Abstract
Background
Distraction osteogenesis (DO) is a powerful reconstructive technique for bone growth and repair. An angiogenic means to enhance the efficacy of this metabolically demanding procedure would be beneficial in expanding its therapeutic potential. We posit that the angiogenic effect of Deferoxamine (DFO), an iron chelator that has been shown to increase angiogenesis, will improve bone regeneration via augmentations in quality and quantity of bone and bone producing cells.
Methods
Two groups of rats (n=12) underwent surgical external fixation and subsequent distraction. During the distraction stage, the experimental DFO group (n=5) was treated with injections into the distraction gap. After 28 days of consolidation, mandibles were harvested and prepared for histological analysis.
Results
We found a proliferation of osteocytes in the DFO treated group when compared to the regenerate (RG) of the control group. DFO effected a significant increase in osteocytes, as well as increase in bone volume fraction with subsequent decreased osteoid volume fraction. The data also demonstrated no significant difference in empty lacunae.
Conclusions
Our study demonstrates the effectiveness of DFO treatment to enhance the number of osteocytes within the RG in a murine mandibular DO model. Maintenance of full lacunae supports our findings of a robust cellular response to DFO therapy. These results suggest that the angiogenic capabilities of DFO translate into an increase in number of bone forming cells in the RG. DFO may have utility in optimizing bone formation in DO and lead to superior reconstructive capabilities for craniofacial surgeons in the future.
Introduction
Distraction osteogenesis is a powerful reconstructive technique that promotes bone induction by applying controlled gradual separation between two osteogenic fronts. Initially developed for long bone lengthening in orthopedic surgery, it has evolved into a conventional reconstructive treatment with a variety of applications including severe craniofacial deformities.1–4 This valuable technique provides advantages over alternative reconstructive methods including avoidance of local, regional or distant donor site morbidity, and concurrent generation of both bone and soft tissue using local endogenous substrate.5,6 Its success has begged the question of how far its inherent regenerative capacity can be stretched and applied in various complex clinical scenarios.
The limitations of DO remain largely unknown and the technique and overall protocols remain the same as when Ilizarov had invented the procedure over 50 years ago.7–9 There is much to gain in optimizing DO beyond its current boundaries allowing for a decreased consolidation time, shorter distraction period, or an expanded distraction gap. Efforts to enhance bone regeneration have largely focused on optimizing the duration of latency and consolidation periods, or altering the rate and rhythm of distraction.10,11 Innovative approaches, including the use of hyperbaric oxygen therapy, cyclic mechanical lengthening and compression, and the addition of several osteogenic factors, have been investigated with varying degrees of success.12–16
Another way to improve upon the DO procedure would be to augment the blood supply to the regenerate (RG) in order to expand and optimize the applications of the technique. DO induces a biological response of skeletal regeneration in a cascade of bone induction and formation processes.6 Angiogenesis plays a significant role during bone regeneration as numerous studies have demonstrated an increase in blood flow in association with increased angiogenesis during DO and bone repair.17-20 In recognition of the importance of vascular supply to skeletal repair, recent studies have focused on pharmacologic interventions to improve blood supply during healing.21
Deferoxamine (DFO) is an FDA approved medication and iron-chelator that has been shown to increase angiogenesis via the hypoxia inducible factor (HIF) pathway. The HIF pathway activates angiogenesis as a regulator of response to hypoxia whose activation is also seen in skeletal repair. HIF-1α is constitutively expressed and rapidly degraded under normoxic conditions. DFO interferes with HIF-1α degradation by its chelation of iron, a necessary cofactor. This allows for accumulations of HIF-1α and activation of responsive genes for angiogenesis.22,23
We have previously shown DFO's ability to increase angiogenesis in a murine model of mandibular DO.24 Despite these findings, the exact mechanisms by which angiogenesis improves bone regeneration in DO have yet to be well defined. We posit that the angiogenic effect of DFO will function to improve bone regeneration in the mandible by augmenting the quality and quantity of bone as well as the number of bone producing cells. Our specific aim is to use quantitative histomorphometry (QHM) to objectively measure the effectiveness of DFO to increase the osteocyte count and bone healing metrics of the RG in DO of the murine mandible.
Materials & Methods
Adult male Sprague-Dawley rats (n=12) weighing approximately 400g were paired in cages and maintained in a pathogen-free environment on a 12-hour light/dark schedule. Rats were fed standard hard chow and water ad libitum during a seven-day acclimation period prior to surgery. All animal procedures were performed in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals and approved by the University of Michigan Animal Care and Use Committee.
Surgical Procedure and Device Placement
Preoperative subcutaneous injections of Gentamycin (5 mg/kg), Buprenorphine (0.03 mg/kg) and Lactated Ringer's solution (25cc/kg) were administered prior to surgery. Anesthesia was achieved with the inhalation of an oxygen/isoflurane mixture. The animals were prepared for surgery, and underwent placement of custom titanium external mandibular fixator devices followed by surgical osteotomy as previously described (Figure 1).25,26
Figure 1.
Radiograph displaying active distraction of the custom external bilateral fixator in a rat.
The critical size defect utilized in our DO model is predicated on our previous experimental experience demonstrating that gradual distraction to 5.1 mm with a 28 day consolidation period consistently resulted in bony healing across the entire regenerate.25 Comparatively, defects created using acute separation to 5.1 mm or greater consistently formed fibrous union.
Postoperative Procedures
Both groups underwent distraction after 4 days of latency. One 180-degree clockwise turn of the distraction screw corresponded to a 0.3-mm separation of the osteotomy fronts. Active distraction began on the evening of post-operative day 4 and went through the evening of postoperative day 12. A total of 17 half-turns were performed on a 12-hour interval, resulting in a 5.1 mm distraction gap. The experimental DFO group (n=5) was treated with a 200 μM DFO injection into the distraction gap every other day during the active distraction period. No analgesic or sedation was required during the distraction.
Postoperative Animal Care
Animals were housed one per cage and fed moist chow with Hill's high-calorie diet (Columbus Serum, Columbus, Ohio) and water ad libitum. Two postoperative doses of Gentamycin (5 mg/kg subcutaneously every 12 hours) were given as well as continuation of Buprenorphine (0.03 mg/kg) and Lactated Ringer's solution (10cc) subcutaneously every 12 hours through postoperative day 4, and as needed thereafter. Weights were monitored daily and diets adjusted as needed. Pin care was performed with Silvadene (Monarch Pharmaceuticals, Inc., Bristol, Tenn.) every other day. Maxillary incisors were clipped weekly due to overgrowth from cross bite and staples were removed by postoperative day 10. Animals were allowed to complete a 40-day recovery period prior to sacrifice.
Tissue Processing
Left hemi-mandibles were harvested and demineralized using Cal-Ex II (Fisher Scientifics; Fairlawn, NJ), a formic acid solution. Specimens were vacuum processed by dehydration and paraffin infiltration for 48 hours (Shandon Hypercenter XP, Pittsburgh, PA), reinfiltrated for 2 hours in a vacuum bath (Leica Embedding Center, model EG1160, Germany), and embedded in paraffin using Paraplast Plus (St Louis, MO). Peel-away embedding molds were used and refrigerated overnight (4°C). Blocks were then sectioned from anterior to posterior into 7-μm coronal sections and mounted on glass slides. A total of 70 to 100 slides per block were obtained. Ten equally spaced slides from within the RG was selected to uniformly represent the distraction gap and stained with Gomori 1-step trichrome. Two midgap representative slides per specimen were chosen for continued evaluation by QHM.
Histomorphometric Evaluation
Point counting of osteocytes and empty lacunae was performed with a light microscope interfaced with a digital camera connected to a computer. Two randomly selected sections from within the regenerate-spanning region were analyzed using the image analysis software program Bioquant NOVA Osteo version 7 (R&M Biometrics, Nashville, Tenn.). Nine high-power field images were randomly selected per region of interest using 16× magnification. The high-power field images measured 295 × 366 pixels and were stored as TIFF files. Point counting of osteocytes and empty lacunae was performed by three independent reviewers.
Image thresholding was also performed with Bioquant software. Using the images obtained previously for point counting, three independent reviewers each obtained the tissue volume (TV), mineralized bone volume (BV), and nonmineralized immature osteoid volume (OV). Mature, mineralized bone color thresholded blue, while osteoid, immature bone color thresholded red. The obtained measurements were then used to calculate ratios for BV/TV and OV/TV.
Statistics
Statistical analysis was performed using SPSS for Windows, version 17.0 (SPSS Inc, Chicago, IL). All data is presented as mean ± SE. Levene's test was used to determine distribution of data. Two-tailed independent samples t-test and Mann-Whitney (M-W) test were used for analyzing the following metrics: osteocytes/HPF and empty lacunae/HPF. Results were accepted as statistically significant at p<0.05. Mann-Whitney results were reported as the median with the interquartile range.
Results
Both groups completed the surgical procedure without incident. Postoperatively, all animals gained weight and maintained normal cage activity. None of the animals experienced device dislodgement and the fixators remained stable until the animals were sacrificed. Both groups did appear to have complete bony bridging. Gross examination of the left hemimandibles revealed an increase in RG formation and surrounding tissue in the experimental group receiving DFO.
Microscopic evaluation confirmed that the DFO treated group generated more dense woven bone in the distraction gap compared with controls (Figure 2). QHM point counting of osteocytes demonstrated a substantial proliferation of osteocytes in the DFO treated animals when compared to the RG of the control group. DFO treated animals saw a statistically significant increase of over 80% in osteocyte count per high powered field when compared to those animals that did not receive DFO (49 ±2.5 vs 27 ±3.1; p<0.001; Levine's test 0.44; M-W 49.8 vs 22.0 [21.3 – 49.2]; Figure 3). No significant difference in the number of empty lacunae between the two groups could be demonstrated via QHM analysis (1.11 ±0.25 vs 0.75 ±0.31; Levine's test 0.86; M-W 0.9 vs 0.7 [0.41 – 1.10]; Figure 3).
Figure 2.
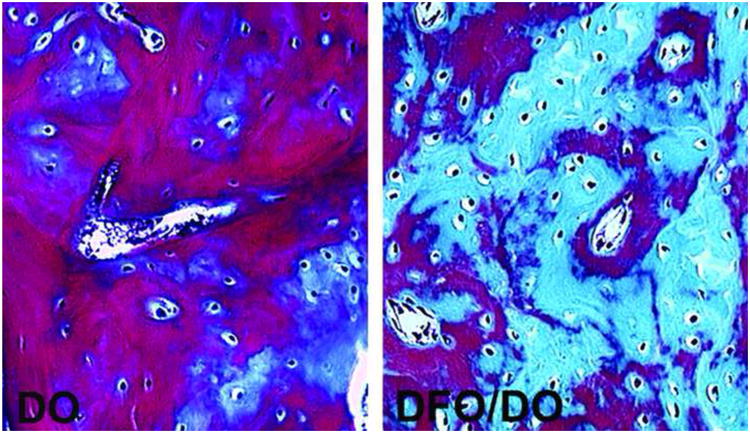
Micrographs of 7micron sections of bone within the regenerate stained with Gomori trichrome taken at 16× magnification. Mature bone thresholded blue and osteoid thresholded red. DO - representative distracted control sample. DFO/DO - representative DFO treated distracted sample.
Figure 3.
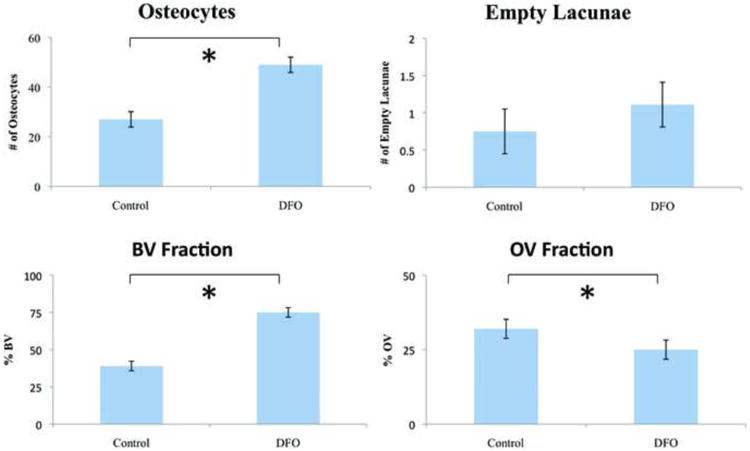
Quantitative histomorphometric data comparing the distracted control group and the DFO treated distracted group. BV – bone volume. OV – osteoid volume.
Gross examination of the slides showed an increase in total volume and apparent BV in the DFO treated group. Color thresholding revealed a significant increase in BV fraction of the DFO treated group compared with that of the control group (0.75 ±0.048 vs 0.39 ±0.032, p<0.05; Levine's test 0.11; M-W 0.79 vs 0.39 [0.39 – 0.76]; Figure 3). There was also a corresponding decrease in nonmineralized, osteoid matrix volume fraction in the DFO treated mandibles compared to the control group (0.25 ±0.048 vs 0.32 ±0.032, p<0.05; Levine's test 0.10; M-W 0.24 vs 0.34 [0.24 – 0.35]; Figure 3).
Discussion
The technique of mandibular DO relies on the recruitment and proliferation of bone progenitor cells.6 The osteogenic process appears to be driven by angiogenesis, which is stimulated through HIF activation whereby increased bone deposition is proportional to an increase in vascularity.6,17,19,20 In the present study, we document the effectiveness of DFO treatment to enhance bone production and the number of osteocytes within the RG of a murine mandibular model of DO. Our in vivo results suggest that these effects are a result of DFO's ability to maintain HIF-1α expression thereby increasing vascularity to the RG. To our knowledge, all other studies using DFO for osteogenic purposes have been completed exclusively in long bones.22,23 Our results in the membranous bone of the murine mandible support and corroborate other recent DFO therapy studies in endochondral long bones showing increased callus size and bone-strength in distraction and fracture repair.22,23
Studies in long bone distraction have shown the biological impact of HIF activation to increase callus size, bone volume, and biomechanical strength.23 Our data in the mandible confirms an increase in bone volume, more specifically, that of increased mineralized bone. Increased bone mineralization was demonstrated by the higher BV/TV ratio and a subsequent decrease in OV/TV ratio in the experimental group when compared with controls. Our data also suggests that the improvement in bone healing metrics as a result of improved angiogenesis may be attributed to a proliferation of osteocytes. A proposed theory for DFO's activity is that the angiogenesis observed in the regenerating bone would serve to increase the number of active bone (re)modeling units and provide a conduit for supply of circulating bone precursor cells and/or delivery of vessel derived factors and cells required for bone formation.23 The results of this study lend substantial support to this theory by demonstrating a substantial expansion in RG cellularity, specifically an 80% increase in osteocyte count. In similar studies using long bones, the proliferation of osteocytes and increase of more dense woven bone reveals a clinical benefit in DO as revealed by increased bone strength.22 Our lab is completing further studies to extend this clinical significant conclusion of increased bone strength with DFO to distraction of intramembranous bones.
The ability to enhance the RG in mandibular DO may allow significant modifications of the conventional applications of the procedure. Future studies of interest will indicate how increased vascularity might decrease the latency and consolidation period or increase the periodicity, allowing for quicker removal of the distractor hardware. Even more promising is the potential to increase the overall length that is achievable using current distraction protocols. Our findings that increased vascularity translates into increased and enhanced bone formation may have further application in scenarios where the vascular environment is sub-optimal such as trauma or radiation injury. In these cases DFO could be utilized as a therapeutic utilized to mitigate the pernicious effects of poor vascularity on new bone formation and promote healing. Our findings also have potential for improvement of current conventional DO protocols as DFO may allow for better consolidation and further maturity of the bone thereby decreasing relapse.
Our treatment protocol of DFO was designed to promote angiogenesis and bone growth while avoiding the adverse effects of iron deficiency on osteoblastogenesis and decreased bone density. Although we focused our efforts during the time of distraction in this report, there could also be a potential benefit for the use of DFO during the consolidation period depending on the outcome that is desired. An important limitation of this study is the limited time frame in which the bone is characterized. Additional studies analyzing the bone at different time points currently underway will provide a more detailed our understanding of the entire remodeling process with DFO treatment. As DFO therapy progresses to clinical treatment, different methods of delivery, timing, and dosage need to be considered and optimized.
Conclusions
Our findings suggest that DFO can improve the quantity, quality, and cellularity of RG formation in the setting of mandibular DO. Our study also raises the possibility that DFO may be more broadly applied to bone repair where vascularity is critical. The ability of DFO to enhance bone formation may have utility in surgeon directed modification of current conventional DO protocols which may in turn lead to superior reconstructive capabilities for craniofacial surgeons in the future.
Acknowledgments
Funding was provided by National Institutes of Health grant RO1 CA 12587-01 to S.R.B. and ASMS/MSF & Synthes CMF Research Grant to A.S.F. The authors thank Elizabeth R. Razdolsky and Aria J. Zehtabzadeh for assistance during surgery and animal care, and Salman Ahsan for assistance with the manuscript. They also thank Charles Roehm and John Baker for technical assistance in the preparation of the external fixator/distractor and the preparation of the tissues for histologic analysis, respectively.
Footnotes
Disclosure/Financial Support: All authors have no competing financial interests to report.
References
- 1.Codivilla A. On the means of lengthening in the lower limbs. Am J Orthop Surg. 1905;2:353–369. [Google Scholar]
- 2.Karp NS, Thorne CH, McCarthy JG, Sissons HA. Bone lengthening in the craniofacial skeleton. Ann Plast Surg. 1990 Mar;24(3):231–7. doi: 10.1097/00000637-199003000-00007. [DOI] [PubMed] [Google Scholar]
- 3.McCarthy JG, Schreiber J, Karp N, Thorne CH, Grayson BH. Lengthening the human mandible by gradual distraction. Plast Reconstr Surg. 1992 Jan;89(1):1–8. [PubMed] [Google Scholar]
- 4.Yu JC, Fearon J, Havlik RJ, Buchman SR, Polley JW. Distraction osteogenesis of the craniofacial skeleton. Plast Reconstr Surg. 2004;114:1E–20E. doi: 10.1097/01.prs.0000128965.52013.95. [DOI] [PubMed] [Google Scholar]
- 5.Holmes SB, Lloyd T, Coghlan KM, Newman L. Distraction osteogenesis of the mandible in the previously irradiated patient. J Oral Maxillofac Surg. 2002;60:305–309. doi: 10.1053/joms.2002.30581. [DOI] [PubMed] [Google Scholar]
- 6.Ai-Aql ZS, Alagl S, Graves DT, Gerstenfeld LC, Einhorn T. Molecular mechanisms controlling bone formation during fracture healing and distraction osteogenesis. J Dent Res. 2008;87(2):107–118. doi: 10.1177/154405910808700215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ilizarov GA. The tension-stress effect on the genesis and growth of tissues: Part I. The influence of stability of fixation and soft tissue preservation. Clin Orthop Relat Res. 1989;238:249–281. [PubMed] [Google Scholar]
- 8.Ilizarov GA. The tension-stress effect on the genesis and growth of tissues: Part II. The influence of the rate and frequency of distraction. Clin Orthop Relat Res. 1989;239:263–285. [PubMed] [Google Scholar]
- 9.Ilizarov GA, Ledyaev VI. The replacement of long tubular bone defects by lengthening distraction osteotomy of one of the fragments. Clin Orthop Relat Res. 1992;280:7–10. [PubMed] [Google Scholar]
- 10.King G, Liu ZJ, Wang LL, Chiu IY, Whelan MF, Huang GJ. Effect of distraction rate and consolidation period on bone density following mandibular osteodistraction in rats. Arch Oral Biol. 2003;48:299–308. doi: 10.1016/s0003-9969(03)00004-9. [DOI] [PubMed] [Google Scholar]
- 11.Troulis MJ, Glowacki J, Perrott DH, Kaban LB. Effects of latency and rate on bone formation in a porcine mandibular distraction model. J Oral Maxillofac Surg. 2000;58:507–513. doi: 10.1016/s0278-2391(00)90012-0. discussion 514. [DOI] [PubMed] [Google Scholar]
- 12.Marx RE, Johnson RP. Studies in the radiobiology of osteoradionecrosis and their clinical significance. Oral Surg Oral Med Oral Pathol. 1987;64(4):379–90. doi: 10.1016/0030-4220(87)90136-8. [DOI] [PubMed] [Google Scholar]
- 13.Lai JP, Wang FS, Hung CM, Wang CJ, Huang CJ, Kuo YR. Extracorporeal shock wave accelerates consolidation in distraction osteogenesis of the rat mandible. J Trauma. 2010;69:1252–1258. doi: 10.1097/TA.0b013e3181cbc7ac. [DOI] [PubMed] [Google Scholar]
- 14.Cillo JE, Jr, Gassner R, Koepsel RR, Buckley MJ. Growth factor and cytokine gene expression in mechanically strained human osteoblast-like cells: Implications for distraction osteogenesis. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2000;90:147–154. doi: 10.1067/moe.2000.107531. [DOI] [PubMed] [Google Scholar]
- 15.Ashinoff RL, Cetrulo CL, Jr, Galiano RD, et al. Bone morphogenic protein-2 gene therapy for mandibular distraction osteogenesis. Ann Plast Surg. 2004;52:585–590. doi: 10.1097/01.sap.0000123023.28874.1e. discussion 591. [DOI] [PubMed] [Google Scholar]
- 16.Kontaxis A, Abu-Serriah M, Ayoub AF, Barbenel JC. Mechanical testing of recombinant human bone morphogenetic protein-7 regenerated bone in sheep mandibles. Proc Inst Mech Eng H. 2004;218:381–388. doi: 10.1243/0954411042632135. [DOI] [PubMed] [Google Scholar]
- 17.Aronson J. Temporal and spatial increases in blood flow during distraction osteogenesis. Clin Orthop Relat Res. 1994;(301):124–31. [PubMed] [Google Scholar]
- 18.Glowacki J. Angiogenesis in fracture repair. Clin Orthop Relat Res. 1998;355(Suppl):S82–S89. doi: 10.1097/00003086-199810001-00010. [DOI] [PubMed] [Google Scholar]
- 19.Fang TD, Salim A, Xia W, et al. Angiogenesis is required for successful bone induction during distraction osteogenesis. J Bone Miner Res. 2005;20(7):1114–24. doi: 10.1359/JBMR.050301. [DOI] [PubMed] [Google Scholar]
- 20.Donneys A, Tchanque-Fossuo CN, Farberg AS, Deshpande SS, Buchman SR. Bone regeneration in distraction osteogenesis demonstrates significantly increased vascularity in comparison to fracture repair in the mandible. J Craniofac Surg. 2012;23:328–332. doi: 10.1097/SCS.0b013e318241db26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Street J, Bao M, deGuzman L, et al. Vascular endothelial growth factor stimulates bone repair by promoting angiogenesis and bone turnover. Proc Natl Acad Sci USA. 2002;99:9656–9661. doi: 10.1073/pnas.152324099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shen X, Wan C, Ramaswamy G, et al. Prolyl hydroxylase inhibitors increase neoangiogenesis and callus formation following femur fracture in mice. J Orthop Res. 2009;27:1298–1305. doi: 10.1002/jor.20886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wan C, Gilbert SR, Cao X, et al. Activation of the hypoxia-inducible factor-1alpha pathway accelerates bone regeneration. Proc Natl Acad Sci USA. 2008;105:686–691. doi: 10.1073/pnas.0708474105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Donneys A, Farberg AS, Tchanque-Fossuo CN, Deshpande SS, Buchman SR. Plast Reconstr Surg. 2012;129:850–856. doi: 10.1097/PRS.0b013e31824422f2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Buchman SR, Ignelzi MA, Jr, Radu C, et al. A unique rodent model of distraction osteogenesis of the mandible. Ann Plast Surg. 2002;49:511–519. doi: 10.1097/00000637-200211000-00012. [DOI] [PubMed] [Google Scholar]
- 26.Tong L, Buchman SR, Ignelzi MA, Jr, Rhee S, Goldstein SA. Focal adhesion kinase expression during mandibular distraction osteogenesis: evidence for mechanotransduction. Plast Reconstr Surg. 2003;111(1):211–22. doi: 10.1097/01.PRS.0000033180.01581.9A. discussion 223–4. [DOI] [PubMed] [Google Scholar]


