Abstract
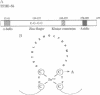
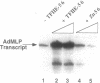
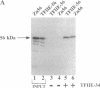
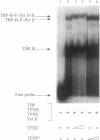
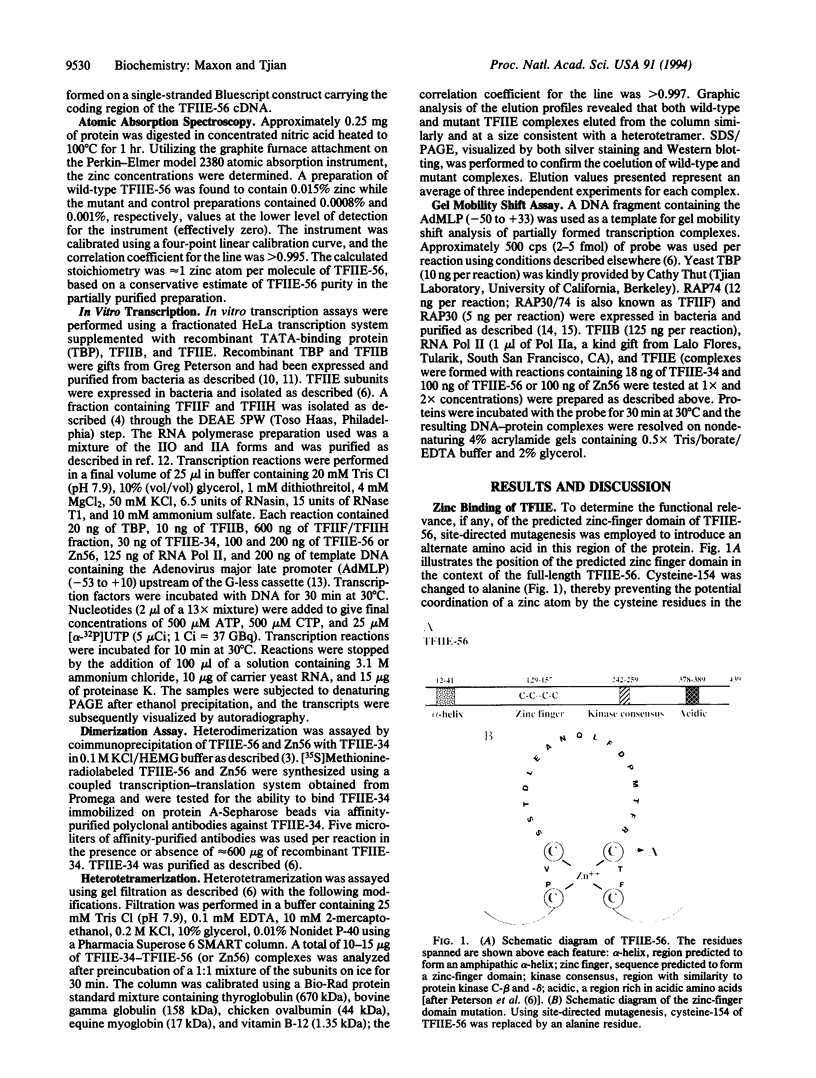
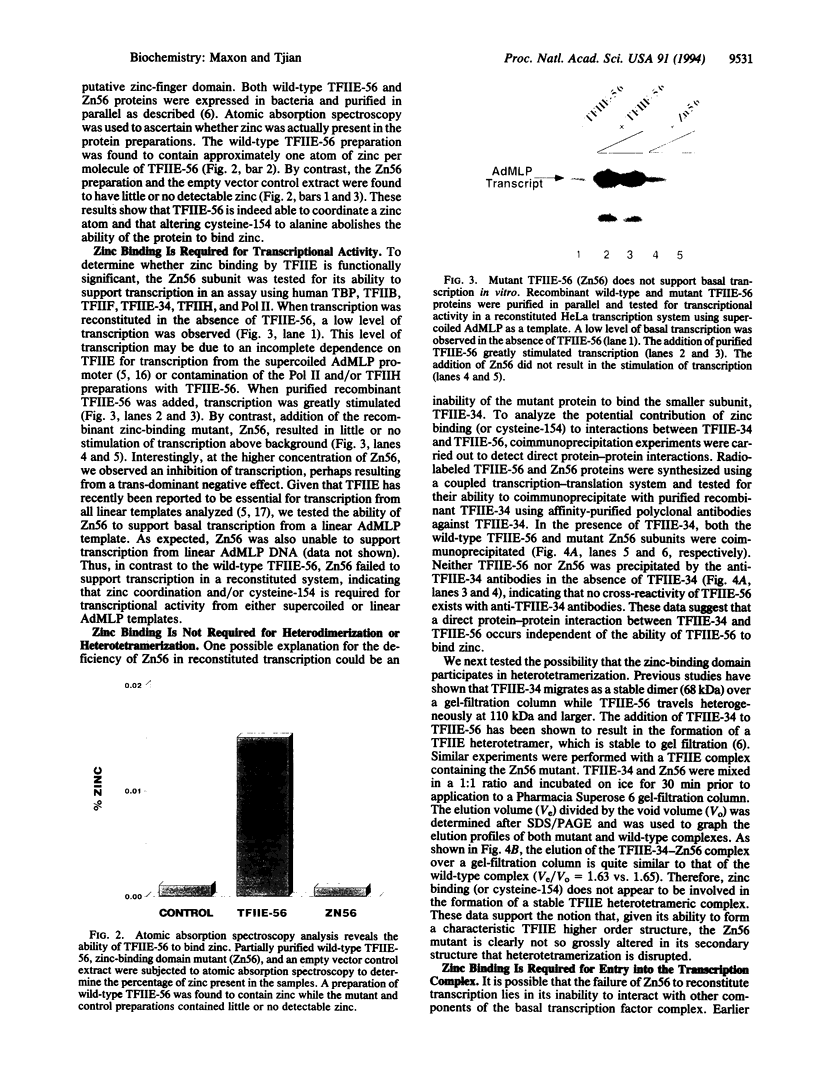
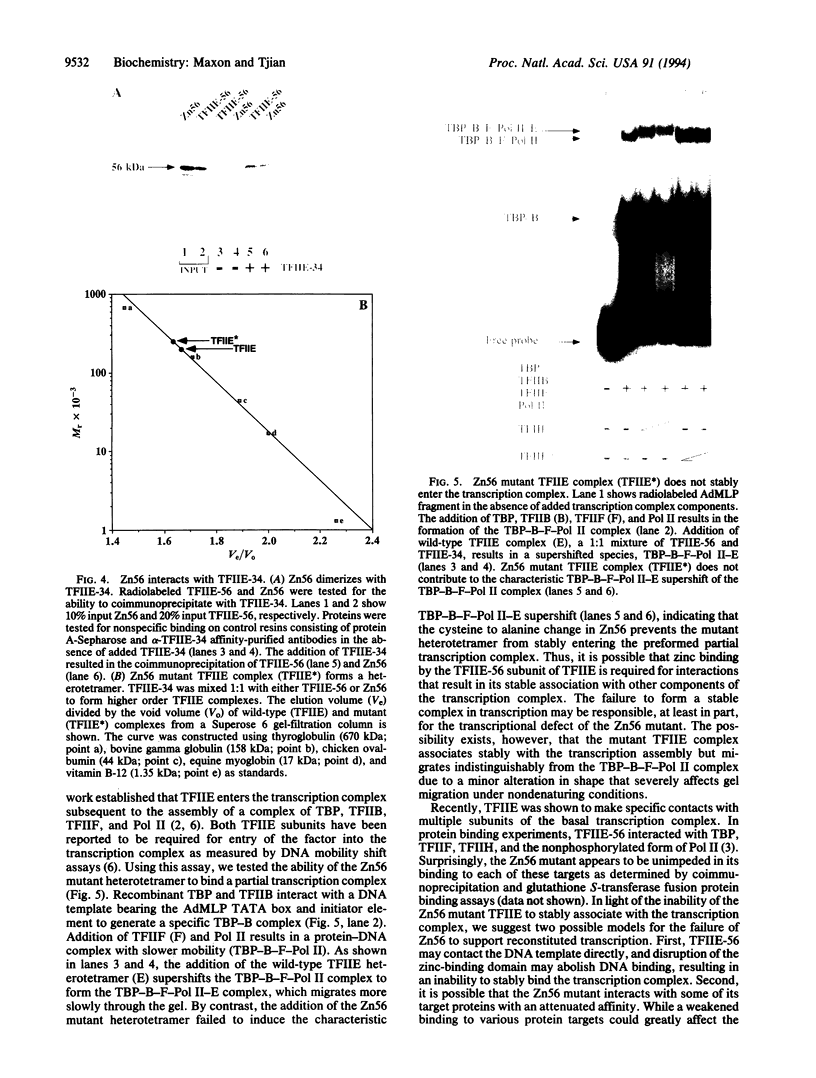
The functions of individual basal transcription factors during the formation of an initiation complex by RNA polymerase II remain largely unknown. Transcription factor IIE (TFIIE) has recently been shown to bind to multiple targets in the initiation complex. To assess the role of zinc binding in basal transcription, we have mutated the predicted zinc-finger domain of human TFIIE. Atomic absorption spectroscopy using purified recombinant proteins revealed that the large subunit, TFIIE-56, is indeed a zinc-binding protein. Mutation of a cysteine residue in the putative zinc-finger domain abolished zinc binding. Moreover, mutant TFIIE-56 failed to support reconstituted basal transcription in vitro, suggesting that zinc binding is required for TFIIE function. However, gel-filtration experiments and protein affinity experiments suggest that mutant TFIIE-56 forms a stable heterotetramer with the small subunit, TFIIE-34, that is similar to wild type. Interestingly, gel mobility shift experiments reveal that loss of transcriptional activity by mutant TFIIE is correlated with its inability to stably assemble into the transcription complex. These findings establish that zinc binding by TFIIE may help form a specific structure that is required for stable entry into the transcription complex.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aso T., Vasavada H. A., Kawaguchi T., Germino F. J., Ganguly S., Kitajima S., Weissman S. M., Yasukochi Y. Characterization of cDNA for the large subunit of the transcription initiation factor TFIIF. Nature. 1992 Jan 30;355(6359):461–464. doi: 10.1038/355461a0. [DOI] [PubMed] [Google Scholar]
- Berg J. M. Zinc fingers and other metal-binding domains. Elements for interactions between macromolecules. J Biol Chem. 1990 Apr 25;265(12):6513–6516. [PubMed] [Google Scholar]
- Coleman J. E. Zinc proteins: enzymes, storage proteins, transcription factors, and replication proteins. Annu Rev Biochem. 1992;61:897–946. doi: 10.1146/annurev.bi.61.070192.004341. [DOI] [PubMed] [Google Scholar]
- Drapkin R., Reardon J. T., Ansari A., Huang J. C., Zawel L., Ahn K., Sancar A., Reinberg D. Dual role of TFIIH in DNA excision repair and in transcription by RNA polymerase II. Nature. 1994 Apr 21;368(6473):769–772. doi: 10.1038/368769a0. [DOI] [PubMed] [Google Scholar]
- Finkelstein A., Kostrub C. F., Li J., Chavez D. P., Wang B. Q., Fang S. M., Greenblatt J., Burton Z. F. A cDNA encoding RAP74, a general initiation factor for transcription by RNA polymerase II. Nature. 1992 Jan 30;355(6359):464–467. doi: 10.1038/355464a0. [DOI] [PubMed] [Google Scholar]
- Flores O., Lu H., Reinberg D. Factors involved in specific transcription by mammalian RNA polymerase II. Identification and characterization of factor IIH. J Biol Chem. 1992 Feb 5;267(4):2786–2793. [PubMed] [Google Scholar]
- Goodrich J. A., Tjian R. Transcription factors IIE and IIH and ATP hydrolysis direct promoter clearance by RNA polymerase II. Cell. 1994 Apr 8;77(1):145–156. doi: 10.1016/0092-8674(94)90242-9. [DOI] [PubMed] [Google Scholar]
- Ha I., Lane W. S., Reinberg D. Cloning of a human gene encoding the general transcription initiation factor IIB. Nature. 1991 Aug 22;352(6337):689–695. doi: 10.1038/352689a0. [DOI] [PubMed] [Google Scholar]
- Inostroza J., Flores O., Reinberg D. Factors involved in specific transcription by mammalian RNA polymerase II. Purification and functional analysis of general transcription factor IIE. J Biol Chem. 1991 May 15;266(14):9304–9308. [PubMed] [Google Scholar]
- Kunkel T. A., Roberts J. D., Zakour R. A. Rapid and efficient site-specific mutagenesis without phenotypic selection. Methods Enzymol. 1987;154:367–382. doi: 10.1016/0076-6879(87)54085-x. [DOI] [PubMed] [Google Scholar]
- Lu H., Flores O., Weinmann R., Reinberg D. The nonphosphorylated form of RNA polymerase II preferentially associates with the preinitiation complex. Proc Natl Acad Sci U S A. 1991 Nov 15;88(22):10004–10008. doi: 10.1073/pnas.88.22.10004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maxon M. E., Goodrich J. A., Tjian R. Transcription factor IIE binds preferentially to RNA polymerase IIa and recruits TFIIH: a model for promoter clearance. Genes Dev. 1994 Mar 1;8(5):515–524. doi: 10.1101/gad.8.5.515. [DOI] [PubMed] [Google Scholar]
- Mazur S. J., Grossman L. Dimerization of Escherichia coli UvrA and its binding to undamaged and ultraviolet light damaged DNA. Biochemistry. 1991 May 7;30(18):4432–4443. doi: 10.1021/bi00232a009. [DOI] [PubMed] [Google Scholar]
- Parvin J. D., Sharp P. A. DNA topology and a minimal set of basal factors for transcription by RNA polymerase II. Cell. 1993 May 7;73(3):533–540. doi: 10.1016/0092-8674(93)90140-l. [DOI] [PubMed] [Google Scholar]
- Peterson M. G., Inostroza J., Maxon M. E., Flores O., Admon A., Reinberg D., Tjian R. Structure and functional properties of human general transcription factor IIE. Nature. 1991 Dec 5;354(6352):369–373. doi: 10.1038/354369a0. [DOI] [PubMed] [Google Scholar]
- Peterson M. G., Tanese N., Pugh B. F., Tjian R. Functional domains and upstream activation properties of cloned human TATA binding protein. Science. 1990 Jun 29;248(4963):1625–1630. doi: 10.1126/science.2363050. [DOI] [PubMed] [Google Scholar]
- Qian X., Gozani S. N., Yoon H., Jeon C. J., Agarwal K., Weiss M. A. Novel zinc finger motif in the basal transcriptional machinery: three-dimensional NMR studies of the nucleic acid binding domain of transcriptional elongation factor TFIIS. Biochemistry. 1993 Sep 28;32(38):9944–9959. doi: 10.1021/bi00089a010. [DOI] [PubMed] [Google Scholar]
- Sawadogo M., Roeder R. G. Factors involved in specific transcription by human RNA polymerase II: analysis by a rapid and quantitative in vitro assay. Proc Natl Acad Sci U S A. 1985 Jul;82(13):4394–4398. doi: 10.1073/pnas.82.13.4394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seeberg E., Steinum A. L. Purification and properties of the uvrA protein from Escherichia coli. Proc Natl Acad Sci U S A. 1982 Feb;79(4):988–992. doi: 10.1073/pnas.79.4.988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyree C. M., George C. P., Lira-DeVito L. M., Wampler S. L., Dahmus M. E., Zawel L., Kadonaga J. T. Identification of a minimal set of proteins that is sufficient for accurate initiation of transcription by RNA polymerase II. Genes Dev. 1993 Jul;7(7A):1254–1265. doi: 10.1101/gad.7.7a.1254. [DOI] [PubMed] [Google Scholar]
- Zawel L., Reinberg D. Initiation of transcription by RNA polymerase II: a multi-step process. Prog Nucleic Acid Res Mol Biol. 1993;44:67–108. doi: 10.1016/s0079-6603(08)60217-2. [DOI] [PubMed] [Google Scholar]