Abstract
Objective
Perhaps the most vexing and exigent problem confronting head and neck cancer reconstruction is overcoming the impediments of collateral damage imposed by radiation therapy (XRT) on normal surrounding tissue. XRT is detrimental to bone and soft tissue repair resulting in an unacceptably high incidence of devastating wound healing complications as well as the associated morbidity of late pathologic fractures, reduced bone healing, and osteoradionecrosis. The consequences of XRT on bone vasculature, long known to be affected by radiation, have been poorly understood. The purpose of this study was to analyze the degree by which irradiation degrades existing bone vascularity using a powerful micro-computed tomography (micro-CT) technique to attain highly precise quantitative metrics of the vascular tree.
Methods
Fourteen 400g male Sprague-Dawley rats underwent 35 Gy of fractionated XRT at 7 Gy/day. The animals were euthanized after 28 days and the left ventricle was fixed and injected with Microfil contrast. Left hemimandibles were dissected and scanned using high-resolution micro-CT (18μ voxels). The vessel number, thickness, separation, connectivity and vessel volume fraction were analyzed for the region of interest (ROI), defined to be the volume behind the third molar spanning a total distance of 5.1 mm.
Results
Stereological analysis and subsequent ANOVA test demonstrated a significant and quantifiable diminution in the irradiated vasculature when compared to control animals. The vessel volume fraction (0.016 vs. 0.032, p≤0.003) and vessel thickness (0.042mm vs. 0.067mm, p≤0.001) were markedly reduced. Interestingly, further analysis demonstrated no significant differences between vessel separation and vessel number.
Conclusion
The results of our study specifically quantify the corrosive affects of XRT on the vasculature of the mandible. The data from this novel technique goes even further and implies retention of blood vessels, but a degradation of their quality and size. Further experiments can now be directed at therapeutic interventions to reverse this process and better understand the underlying mechanism of XRT-induced bone injury.
Keywords: Radiation, osteoradionecrosis, head and neck cancer, human equivalent dose, Mandible
Introduction
The dangers and pernicious side-effects of adjuvant radiation therapy (XRT) have been long-known and are well documented. While the curative role of XRT as a treatment for cancer is undeniable—fractionated regimens have been shown to decrease local tumor recurrence1 and increase overall cancer survival rates2—adjuvant radiotherapy carries a high degree of risk to the patient. Typical side effects of XRT for head and neck cancer patients include myelosuppression, nausea and vomiting, mucositis, radiation ulceration, alopecia, diarrhea, and dermatitis.3-6 Even more problematic are a segment of patients who experience significantly more devastating morbidities such as wound healing complications, late pathologic fractures, and osteoradionecrosis.
Spanning back to 1971, it has long been suspected that the application of radiation can diminish local vascularity.7,8 This hypovascularity has been implicated in many of radiation's devastating sequelae, such as osteoradionecrosis of the mandible.9 However, the literature has heretofore not been able to demonstrate a clear and quantitative degradation of bone microvasculature. Previous methodologies have relied on either physical histological counts, which are prone to human or 3D sampling errors, or flow studies which measure changes in blood flow but do not directly determine the changes in the vessel number or other stereologic parameters of the vascular tree.
Recent reports have utilized a novel technique to examine microvasculature of the liver, retina, kidney, and femur.10-13 This technique involves the injection of a radio-opaque compound into the vascular tree, followed by micro-CT(after tissue demineralization, in the case of bone samples) with subsequent stereological analysis. This procedure is capable of attaining precise, quantitative total vascularity measurements of a given structure, organ, or region. Curiously, this powerful new methodology has never been applied to the craniofacial skeleton in order to determine the effect of radiation on the associated vascular microarchitecture. This has led our laboratory to inquire upon radiation-induced hypovascularity in the cortical flat bone of the craniofacial skeleton, specifically the mandible.
Our global hypothesis is that a human-equivalent radiation dose will result in a significant, demonstrable, and quantifiable degradation of the overall microvascular networks in the murine membranous mandible. Furthermore, we postulate that radiation exerts its damage through one of two mechanisms. The first is a mechanism of vascular obliteration, where vessel number is significantly decreased and vessel separation is significantly increased. The second is a mechanism of vascular contraction, in which case vessel thickness will be significantly diminished. These findings cannot be elucidated using conventional flow studies. The specific aim of this paper is to ascertain, measure, and define these destructive effects of radiation on the vascular microarchitecture of the mandible utilizing vascular perfusion. By determining and quantifying these parameters we will be able to utilize our findings to measure the efficacy of therapies aimed at remediating radiation-induced vascular damage to the craniofacial skeleton in the future.
Methods
Fourteen adult male Sprague-Dawley rats weighing approximately 400g were paired in cages and maintained in a pathogen-free environment on a 12-hour light/dark schedule. Rats were fed standard hard chow and water ad libitum during a seven-day acclimation. After seven days of acclimation, the animals were randomly divided into two groups. Group 1 served as a control group (n = 6). Group 2 was the experimental group (XRT, n = 8).
Radiation Protocol
The XRT group underwent 35 Gy of fractionated radiation over 5 days, at 7 Gy / day. Subsequent to the start of radiation treatment, all animals were fed a standard soft chow diet. Rat hemi-mandibles were irradiated using a Pantak DXT 300 orthovoltage unit fractionating the dose at approximately 3.72 Gy/min over 5 days for a 35 Gray total, in the Irradiation Core at the University of Michigan Cancer Center. A previous study in our laboratory successfully demonstrated both the method and fractionation regimen and showed this to be a human-equivalent dose with a therapeutic equivalence of 70 Gy of radiation.14 X-Rays are utilized since they taper off quickly, affecting only the one side of the mandible, thus obviating the need for any intra-oral shield. They provide the same physiologic effect on bone and soft tissue as gamma radiation does. Rats will be anesthetized with Isofluorane, then placed right side down so only the left hemi mandible will be irradiated. A lead shield with rectangular window will protect the pharynx, brain and the remainder of the animal. Dosimetry will be carried out using an ionization chamber connected to an electrometer system, which is directly traceable to a National Institute of Standards and Technology calibration. The rats are maintained on soft chow concomitant with the start of radiation.
All animal procedures were performed in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals and approved by the University of Michigan Committee on the Use and Care of Animals. Maxillary incisors were clipped weekly due to overgrowth. Animals completed a 54-day recovery period prior to perfusion and sacrifice.
Perfusion Protocol
The perfusion protocol for this murine mandible model has been published previously.15, 16 Briefly, all rats were anesthetized prior to performing a thoracotomy and left cardio-ventricular catheterization. Perfusion with heparinized normal saline followed by pressure fixation with normal buffered formalin solution ensured euthanasia. After fixation, the vasculature was injected with Microfil (MV-122, Flow Tech, Carver, MA). Mandibles were harvested and demineralized using Cal-Ex II (Fisher Scientifics; Fairlawn, NJ), a formic acid solution. Perfusion was verified by coloration of the dissected mandible and by subsequent micro-CT maximal intensity projection (MIP). Leeching of mineral was confirmed with serial radiographs to ensure adequate demineralization prior to scanning.
Micro-Computed Tomography
Specimens were scanned at 18μm voxel size with micro-CT. We previously utilized this voxel size and found it optimal to adequately resolve small, murine mandibular vessel networks. Other laboratories have utilized similar resolutions in mouse tibia vasculature studies, and have found these resolutions adequate as well. The region of interest was defined as a distance measuring 5.1 mm after the third molar of the left hemimandible. At an 18μm resolution, this is equivalent to 283 slices. Analysis of the region of interest (ROI) was then performed with Micro View 2.2 software (GE Healthcare, Milwaukee, WI.). Contours were defined highlighting the ROIs using the spline function. Analysis of vascularity in this region is accomplished by setting a global grayscale threshold of 1000 to differentiate vessels from surrounding tissue, as previously described.17 Using this information, MicroView allocates only the number of voxels above this threshold and uses stereologic algorithms to assign relative densities to the voxels based on the contrast content within the vessels. MicroView's analysis of the ROI reported four metrics, all of which controlled for total size of the ROI: vessel volume fraction (VVF), vessel number (VN), vessel thickness (VT), and vessel separation (VS). VVF depicts the fraction of bone occupied by the volume of vessels within the ROI tissue. It is calculated based on the voxel size and the number of segmented voxels in the 3-D image after application of the binarization threshold. VN is a reflection of the actual number of vessels per millimeter within the ROI. To calculate vessel number, a segmented volume is skeletonized, leaving just the voxels above our binarization threshold at the midaxes of the vascular structures. The vessel number is defined as the inverse of the mean spacing between the midaxes of the structures in the segmented volume. VT is measured by calculating an average of the local voxel thicknesses within the vessel. Local voxel thickness is defined as the diameter of the largest sphere that both contains the point regardless of position within the sphere, and is completely within the structure of interest. This distinguishes the blood vessel from the background space surrounding the object.18 It is worth noting that VT, in this case, defines the intraluminal diameter of the vessel and not the thickness of the vessel wall. In addition, for qualitative comparison, 3-Dvisualization of vascular anatomy was accomplished utilizing maximal intensity projections. This enables instant volume rendering of a volumetric data set, yielding a 3-D representation of the scanned specimen.
Statistical Analysis
The independent samples t-test was used to analyze differences between group means (PASW version 18.0; SPSS, Inc., Chicago, IL.). Vascular metrics were compared between groups and reported as their respective means with significance indicated at p ≤ .05.
Results
All XRT rats tolerated the radiation with no major complications, though alopecia was reported in all XRT animals. All XRT animals underwent successful perfusions (Figure 1.), however, two control animals were excluded due to incomplete perfusions. Stereological analysis and subsequent independent samples t-test demonstrated a significant and quantifiable diminution of XRT vasculature, compared to the control.
Figure 1.
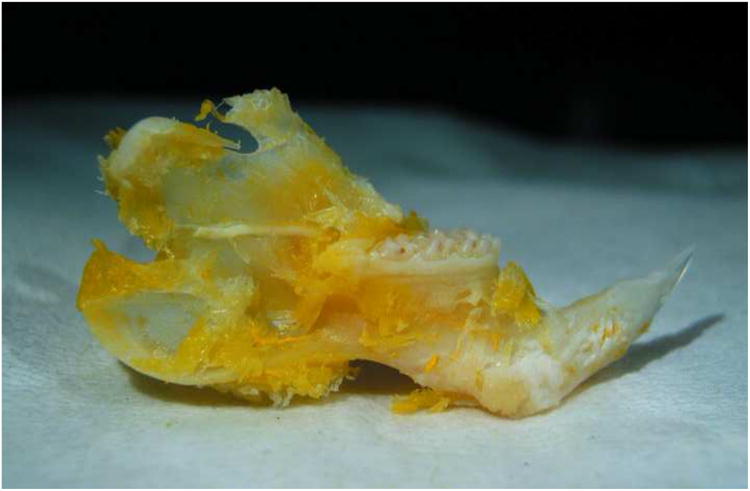
Human-equivalent irradiated left hemimandible that underwent successful Microfil perfusion. Note the yellow discoloration of the bone, indicating complete perfusion by the Microfil.
Discussion
A number of studies have been completed which attempt to correlate a wide variety of outcome measures examining the effect of radiation on the vascular microarchitecture of bone. Cutright and Brady attempted to quantitatively measure the effect of radiation on the murine humerus7 by perfusing the bone with a chromium radioisotope, then measuring the total amount of radioactive material in the bone.7 This method is suspect primarily because it does not control for size or anatomical differences between animals, and secondarily because it only provides data on the gross vascularity of the bone without detail on the microarchitecture of the bone. Other studies, such as those performed by King et al, attempt to correlate histological findings to overall bone vascularity.19 These studies are limited by the fact that they examine random samples of the bone in two dimensions and do not quantitatively examine the bone as a whole. Furthermore, the histological methodology they utilize cannot provide quantitative data on the nature of the vessels in the bone, as it does not take into account the serpentine nature of blood vessels. As seen in Figure 2, vessels often deviate back and forth along their trajectory, which may result in double-counting using a planar, histology-based methodology.
Figure 2.
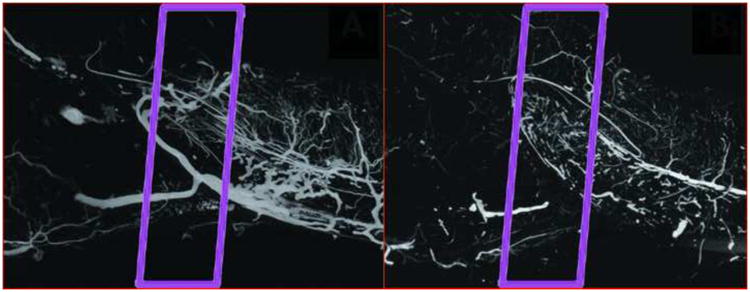
Representative Maximum Intensity Projections (MIPs) of Control vs. Radiated animals. Left: MIP of a control animal, with the ROI outlined. Right: MIP of a XRT animal, with the ROI outlined.
VVF showed a significant decrease from control to XRT (0.032±0.008 vs. 0.019±0.004), p≤0.005), thereby confirming our global hypothesis and corroborating the indirect evidence presented previously in the literature. VS slightly increased (2.081±0.28 mm vs. 2.327±0.683 mm, p=0.422) and VN slightly decreased (0.472±0.064 mm-3 vs. 0.449±0.119 mm-3, p=0.699), but neither achieved significance. However, VT (0.067±0.016 mm vs. 0.042±0.005 mm, p≤0.003) was markedly and significantly reduced.
Along with histology, another common method of bony vascular analysis is laser Doppler flowmetry. Hellem, et al, pioneered the use of Doppler flowmetry for the analysis of bony vasculature.20 This was then applied by Okunieff, et al, to an irradiated mouse long bone model where they found that radiation has a severe anti-angiogenic effect.21 The methodology, however, was hindered by the fact that Doppler flowmetry is unable to specify the extent of vascular depletion. While Doppler flowmetry is often the most clinically feasible (as it does not require sacrificing the patient), it is by no means the most accurate, making it non-ideal for an animal study. As Doppler flowmetry relies on measuring the backscatter caused by passing erythrocytes and attempts to correlate it to overall vascular function, rather than directly evaluating the vascularity of the bone, it is only able to give qualitative rather than quantitative results. Compounding this issue, laser Doppler flowmetry results vary depending on a number of physiologic factors such as device placement, blood pressure, and respiration. As such, it is ill-suited as a technique to define and measure the degree of radiation-induced damage upon the actual vascular microarchitecture. Despite these significant drawbacks, it remains the current state-of-the-art and clinical gold standard.
Our study specifically quantifies the corrosive effects of XRT on mandibular vasculature. The data from this novel technique implies retention of blood vessels but diminished vessel size. Given this, it was expected that there would not be changes in either vessel number or separation. As expected, there were no significant differences in these two metrics. The resultant p-values (VS=0.422, VN=0.699) force our acceptance of the null hypothesis, that radiation does not alter the vessel separation and number of murine mandibular vascular networks. It is of note that some microvasculature can have intraluminal diameters smaller than our scanning resolution for micro-CT. Studies have previously been performed examining murine microvasculature, where they found that there was no significant difference between scans of 8 μm and 16 μm.13 Given these findings and the massive technical complications associated with higher resolution scanning, we elected to use a high- rather than an ultra-high scanning resolution.
Although some of the sequelae of radiation therapy, such as osteoradionecrosis or pathologic fracture, can occur years or even decades after radiotherapy treatment, the inciting damage predisposing those conditions occurs soon after radiation is completed, as seen in the decreassed vascularity evidenced in our model. It is our hope to develop treatment strategies to predictably and successfully mitigate the documented damage and thereby lessen the likelihood of long-term consequences such as pathologic fracture or osteoradionecrosis.
In conclusion, these results present an easily-replicated, quantifiable examination of radiation-induced mandibular hypovascularity, along with its means (vascular constriction). Our findings—that vessel volume fraction and vessel thickness are definitively and significantly decreased in a mandibular XRT model—both validate and quantify assertions made previously in the literature regarding the pernicious effects of radiation on the mandibular vascular microarchitecture, thereby providing an excellent experimental model for the study of diseases such as osteoradionecrosis and pathologic fracture. This information (irradiated bone's vascular density) is essential to understand the capacity of the mandible to satisfy the metabolic needs of repair processes in the bone. For example, the knowledge that an irradiated mandible will have on average a 40.6% decrease in overall vascularity and a 37.3% decrease in vessel thickness could have considerable clinical impact on surgical strategies and patient management. Further experiments will be directed at developing therapeutic interventions to either prevent or reverse this process and to better understand the mechanism of XRT-induced injury, with the eventual goal of developing a cancer treatment regimen free of the current, devastating biomedical burden of XRT-induced bone pathology.
Figure 3.
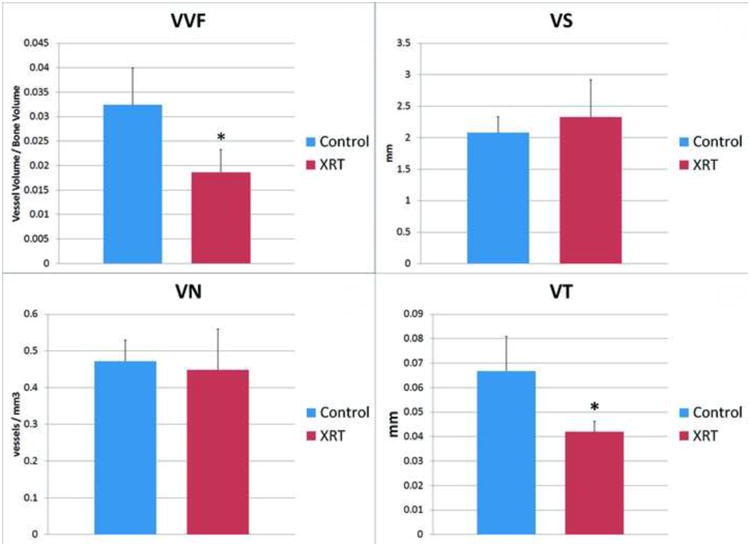
Graphs showing differences in four stereologic metrics of bone vascularity. Top Left: Significant decrease in vessel volume fraction with XRT. Top Right: Non-significant increase in vessel separation with XRT. Bottom Left: Non-significant decrease in vessel number with XRT. Bottom Right: Significant decrease in vessel thickness with XRT. (*) indicates significance. Significance taken at p < 0.005.
Acknowledgments
Funding was provided by National Institutes of Health grant RO1 CA 12587-01 to Steven R. Buchman, M.D. The authors thank Aria J. Zehtabzadeh for technical assistance during surgery, animal care, and micro–CT analysis.
References
- 1.Vinh-Hung V, Verschraegen C. Breast-Conserving Surgery With or Without Radiotherapy: Pooled-Analysis for Risks of Ipsilateral Breast Tumor Recurrence and Mortality. [Accessed July 31, 2010];JNCI Journal of the National Cancer Institute. 2004 96(2):115–121. doi: 10.1093/jnci/djh013. Available at: http://jnci.oxfordjournals.org/cgi/content/abstract/96/2/115. [DOI] [PubMed] [Google Scholar]
- 2.Folkesson J, Birgisson H, Pahlman L, et al. Swedish Rectal Cancer Trial: long lasting benefits from radiotherapy on survival and local recurrence rate. [Accessed July 18, 2010];Journal of clinical oncology: official journal of the American Society of Clinical Oncology. 2005 23(24):5644–50. doi: 10.1200/JCO.2005.08.144. Available at: http://jco.ascopubs.org/cgi/content/abstract/23/24/5644. [DOI] [PubMed] [Google Scholar]
- 3.Wood AJJ, Shapiro CL, Recht A. Side Effects of Adjuvant Treatment of Breast Cancer. New England Journal of Medicine. 2001;344(26):1997–2008. doi: 10.1056/NEJM200106283442607. Available at: http://dx.doi.org/10.1056/NEJM200106283442607. [DOI] [PubMed] [Google Scholar]
- 4.Cooper J, Fu K, Marks J, Silverman S. Late effects of radiation therapy in the head and neck region. [Accessed January 12, 2011];International Journal of Radiation OncologyBiologyPhysics. 1995 31(5):1141–1164. doi: 10.1016/0360-3016(94)00421-G. Available at: http://dx.doi.org/10.1016/0360-3016(94)00421-G. [DOI] [PubMed] [Google Scholar]
- 5.Forastiere A, Koch W, Trotti A, Sidransky D. Head and Neck Cancer. New England Journal of Medicine. 2001;345(26):1890–1900. doi: 10.1056/NEJMra001375. Available at: http://dx.doi.org/10.1056/NEJMra001375. [DOI] [PubMed] [Google Scholar]
- 6.Wasserman TH, Brizel DM, Henke M, et al. Influence of intravenous amifostine on xerostomia, tumor control, and survival after radiotherapy for head-and- neck cancer: 2-year follow-up of a prospective, randomized, phase III trial. [Accessed January 12, 2011];International journal of radiation oncology, biology, physics. 2005 63(4):985–90. doi: 10.1016/j.ijrobp.2005.07.966. Available at: http://dx.doi.org/10.1016/j.ijrobp.2005.07.966. [DOI] [PubMed] [Google Scholar]
- 7.Cutright DE, Brady JM. Long-Term Effects of Radiation on the Vascularity of Rat Bone: Quantitative Measurements with a New Technique. [Accessed August 23, 2010];Radiation Research. 1971 48(2):402–408. Available at: http://www.jstor.org/stable/3573324. [PubMed] [Google Scholar]
- 8.Pitkänen MA, Hopewell JW. Functional changes in the vascularity of the irradiated rat femur. Implications for late effects. [Accessed January 12, 2011];Acta radiologica Oncology. 1983 22(3):253–6. doi: 10.3109/02841868309134038. Available at: http://www.ncbi.nlm.nih.gov/pubmed/6312765. [DOI] [PubMed] [Google Scholar]
- 9.Marx RE. A new concept in the treatment of osteoradionecrosis. [Accessed January 12, 2011];Journal of Oral and Maxillofacial Surgery. 1983 41(6):351–357. doi: 10.1016/s0278-2391(83)80005-6. Available at: http://dx.doi.org/10.1016/S0278-2391(83)80005-6. [DOI] [PubMed] [Google Scholar]
- 10.Bentley MD, Ortiz MC, Ritman EL, Romero JC. The use of microcomputed tomography to study microvasculature in small rodents. [Accessed January 12, 2011];American journal of physiology Regulatory, integrative and comparative physiology. 2002 282(5):R1267–79. doi: 10.1152/ajpregu.00560.2001. Available at: http://ajpregu.physiology.org/cgi/content/abstract/282/5/R1267. [DOI] [PubMed] [Google Scholar]
- 11.Bek T, Jensen PK. Three-dimensional structure of human retinal vessels studied by vascular casting. [Accessed January 12, 2011];Acta Ophthalmologica. 2009 71(4):506–513. doi: 10.1111/j.1755-3768.1993.tb04627.x. Available at: http://doi.wiley.com/10.1111/j.1755-3768.1993.tb04627.x. [DOI] [PubMed] [Google Scholar]
- 12.Ortiz MC, García-Sanz A, Bentley MD, et al. Microcomputed tomography of kidneys following chronic bile duct ligation. [Accessed January 12, 2011];Kidney international. 2000 58(4):1632–40. doi: 10.1111/j.1523-1755.2000.00324.x. Available at: http://www.ncbi.nlm.nih.gov/pubmed/11012897. [DOI] [PubMed] [Google Scholar]
- 13.Duvall CL, Taylor WR, Weiss D, Guldberg RE. Quantitative microcomputed tomography analysis of collateral vessel development after ischemic injury. [Accessed January 12, 2011];American journal of physiology Heart and circulatory physiology. 2004 287(1):H302–10. doi: 10.1152/ajpheart.00928.2003. Available at: http://ajpheart.physiology.org/cgi/content/abstract/287/1/H302. [DOI] [PubMed] [Google Scholar]
- 14.Monson LA, Farberg A, Jing L, Buchman S. Human Equivalent Radiation Dose Response in the Rat Mandible. [Accessed August 25, 2010];Plastic and Reconstructive Surgery. 2009 124(4S):2. Available at: http://journals.lww.com/plasreconsurg/Abstract/2009/10002/Human_Equivalent_Radiation_Dose_Response_in_the.3.aspx. [Google Scholar]
- 15.Jing XL, Farberg AS, Monson LA, et al. 204A: A Quantitative Analysis of Angiogenesis in Mandibular Distraction Osteogenesis. Plastic and Reconstructive Surgery. 2010;125(6) Available at: http://journals.lww.com/plasreconsurg/Fulltext/2010/06002/204A__A_Quantitative_Analysis_of_Angiogenesis_in.206.aspx. [Google Scholar]
- 16.Donneys A, Tchanque-Fossuo C, Farberg A, et al. Quantitative Analysis of Vascular Response after Mandibular Fracture Repair Using Microcomputed Tomography with Vessel Perfusion. Plastic & Reconstructive Surgery. 2011;127(4):1–7. doi: 10.1097/PRS.0b013e318208f3c1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jing X, Farberg A, Monson L, SR Radiomorphometric Quantitative Analysis of Vasculature in Rat Mandible Utilizing Microcomputed Tomography. [Accessed October 17, 2010];Plastic and Reconstructive Surgery. 2009 124(4S):26–27. Available at: http://journals.lww.com/plasreconsurg/Citation/2009/10002/Radiomorphometric_Quantitative_Analysis_of.32.aspx. [Google Scholar]
- 18.Duvall CL, Taylor WR, Weiss D, Guldberg RE. Quantitative microcomputed tomography analysis of collateral vessel development after ischemic injury. American journal of physiology Heart and circulatory physiology. 2004;287(1):H302–10. doi: 10.1152/ajpheart.00928.2003. Available at: http://www.ncbi.nlm.nih.gov/pubmed/15016633. [DOI] [PubMed] [Google Scholar]
- 19.King MA, Casarett GW, Weber DA. A Study of Irradiated Bone: I Histopathologic and Physiologic Changes. [Accessed January 12, 2011];J Nucl Med. 1979 20(11):1142–1149. Available at: http://jnm.snmjournals.org/cgi/content/abstract/20/11/1142. [PubMed] [Google Scholar]
- 20.Hellem S, Jacobsson L, Nilsson G, Lewis D. Measurement of microvascular blood flow in cancellous bone using laser Doppler flowmetry and 133Xe-clearance. [Accessed January 12, 2011];International Journal of Oral Surgery. 1983 12(3):165–177. doi: 10.1016/s0300-9785(83)80063-5. Available at: http://dx.doi.org/10.1016/S0300-9785(83)80063-5. [DOI] [PubMed] [Google Scholar]
- 21.Okunieff P, Wang X, Rubin P, et al. Radiation-induced changes in bone perfusion and angiogenesis. [Accessed January 12, 2011];International Journal of Radiation OncologyBiologyPhysics. 1998 42(4):885–889. doi: 10.1016/s0360-3016(98)00339-3. Available at: http://dx.doi.org/10.1016/S0360-3016(98)00339-3. [DOI] [PubMed] [Google Scholar]
- 22.Wahl JH, Goodlett DR, Udseth HR, Smith RD. Use of small-diameter capillaries for increasing peptide and protein detection sensitivity in capillary electrophoresis-mass spectrometry. Eletrophoresis. 1993;14(1):448–457. doi: 10.1002/elps.1150140170. Available at: http://dx.doi.org/10.1002/elps.1150140170. [DOI] [PubMed] [Google Scholar]


