rhizopus arrhizus |
Negative Rhizopus arrhizus |
| Diagnostic delay (days) | 57 | 15 | 7 | 2 | 7 |
| Ampho B duration (days) Ampho B side effects Surgery |
46 ARF Yes |
34 Hypokalemia; ARF No |
21 None Yes |
15 None No |
4 None No |
| Outcome | Sequelae/PFP | Sequelae/PFP | Local improvement death/shok-multiorgan failure | Death/ARDS | Death/brain hemorrhage |
F: female, M: male; PFP: peripheral facial paralysis; ampho B: amphotericin B;
ARF: acute renal failure; ARDS: Acute respiratory distress syndrome.
The patiuents were four men and one woman, mean age 60 years (25–77 years). Three patients had type 2 diabetes, one patient had acute leukemia (AL) in treatment failure with prolonged neutropenia and one patient was immunocompetent. Mucormycosis was rhino-cerebral, rhino-orbital, auricular (left otitis media), pulmonary and cutaneous. The mean duration of symptoms was 11 days (2–30 days).
Clinical signs included fever (all cases), peripheral facial palsy (PFP) and an orbital edema (two cases each), subcutaneous frontal abscess (Fig. 1) with mental confusion, ear pain with purulent otorrhea and hearing loss left, productive cough with dyspnea and necrotizing fasciitis of the left leg. Ketotic decompensation was noted in three patients with diabetes. All patients had received antibiotics before diagnosis of mucormycosis. The diagnosis of mucormycosis was made after average 17 days of hospitalization (2–57 days).
Fig. 1.
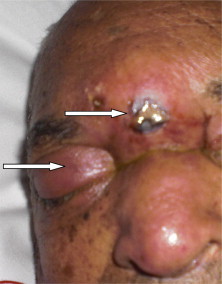
Frontal abscess with right orbital subcutaneous edema in one patient with mucormycosis.
Imaging and laboratory data
Computed tomography (CT) of the facial bones showed pansinusitis in two patients, associated with subcutaneous frontal abscess in a patient.
CT showed a rocks filling the mastoid cells in a patient.
Chest CT showed bilateral alveolar-interstitial infiltrate with alveolar consolidation in the right lower lobe (Fig. 2). The magnetic resonance imaging (MRI) showed bilateral predominantly frontal right lesion hyperintense on T2-Flair in a patient (Fig. 3).
Fig. 2.
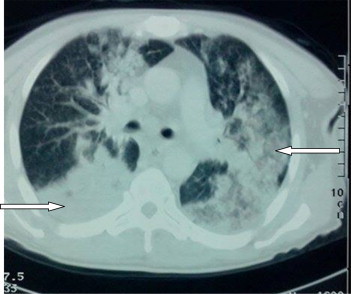
Chest CT. Bilateral iterstitiel and alveolar interstitial with lower right lobar alveolar consolidation.
Fig. 3.
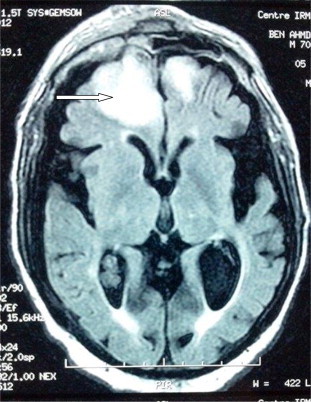
MRI Mucorales brain abscess: bilateral, predominantly frontal right lesion on T2-Flair.
The direct examination showed live mycological wide non septate hyphae with irregular diameter, indicative of Mucorales, in three cases. Culture was positive in all cases. Mucorales were isolated from the following samples: pus from the subcutaneous frontal abscess obtained by needle puncture, pus ear swab obtained on five different samples spaced several days, sinus biopsy, bronchial biopsy and intraoperative biopsy the soft tissue. It was identified as Lichteimia corymbifera (formerly Absidia corymbifera) in three cases and Rhizopus arrhizus in two cases.
Treatment – Evolution
All patients were treated with intravenous amphotericin B 0.7 to 1 mg / kg / day. Apart from one patient who died after four days of hospitalization, the mean duration of treatment was 32 days (15–46). Hypokalemia was observed in one patient in the course of moderate acute renal failure at the end of treatment in two patients. In two cases, the treatment was continued with correction of hypokalemia and hydration.
The evolution was marked by the early death in two patients (inter- hemispheric cerebral hematoma complicated with coma and respiratory distress in one case, and extensive pneumonia with respiratory distress in one case) and persistent sequelae in two other patients (PFP in two cases and hearing loss in one case). In one patient who had AL, treatment with amphotericin B and surgical debridement resulted in a local improvement, but the patient died one month after his discharge due to his underlying disease.
Discussion
In our study, mucormycosis was observed more often in immunocompromised patients (four out of five cases) and the most common sites were rhino-cerebral and rhino-orbital. These data are consistent with the literature data. Indeed, in 90% of cases, mucormycosis occur in immunocompromised patients, mainly diabetic ketosis or hematological malignancy with neutropenia. The naso-orbito-cerebral, lung and skin are the most common [1,2].
The observed auricular localization in one of our patients, immunocompetent is exceptional [3]. A Tunisian retrospective study compilING 17 cases of mucormycosis between 1992 and 2007, diabetes and rhino-orbitofrontal cerebral localization was noted in all patients [4].
In rhino-sinus mucormycosis, CT is the investigation of choice to study the invasion of bone and soft tissue abscesses, or hematoma, and extension to the central nervous system. MRI is more sensitive than CT for the investigation of possible cerebrovascular thrombosis. In pulmonary mucormycosis, chest radiograph or better chest CT typically show alveolar condensations sometimes excavated or nodular infiltrates frosted glass with or without halo sign [5]. These nose and brain damage and lung characteristics were observed in our patients.
Since imaging lesions are not specific of mucormycosis, a mycological diagnosis is necessary. The reference method is the direct examination and culturing of the pathological product: puncture fluid (pus, serous fluid), tissue biopsy. Mucorales hyphae are short, little or no septate, thick-walled and often branched at right angles.
The identification of the genus and species is of epidemiological interest but does not guide antifungal therapy. It is based on macroscopic and microscopic cultural characters. These characters are not specific where the recent use in specialized laboratories in molecular biology techniques such as PCR improved the etiological diagnostic [6]. The most common mucormycosis genera are Rhizopus (47%) and Mucor (18%). Lichteimia is responsible for 5% of the cases [2]. In a previous Tunisian study, Rhizopus had been isolated in 70% of patients [4]. Here, Lichteimia was isolated in three of five patients. In the literature, this type is more frequently isolated from male patients. There is no particular geographic distribution [2].
In addition to the mycological diagnosis, histological study of biopsy fragments are useful for the diagnosis of mucormycosis and allows the confirmation in case of Mucorales filament presence in tissues and vessels where they are responsible for thrombosis with infarction and hemorrhage [7].
In our study, the Mucorales were isolated from pus obtained by percutaneous puncture or multiple swab samples in two cases, and on biopsy fragments in three cases. Culture was positive in all cases. The two isolated genera were Rhizopus and Lichteimia. The predominance of the latter would be explained by the male in our study.
The treatment of mucormycosis is mainly based on antifungal and surgical debridement. The rapid equilibration of ketoacidosis in diabetics, transfusion of hematopoietic growth factors in long-term neutropenia and hyperbaric oxygen therapy may be useful [8]. Reference antifungal therapy is liposomal amphotericin B, 5 to 10 mg / kg / day. Amphotericin B deoxycholate should no longer be used because of its nephrotoxicity [8]. Other antifungal posaconazole or caspofungin can be used in combination with liposomal amphotericin B in case of treatment failure or as a substitute for serious side effects [8]. Fluconazole, voriconazole and itraconazole have no activity on Mucorales [9].
Mucormycosis has poor prognosis with a mortality rate of 17–51% [10]. Mortality is higher in case of diagnostic delay of more than five days and monocytopenia in patients with active malignant blood diseases. Surgical treatment associated with antifungals improves prognosis [2,10]. The genus or species of Mucorales offending does not appear to influence the outcome [10,11]. In a previous Tunisian study, mortality was 65% [4].
In our study, all patients were treated with amphotericin B deoxycholate as liposomal amphotericin B is not available in our country and two patients had surgical excision. Moderate renal impairment occurred in two patients. A late diagnosis> five days was noted in four patients. Two patients died and two kept sequelae.
Conclusion
The diagnosis of mucormycosis should be suspected in any diabetic patient with neutropenia or neutropenic presenting with rhino-orbitofrontal brain or lung unimproved by appropriate antibiotic therapy. Other locations are less characteristic. Diagnosis is suspected on clinical and radiological features and confirmed by mycological and pathological examination. Treatment consists of amphotericin B combined with surgery. Morbidity and mortality are high due to the invasive nature of the frequent underlying malignancy, hence the importance of early and appropriate management.
Conflict of interest
None.
References
- 1.Petrikkos G., Skiada A., Lortholary, Roilides E., Walsh T.J., Kontoyiannis D.P. Epidemiology and Clinical Manifestations of Mucormycosis. Clin Infect Dis. 2012;54(S1):S23–S34. doi: 10.1093/cid/cir866. [DOI] [PubMed] [Google Scholar]
- 2.Roden M.M., Zaoutis T.E., Buchanan W.L. Epidemiology and outcome of mucormycosis: a review of 929 reported cases. Clin Infect Dis. 2005;41:634–653. doi: 10.1086/432579. [DOI] [PubMed] [Google Scholar]
- 3.Hazarika P, Zachariah J, Victor J, John M, Devi C, Abraham P. Mucormycosis of the Middle Ear: A Case Report with Review of Literature. [DOI] [PMC free article] [PubMed]
- 4.Anane S., Kaouech E., Belhadj S., Ammari L., Abdelmalek R., Ben Chaabane T. Rhino-orbito-cerebral mucormycosis in the diabetic: a better known pathology in Tunisia. Ann Biol Clin. 2009;67(3):325–332. doi: 10.1684/abc.2009.0323. [DOI] [PubMed] [Google Scholar]
- 5.Trifilio S.M., Bennett C.L., Yarnold P.R. Breakthrough mucormycosis after voriconazole administration among patients with hematologic malignancies who receive hematopoietic stem-cell transplants or intensive chemotherapy. Bone Marrow Transplant. 2007;39:425–429. doi: 10.1038/sj.bmt.1705614. [DOI] [PubMed] [Google Scholar]
- 6.Petrikkos G.L., Skiada A., Sambatakou H. Mucormycosis: ten year experience in a tertiary-care centre in Greece. Eur J Clin Microbiol Infect Dis. 2003;22:753–756. doi: 10.1007/s10096-003-1035-y. [DOI] [PubMed] [Google Scholar]
- 7.Nosari A., Oreste P., Montillo M. Mucormycosis in hematologic malignancies: an emerging fungal infection. Haematologica. 2000;85:1068–1071. [PubMed] [Google Scholar]
- 8.Cornely O.A., Arikan-Akdagli S., Dannaoui E., Groll A.H., Lagrou K., Chakrabarti A. ESCMID and ECMM joint clinical guidelines for the diagnosis and management of mucormycosis 2013. Clin Microbiol Infect. 2014;20(Suppl. 3):5–26. doi: 10.1111/1469-0691.12371. [DOI] [PubMed] [Google Scholar]
- 9.Sun Q.N., Fothergill A.W., McCarthy D.I. In vitro activities of posaconazole, itraconazole, voriconazole, amphotericin B, and fluconazole against 37 clinical isolates of zygomycetes. Antimicrob Agents Chemother. 2002;46:1581–1582. doi: 10.1128/AAC.46.5.1581-1582.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chamilos G., Lewis R.E., Kontoyiannis D.P. Delaying amphotericin B-based frontline therapy significantly increases mortality among patients with hematologic malignancy who have zygomycosis. Clin Infect Dis. 2008;47:503–509. doi: 10.1086/590004. [DOI] [PubMed] [Google Scholar]
- 11.Yohai R.A., Bullock J.D., Aziz A.A., Markert R.J. Survival factors in rhino-orbital-cerebral mucormycosis. Surv Ophtalmol. 1994;39:3–22. doi: 10.1016/s0039-6257(05)80041-4. [DOI] [PubMed] [Google Scholar]


