Abstract
Background
While the current methodology for determining fibrous cap (FC) thickness of lipid plaques is based on manual measurements of arbitrary points, which could lead to high variability and decreased accuracy, it ignores the three-dimensional (3-D) morphology of coronary artery disease.
Objective
To compare, utilizing optical coherence tomography (OCT) assessments, volumetric quantification of FC, and macrophage detection using both visual assessment and automated image processing algorithms in non-culprit lesions of STEMI and stable angina pectoris (SAP) patients.
Methods
Lipid plaques were selected from 67 consecutive patients (1 artery/patient). FC was manually delineated by a computer-aided method and automatically classified into three thickness categories: FC < 65 μm (i.e., thin-cap fibroatheroma [TCFA]), 65–150 μm, and >150 μm. Minimum thickness, absolute categorical surface area, and fractional luminal area of FC were analyzed. Automated detection and quantification of macrophage was performed within the segmented FC.
Results
A total of 5,503 cross-sections were analyzed. STEMI patients when compared with SAP patients had more absolute categorical surface area for TCFA (0.43 ± 0.45 mm2 vs. 0.15 ± 0.25 mm2; P = 0.011), thinner minimum FC thickness (31.63 ± 17.09 μm vs. 47.27 ± 26.56 μm, P = 0.012), greater fractional luminal area for TCFA (1.65 ± 1.56% vs. 0.74 ± 1.2%, P = 0.046), and greater macrophage index (0.0217 ± 0.0081% vs. 0.0153 ± 0.0045%, respectively, P< 0.01).
Conclusion
The novel OCT-based 3-D quantification of the FC and macrophage demonstrated thinner FC thickness and larger areas of TCFA coupled with more inflammation in non-culprit sites of STEMI compared with SAP.
Keywords: optical coherence tomography, fibrous cap, macrophage, ST-segment elevation myocardial infarction, stable angina, thin-cap fibroatheroma, atherosclerosis
INTRODUCTION
Atherosclerotic plaques that have a higher likelihood of rupture (i.e., unstable plaques) have been characterized by hystopathology by the presence of large lipid cores covered with a thin fibrous cap (FC) (i.e., thin-cap fibroatheroma [TCFA]) and inflammation [1–3]. Notwithstanding the paramount contribution of these studies aiming at elucidating the complex pathophysiological process of plaque rupture and thrombus formation, the ultimate goal of identifying “rupture-prone” plaques in vivo has been hampered by the previous insufficient resolution of intravascular imaging systems. Due to its unprecedentedly high axial resolution (~10 μm), intravascular optical coherence tomography (OCT) enables comprehensive assessments of plaque morphology [4], being the only imaging modality available for clinical use capable of quantifying FC thickness and macrophage infiltration, a potential marker of plaque inflammation [5–9].
While thinner FC has been more frequently demonstrated in ST-segment elevation myocardial infarction (STEMI) compared with stable angina pectoris (SAP) patients [10], macrophage infiltration in the FC, which plays an important role in the pathogenesis of plaque rupture, is also more commonly found in coronary artery specimens obtained from patients suffering from acute coronary syndromes compared with SAP [2,9]. The current methodology for determining FC thickness in vivo, however, is based on manual individual measurements of arbitrary points (i.e., the thinnest regions determined by visual assessment), which could lead to high variability and reduced accuracy. Importantly, such planar, one-dimensional evaluation of FC thickness ignores the three-dimensional (3-D) morphology of coronary artery disease [7,11–17]. Therefore, in order to advance our understanding and ability to determine the characteristics of unstable plaques, a more comprehensive assessment, which takes into account the complex 3-D nature of coronary artery disease, is required.
Our group previously developed a semi-automated method that allows comprehensive quantification of FC thickness and 3-D visualization of its longitudinal and circumferential distribution along the vessel [18,19]. In addition, the feasibility of macrophage quantification by means of OCT in murine aorta with high histological correlation has been demonstrated [20]. Therefore, in the present study, we compared FC 3-D quantification and macrophage detection capitalizing on OCT imaging assessment using automated processing algorithms in non-culprit lesions of STEMI and SAP patients.
METHODS
Consecutive patients with STEMI and SAP who underwent OCT evaluation of target coronary arteries after stent implantation to the culprit lesion were selected from the database of the Cardiovascular Imaging Core Laboratory, University Hospitals Case Medical Center, Cleveland, OH, USA. The OCT imaging was performed whenever the operator judged it was necessary to optimize the stent result. The region of interest (ROI) was defined as the proximal and distal segments visualized by OCT within 5 mm out of the stent edges. All OCT images were acquired by a commercial Fourier-Domain OCT system (C7-XR™ OCT Intravascular Imaging System, St. Jude Medical, St. Paul, MN) after intracoronary administration of 200 μg nitroglycerin through conventional guiding catheters. OCT catheter was advanced through a 0.014″ guidewire to the most distal segment of the artery. The entire length of the ROI was scanned using an integrated automated pullback device at 20 mm/sec. During image acquisition, blood was displaced by the injection of iso-osmolar contrast (Iodixanol 370, Visipaque™, GE Health Care, Ireland) with a power injector through conventional guiding catheters. All images were digitally stored and submitted to Core Laboratory offline evaluation and subsequent analysis using proprietary software (St. Jude Medical). The images were analyzed by two experienced OCT analysts blinded to clinical presentation. All cross-sectional images were initially screened for quality assessment and excluded from analysis if any portion of the image was out of the screen, a side branch occupied >45° of the cross-section, or the image had poor quality caused by residual blood or sew-up artifact [4]. Luminal areas and diameters were measured every 1 mm [4,8]. Qualitative plaque assessment was performed by dividing the cross-sections into four quadrants, with each quadrant labeled according to its most prevalent component, as follows: (a) normal: three layered architecture, comprising intima, media, and adventitia, (b) fibrous plaque: homogeneous, highly backscattering regions with intimal thickness ≥ 300 μm, (c) calcified plaque: low scattering regions with sharply delineated borders, and (d) lipid plaque: signal-poor regions with diffuse borders and high attenuation [8]. Plaque components were then quantified and expressed as percentages. Aiming at assessing the reproducibility of our methodology for qualitative assessment of plaque characteristics, 150 cross-sections were analyzed by two different OCT analysts with an interval of 60 days. Intra- and inter-observer variability was assessed.
Lipid plaques were identified and all the correspondent frames were analyzed. FC was delineated by a luminal boundary, coinciding with the vessel lumen contour, and an abluminal boundary, commonly described as the “diffuse border” created by the interface between the FC and the underlying lipid pool. We applied a computer-aided method to segment the FC boundaries. The method has been previously validated and has been found to be highly accurate, yet more consistent than human experts [18]. With the fully segmented FC, we were able to quantify the thickness at each point of its luminal boundary as the minimum distance from this point to the abluminal boundary. The mean and minimum thickness was then obtained. The FC surface area of a lesion was calculated as the product of the frame interval and the arc length of FC summed over involved frames. The absolute and fractional FC categorical surface area of a lesion was calculated as the absolute and relative FC area in a thickness category. We classified FC thickness into three categories: <65 μm, 65–150 μm, and >150 μm. This definition was based on both histopathology and in vivo clinical studies using OCT [2,12]. FC fractional luminal area was defined as the percentage of luminal area occupied by the FC. Categorical FC fractional luminal area was defined in a similar way but only counting the area in a thickness category [18]. TCFA was defined as a FC thickness of <65 μm overlying a low-intensity area with an unclear border (i.e., lipid plaque).
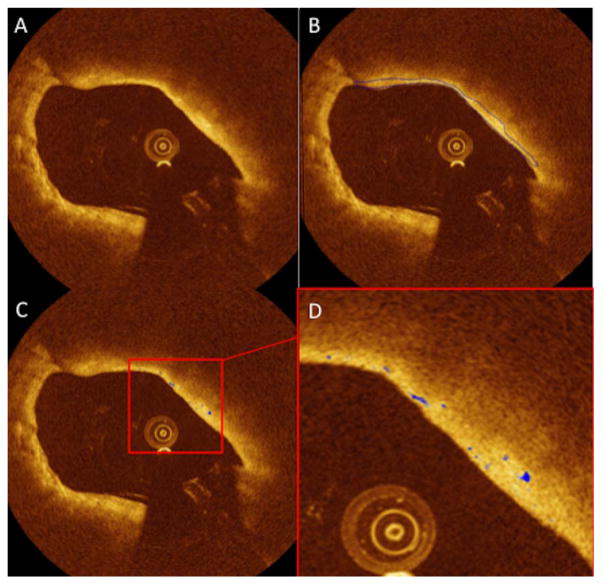
Macrophage quantification was performed within the segmented FC using the linear-scale OCT images for feature extraction and classification as suggested by Tearney et al. [5]. We have previously demonstrated a decision tree-based classification method for macrophage quantification in murine aorta and have found good correlation (r = 0.92) with histology [20]. We hereby extend the work and apply it to human macrophage quantification. First, the features of every pixel inside the FC were extracted, including: intensity, standard deviation of surrounding neighborhood (denoted by STD), the intensity difference of the central pixel and the median of its surrounding window (denoted by MID), and the attenuation of the 75 μm A-line segment behind the pixel. STD and MID were extracted from windows of different sizes (50 × 50, 85 × 85, and 120 × 120 μm2) to capture the heterogeneous texture at different scales. Training data was provided by an independent interventional cardiologist by carefully segmenting the macrophages manually from a different dataset, consisting of 22 images from 10 vessels. Features were selected on the training data with 5-fold cross validation using a Wrapper model and greedy step-wise evaluation procedure in the software Weka [21] and were then used to build a C4.5 decision tree [22], which was then applied to the test data. Bagging [23] was used to reduce the variance of the data. Once the macrophages inside the FC were classified, we can calculate the macrophage index, defined as the ratio between the macrophage area and the FC area in the same cross-section (Fig. 1).
Fig. 1.
Illustration of FC segmentation and macrophage quantification performed on the same OCT cross-section, enabling the quantification of the thickness at each point of the FC as the surface area. A: Original image of a lipid-rich plaque with high signal attenuation of light; (B) Automated segmentation of FC; (C) Macrophage area (showed in blue); (D) Zoom highlighting the macrophage areas.
All statistical analyses were performed using SAS 9.2 (SAS Institute Inc., Cary, NC). Categorical data were compared using either Chi-square test or Fisher’s Exact Test as appropriate. Continuous variables were expressed as mean ± std deviation. Non-normally distributed variables were compared with non-parametric statistics using the Wilcoxon two-sample test. Normally distributed variables were compared using the t-test. In order to take into account multiple plaque measurement per subject, plaque level analysis was performed using generalized estimating equations.
RESULTS
Baseline clinical characteristics were similar between the groups (Table I). Sixty-seven non-consecutive patients (30 with STEMI and 37 with SAP) were included. A total of 5,503 cross-sections from 67 arteries (1 artery per patient) were analyzed. Thirty-five cross-sections (0.35%) were excluded due to residual blood impairing analysis. Overall, the length of the ROI in both groups was equivalent (Table II). Plaque components in both groups were also similar (Table II). Low inter- (kappa = 0.92, 95% confidence interval [0.89, 0.96]) and intra-observer (observer 1: kappa = 0.95, 95% confidence interval [0.93, 0.98]); observer 2: kappa = 0.97, 95% confidence interval [0.95, 0.99]) variability for the assessment of plaque components were revealed. In addition, lipid plaques were equally distributed proximally and distally to the stents in both groups (Table II).
TABLE I.
Demographics and Clinical Characteristics
| STEMI (N = 30) | SAP (N = 37) | P-value | |
|---|---|---|---|
| Age | 62.19 ± 7.56 | 64.37 ± 10.63 | 0.33 |
| Male | 15 (40.54%) | 19 (63.33%) | 0.07 |
| Hypertension | 35 (94.59%) | 28 (93.33%) | 0.83 |
| Hyperlipidemia | 34 (91.89%) | 28 (93.33%) | 0.82 |
| Diabetes mellitus | 8 (21.62%) | 12 (40.00%) | 0.1 |
| Smoking | 19 (51.35%) | 18 (60.00%) | 0.48 |
| Prior CABG | 2 (5.41%) | 3 (10.00%) | 0.48 |
| Prior MI | 4 (10.81%) | 6 (20.00%) | 0.29 |
| Prior PCI | 5 (13.51%) | 8 (26.67%) | 0.72 |
| Prior CVA | 1 (2.7%) | 1 (3.33%) | 0.88 |
| PAD | 3 (8.11%) | 6 (20.00%) | 0.16 |
Values are given as n, %, or mean ± SD.
STEMI = ST elevation myocardium infarction; SAP = stable angina pectoris; CABG = coronary artery bypass grafting; MI = myocardium infarction; PCI = percutaneous coronary intervention; CVA = cerebral vascular accident; PAD = peripheral artery disease.
TABLE II.
Quantitative and Semi-Quantitative Assessment of Plaque
| STEMI (N = 30) | SAP (N = 37) | P-value | |
|---|---|---|---|
| Total frames analyzed (n) | 2717.00 | 2786.00 | 0.51 |
| Distal segment | 44.83 ± 29.95 | 39.38 ± 25.84 | 0.72 |
| Proximal segment | 45.73 ± 44.51 | 34.30 ± 37.01 | 0.34 |
| Total length (mm) | |||
| Distal segment | 8.97 ± 5.99 | 8.20 ± 5.44 | 0.72 |
| Proximal segment | 9.15 ± 8.90 | 6.86 ± 7.40 | 0.34 |
| Quantitative assessment (mm2) | |||
| Lumen area | 7.48 ± 2.80 | 6.85 ± 2.85 | 0.84 |
| Lumen diameter | 3.03 ± 0.59 | 2.83 ± 0.61 | 0.74 |
| Plaque characteristic (% of Total) | |||
| Calcified | 4.97 | 8.40 | 0.17 |
| Fibrotic | 47.13 | 42.68 | 0.40 |
| Lipidic | 14.89 | 10.59 | 0.20 |
| Normal | 27.35 | 34.78 | 0.23 |
| N/A | 5.66 | 3.55 | 0.22 |
| Vessel (%) | 0.35 | ||
| LAD | 43.33 | 54.05 | |
| LCX | 23.33 | 21.62 | |
| RCA | 33.33 | 24.32 | |
Values are given as n, %, or mean ± SD.
STEMI = ST elevation myocardium infarction; SAP = stable angina pectoris; N/A = non analyzable; ROI = region of interest; LAD = left anterior descendent; LCX = left circumflex; RCA = right coronary artery.
A total of 69 lipid plaques were identified: 38 plaques in 18 STEMI patients (2.11 plaques/patient) and 31 plaques in 20 SAP patients (1.55 plaques/patient). The absolute and fractional FC categorical surface area of the three thickness categories (FC <65 μm, 65–150 μm, and >150 μm) were compared in both groups (Table III). The STEMI compared with SAP patients had significantly more absolute categorical surface area for TCFA (0.43 ± 0.45 mm2 and 0.15 ± 0.25 mm2; P = 0.011) and thinner minimum FC thickness (31.63 ± 17.09 μm and 47.27 ± 26.56 μm, respectively, P = 0.012) (Table III). Furthermore, STEMI patients revealed significantly greater categorical FC fractional luminal area for TCFA compared with SAP patients (1.65 ± 1.56% and 0.74 ± 1.20%, respectively, P = 0.046), likewise for thickness category of 65–150 μm (16.71 ± 6.56% and 12.14 ± 8.36%, respectively, P = 0.013) (Table III).
TABLE III.
Fibrous Cap: Plaque Level Analysis
| STEMI (n = 38) | SAP (n = 31) | P-value | |
|---|---|---|---|
| Lipid-rich plaque distribution (%) | 0.15 | ||
| Distal segment | 36.84 | 54.84 | |
| Proximal segment | 63.16 | 45.16 | |
| Fibrous cap measurement | |||
| Total area of fibrous cap (mm2) | 8.43 ± 7.22 | 5.46 ± 5.00 | 0.06 |
| Total fractional area (mm2) | 31.19 ± 10.25 | 24.52 ± 8.09 | 0.02 |
| Minimum cap thickness (μm) | 31.63 ± 17.09 | 47.27 ± 26.56 | 0.01 |
| Mean cap thickness (μm) | 144.37 ± 27.05 | 165.30 ± 52.46 | 0.06 |
| Fibrous cap categories | |||
| Thickness <65 μm | |||
| Surface area (mm2) | 0.43 ± 0.45 | 0.15 ± 0.25 | 0.01 |
| Fractional area (%) | 1.65 ± 1.56 | 0.74 ± 1.20 | 0.05 |
| Thickness 65–150 μm | |||
| Surface area (mm2) | 4.5 ± 4.07 | 2.96 ± 4.04 | 0.12 |
| Fractional area (%) | 16.71 ± 6.56 | 12.14 ± 8.36 | 0.01 |
| Thickness >150 μm | |||
| Surface area (mm2) | 3.5 ± 3.13 | 2.35 ± 1.93 | 0.09 |
| Fractional area (%) | 12.83 ± 7.22 | 11.64 ± 7.72 | 0.48 |
Values are given as n, %, or mean ± SD.
The macrophage area, as well as the macrophage index constrained within the FC regions of lipid plaques were significantly larger in STEMI patients compared with SAP patients (0.0024 ± 0.0009 mm2 vs. 0.0015 ± 0.0004 mm2; P = 0.01, and 0.0217 ± 0.0081% vs. 0.0153 ± 0.0045%, respectively; P <0.01).
DISCUSSION
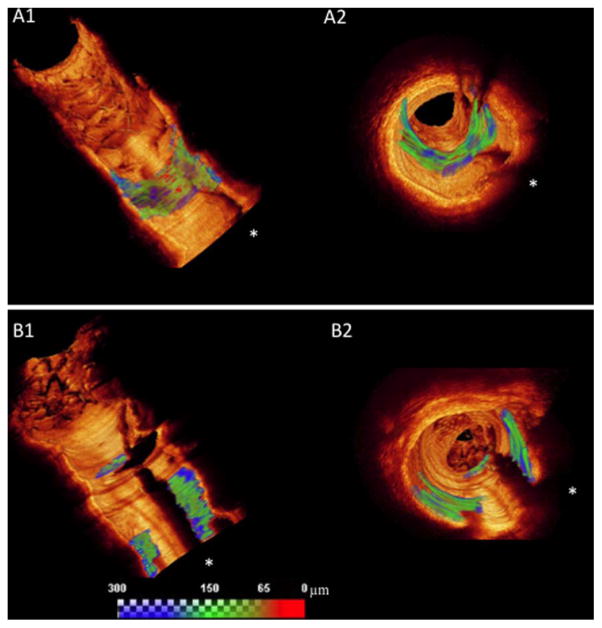
This is the first in vivo study to perform a comprehensive 3-D volumetric quantification of FC morphology and inflammation in non-culprit plaques of patients with two different clinical presentations: STEMI and SAP. Our main findings were: (1) overall plaque composition proximal and distal to the implanted stent was similar in STEMI and SAP patients; (2) STEMI- had larger thin-cap surface areas and thinner minimum FC thickness compared with SAP-patients (Fig. 2); (3) A large proportion of non-culprit plaques in both STEMI and SAP patients had FC thickness <65 μm; and (4) Macrophage index (i.e., surrogate for FC inflammation) determined by automated quantification was higher in STEMI compared with SAP patients.
Fig. 2.
Representative cases of segmented FC in 3-D rendering with a continuous color map, from blue (>150 μm) to green (65–150 μm) to red (FC < 65 μm). Regardless of similar FC surface area, the morphological differences between the cases are easily demonstrated, as follows: the STEMI patient has a larger area of FC < 65 μm (red areas) than SAP patient. A1: Represents a longitudinal view of the ROI distal to the stented (white arrow) region showing FC area with TCFA; in (A2) a fly throw view of the same FC in the STEMI patient is depicted. B1: A longitudinal view of the ROI of SAP patient depicts FC area with TCFA, while (B2) shows a fly through view of the same FC. White asterisks represent guide wire shadow.
The FC overlying a lipid plaque has a very complex architecture that interacts with mechanical, biological, chemical, and inflammatory triggers [24,25]. Performing random measurements in a single or few cross-sections will neither capture the complex 3-D nature of the FC, nor its changes and responses to different microenvironments. While the mechanisms associated with FC rupture remain elusive, it is possible that its mechanical stability may not only depend on the thickness of a focal point, but rather on coalescent thin regions. The automated quantification of FC is, therefore, desirable because it may help elucidate the morphological and mechanical properties that lead to plaque changes and rupture, minimizing inter-observer and intra-observer variability [18].
Autopsy studies were instrumental for the current knowledge on the role of FC thickness as one of the potential triggers of acute coronary syndromes. While FC thickness of <65 μm was demonstrated a strong predictor of plaque rupture in those studies, the inherent limitations of histological assessments (i.e., sample shrinkage, limited sampling, selection bias, post-mortem rather than in vivo) hampered a complete elucidation of this complex scenario [2,3]. In vivo studies that benefit from the unique high-axial resolution delivered by OCT, which enables FC measurements, could help filling the gap left by the autopsy studies. Indeed, such studies demonstrated that FC thickness varies according to the clinical presentation, as follows: STEMI patients exhibited significantly thinner FC (47 μm) in comparison with non-STEMI (53.8 μm) and SAP (102.6 μm) [10], while TCFAs identified in non-culprit sites were significantly more common in STEMI patients [26]. Furthermore, patients with acute coronary syndrome symptoms and plaque rupture at rest demonstrated thinner FC compared with those with similar clinical presentation while in exertion (50 μm vs. 90 μm) [27]. Taken together, these data suggest the importance of properly quantifying FC thickness in order to better characterize the complex pathophysiology of the rupture-prone plaques, therefore, the rationale for utilizing a more comprehensive methodology as herewith described.
Importantly, plaque ruptures also occur in fibroatheromas with thicker FC. In fact, Tanaka et al. demonstrated that 33% of ruptured plaques in acute coronary syndrome patients demonstrated FC thickness >70 μm [27], while Toutouzas et al. demonstrated a broad range of FC thickness that ruptured in STEMI patients [28]. While the great variability in FC thickness demonstrated across the studies may reflect the complex nature of this multifactorial pathological process, in which different ranges of FC thickness could be prone to rupture as long as they are combined with other risk factors (i.e., patient activity at the onset of rupture) [27], another potential explanation for such variability observed in similar clinical scenarios may be a consequence of methodological limitations demonstrated by previous studies, in which only few regions (i.e., chosen by visual estimation as the thinnest points) [15,17,28] rather than the entirety of the FC, (i.e., as demonstrated by our methodology) were measured [18,19]. Kubo et al. previously described thinner minimum FC thickness and higher proportion of TCFA in non-culprit lesions of STEMI vs. SAP patients [26]. While we confirmed those results in our study, we expanded their findings by showing that STEMI patients had larger thin-cap surface areas compared with SAP patients, which might be a potential refined indicator of the higher vulnerability of certain individuals to plaque rupture. We believe, therefore, that our methodology might have the potential to re-group individuals that are, indeed, at potential risk for plaque rupture and adverse coronary events. Nonetheless, we acknowledge that further investigation is warranted to completely elucidate the clinical impact of our findings.
Previous studies had already observed that as a function of their size (20–30 μm) and the refractive index difference between the phagolysosomes and the surrounding intracellular fluid, macrophages may have a higher OCT signal than the surrounding tissue [5,9]. Our study showed that macrophage area was larger in non-culprit sites of STEMI- compared with SAP-patients. This is consistent with the findings of previous investigations, although the methods to compute the macrophage density among the studies were fundamentally different [6,9]. Although plaque vulnerability depends on several factors other than macrophage infiltration and FC thickness, it is known that macrophages produce proteolytic enzymes, such as matrix metalloproteinases, which digest extracellular matrix compromising the integrity of the FC [29–31]. This inflammatory process in the FC has been shown to be present in the immediate site of the plaque rupture or erosion [31]. Therefore, accurate and reproducible automated quantification of FC thickness and inflammation are desirable as they provide a potential opportunity to further elucidate the mechanisms involved in the transition from stable to unstable coronary artery disease. In addition, the automated methodology herewith described could further clarify the role of certain drugs such as the statins in reducing FC inflammation while promoting plaque stabilization [32,33]. Additional prospective studies utilizing our methodology are required to establish the clinical relevance of our findings.
Limitations
The study population was relatively small and the analyses were performed in a retrospective fashion; nonetheless, we used a novel methodology to automatically quantify FC thickness and macrophage infiltration that can be applied in future prospective studies. Although plaque instability may exist in multiple points in the coronary tree [34], we evaluated only one coronary artery per patient in each group, as the study was not prospectively designed to assess other sites. Despite the promising applications of our technology for a more comprehensive elucidation of coronary artery disease, OCT imaging is invasive and requires contrast injection to be performed, however, its high safety rates are reassuring.
We have no information on serum markers of inflammation such as hs-CRP, known to be associated with the inflammatory activities of coronary plaques. Although previously demonstrated in murine aorta with very good correlation with histology (r = 0.92) [20], the decision tree-based classification method for macrophage quantification has not been correlated with histology in humans. Finally, the analyses were focused on regions proximal and distal to the culprit site where the stent was implanted, which may serve as an indicator of overall plaque vulnerability.
CONCLUSION
The 3-D quantification of the entire FC of non-culprit lesions of STEMI and SAP patients demonstrated that despite similar amount of lipid-rich plaque area, STEMI patients had a larger area of TCFA with more macrophage infiltration. This novel method refines the in vivo assessment of atherosclerosis and may potentially shed light on the investigation of rupture-prone plaques.
Footnotes
Conflict of interest: Drs. Attizzani, Bezerra, and Costa receive consulting and lecture fees from St Jude Medical, Inc.
References
- 1.Virmani R, Burke AP, Farb A, Kolodgie FD. Pathology of the unstable plaque. Prog Cardiovasc Dis. 2002;44:349–356. doi: 10.1053/pcad.2002.122475. [DOI] [PubMed] [Google Scholar]
- 2.Virmani R, Kolodgie FD, Burke AP, Farb A, Schwartz SM. Lessons from sudden coronary death: A comprehensive morphological classification scheme for atherosclerotic lesions. Arterioscler Thromb Vasc Biol. 2000;20:1262–1275. doi: 10.1161/01.atv.20.5.1262. [DOI] [PubMed] [Google Scholar]
- 3.Narula J, Nakano M, Virmani R, Kolodgie FD, Petersen R, Newcomb R, Malik S, Fuster V, Finn AV. Histopathologic characteristics of atherosclerotic coronary disease and implications of the findings for the invasive and noninvasive detection of vulnerable plaques. J Am Coll Cardiol. 2013;61:1041–1051. doi: 10.1016/j.jacc.2012.10.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bezerra HG, Costa MA, Guagliumi G, Rollins AM, Simon DI. Intracoronary optical coherence tomography: A comprehensive review clinical and research applications. JACC Cardiovasc Interv. 2009;2:1035–1046. doi: 10.1016/j.jcin.2009.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tearney GJ, Yabushita H, Houser SL, Aretz HT, Jang I-K, Schlendorf KH, Kauffman CR, Shishkov M, Halpern EF, Bouma BE. Quantification of macrophage content in atherosclerotic plaques by optical coherence tomography. Circulation. 2003;107:113–119. doi: 10.1161/01.cir.0000044384.41037.43. [DOI] [PubMed] [Google Scholar]
- 6.MacNeill BD, Jang IK, Bouma BE, Iftimia N, Takano M, Yabushita H, Shishkov M, Kauffman CR, Houser SL, Aretz HT, et al. Focal and multi-focal plaque macrophage distributions in patients with acute and stable presentations of coronary artery disease. J Am Coll Cardiol. 2004;44:972–979. doi: 10.1016/j.jacc.2004.05.066. [DOI] [PubMed] [Google Scholar]
- 7.Kume T, Akasaka T, Kawamoto T, Okura H, Watanabe N, Toyota E, Neishi Y, Sukmawan R, Sadahira Y, Yoshida K. Measurement of the thickness of the fibrous cap by optical coherence tomography. Am Heart J. 2006;152:755.e1–755 e4. doi: 10.1016/j.ahj.2006.06.030. [DOI] [PubMed] [Google Scholar]
- 8.Tearney GJ, Regar E, Akasaka T, Adriaenssens T, Barlis P, Bezerra HG, Bouma B, Bruining N, Cho JM, Chowdhary S, et al. Consensus standards for acquisition, measurement, and reporting of intravascular optical coherence tomography studies: A report from the International Working Group for Intravascular Optical Coherence Tomography Standardization and Validation. J Am Coll Cardiol. 2012;59:1058–1072. doi: 10.1016/j.jacc.2011.09.079. [DOI] [PubMed] [Google Scholar]
- 9.Tanaka A, Tearney GJ, Bouma BE. Challenges on the frontier of intracoronary imaging: atherosclerotic plaque macrophage measurement by optical coherence tomography. J Biomed Opt. 2010;15:011104. doi: 10.1117/1.3290810. [DOI] [PubMed] [Google Scholar]
- 10.Jang IK, Tearney GJ, MacNeill B, Takano M, Moselewski F, Iftima N, Shishkov M, Houser S, Aretz HT, Halpern EF, et al. In vivo characterization of coronary atherosclerotic plaque by use of optical coherence tomography. Circulation. 2005;111:1551–1555. doi: 10.1161/01.CIR.0000159354.43778.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kubo T, Imanishi T, Takarada S, Kuroi A, Ueno S, Yamano T, Tanimoto T, Matsuo Y, Masho T, Kitabata H, et al. Assessment of culprit lesion morphology in acute myocardial infarction: ability of optical coherence tomography compared with intravascular ultrasound and coronary angioscopy. J Am Coll Cardiol. 2007;50:933–939. doi: 10.1016/j.jacc.2007.04.082. [DOI] [PubMed] [Google Scholar]
- 12.Yonetsu T, Kakuta T, Lee T, Takahashi K, Kawaguchi N, Yamamoto G, Koura K, Hishikari K, Iesaka Y, Fujiwara H, et al. In vivo critical fibrous cap thickness for rupture-prone coronary plaques assessed by optical coherence tomography. Eur Heart J. 2011;32:1251–1259. doi: 10.1093/eurheartj/ehq518. [DOI] [PubMed] [Google Scholar]
- 13.Ino Y, Kubo T, Tanaka A, Kuroi A, Tsujioka H, Ikejima H, Okouchi K, Kashiwagi M, Takarada S, Kitabata H, et al. Difference of culprit lesion morphologies between ST-segment elevation myocardial infarction and non-ST-segment elevation acute coronary syndrome: An optical coherence tomography study. JACC Cardiovasc Interv. 2011;4:76–82. doi: 10.1016/j.jcin.2010.09.022. [DOI] [PubMed] [Google Scholar]
- 14.Rathore S, Terashima M, Matsuo H, Kinoshita Y, Kimura M, Tsuchikane E, Nasu K, Ehara M, Asakura Y, Katoh O, et al. In-vivo detection of the frequency and distribution of thin-cap fibroatheroma and ruptured plaques in patients with coronary artery disease: an optical coherence tomographic study. Coron Artery Dis. 2011;22:64–72. doi: 10.1097/MCA.0b013e32833e1c36. [DOI] [PubMed] [Google Scholar]
- 15.Tanaka A, Imanishi T, Kitabata H, Kubo T, Takarada S, Kataiwa H, Kuroi A, Tsujioka H, Tanimoto T, Nakamura N, et al. Distribution and frequency of thin-capped fibroatheromas and ruptured plaques in the entire culprit coronary artery in patients with acute coronary syndrome as determined by optical coherence tomography. Am J Cardiol. 2008;102:975–979. doi: 10.1016/j.amjcard.2008.05.062. [DOI] [PubMed] [Google Scholar]
- 16.Sawada T, Shite J, Garcia-Garcia HM, Shinke T, Watanabe S, Otake H, Matsumoto D, Tanino Y, Ogasawara D, Kawamori H, et al. Feasibility of combined use of intravascular ultrasound radiofrequency data analysis and optical coherence tomography for detecting thin-cap fibroatheroma. Eur Heart J. 2008;29:1136–1146. doi: 10.1093/eurheartj/ehn132. [DOI] [PubMed] [Google Scholar]
- 17.Tian J, Ren X, Vergallo R, Xing L, Yu H, Jia H, Soeda T, McNulty I, Hu S, Lee H, et al. Distinct morphological features of ruptured culprit plaque for acute coronary events compared to those with silent rupture and thin-cap fibroatheroma: A combined optical coherence tomography and intravascular ultrasound study. J Am Coll Cardiol. 2014;63:2209–2216. doi: 10.1016/j.jacc.2014.01.061. [DOI] [PubMed] [Google Scholar]
- 18.Wang Z, Chamie D, Bezerra HG, Yamamoto H, Kanovsky J, Wilson DL, Costa MA, Rollins AM. Volumetric quantification of fibrous caps using intravascular optical coherence tomography. Biomed Opt Express. 2012;3:1413–1426. doi: 10.1364/BOE.3.001413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bezerra HG, Attizzani GF, Costa MA. Three-dimensional imaging of fibrous cap by frequency-domain optical coherence tomography. Catheter Cardiovasc Interv. 2013;81:547–549. doi: 10.1002/ccd.23323. [DOI] [PubMed] [Google Scholar]
- 20.Tahara S, Morooka T, Wang Z, Bezerra HG, Rollins AM, Simon DI, Costa MA. Intravascular optical coherence tomography detection of atherosclerosis and inflammation in murine aorta. Arterioscler Thromb Vasc Biol. 2012;32:1150–1157. doi: 10.1161/ATVBAHA.111.243626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Frank E, Hall M, Trigg L, Holmes G, Witten IH. Data mining in bioinformatics using Weka. Bioinformatics. 2004;20:2479–2481. doi: 10.1093/bioinformatics/bth261. [DOI] [PubMed] [Google Scholar]
- 22.Quinlan JR. C4. 5: Programs for Machine Learning. San Diego: Morgan Kaufmann; 1993. [Google Scholar]
- 23.Breiman L. Bagging predictors. Mach Learn. 1996;24:123–140. [Google Scholar]
- 24.Vengrenyuk Y, Carlier S, Xanthos S, Cardoso L, Ganatos P, Virmani R, Einav S, Gilchrist L, Weinbaum S. A hypothesis for vulnerable plaque rupture due to stress-induced debonding around cellular microcalcifications in thin fibrous caps. Proc Natl Acad Sci USA. 2006;103:14678–14683. doi: 10.1073/pnas.0606310103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ohayon J, Finet G, Gharib AM, Herzka DA, Tracqui P, Heroux J, Rioufol G, Kotys MS, Elagha A, Pettigrew RI. Necrotic core thickness and positive arterial remodeling index: emergent biomechanical factors for evaluating the risk of plaque rupture. Am J Physiol Heart Circ Physiol. 2008;295:H717–H727. doi: 10.1152/ajpheart.00005.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kubo T, Imanishi T, Kashiwagi M, Ikejima H, Tsujioka H, Kuroi A, Ishibashi K, Komukai K, Tanimoto T, Ino Y, et al. Multiple coronary lesion instability in patients with acute myocardial infarction as determined by optical coherence tomography. Am J Cardiol. 2010;105:318–322. doi: 10.1016/j.amjcard.2009.09.032. [DOI] [PubMed] [Google Scholar]
- 27.Tanaka A, Imanishi T, Kitabata H, Kubo T, Takarada S, Tanimoto T, Kuroi A, Tsujioka H, Ikejima H, Ueno S, et al. Morphology of exertion-triggered plaque rupture in patients with acute coronary syndrome: An optical coherence tomography study. Circulation. 2008;118:2368–2373. doi: 10.1161/CIRCULATIONAHA.108.782540. [DOI] [PubMed] [Google Scholar]
- 28.Toutouzas K, Tsiamis E, Karanasos A, Drakopoulou M, Synetos A, Tsioufis C, Tousoulis D, Davlouros P, Alexopoulos D, Bouki K, et al. Morphological characteristics of culprit atheromatic plaque are associated with coronary flow after thrombolytic therapy: New implications of optical coherence tomography from a multicenter study. JACC Cardiovasc Interv. 2010;3:507–514. doi: 10.1016/j.jcin.2010.02.010. [DOI] [PubMed] [Google Scholar]
- 29.Lee RT, Schoen FJ, Loree HM, Lark MW, Libby P. Circumferential stress and matrix metalloproteinase 1 in human coronary atherosclerosis. Implications for plaque rupture. Arterioscler Thromb Vasc Biol. 1996;16:1070–1073. doi: 10.1161/01.atv.16.8.1070. [DOI] [PubMed] [Google Scholar]
- 30.Davies MJ, Richardson PD, Woolf N, Katz DR, Mann J. Risk of thrombosis in human atherosclerotic plaques: role of extracellular lipid, macrophage, and smooth muscle cell content. Br Heart J. 1993;69:377–381. doi: 10.1136/hrt.69.5.377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.van der Wal AC, Becker AE, van der Loos CM, Das PK. Site of intimal rupture or erosion of thrombosed coronary atherosclerotic plaques is characterized by an inflammatory process irrespective of the dominant plaque morphology. Circulation. 1994;89:36–44. doi: 10.1161/01.cir.89.1.36. [DOI] [PubMed] [Google Scholar]
- 32.Kataoka Y, Puri R, Hammadah M, Duggal B, Uno K, Kapadia SR, Tuzcu EM, Nissen SE, Nicholls SJ. Frequency-domain optical coherence tomographic analysis of plaque microstructures at nonculprit narrowings in patients receiving potent statin therapy. Am J Cardiol. 2014;114:549–554. doi: 10.1016/j.amjcard.2014.05.035. [DOI] [PubMed] [Google Scholar]
- 33.Chia S, Raffel OC, Takano M, Tearney GJ, Bouma BE, Jang IK. Association of statin therapy with reduced coronary plaque rupture: An optical coherence tomography study. Coron Artery Dis. 2008;19:237–242. doi: 10.1097/MCA.0b013e32830042a8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kato K, Yonetsu T, Kim SJ, Xing L, Lee H, McNulty I, Yeh RW, Sakhuja R, Zhang S, Uemura S, et al. Nonculprit plaques in patients with acute coronary syndromes have more vulnerable features compared with those with non-acute coronary syndromes: A 3-vessel optical coherence tomography study. Circ Cardiovasc Imaging. 2012;5:433–440. doi: 10.1161/CIRCIMAGING.112.973701. [DOI] [PubMed] [Google Scholar]