Abstract
Importance
Increasing access to care may be insufficient to improve health for diabetes patients with unmet basic needs. However, how specific material need insecurities relate to clinical outcomes and care utilization in a setting of near-universal care access is unclear.
Objective
To determine the association of food insecurity, cost-related medication underuse, housing instability, and energy insecurity with diabetes control and healthcare utilization.
Design
Cross-sectional(data collected June 2012 -- October 2013).
Setting
One academic primary care clinic, two community health centers and one specialty diabetes center in Massachusetts.
Participants
Random sample, stratified by clinic, of adult(age >20 years) diabetes patients. 411 patients were included(response rate: 62.3%).
Main Outcome(s) and Measure(s)
The pre-specified primary outcome was a composite indicator of poor diabetes control(Hemoglobin A1c >9.0%, LDL cholesterol >100mg/dL, or blood pressure >140/90mm/Hg). Pre-specified secondary outcomes included outpatient visits and emergency department visits/acute care hospitalizations (ED/inpatient).
Results
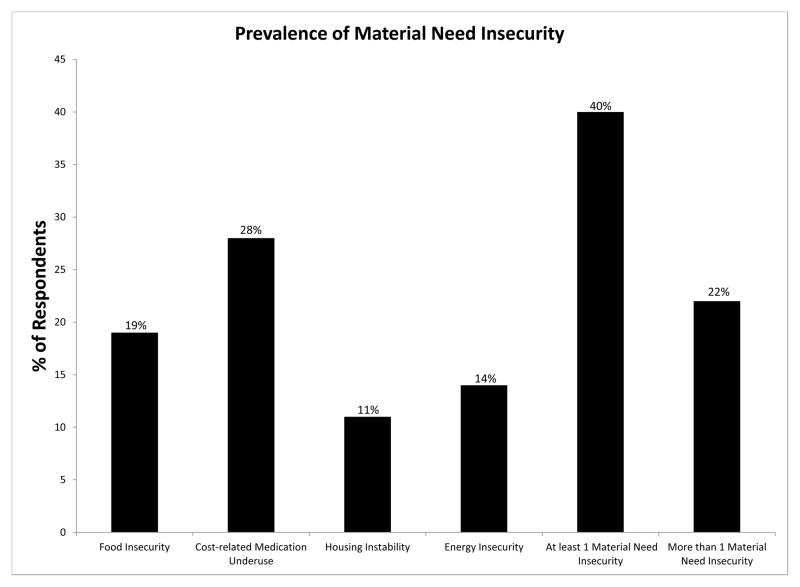
Overall, 19% of respondents reported food insecurity, 28% cost-related medication underuse, 11% housing instability, and 14% energy insecurity; 40% reported at least one material need insecurity. Forty-two percent of respondents had poor diabetes control. In multivariable models, food insecurity was associated with greater odds of poor diabetes control(adjusted Odds Ratio[OR] 1.97, 95% confidence interval[95%CI]1.58 – 2.47) and increased outpatient visits(adjusted Incident Rate Ratio[IRR] 1.19 95%CI 1.05 – 1.36), but not increased ED/inpatient visits(IRR 1.00 95%CI 0.51 – 1.97). Cost-related medication underuse was associated with poor diabetes control(OR 1.91 95%CI 1.35 – 2.70) and greater ED/inpatient utilization(IRR 1.68 95%CI 1.21 – 2.34), but not outpatient visits(IRR 1.07 95%CI 0.95 – 1.21). Housing instability(IRR 1.31 95%CI 1.14– 1.51) and energy insecurity(IRR 1.12 95%CI 1.00 – 1.25) were both associated with increased outpatient utilization, but not diabetes control(OR 1.10 95%CI 0.60 – 2.02, OR 1.27 95%CI 0.96 – 1.69) or ED/inpatient utilization(IRR 1.49 95%CI 0.81 – 2.73, IRR 1.31 95%CI 0.80 – 2.13), respectively. Increasing number of insecurities was associated with poor diabetes control(OR for each additional need 1.39 95%CI 1.18 – 1.63) and increased utilization(IRR for outpatient visits 1.09 95%CI 1.03 – 1.15; IRR for ED/inpatient 1.22 95%CI 0.99 – 1.51).
Conclusions and Relevance
Material need insecurities were common among diabetes patients, and had varying but generally adverse associations with diabetes control and care utilization. Material need insecurities may be important targets for improving diabetes care.
The expansion of health insurance coverage offered by the Patient Protection and Affordable Care Act (ACA)1 will increase access to healthcare for patients with diabetes. However, recent randomized trial results have demonstrated that increasing access to care may not improve diabetes control2 in low-income patients. This discrepancy may be due to social determinants of health3, 4 that are outside the scope of standard medical interventions5, such as difficulty paying for food6–9, medications10–14, housing15, or utilities16, 17.
Recognition that social determinants of health may be key to improving health outcomes and optimizing the use of healthcare resources has led to interest in management strategies that address the relevant material need insecurities of patients18–20. However, the knowledge base for this approach within healthcare systems remains limited. Most prior clinical epidemiology studies have focused on single needs in isolation6, 8, 13, in settings with significant numbers of uninsured patients. In diabetes, a condition where successful self-management carries significant out-of-pocket costs even among the insured21, 22, the relationship between material need insecurities and diabetes outcomes is likely to be complex. Patients with one insecurity may have others, and the effect of each may be different when considering clinical and care utilization outcomes. Further, patients’ specific needs may offer targets for intervention.
To strengthen the knowledge base regarding material need insecurities and diabetes, we simultaneously evaluated several potentially modifiable material need insecurities and their relationship with diabetes control and healthcare utilization. Specifically, based on prior work15, 23, we hypothesized that difficulty paying for food and medications would be associated with poor diabetes control and greater healthcare utilization even when accounting for other material need insecurities.
Methods
Study Setting and Sample
This study was conducted among patients linked to one of four clinics within a practice-based research network24: two community health centers (Revere HealthCare Center and Charlestown HealthCare Center), one academic general internal medicine practice (Internal Medicine Associates at Massachusetts General Hospital [MGH]), and one specialty diabetes clinic (MGH Diabetes Treatment Center), all in the Boston, Massachusetts metropolitan area. The community health centers are academically-affiliated clinics located in two different suburbs of Boston and comprise part of the healthcare ‘safety-net’ for their communities. The academic general internal medicine practice and specialty clinic are hospital-based. All clinics accept Medicaid and self-pay patients. Massachusetts has had near-universal health insurance coverage for almost 10 years25, with plan coverage requirements similar to those being enacted nationally under the ACA1. All adults (age > 20 years) with diabetes, defined by a previously validated electronic algorithm26 were eligible to participate. We selected a random sample of patients, stratified by clinic, to complete a survey on material need insecurity. A trained interviewer administered all surveys over the telephone or in-person at a regularly scheduled clinic visit. Validated instruments were available only in English and Spanish, so we excluded patients who could not complete the survey in one of those languages, along with patients who could not complete it due to disabling conditions, such as dementia.
The Partners Health Care Institutional Review Board approved this study, and all patients provided verbal informed consent.
Measures of Material Need Insecurity
We collected data using a standardized questionnaire (eSurvey 1) with previously validated instruments15, 16, 23, 27, 28 on four different material need insecurities. The four were: 1) food insecurity, defined as limited or uncertain availability of food due to cost27, 2) cost-related medication underuse, 3) housing instability, which could include homelessness as an extreme form, evictions and frequent moves, or ‘doubling up’, defined as moving in with relatives to share living expenses in the past 12 months15, 28, and 4) energy insecurity, which is difficulty affording household heating or cooling16. While distinct concepts, each of the four material need insecurities is similar in that it represents difficulties meeting basic needs due to cost. All scales used the same 12-month ‘look back’ period for patient report. Study data were collected and managed using REDCap electronic data capture tools hosted at Partners Healthcare29.
Outcomes
Because a major goal of outpatient care30, 31, and especially population management programs19, 20, is both to improve clinical outcomes and optimize the use of healthcare resources, we evaluated several outcomes relevant to these goals. We collected data for laboratory, clinical measurements, and utilization from an electronic data repository32 during the 12-month look-back period, and linked this to survey responses. The electronic data repository contains data from 18 clinics in a practice-based research network, along with emergency department and inpatient information from MGH.
The pre-specified primary outcome was a composite measure of poor diabetes control: any of Hemoglobin A1c (HbA1c) > 9.0%, low-density lipoprotein (LDL) cholesterol > 100 mg/dL, systolic blood pressure (SBP) > 140 mm/Hg, or diastolic blood pressure (DBP) > 90 mm/Hg, using the most recent values from within the 12-month look-back period. These values were selected on the basis of conferring roughly equivalent risks of diabetes complications33, 34, and because they are commonly used in quality reporting35 and endorsed in clinical guidelines36. Pre-specified secondary clinical outcomes included separate evaluation of the components of poor clinical diabetes control: HbA1c > 9.0%, LDL > 100mg/dL, and poor blood pressure control (SBP > 140 mm/Hg or DBP > 90 mm/Hg).
We also evaluated 3 pre-specified healthcare utilization outcomes, all occurring during the same 12-month look-back period. The first was outpatient visits. The second was emergency department visits and inpatient acute care hospitalizations (ED/inpatient). We created the combined ED/inpatient indicator because diabetes is commonly considered a condition for which improving ambulatory can reduce both types of utilization37–39. We separated the evaluation of outpatient visits and ED/inpatient to reflect different priorities for these types of visits within the healthcare system. Although a goal of chronic disease management is to minimize ED/inpatient visits, this may not be a goal with outpatient visits, especially if extra outpatient visits could prevent an inpatient admission. Despite this, if outpatient utilization is high and diabetes is poorly controlled, or ED/inpatient utilization is also high, it may suggest that standard outpatient care is not producing the desired effect of better health. Finally, among the subset of patients who had at least one acute care hospital admission in the study period, we examined 30-day readmission rates.
Covariates
We collected information regarding education, nativity, and years living in the United States if born abroad. For health literacy40, we considered a response of ‘extremely’ or ‘quite a bit’ to the question “How confident are you filling out medical forms by yourself?” to indicate adequate health literacy. We also asked whether patients had prescription drug coverage and the duration of their diabetes. From the electronic data repository, we extracted data regarding age, self-reported race/ethnicity, health insurance (commercial, Medicare, Medicaid, Massachusetts Health Safety Net [‘Free Care’—a non-Medicaid health benefit for essential medical care services among Massachusetts residents41], and no insurance/self-pay), and Charlson comorbidity score, using previously validated algorithms24. For medications, we constructed binary indicator variables by class for glycemic, cholesterol, and blood pressure medications: metformin, sulfonylurea/meglitinide, thiazolidinediones, dipeptidyl peptidase-4 inhibitors, insulin, 3-hydroxy-3-methyl-glutaryl-CoA reductase inhibitors, thiazide diuretics, angiotensin converting enzyme (ACE) inhibitors and angiotensin receptor blockers, calcium-channel blockers, and beta-blockers.
Statistical analysis
Material need insecurity scales were dichotomized as ‘secure’ or ‘insecure’ following scoring similar to that used in prior studies of these needs (eAppendix 1). For most models, we considered the needs as separate, dichotomous variables. Because material need insecurities may cluster, and may have an additive effect on health, we also created a variable summing the number of insecurities (which assumes an additive effect). To account for the stratified random sample design, we used inverse probability weighting (weighted by number of diabetes patients in the clinic) to produce prevalence estimates. We used the American Association of Public Opinion Reporting definition #342 to calculate response rate, which accounts for both eligible patients who decline to participate and patients of unknown eligibility, among those who could not be contacted. We compared demographic information of non-responders to responders using electronic health record data. We conducted unadjusted analyses using chi-squared tests for categorical variables and Wilcoxon tests for non-normally distributed continuous variables. We then conducted multivariable logistic regression for our clinical outcomes, which also accounted for the design effect and correlations within each clinic (SAS PROC GENMOD). For utilization outcomes, we conducted multivariable negative binomial regression, again clustered by clinic. When we did not have sufficient events to include all possible covariates in the multivariable regression models, we removed covariates that were not associated with the outcome in bivariable analyses and that did not demonstrate evidence of confounding the effect estimate for any material need insecurity term (change in Beta < 10% when removed). A p-value of <0.05 was taken to indicate statistical significance for association of a material need insecurity with the primary composite outcome of poor diabetes control. All analyses were conducted using SAS version 9.3 (SAS Institute, Cary, NC).
Results
Overall, 1000 potential participants were initially identified, 270 patients were found to be ineligible for the survey, 206 could not be contacted, 113 refused to participate, and 411 completed the survey (response rate 62.3% by AAPOR definition42). Compared with patients who did not participate in the survey, respondents were younger (mean age 62 vs. 65 years, p < 0.001), but were similar with regards to gender, insurance coverage, educational attainment, and Hispanic and non-Hispanic Black race/ethnicity. The mean age of participants was 62 years, 48% were women, and 75% were non-Hispanic white (Table 1). In general, patients with any material need insecurity were more likely to be younger, from a racial/ethnic minority group, and have low health literacy. Reflecting the Massachusetts setting, health insurance coverage was high (only 4% had no insurance or were self-pay) and 3% reported lacking prescription medication coverage. Overall, prevalence of material need insecurity was high (Figure 1), and there was modest overlap between the presence of one material need insecurity and others (eTable A).
Table 1.
Demographics and comparisons of patients with and without material need insecurity
| Overall N=411 |
Food Insecurity N=80 |
Cost-related Medication Underuse N=104 |
Housing Instability N=44 |
Energy Insecurity N=72 |
|
|---|---|---|---|---|---|
| Age (y) mean (SD) | 62.0 (11.0) | 56.4 (10.6) | 57.4 (11.5) | 55.0 (13.5) | 57.1 (10.8) |
| Female (%) | 47.5 | 51.3 | 47.1 | 52.3 | 47.2 |
| Race/ethnicity (%) | |||||
| NH white | 78.6 | 65.0 | 65.4 | 47.7 | 59.7 |
| NH black | 7.8 | 15.0 | 14.4 | 15.9 | 16.6 |
| Hispanic | 9.3 | 16.3 | 15.4 | 34.1 | 18.0 |
| Asian/other | 4.4 | 3.8 | 4.8 | 2.3 | 5.6 |
| Education (%) | |||||
| <HS diploma | 14.4 | 21.3 | 26.0 | 22.7 | 20.8 |
| HS diploma | 26.8 | 18.8 | 23.1 | 27.3 | 26.4 |
| >HS diploma | 58.9 | 60.0 | 51.0 | 50.0 | 52.8 |
| Insurance (%) | |||||
| Commercial | 50.6 | 38.5 | 38.2 | 35.7 | 37.5 |
| Medicare | 26.9 | 29.5 | 35.5 | 31.0 | 26.4 |
| Medicaid | 13.3 | 21.8 | 22.6 | 16.7 | 22.2 |
| Free care | 4.6 | 7.7 | 9.8 | 16.7 | 9.7 |
| None/self-pay | 4.1 | 2.6 | 3.9 | 0.0 | 4.2 |
| Low health literacy(%) | 28.1 | 38.2 | 43.3 | 45.4 | 33.3 |
| Born outside U.S. (%) | 20.4 | 30.0 | 29.8 | 40.9 | 31.5 |
| Spanish survey (%) | 5.1 | 8.8 | 7.7 | 20.5 | 9.7 |
| Charlson score, mean (SD) | 4.9 (3.0) | 5.2 (3.3) | 4.8 (2.9) | 4.2 (2.8) | 5.1 (3.1) |
| HbA1c tests/year, mean (SD) | 2.6 (1.1) | 2.6 (1.09) | 2.6 (1.1) | 2.8 (1.1) | 2.5 (1.1) |
| Age at diabetes diagnosis, mean (SD) | 50.0 (14.2) | 45.9 (14.0) | 45.8 (15.1) | 43.8 (15.4) | 45.6 (10.5) |
| Number of ‘insecurities’ (%) | |||||
| 0 | 60.3 | -- | -- | -- | -- |
| 1 | 17.4 | -- | -- | -- | -- |
| 2 | 12.9 | -- | -- | -- | -- |
| 3 | 7.2 | -- | -- | -- | -- |
| 4 | 2.2 | -- | -- | -- | -- |
Bold values represent p<0.05 in ‘insecure’ category compared with ‘secure’ counterpart
Figure 1.
Prevalence of Material Need Insecurity
In unadjusted analyses, patients with food insecurity, cost-related medication underuse, and housing instability were more likely to have poor diabetes control, compared with their secure counterparts (Table 2). For example, 64% of patients reporting food insecurity had poor diabetes control, compared with 42% of food secure patients (p=0.001). The relationship was similar, though the difference did not meet statistical significance, for those with energy insecurity. All four material need insecurities were associated with increased outpatient visits, but only cost-related medication underuse was associated with ED/inpatient utilization.
Table 2.
Unadjusted comparisons of material need insecurity with diabetes control and healthcare utilization
| Prevalence of Poor Diabetes Control* | p | Median Outpatient Visits (IQR) | p | Median ED/Inpatient Visits (IQR) | p | |
|---|---|---|---|---|---|---|
| Food Insecurity | 64.1% | .001 | 8 (5—11) | .003 | 0 (0—1) | .05 |
| No Food Insecurity | 41.6% | 6 (4—10) | 0 (0—1) | |||
|
| ||||||
| Cost-related Medication Underuse | 62.0% | .001 | 8 (5—11) | .047 | 0 (0—1) | .02 |
| No Cost-related Medication Underuse | 40.2% | 7 (4—10) | 0 (0—1) | |||
|
| ||||||
| Housing Instability | 60.5% | .04 | 9 (6—13) | .003 | 0 (0—2) | .16 |
| No Housing Instability | 43.9% | 7 (4—10) | 0 (0—1) | |||
|
| ||||||
| Energy Insecurity | 56.7% | .06 | 8 (5—11) | .01 | 0 (0—1) | .20 |
| No Energy Insecurity | 43.5% | 7 (4—10) | 0 (0—1) | |||
Composite of HbA1c > 9.0%, LDL cholesterol > 100mg/dL, or blood pressure > 140/90 mm/Hg
In multivariable models including each material need insecurity individually, and accounting for age, gender, race/ethnicity, education, insurance, health literacy, survey language, nativity, duration of diabetes, medications, and clustering by clinic, (Table 3), food insecurity was associated with poor diabetes control (Odds Ratio [OR] 1.97, 95% confidence interval[95%CI] 1.58 – 2.47, referent food secure) and increased outpatient visits (Incidence Rate Ratio in visits [IRR] 1.19 95%CI 1.05 – 1.36), but not increased ED/inpatient utilization (IRR 1.00 95% CI 0.51 – 1.97) (Full models in eTables B–G). By contrast, cost-related medication underuse was associated with poor diabetes control (OR 1.91 95% CI 1.35 – 2.70, referent no cost-related medication underuse), and increased ED/inpatient utilization (IRR 1.68 95% CI 1.21 – 2.34), but not increased outpatient visits (IRR 1.07 95% CI 0.95– 1.21).
Table 3.
Adjusted comparisons of material need insecurity with diabetes control and healthcare utilization
| Poor Diabetes Controla ORb (95% CI) |
Outpatient Visits IRRc (95% CI) |
ED/Inpatient Visits IRRc (95% CI) |
|
|---|---|---|---|
| Food Insecurity | 1.97 (1.58 – 2.47) | 1.19 (1.05 – 1.36) | 1.00 (0.51 – 1.97) |
| No Food Insecurity | 1.00 (--) | 1.0 (--) | 1.0 (--) |
|
| |||
| Cost-related Medication Underuse | 1.91 (1.35 – 2.70) | 1.07 (0.95 – 1.21) | 1.68 (1.21 – 2.34) |
| No Cost-related Medication Underuse | 1.00 (--) | 1.0 (--) | 1.0 (--) |
|
| |||
| Housing Instability | 1.10 (0.60 – 2.02) | 1.31 (1.14 – 1.51) | 1.49 (0.81 – 2.73) |
| No Housing Instability | 1.00 (--) | 1.0 (--) | 1.0 (--) |
|
| |||
| Energy Insecurity | 1.27 (0.96 – 1.69) | 1.12 (1.00– 1.25) | 1.31 (0.80 – 2.13) |
| No Energy Insecurity | 1.0 (--) | 1.0 (--) | 1.0 (--) |
Composite of HbA1c > 9.0%, LDL cholesterol > 100mg/dL, or blood pressure > 140/90 mm/Hg
Odds Ratio (OR) adjusted for age, gender, race/ethnicity, education, insurance, health literacy, survey language, nativity, duration of diabetes, glycemic, cholesterol, and blood pressure medications, and clustering by clinic
Incidence Rate Ratio (IRR) adjusted for age, gender, race/ethnicity, education, insurance, health literacy, survey language, nativity, duration of diabetes, Charlson score, and clustering by clinic
In multivariable models accounting for the same covariates, but considering the cumulative number of material need insecurities, increasing number was associated with increased odds of poor diabetes control: a 39% increase in odds of poor diabetes control for each additional material need insecurity (OR 1.39 (95% CI 1.18 – 1.63). Results were similar for outpatient visits: a 9% increase in rate of visits (IRR 1.09 95% CI 1.03 – 1.15), and ED/inpatient visits: a 22% increase (IRR 1.22 95% CI 0.99 – 1.51), for each additional material need insecurity.
In multivariable analyses looking at the individual components of diabetes control (Table 4, full models in eTables H–J), food insecurity was associated with poor glycemic control (OR 2.05 95% CI 1.61 – 2.60), and poor cholesterol control (OR 1.49 95% CI 1.13 – 1.98). Food insecurity was not associated with poor blood pressure control (OR 1.58 95% CI 066 – 3.76). Cost-related medication underuse was associated with increased odds of poor glycemic (OR 2.08 95%CI 1.11 – 3.88) cholesterol (OR 1.80 95% CI 1.60 – 2.02) and blood pressure (1.82 95% CI 1.03 – 3.22) control. Models including all four material need insecurities together are presented in the appendix (eTables K–P).
Table 4.
Adjusted comparisons of material need insecurity and components of diabetes control
| Hemoglobin A1c > 9.0% ORa (95% CI) |
LDL cholesterol > 100 mg/dL ORb (95% CI) |
Blood Pressure > 140/90 mm/Hg ORc (95% CI) |
|
|---|---|---|---|
| Food Insecurity | 2.04 (1.61 – 2.60) | 1.49 (1.13 – 1.98) | 1.58 (0.66 – 3.76) |
| No Food Insecurity | 1.00 (--) | 1.0 (--) | 1.0 (--) |
|
| |||
| Cost-related Medication Underuse | 2.08 (1.11– 3.88) | 1.80 (1.60 – 2.02) | 1.82 (1.03 – 3.22) |
| No Cost-related Medication Underuse | 1.00 (--) | 1.0 (--) | 1.0 (--) |
|
| |||
| Housing Instability | 1.77 (0.64– 4.88) | 0.99 (0.47 – 2.10) | 0.93 (0.63 – 1.37) |
| No Housing Instability | 1.00 (--) | 1.0 (--) | 1.0 (--) |
|
| |||
| Energy Insecurity | 1.16 (0.60 – 2.23) | 1.11 (0.75 – 1.64) | 1.29 (0.76 – 2.21) |
| No Energy Insecurity | 1.0 (--) | 1.0 (--) | 1.0 (--) |
Odds Ratio (OR) adjusted for age, gender, race/ethnicity, education, insurance, Charlson score, survey language, nativity, duration of diabetes, insulin use, and clustering by clinic
OR adjusted for age, gender, race/ethnicity, education, insurance, health literacy, survey language, nativity, Charlson score, statin use and clustering by clinic
OR adjusted for age, gender, race/ethnicity, education, insurance, health literacy, survey language, nativity, blood pressure medication use, and clustering by clinic
Finally, in unadjusted analyses among patients with at least 1 inpatient admission, both food insecurity (20% in food insecure vs. 7% in food secure, p < .0001) and housing instability (20% in patients with unstable housing vs. 9% in those with stable housing, p = 0.03) were associated with increased 30-day readmissions. Cost-related medication underuse and energy insecurity were not associated with increases. There were too few events to produce adjusted models for this outcome.
Discussion
Material need insecurities were common among respondents in this study, despite high levels of overall health insurance and prescription drug coverage. Although all material need insecurities had some, generally moderate, association with poor clinical control or increased utilization, no single insecurity was associated with all outcomes. For example, food insecurity was strongly and independently associated with glycemic control and outpatient utilization, while cost-related medication underuse was associated with poor glycemic, cholesterol, and blood pressure control, along with ED/inpatient utilization. In addition to their individual associations, increasing number of material need insecurities was associated with worse clinical and utilization outcomes. These results support the hypothesis that food insecurity and cost-related medication underuse are independently associated with poor diabetes control and increased healthcare utilization. Further, they support the hypothesis that there is an additive relationship among multiple material need insecurities in diabetes care.
Education and/or income are often used to indicate socioeconomic status in health research in order to capture aspects of prestige, power, and economic resources43. In this study, we measured and adjusted for education in our analyses, but, rather than measuring income, we measured specific material need insecurities. We did this because a given level of income may be sufficient for one person, but insufficient for another, based on factors such as wealth, expenses, number of people supported and their needs, and local cost of living. Determining what it is specifically that a patient cannot afford, and relating that to health and utilization outcomes, gives clinicians greater understanding of their patients’ circumstances, and helps guide interventions with more precision than measuring income would allow. For example, knowing that a patient’s income is below a poverty threshold suggests only that income assistance, such as a cash transfer, may be useful, and may miss patients whose income is greater than a poverty threshold yet is nevertheless insufficient to meet his or her needs. However, if a patient reports food insecurity, a clinician knows that resources are insufficient, whatever the level of income, Further, this suggests additional areas for intervention beyond cash transfers, including providing resources that can only be used for food (such as ‘food stamp’-like programs or nutritional prescriptions), direct provision of food, or education and skill building programs to use available food resources more effectively. Moreover, without identifying food and addressing insecurity, simple referral for medical nutrition therapy for people with uncontrolled diabetes, a current standard of care36, is likely to be fruitless.
The differential associations we observe between material need insecurities and several components of high-value care suggest that the relationship with health and healthcare outcomes is nuanced and complex. In the future, approaches that consider only global indicators of economic distress, such as poverty, or only single needs in isolation may be less useful for improving health in patients with material need insecurity. Instead, we advocate approaches that build on prior work to examine multiple material need insecurities simultaneously44, 45. This may be particularly relevant when creating population health programs. High outpatient utilization with poorly controlled diabetes may point to a group of patients with several material need insecurities for which the healthcare system is currently under-prepared to intervene; patients and clinicians could be ‘spinning their wheels’ when social needs remain unaddressed.
This study is consistent with and expands upon prior investigations. Previous studies have established an association between food insecurity6–8 and cost-related medication underuse11–13 with diabetes control when examining their specific unmet need of interest in isolation. This study builds on these by examining material need insecurity in a broader context of both competing material need insecurities and non-economic social circumstances, including health literacy and nativity. This more closely approximates the real-world conditions clinicians face when attempting to improve care for vulnerable patients.
Additionally, the differential associations we observe suggest possible mechanisms that may help further refine our understanding of how adverse economic circumstances affect health, the quality side of the value equation. We observe that food insecurity was more strongly associated with LDL and glycemic control than with blood pressure, which is consistent with prior observations regarding the importance of improving diet in patients with very high blood glucose46, and factor analyses suggesting similar physiological processes underlying insulin resistance and dyslipidemia, with blood pressure linked to different metabolic mechanisms47. Similarly, the association between cost-related medication underuse and blood pressure control suggests that medication adherence may be of primary importance for this outcome. Furthermore, unstable access to medications could lead to acute episodes, such as both hypo- and hyperglycemic crises or cardiovascular events, which result in emergency department visits or hospitalization48. These possible mechanisms are speculative, but may provide direction for future research in this area.
In addition to suggesting possible mechanisms, this study has several implications for future interventions. First, multi-factorial interventions addressing different material need insecurities may be more effective than addressing only one. For example, addressing both food and medication access may be important to improve diabetes clinical control. Several evidence-based strategies might be employed for this. Healthcare systems could seek to increase linkages between their system, government programs, and community resources49, and community health worker and peer support interventions may help patients improve their use of available resources50, 51. Second, making key medications available at very low or no out-of-pocket cost for patients, through value-based insurance design, may improve clinical outcomes48 and disparities52. For diabetes care, development of a cost-effective ‘bundle’ of selected medications, such as metformin, generic statins, and generic ACE inhibitors, available without out-of-pocket cost for diabetes patients, may be a successful approach to reducing cost-related medication underuse. Next, direct supplementation of healthy foods may reduce food insecurity and improve clinical outcomes. The PREDIMED study, though not conducted with the goal of reducing food insecurity, did demonstrate that direct supplementation of healthy food was effective in reducing cardiovascular events in a population with high rates of diabetes.53 Finally, with increasing attention paid to 30-day readmissions, some of the material need insecurities we identified may be useful to consider when designing programs to reduce this outcome, especially in light of prior work highlighting socioeconomic barriers to avoiding readmission54.
The results of this study should be interpreted in the context of several limitations. First, the data are cross-sectional, and we were unable to evaluate time-ordering of exposures and outcomes. Although it is certainly plausible that food insecurity, by incenting increased consumption of cheap, calorie-dense, highly processed foods55, and cost-related medication underuse, through reduced adherence56, can worsen diabetes outcomes and increase utilization, the possibility of reverse causation remains. Despite this, it is difficult to imagine significant improvements in diabetes control while these factors remain unaddressed. Moreover, if material need insecurities do have an effect on diabetes care, the temporal relationship between material need insecurities and healthcare outcomes would be important to study; it may differ among the various needs. A second limitation is that this study was conducted in a single healthcare system, with a sample that had less racial/ethnic diversity, greater educational attainment, and older mean age than national averages for diabetes patients57. Material need insecurities may be even more prevalent in more diverse populations. However, the results of this study do indicate that material need insecurities are common among even among the relatively ‘well-off’. Next, with regards to utilization data, we were unable to capture utilization that occurred outside our system. Although we used a validated ‘linkage’ algorithm to capture patients who usually receive care, and especially primary care, in our system24, we do not know what proportion of patents had visits to providers in other institutions. However, this would only change our qualitative interpretation if well-off patients were differentially seen more often in outside clinics, which there is no reason to suspect. Next, while this study was adequately powered for its primary endpoint, some exploratory sub-analyses likely lacked power. This is particularly evident when examining the different components of diabetes control, where confidence intervals were quite wide did not always exclude a clinically relevant increase in risk. In addition, there was relatively low ED/inpatient utilization in this group, and we may have observed different associations between material need insecurities and ED/inpatient utilization in a cohort with greater utilization. Finally, with regard to housing instability, there are several types of housing instability that may not have been captured by our items, but may still be of clinical consequence. Specifically, frequent moves that did not meet our threshold, living in a single room occupancy hotel, living in resident treatment or supervised housing that may be only temporary, living with the threat of eviction, or paying more than 50% of monthly income in rent may all be relevant forms of housing instability not captured. Thus our data likely underestimate the true prevalence of housing instability. Furthermore, whether the addition of these other forms of housing instability would change the associations we observed is unknown.
These limitations were balanced by several strengths. The study included both English and Spanish speaking patients; we used objective utilization data, and had access to detailed information on medications, clinical characteristics of diabetes, and social circumstances beyond economic factors. In addition, this study was conducted in Massachusetts, which has near-universal health insurance coverage. Increasing access to care for patients with material need insecurities may not be sufficient to eliminate disparities in health outcomes.
Healthcare systems are increasingly accountable for health outcomes that have roots outside of clinical care. Because of this, it may be reasonable to couple strategies that increase care access with those that address social determinants of health, including material need insecurities. In particular, food insecurity and cost-related medication underuse may be promising targets for real-world diabetes management.
Supplementary Material
Acknowledgments
The authors gratefully acknowledge Children’s Healthwatch for making housing and energy insecurity items available, and Ms. Emma Wise, BA, of the Tufts University Community Health program and Ms. Sarah Ackroyd, MPH, medical doctoral candidate at the University of Rochester for their uncompensated assistance with data collection.
Seth A. Berkowitz conducted the analyses. Seth A. Berkowitz had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Funding Support: Seth A. Berkowitz was supported by Institutional National Research Service Award #T32HP10251, the Ryoichi Sasakawa Fellowship Fund, and by the Division of General Internal Medicine at Massachusetts General Hospital. Dr. Wexler received funding from the National Institutes of Health under award R03DK090196-01A1 and U01DK098246. Dr. Meigs was supported in part by K24DK080140. The funding organizations had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; and preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Footnotes
Seth A. Berkowitz conceived of the study and drafted the manuscript. Deborah J. Wexler and James B. Meigs conceived of the study and revised the manuscript critically for important intellectual content. Darren DeWalt, Hilary K. Seligman and Steven J. Atlas made substantial contributions to the design of the study and revised the manuscript critically for important intellectual content. Oliver-John M. Bright, Lily S. Barnard, and Marie Schow made substantial contributions to acquisition of the data and revised the manuscript critically for important intellectual content. All authors give final approval of the version to be published and agree to accountability.
Conflict of Interest Disclosures: All authors declare they have no conflicts of interest to report.
References
- 1. [Accessed 17 Mar 2014];The Patient Protection and Affordable Care Act, P.L. 111–148. 2010 Mar 23; http://www.gpo.gov/fdsys/pkg/PLAW-111publ148/pdf/PLAW-111publ148.pdf.
- 2.Baicker K, Taubman SL, Allen HL, et al. The Oregon experiment--effects of Medicaid on clinical outcomes. N Engl J Med. 2013 May 2;368(18):1713–1722. doi: 10.1056/NEJMsa1212321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hill JO, Galloway JM, Goley A, et al. Scientific statement: Socioecological determinants of prediabetes and type 2 diabetes. Diabetes Care. 2013 Aug;36(8):2430–2439. doi: 10.2337/dc13-1161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Walker RJ, Smalls BL, Campbell JA, Strom Williams JL, Egede LE. Impact of social determinants of health on outcomes for type 2 diabetes: a systematic review. Endocrine. 2014 Feb 15; doi: 10.1007/s12020-014-0195-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pincus T, Esther R, DeWalt DA, Callahan LF. Social conditions and self-management are more powerful determinants of health than access to care. Ann Intern Med. 1998 Sep 1;129(5):406–411. doi: 10.7326/0003-4819-129-5-199809010-00011. [DOI] [PubMed] [Google Scholar]
- 6.Berkowitz SA, Baggett TP, Wexler DJ, Huskey KW, Wee CC. Food insecurity and metabolic control among U.S. adults with diabetes. Diabetes Care. 2013 Oct;36(10):3093–3099. doi: 10.2337/dc13-0570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Seligman HK, Bindman AB, Vittinghoff E, Kanaya AM, Kushel MB. Food insecurity is associated with diabetes mellitus: results from the National Health Examination and Nutrition Examination Survey (NHANES) 1999–2002. J Gen Intern Med. 2007 Jul;22(7):1018–1023. doi: 10.1007/s11606-007-0192-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Seligman HK, Jacobs EA, Lopez A, Tschann J, Fernandez A. Food insecurity and glycemic control among low-income patients with type 2 diabetes. Diabetes Care. 2012 Feb;35(2):233–238. doi: 10.2337/dc11-1627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Seligman HK, Laraia BA, Kushel MB. Food insecurity is associated with chronic disease among low-income NHANES participants. J Nutr. 2010 Feb;140(2):304–310. doi: 10.3945/jn.109.112573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Berkowitz SA, Seligman HK, Choudhry NK. Treat or Eat: Food insecurity, cost-related medication underuse and unmet needs. Am J Med. 2014 Jan 16; doi: 10.1016/j.amjmed.2014.01.002. [DOI] [PubMed] [Google Scholar]
- 11.Ngo-Metzger Q, Sorkin DH, Billimek J, Greenfield S, Kaplan SH. The effects of financial pressures on adherence and glucose control among racial/ethnically diverse patients with diabetes. J Gen Intern Med. 2012 Apr;27(4):432–437. doi: 10.1007/s11606-011-1910-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Heisler M, Choi H, Rosen AB, et al. Hospitalizations and deaths among adults with cardiovascular disease who underuse medications because of cost: a longitudinal analysis. Med Care. 2010 Feb;48(2):87–94. doi: 10.1097/MLR.0b013e3181c12e53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Piette JD, Wagner TH, Potter MB, Schillinger D. Health insurance status, cost-related medication underuse, and outcomes among diabetes patients in three systems of care. Med Care. 2004 Feb;42(2):102–109. doi: 10.1097/01.mlr.0000108742.26446.17. [DOI] [PubMed] [Google Scholar]
- 14.Booth GL, Bishara P, Lipscombe LL, et al. Universal drug coverage and socioeconomic disparities in major diabetes outcomes. Diabetes Care. 2012 Nov;35(11):2257–2264. doi: 10.2337/dc12-0364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Vijayaraghavan M, Jacobs EA, Seligman H, Fernandez A. The association between housing instability, food insecurity, and diabetes self-efficacy in low-income adults. J Health Care Poor Underserved. 2011 Nov;22(4):1279–1291. doi: 10.1353/hpu.2011.0131. [DOI] [PubMed] [Google Scholar]
- 16.Cook JT, Frank DA, Casey PH, et al. A brief indicator of household energy security: associations with food security, child health, and child development in US infants and toddlers. Pediatrics. 2008 Oct;122(4):e867–875. doi: 10.1542/peds.2008-0286. [DOI] [PubMed] [Google Scholar]
- 17.Frank DA, Casey PH, Black MM, et al. Cumulative hardship and wellness of low-income, young children: multisite surveillance study. Pediatrics. 2010 May;125(5):e1115–1123. doi: 10.1542/peds.2009-1078. [DOI] [PubMed] [Google Scholar]
- 18.Doran KM, Misa EJ, Shah NR. Housing as health care--New York’s boundary-crossing experiment. N Engl J Med. 2013 Dec 19;369(25):2374–2377. doi: 10.1056/NEJMp1310121. [DOI] [PubMed] [Google Scholar]
- 19.Garg A, Jack B, Zuckerman B. Addressing the social determinants of health within the patient-centered medical home: lessons from pediatrics. JAMA. 2013 May 15;309(19):2001–2002. doi: 10.1001/jama.2013.1471. [DOI] [PubMed] [Google Scholar]
- 20.Eggleston EM, Finkelstein JA. Finding the role of health care in population health. JAMA. 2014 Feb 26;311(8):797–798. doi: 10.1001/jama.2014.163. [DOI] [PubMed] [Google Scholar]
- 21.Cunningham P, Carrier E. Trends in the financial burden of medical care for nonelderly adults with diabetes, 2001 to 2009. Am J Manag Care. 2014 Feb;20(2):135–142. [PubMed] [Google Scholar]
- 22.Li R, Barker LE, Shrestha S, et al. Changes Over Time in High Out-of-Pocket Health-Care Burden in U.S. Adults With Diabetes, 2001–2011. Diabetes Care. 2014 Apr 10; doi: 10.2337/dc13-1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Berkowitz SA, Seligman HK, Choudhry NK. Treat or Eat: Food Insecurity, Cost-related Medication Underuse, and Unmet Needs. Am J Med. 2014 Apr;127(4):303–310. e303. doi: 10.1016/j.amjmed.2014.01.002. [DOI] [PubMed] [Google Scholar]
- 24.Atlas SJ, Grant RW, Ferris TG, Chang Y, Barry MJ. Patient-physician connectedness and quality of primary care. Ann Intern Med. 2009 Mar 3;150(5):325–335. doi: 10.7326/0003-4819-150-5-200903030-00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Altman SH, Doonan M. Can Massachusetts lead the way in health care reform? N Engl J Med. 2006 May 18;354(20):2093–2095. doi: 10.1056/NEJMp068103. [DOI] [PubMed] [Google Scholar]
- 26.Grant RW, Wexler DJ, Ashburner JM, Hong CS, Atlas SJ. Characteristics of “complex” patients with type 2 diabetes mellitus according to their primary care physicians. Arch Intern Med. 2012 May 28;172(10):821–823. doi: 10.1001/archinternmed.2012.1229. [DOI] [PubMed] [Google Scholar]
- 27.Bickel G, Nord M, Price C, Hamilton W, Cook J. U.S. Department of Agriculture Food and Nutrition Service, editor. Guide to Measuring Household Food Security, Revised 2000. Alexandria VA: 2000. [Google Scholar]
- 28.Children’s HealthWatch. [Accessed 30 April 2014];Children’s HealthWatch Interview. 2013 http://www.childrenshealthwatch.org/methods/our-survey/
- 29.Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)--a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009 Apr;42(2):377–381. doi: 10.1016/j.jbi.2008.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Berwick DM, Nolan TW, Whittington J. The triple aim: care, health, and cost. Health Aff (Millwood) 2008 May-Jun;27(3):759–769. doi: 10.1377/hlthaff.27.3.759. [DOI] [PubMed] [Google Scholar]
- 31.Lee TH. Putting the value framework to work. N Engl J Med. 2010 Dec 23;363(26):2481–2483. doi: 10.1056/NEJMp1013111. [DOI] [PubMed] [Google Scholar]
- 32.Nalichowski R, Keogh D, Chueh HC, Murphy SN. Calculating the benefits of a Research Patient Data Repository. AMIA Annu Symp Proc. 2006:1044. [PMC free article] [PubMed] [Google Scholar]
- 33.Currie CJ, Peters JR, Tynan A, et al. Survival as a function of HbA(1c) in people with type 2 diabetes: a retrospective cohort study. Lancet. 2010 Feb 6;375(9713):481–489. doi: 10.1016/S0140-6736(09)61969-3. [DOI] [PubMed] [Google Scholar]
- 34.Yudkin JS, Richter B, Gale EA. Intensified glucose lowering in type 2 diabetes: time for a reappraisal. Diabetologia. 2010 Oct;53(10):2079–2085. doi: 10.1007/s00125-010-1864-z. [DOI] [PubMed] [Google Scholar]
- 35.National Committee for Quality Assurance. [Accessed 30 April 2014];HEDIS & Performance Measurement. 2014 http://www.ncqa.org/HEDISQualityMeasurement.aspx.
- 36.American Diabetes Association. Standards of medical care in diabetes--2014. Diabetes Care. 2014 Jan;37( Suppl 1):S14–80. doi: 10.2337/dc14-S014. [DOI] [PubMed] [Google Scholar]
- 37.Bindman AB, Chattopadhyay A, Auerback GM. Interruptions in Medicaid coverage and risk for hospitalization for ambulatory care-sensitive conditions. Ann Intern Med. 2008 Dec 16;149(12):854–860. doi: 10.7326/0003-4819-149-12-200812160-00004. [DOI] [PubMed] [Google Scholar]
- 38.Johnson PJ, Ghildayal N, Ward AC, Westgard BC, Boland LL, Hokanson JS. Disparities in potentially avoidable emergency department (ED) care: ED visits for ambulatory care sensitive conditions. Med Care. 2012 Dec;50(12):1020–1028. doi: 10.1097/MLR.0b013e318270bad4. [DOI] [PubMed] [Google Scholar]
- 39.Oster A, Bindman AB. Emergency department visits for ambulatory care sensitive conditions: insights into preventable hospitalizations. Med Care. 2003 Feb;41(2):198–207. doi: 10.1097/01.MLR.0000045021.70297.9F. [DOI] [PubMed] [Google Scholar]
- 40.Sarkar U, Schillinger D, Lopez A, Sudore R. Validation of self-reported health literacy questions among diverse English and Spanish-speaking populations. J Gen Intern Med. 2011 Mar;26(3):265–271. doi: 10.1007/s11606-010-1552-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Executive Office of Health and Human Services. [Accessed 28 May 2014];Health Safety Net. 2014 http://www.mass.gov/eohhs/consumer/insurance/more-programs/health-safety-net/
- 42.The American Association for Public Opinion Research. Standard Definitions: Final Dispositions of Case Codes and Outcome Rates for Surveys. 7. AAPOR; 2011. [Google Scholar]
- 43.Braveman PA, Cubbin C, Egerter S, et al. Socioeconomic status in health research: one size does not fit all. JAMA. 2005 Dec 14;294(22):2879–2888. doi: 10.1001/jama.294.22.2879. [DOI] [PubMed] [Google Scholar]
- 44.Kushel MB, Gupta R, Gee L, Haas JS. Housing instability and food insecurity as barriers to health care among low-income Americans. J Gen Intern Med. 2006 Jan;21(1):71–77. doi: 10.1111/j.1525-1497.2005.00278.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kersey MA, Beran MS, McGovern PG, Biros MH, Lurie N. The prevalence and effects of hunger in an emergency department patient population. Acad Emerg Med. 1999 Nov;6(11):1109–1114. doi: 10.1111/j.1553-2712.1999.tb00112.x. [DOI] [PubMed] [Google Scholar]
- 46.Coppell KJ, Kataoka M, Williams SM, Chisholm AW, Vorgers SM, Mann JI. Nutritional intervention in patients with type 2 diabetes who are hyperglycaemic despite optimised drug treatment--Lifestyle Over and Above Drugs in Diabetes (LOADD) study: randomised controlled trial. BMJ. 2010;341:c3337. doi: 10.1136/bmj.c3337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Meigs JB, D’Agostino RB, Sr, Wilson PW, Cupples LA, Nathan DM, Singer DE. Risk variable clustering in the insulin resistance syndrome. The Framingham Offspring Study. Diabetes. 1997 Oct;46(10):1594–1600. doi: 10.2337/diacare.46.10.1594. [DOI] [PubMed] [Google Scholar]
- 48.Choudhry NK, Avorn J, Glynn RJ, et al. Full coverage for preventive medications after myocardial infarction. N Engl J Med. 2011 Dec 1;365(22):2088–2097. doi: 10.1056/NEJMsa1107913. [DOI] [PubMed] [Google Scholar]
- 49.Peek ME, Ferguson M, Bergeron N, Maltby D, Chin MH. Integrated community-healthcare diabetes interventions to reduce disparities. Curr Diab Rep. 2014 Mar;14(3):467. doi: 10.1007/s11892-013-0467-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Long JA, Jahnle EC, Richardson DM, Loewenstein G, Volpp KG. Peer mentoring and financial incentives to improve glucose control in African American veterans: a randomized trial. Ann Intern Med. 2012 Mar 20;156(6):416–424. doi: 10.1059/0003-4819-156-6-201203200-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Tang TS, Funnell M, Sinco B, et al. Comparative Effectiveness of Peer Leaders and Community Health Workers in Diabetes Self-management Support: Results of a Randomized Controlled Trial. Diabetes Care. 2014 Apr 10; doi: 10.2337/dc13-2161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Choudhry NK, Bykov K, Shrank WH, et al. Eliminating medication copayments reduces disparities in cardiovascular care. Health Aff (Millwood) 2014 May;33(5):863–870. doi: 10.1377/hlthaff.2013.0654. [DOI] [PubMed] [Google Scholar]
- 53.Estruch R, Ros E, Salas-Salvado J, et al. Primary prevention of cardiovascular disease with a Mediterranean diet. N Engl J Med. 2013 Apr 4;368(14):1279–1290. doi: 10.1056/NEJMoa1200303. [DOI] [PubMed] [Google Scholar]
- 54.Kangovi S, Barg FK, Carter T, et al. Challenges faced by patients with low socioeconomic status during the post-hospital transition. J Gen Intern Med. 2014 Feb;29(2):283–289. doi: 10.1007/s11606-013-2571-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Seligman HK, Schillinger D. Hunger and socioeconomic disparities in chronic disease. N Engl J Med. 2010 Jul 1;363(1):6–9. doi: 10.1056/NEJMp1000072. [DOI] [PubMed] [Google Scholar]
- 56.Egede LE, Gebregziabher M, Dismuke CE, et al. Medication nonadherence in diabetes: longitudinal effects on costs and potential cost savings from improvement. Diabetes Care. 2012 Dec;35(12):2533–2539. doi: 10.2337/dc12-0572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ali MK, Bullard KM, Saaddine JB, Cowie CC, Imperatore G, Gregg EW. Achievement of goals in U.S. diabetes care, 1999–2010. N Engl J Med. 2013 Apr 25;368(17):1613–1624. doi: 10.1056/NEJMsa1213829. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.