Abstract
Introduction
Among individuals with schizophrenia, those who have persistent and clinically significant negative symptoms (PNS) have the poorest functional outcomes and quality of life. The NIMH-MATRICS Consensus Statement indicated that these symptoms represent an unmet therapeutic need for large numbers of individuals with schizophrenia. No psychosocial treatment model addresses the entire constellation of PNS.
Method
51 patients with PNS were randomized into one of two groups for a period of 9 months: 1) MOtiVation and Engagement (MOVE) or 2) Treatment as usual. MOVE is a home based, manual-driven, multi-modal treatment that employs a number of cognitive and behavioral principles to address the broad range of factors contributing to PNS and their functional consequences. Components of MOVE include: Environmental supports to prompt initiation and persistence, in-vivo skills training to ameliorate deficits and encourage interaction, cognitive behavioral techniques to address self-defeating attitudes, in-vivo training in emotional processing to address affective blunting and problems in identifying emotions, and specific techniques to address the deficits in anticipatory pleasure. Patients were assessed at baseline and each 3 months with multiple measures of negative symptoms.
Results
Repeated measures analyses of variance for mixed models indicated significant Group by Time effects for the Negative Symptom Assessment (NSA; p<.02) and the Clinical Assessment Interview for Negative Symptoms (CAINS p<.04). Group differences were not significant until 9 months of treatment and were not significant for the Brief Negative Symptom Scale (BNSS).
Conclusion
Further investigation of a comprehensive treatment for PNS, such as MOVE, is warranted.
Keywords: schizophrenia, negative symptoms, Motivation and Enhancement Training (MOVE), Negative Symptom Assessment, Clinical Assessment Interview for Negative Symptoms
Introduction
The negative symptoms of schizophrenia are major contributors to lost productivity, poor quality of life, social deficits, poor occupational attainment and generally poor outcomes (Buchanan, 2007b; Kirkpatrick et al., 2006; Kirkpatrick et al., 2001; Kurtz et al., 2005; Milev et al., 2005). Dimensions of negative symptoms include restricted affect, diminished emotional range, poverty of speech, decreased motivation and interests, diminished sense of purpose and diminished social drive. In contrast to the positive symptoms of schizophrenia, negative symptoms are more difficult to treat and often persist long after positive symptoms have resolved or been substantially reduced (Buchanan, 2007a). Negative symptoms have been found to be more predictive of concurrent and future functioning in the community than positive symptoms (Breier et al., 1991; Ho et al., 1999; Milev et al., 2005; Mueser et al., 1990; Velligan et al., 1997). Recent factor analyses of many negative symptom instruments are composed of 2 factors, emotion expression and anhedonia/amotivation (CAINS; Forbes et al., 2010; BNSS; Kirkpatrick et al., 2011.)
The NIMH-MATRICS Consensus Statement on Negative Symptoms indicated that persistent negative symptoms (PNS) are a distinct therapeutic indication and represent an unmet therapeutic need for large numbers of individuals with schizophrenia (Alphs, 2006; Buchanan, 2007b; Kirkpatrick et al., 2006). According to the 2009 update of the Schizophrenia Patient Outcomes Research Team (PORT) Treatment Recommendations (Kreyenbuhl et al., 2010) there is not sufficient evidence to recommend any current pharmacologic agent for the treatment of deficit or persistent negative symptoms in schizophrenia. With respect to psychosocial treatments, recent work on CBT has demonstrated improvements in measures of negative symptoms (Riggs et al., 2012). Studies of Cognitive Adaptation Training (the use of environmental supports to bypass cognitive and motivational problems underlying functional impairment) have demonstrated improvements on the Motivation factor of the Negative Symptom Assessment (Alphs et al., 1989; Velligan et al., 2000a; Velligan et al., 2008a, 2008b), with effect sizes in the moderate range. However, with respect to both CBT and CAT, design features essential to prove efficacy for PNS have not been followed (Buchanan, 2007).
To address the need for novel treatments we developed MOtiVation and Engagement (MOVE) Training. MOVE is based upon techniques from a variety of interventions that each address a piece of the negative syndrome presentation. Our theoretical model of negative symptoms supporting the MOVE intervention has previously been published (Velligan et al., 2014). Briefly, negative symptoms are thought to be related to disruptions in ventral striatal reward systems (Goldstein and Volkow, 2002; Juckel et al., 2006; Wise, 1982). Beck et al. (2009) have proposed that negative symptoms may emerge during the early experience of psychosis as a psychological defense against experiencing distress beyond one’s capacity to cope. Once negative symptoms are present, our model proposes that the avolition leads to increasing difficultly for an individual to initiate action (Frith, 1992; Maples and Velligan, 2008), if prompted to do something or if able to generate a plan, negative cognitions about the possibility of failure may prevent the individual from executing or persisting at the behavior (Beck et al., 2009; Granholm et al., 2009). Moreover, deficits in anticipatory pleasure may prevent the individual from perceiving that they will enjoy the activity sufficiently to make it worth the effort (Gard et al., 2007). Negative thoughts as well as the atrophy of previously mastered social and work skills, may make failure more likely when a behavior is attempted (Bellack, 2004), and repeated negative consequences following the initiation of various activities may further prevent initiation (Beck et al., 2009).
MOtiVation and Enhancement Training (MOVE)
MOVE is a home-based manual driven treatment described in detail in the Manual available upon request from the first author (Velligan et al., 2014). Procedures include an initial assessment of negative symptoms, basic cognition, defeatist attitudes, and social skills completed during the first month and ½ of treatment. MOVE treatment plans are developed in a collaborative manner with the client. Interventions are, agreed upon, explained, maintained and altered as necessary on weekly visits from a MOVE trainer. MOVE sessions are conducted in person’s home or community (e.g restaurants, stores, activities) and last for approximately 1.25 hours once weekly. Conducting sessions in the clients environment reduces the demand for generalizability and has been found to be extremely effective in improving adaptive behavior (Velligan et al., 2000a; 2008a; 2008b,Velligan et al., 2009). Homework assignments to practice or do specific activities are often suggested.
MOVE includes 5 companion interventions, antecedent control, anticipatory pleasure, emotional processing and expression, CBT to address self-defeating thoughts and skill building. These interventions build upon one another to address both the emotion expression and anhedonia/amtovation domains of negative symptoms. These 5 components are discussed below.
Antecedent Control involves the use of environmental supports such as signs, alarms, and the appropriate placement of supplies to cue specific behaviors in the home environment. This aspect of treatment allows small behavioral changes with initial success that forms the foundation for later changes. When behavior is externally cued (e.g. “call Susan about going out for coffee”), the individual does not have to generate an idea or plan. (Velligan et al., 2009b; Velligan et al., 2000; Velligan et al., 2008a). Once the behavior is initiated, ensuring clear behavioral goals by outlining the process through a step-by-step checklist or an audio tape, and decreasing the number of steps required to complete each task increases the likelihood that a task will be successfully completed. As pointed out by Gard and colleagues (2014) and Strauss and colleagues (2014) motivational deficits are a major contributor to negative symptoms. External cues are designed to make behaviors more automatic, (answering a ringing phone) bypassing motivational impairments (Velligan, 2012). In addition, by making the task easier with a decreased number of steps, the perceived effort is reduced. Cues are also used throughout treatment to prompt homework assignment.
Anticipatory Pleasure—Deficits in anticipatory pleasure prevent the individual from perceiving that they will enjoy an activity sufficiently to make it worth the effort, leading to deficits in initiating conversations and social and leisure activities (Gard et al., 2007). In treatment, the degree of pleasure anticipated by the client prior to an activity is elicited on a 10 point scale. The MOVE trainer accompanies the person to the activity and then the client rates the enjoyment during the actual activity. The discrepancy between what the patient anticipated and the level of enjoyment actually experienced enables a discussion of how this problem may interfere with planning activities that may improve the quality of life. Ratings, client’s statements of enjoyment during activities and pictures are all saved to visual or digital media which show the entire process from anticipation through enjoyment with a cue to plan the next activity.
Emotional Processing and Expression—For clients who have difficulty identifying and expressing their own feeling states, MOVE therapists use a series of graded check-ins in which principles of errorless learning and physiological attunement help clients to develop increasingly fine-grained ability to label their own emotions. To address problems in identifying other’s emotion in voice tone and facial expression, computerized emotion perception exercises are adapted from existing social cognition training programs to help individuals to identify the specific facial expressions and voice modulations associated with different emotions and to help the client to make better judgments about the feeling states of others (Roberts and Penn, 2006). To generalize basic emotion processing skills to real life, emotions are explicitly discussed during and immediately following various work, leisure, and social activities. Photographs and tape recorded segments of conversation are used to illustrate various emotions that were displayed during these activities. Clients also receive coaching in how to use expression of emotion in conversation. Practice in front of a mirror, and video and audio recordings of facial expression and voice pitch changes are used to train expression of internal feeling states. Compensatory skills taught in MOVE to help with eliciting the emotions of others and expression include reminder cards to prompt clients to ask others, “Are you angry?” or “Are you enjoying this?” and to prompt them to use expressive skills previously taught, “Show your excitement in your voice”. Additional strategies used to address emotion processing difficulties in longer-term relationships include coaching clients to disclose difficulties (e.g. in hearing pitch differences that identify emotion or difficulties with expression) with requests by the patient asking that others communicate verbally about their feelings and remind the patient to “show it on your face.”
CBT to address self-defeating thoughts—Negative cognitions that prevent initiation and engagement are actively addressed using CBT techniques as described by (Beck et al., 2009).
Skill Building—Skill building techniques for social and independent living tasks use role plays to increase the likelihood of success when initiating unfamiliar tasks (Bellack, 2004; Liberman et al., 2002). Specific areas relevant to the client’s day-to-day experience are the focus (e.g. returning an item to a store,). Steps are written out and rehearsed. Behavioral techniques such as over-learning, modeling, and shaping are used.
In sum, these five intervention techniques work together to improve initiation, enjoyment, success, and improvement in adaptive behaviors, and address both higher order factors associated with negative symptoms emotion expression and anhedonia/amotivation. MOVE techniques lead to a positive feedback loop in which there is an increased willingness to initiate novel behavior, increased opportunity to experience situations likely to provoke joy, anxiety, pride, and other emotions, and increased willingness to follow through with plans in the present and to make plans for the future. The mechanisms of action of move are explained in more detail in another paper (Velligan et al…..).
We conducted a randomized, rater-blinded trial examining MOVE compared to treatment as usual in a sample of 51 individuals meeting criteria for severe and persistent negative symptoms.
Methods
Study Design
Outpatients with schizophrenia/schizoaffective disorder meeting eligibility criteria were followed for 1 month to ensure stability of negative symptoms, positive symptoms, depression and movement side effects. After this screening period, if participants continued to meet all eligibility criteria, they continued with medication follow-up provided by their community health center and were randomly assigned to one of two treatment groups 1) MOVE or 2) treatment as usual (TAU) for a period of 9 months. Patients were assessed at 3, 6, and 9 months following randomization with multiple measures of negative symptoms.
Participants
Eligibility criteria for the study were set up according to recommendations of the MATRICS Consensus Statement and follow-up articles (Buchanan et al., 2005, 2011; Nuechterlein, Green, Kern, 2008). Participants were 92 outpatients with schizophrenia from community clinics in Bexar and Travis Counties in Texas. They were recruited from July 2011 to January 2014.
In addition to meeting diagnostic criteria, participants were required to have been clinically stable with respect to positive symptoms, negative symptoms, depression and movement side effects for a minimum of 5 months prior to screening (based upon chart review and clinical interview), have clinically meaningful negative symptoms (as evidenced by a score 4 or higher on at least 2 of 6 of the following symptom domains as rated by the NSA-16 (restricted affect, diminished emotional range, poverty of speech, curbing of interest, diminished sense of purpose, and diminished social drive), have no more than moderate positive symptoms (as evidenced by a score of 5 or lower on BPRS-E items measuring delusions (unusual thought content) and hallucinations and no more than a 4 or higher on conceptual disorganization), have no more than mild depression (as evidenced by a score of 4 or lower on the BPRS depression item), have no significant movement disorder (as evidenced by a score of 3 or lower on the ESRS-A), and be able to provide evidence of a stable living environment with no plans to move in the next year. Participants had to continue to meet eligibility criteria after a one-month prospective period prior to randomization. We excluded individuals with a documented history of significant head trauma, seizure disorder, or mental retardation, alcohol or drug abuse or dependence within the past 3 months or a history of violence in the past one year period.
Subjects were identified through chart reviews by research staff credentialed at participating sites in accordance with HIPAA requirements. All participants signed a written consent form approved by an Institutional Review Board and procedures were consistent with internationally recognized standards for ethical conduct of human research.
Of the 94 individuals screened, 51 were eligible for randomization based upon stability criteria. 34 subjects were male and 17 were female. 25 were Hispanic, 12 were Anglo, and 14 were African-American. Mean age of participants was 41.6 (S.D. 11.3). All patients were on second generation antipsychotic medications. At baseline, mean negative symptoms were in the mild range, while specific symptoms had to be scored in at least the moderate range (NSA M=3.05 S.D. =0.62). There were no significant differences in demographics for participants not making it to randomization versus those randomized to treatment (all p’s >.20).
Treatment Groups
MOVE treatment is described above. MOVE therapists were trained using a serious of didactic and in vivo sessions at patient homes. Supervision was conducted weekly and all sessions were audiotape recorded. Twenty percent were assessed with a MOVE fidelity measure. Treatment as usual which included medication follow-up and case management provided by the local community mental health authority
Assessments
Diagnosis
The Structured Clinical Interview for the Diagnostic and Statistical Manual for Mental Disorders were utilized to make DSM-IV diagnoses. Prior to administering this interview, all raters were trained to a reliability of .95 Kappa statistics for a diagnosis of schizophrenia or schizoaffective disorder versus all other diagnoses.
Negative Symptoms
Negative symptoms were rated using the Negative Symptom Assessment (NSA-16; Axelrod, Goldman, Alphs, 1989), the Clinical Assessment Interview for Negative Symptoms (CAINS; Forbes et al., 2010) and the Brief Negative Symptom Scale (BNSS; Kirkpatrick et al., 2011). Higher scores indicate a higher level of negative symptoms on all scales. The NSA was the primary outcome measure for the study. Raters were trained to a reliability of .80 intraclass correlation coefficient on all assessments before making ratings for the study and participated in regular rater drift meetings as recommended by (Ventura et al., 1993).
Treatment Blinds
In an effort to maintain treatment blinds, all participants were asked at the beginning of each assessment neither to divulge information about any visits made by staff of the research project nor to refer to any items they may have received as part of the study. If blinds were broken, alternative raters blind to group assignment completed the remaining assessments.
Data Analysis
We examined group differences in negative symptoms (NSA, CAINS, BNSS) over time (3, 6, 9, months) by treatment group (MOVE, Treatment as Usual) using mixed effects regression with repeated measures (SAS PROC MIXED). Baseline scores were used as covariates in the model. To investigate whether statistically significant effects were clinically meaningful, effect sizes were calculated utilizing the standard deviation for the outcome variable in the control group pooled over time.
Results
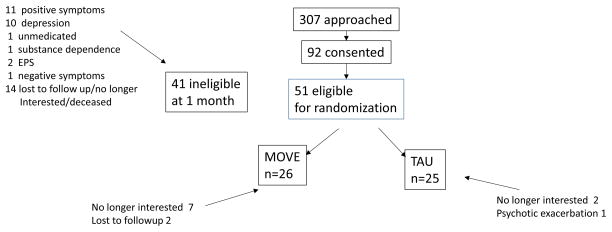
Table 2, presents the demographic and baseline variables by treatment group. There were no statistically significant group differences with respect to demographic or baseline data. The consort diagram appears in Figure 1. There was a trend for individuals in MOVE to be more likely to drop out of the study ((X2(1) =3.36; p<.06). During the active treatment phase, 9 of 26 individuals dropped in MOVE treatment and 3 out 25 dropped in TAU.
Table 2.
Baseline Characteristics by Treatment Group
| Treatment As Usual (n=25) | MOVE n = (26) | |
|---|---|---|
| % male | 68.00 (n=17) | 65.38 (n=17) |
| % Hispanic | 44.00 (n=11) | 53.85 (n=14) |
| % Non-Hispanic White | 28.00 (n=7) | 19.23 (n=5) |
| Age | 42.32(SD=11.10) | 41.04(SD=11.48) |
| Education | 11.74(SD=2.26) | 12.27(SD=1.51) |
| BPRS Psychosis Factor | 3.04(SD=1.10) | 2.65(SD=0.86) |
| Negative Symptom Assessment | 2.75(SD=0.92) | 2.37(SD=0.75) |
Figure 1.
Subject recruitment and retention
Negative Symptom Outcomes
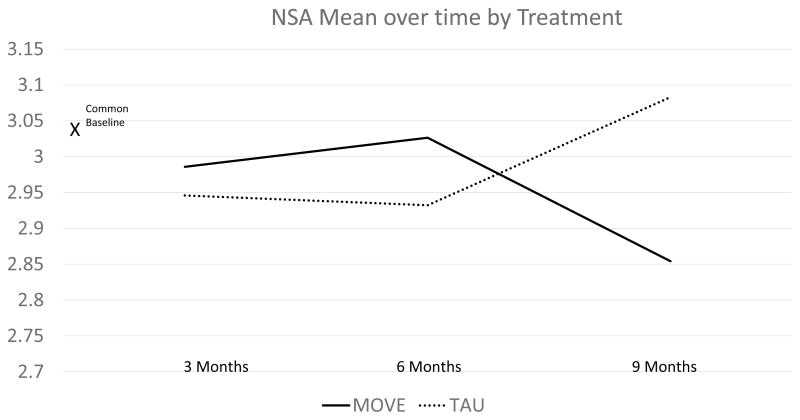
The mixed effects regression model examining the NSA mean score yielded no significant effects of group (F (1,38)=.06 p<.80) and time (F(2,76)=0.03 p<.97) and a significant group by time interaction (F(2,76) =4.09 p<.02) indicating that the effects of the treatment differed by group over time. An inspection of means indicates improvement in MOVE and worsening of negative symptoms in TAU. Figure 2 depicts estimated means derived from the regression model at specified time points by treatment group.
Figure 2.
Differences were primarily noted at the 9 month time point at the end of treatment. The effect size at 9 months for MOVE compared to treatment as usual was .5. According to Cohen’s conventions, this represents a moderate treatment effect. To examine whether domains of negative symptoms differed, we conducted the same analysis in a post hoc fashion for the NSA factor scores. Results indicated that there were strong trends for group by time only for socialization and motivation factors (P<.06). There were no trends nor significant effects for emotion, psychomotor speed, or communication (all p’s>.20).
In secondary analyses, we also examined negative symptoms as rated by two newer negative symptom assessments the CAINS and the BNSS. With respect to the CAINS, the mixed effects regression model yielded non-significant main effects of group (F(1,39)=0.23; P<.63 and time (F(2,77)=0.35; p>.70) and a significant group by time interaction (F(1,77)=3.30; p<.05). Patients in MOVE had significantly fewer negative symptoms, again this effect was most pronounced a 9 months. The effect size for end of treatment for MOVE versus TAU was .5. Post-hoc analyses revealed significant group differences for recreation (p<.02) and sociality (p<.03) only, With respect to the BNSS, the mixed effects regression model yielded non-significant effects of group, time and group by time (all F’s <2.1; all p’s>.14).
Discussion
Findings indicated that MOVE potentially has a positive impact on negative symptoms for patients meeting criteria described in guidelines for trials specifically designed to test treatments that improve negative symptoms. This is the first study to date that has demonstrated improvement in negative symptoms while utilizing the strict entry criteria recommended by the MATRICS negative symptom initiative (Buchanan et al., 2005). While previous studies of CBT for negative symptoms have demonstrated improvements, the criteria for entry were not sufficient to eliminate pseudo specificity in which negative symptoms improve as a consequence of improvements in other symptoms. That being said, effects were very modest in this pilot study, and were driven by both improvement in the MOVE group and worsening in the TAU group. Moreover, not all domains of negative symptoms were impacted as hypothesized.
While it did not reach statistical significance, more patients in MOVE decided to drop out of the treatment which was designed to get them more engaged in life. It makes sense that individuals with high levels of these symptoms would find it difficult to participate in an activating program. Including additional incentives may be needed to help individuals with long histories of withdrawal to re-engage. This difficulty in engaging individuals is evident in our recruiting success as well. In our previous studies not designed for negative symptoms, nearly 50% agreed to participate (Velligan et al., 2008b and 2009b). In the current study we had less than a 33% success rate in consenting.
The screen failure rate of 44% after a month is also a concern. This rate is slightly higher than that of pharmaceutical trials in the same population with similar entry criteria (Buchanan et al., 2007; Buchanan et al., 2012; Umbricht et al., 2014). The primary reasons for screen failure at one month were inability to contact the patient and increases in positive symptoms such that the patient no longer met entry criteria. Negative symptoms remained stable in all but one patient who were initially included.
It is possible that MOVE would have a more substantial impact upon negative symptoms if it were used in conjunction with a pharmacotherapy that targeted PNS. Currently, no such medication is available, but clinical trials to address this unmet clinical need are in process. The synergy between psychopharmacological and psychosocial treatments may be needed to make a clinically significant difference in negative symptoms.
The impact of MOVE was principally on motivation and social engagement. Although emotional expression was targeted we did not find a significant impact of MOVE on these symptom dimensions. It is possible that more time is needed to impact these measures.
Significant group differences were found on the the NSA-16 and CAINS but not the BNSS. This may suggest that the former instruments are more sensitive to change. If replicated, these findings may have implications for choice of assessment methods in future studies for treatments of negative symptoms.
The findings in this study must be interpreted in light of a number of methodological weaknesses including the recruitment and screen failure rate discussed above. In addition, this was a pilot study with a relatively small sample of patients and more comprehensive measures outcome were not included.
Despite these limitations, the data suggest impact on a symptom complex which is very difficult to move in treatment. While the treatment studied here was labor intensive and took well trained master’s level clinicians, it may be cost effective if functional outcomes can be improved. Results justify further study of MOVE. Future studies should focus on combining MOVE with promising pharmacotherapies to address PNS in schizophrenia.
Table 1.
Specific Interventions for Negative Symptoms
| Negative Symptom Targeted | Intervention Strategy |
|---|---|
| Reduced daily activity | Use of a blank checklist to illustrate to the therapist and client what was actually being done each day. Discussion of the importance of a daily routine; discussion of the importance of increasing daily activity such that she could develop the initiation and stamina to persist with demand of employment; goal setting to determine appropriate daily activities including: medication regimes, hygiene schedule, household chores, exercise, and a leisure activity; Use of checklists, signs and an alarm to prompt these; Efforts to streamline steps to completing household tasks; initial incentives ($5 gift card to Walmart) for successful goal achievement with house-keeping tasks. |
| Reduced affective display and emotional range | Identification and processing of thoughts and emotions related to both reaching and failing to reach weekly goals; Practice identifying emotions in role plays with the therapist and in magazine pictures; Practice making specific facial expressions in a mirror. |
| Reduced social drive | Social skills training to include: making greetings, maintaining conversations, closing conversations, reading and demonstrating non-verbal communication, importance of initiation, and practice in assertive communication; goals set to initiate phone calls to family; goal to attend church and to practice greeting people following service |
| Reduced interests | Development of leisure skills; goals set to attend church regularly, therapist accompanied her on outings to craft store to select hobby supplies, regular visits to family using public transportation, and daily walking for exercise |
| Reduced sense of purpose | Identification of both short- and long-term goals; practice breaking down large goals into smaller steps; practice with goal-setting and goal modification when necessary |
Acknowledgments
We wish to thank the participants and staff from the Center for Health Care Services (Executive Director: Leon Evans) and Austin Travis County Integrated Care (Executive Director: David Evans) for their ongoing support of our research program.
The National Institute of Health was the sole provider of funds for this project, awarding Dawn Velligan, Ph.D the 3R34MH093483 grant.
Footnotes
Dr. Velligan was the principle investigator for this project; she wrote the protocol and designed the study along with Dr. Roberts. She also wrote the first draft of the manuscript. Dr. Mintz provided oversight of data management as well as supervision of statistical staff. He and Xueying Li conducted data analyses. Natalie Maples participated in study design and literature searches. Elisa Medellin participated in designing the study and conducted literature searches. Matt Brown contributed to the study design. All authors have contributed to and have approved the final manuscript.
There are no conflicts of interest to report.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Alphs L. An industry perspective on the NIMH consensus statement on negative symptoms. Schizophr Bull. 2006;32(2):225–30. doi: 10.1093/schbul/sbj056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alphs L, Summerfelt A, Lann H, Muller RJ. The negative symptom assessment: A new instrument to assess negative symptoms of schizophrenia. Psychopharmacol Bull. 1989;25(2):159–63. [PubMed] [Google Scholar]
- Axelrod BN, Goldman RS, Alphs LD. Validation of the 16-item negative symptom assessment. J Psychiatr Res. 1993;27(3):253–258. doi: 10.1016/0022-3956(93)90036-2. [DOI] [PubMed] [Google Scholar]
- Beck AT, Rector NA, Stolar N, Grant P. Schizophrenia: Cognitive Theory, Research, and Therapy. New York: The Guilford Press; 2009. [Google Scholar]
- Bellack AS, Mueser KT, Gingerich S, Agresta J. Social skills training for schizophrenia: A step-by-step guide. 2. New York: The Guilford Press; 2004. [Google Scholar]
- Breier A, Schreiber JL, Dyer J, Pickar D. National Institute of Mental Health longitudinal study of chronic schizophrenia: Prognosis and predictors of outcome. Arch Gen Psychiatry. 1991;48:239–46. doi: 10.1001/archpsyc.1991.01810270051007. [DOI] [PubMed] [Google Scholar]
- Buchanan R. Persistent negative symptoms in schizophrenia: An overview. Schizophr Bull. 2007;33(4):1013–22. doi: 10.1093/schbul/sbl057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchanan RW, Davis M, Goff D, et al. A summary of the FDA-NIMH-MATRICS workshop on clinical trial design for neurocognitive drugs for schizophrenia. Schizophr Bull. 2005;31:5–19. doi: 10.1093/schbul/sbi020. [DOI] [PubMed] [Google Scholar]
- Buchanan RW, Javitt DC, Marder SR, Schooler NR, Gold JM, McMahon RP, Heresco-Levy U, Carpenter WT. The Cognitive and Negative Symptoms in Schizophrenia Trial (CONSIST): The efficacy of glutamatergic agents for negative symptoms and cognitive impairments. Am J Psychiatry. 2007b;164(10):1593–1602. doi: 10.1176/appi.ajp.2007.06081358. [DOI] [PubMed] [Google Scholar]
- Buchanan RW, Keefe RSE, Umbricht D, et al. The FDA-NIMH-MATRICS guidelines for clinical trial design of cognitive-enhancing drugs: what do we know 5 years later? Schizophr Bull. 2011;37(6):1209–1217. doi: 10.1093/schbul/sbq038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchanan RW, Panagides J, Zhao J, Phiri P, den Hollander WM, Xianwei H, et al. Asenapine versus olanzapine in people with persistent negative symptoms of schizophrenia. Journal of clinical psychopharmacology. 2012;32(1):36–45. doi: 10.1097/JCP.0b013e31823f880a. [DOI] [PubMed] [Google Scholar]
- Cohen J. Statistical power for the behavioral sciences. 2. Lawrence Erlbaum; Hillsdale, NJ: 1988. [Google Scholar]
- Forbes C, Blanchard JJ, Bennett M, et al. Initial development and preliminary validation of a new negative symptom measure: the Clinical Assessment Interview for Negative Symptoms (CAINS) Schizophr Res. 2010;124(1–3):36–42. doi: 10.1016/j.schres.2010.08.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frith CD. The cognitive neuropsychology of schizophrenia. East Sussex, United Kingdom: Erlbaum Taylor & Francis; 1992. [Google Scholar]
- Gard DE, Kring AM, Germans Gard M, Horan WP, Green MF. Anhedonia in schizophrenia: Distinctions between anticipatory and consummatory pleasure. Schizophr Res. 2007;93:253–60. doi: 10.1016/j.schres.2007.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gard DE, Sanchez AH, Starr J, Cooper S, Fishers M, Rowlands A, Vinogradov S. Using self-determination theory to understand motivation deficits in schizophrenia: the “why” of motivated behavior. Schizophr Res. 2014;156:217–222. doi: 10.1016/j.schres.2014.04.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein R, Volkow N. Drug addiction and its underlying neurobiological basis: Neuroimaging evidence for the involvement of the frontal cortex. Am J Psychiatry. 2002;159(10):1642–52. doi: 10.1176/appi.ajp.159.10.1642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Granholm E, Ben-Zeev D, Link PC. Social disinterest attitudes and group cognitive-behavioral social skills training for functional disability in schizophrenia. Schizophr Bull. 2009;35(5):874–83. doi: 10.1093/schbul/sbp072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho B, Nopoulos P, Flaum M, Arndt S, Andreasen NC. Two-year outcome in first-episode schizophrenia: Predictive value of symptoms for quality of life. Am J Psychiatry. 1998;155:1196–1201. doi: 10.1176/ajp.155.9.1196. [DOI] [PubMed] [Google Scholar]
- Juckel G, Schlagenhauf F, Koslowski M, Wustenberg T, Villringer A, Knutson B, Wrase J, Hein A. Dysfunction of ventral striatal reward prediction in schizophrenia. Neuroimage. 2006;29:409–16. doi: 10.1016/j.neuroimage.2005.07.051. [DOI] [PubMed] [Google Scholar]
- Kirkpatrick B, Buchanan R, Ross D, Carpenter W. A separate disease within the syndrome of schizophrenia. Arch Gen Psychiatry. 2001;58(2):165–71. doi: 10.1001/archpsyc.58.2.165. [DOI] [PubMed] [Google Scholar]
- Kirkpatrick B, Fenton WS, Carpenter WT, Jr, Marder SR. The NIMH-MATRICS consensus statement on negative symptoms. Schizophr Bull. 2006;32(2):214–219. doi: 10.1093/schbul/sbj053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirkpatrick B, Hack G, Higginbottom E, Hoffacker D, Fernandez-Egea E. Palate and dentition in schizophrenia. Schizophr Res. 2007;91(1–3):187–91. doi: 10.1016/j.schres.2006.12.022. [DOI] [PubMed] [Google Scholar]
- Kirkpatrick B, Strauss GP, Nguyen L, et al. The Brief Negative Symptom Scale: Psychometric Properties. Schizophr Bull. 2011;37(2):300–305. doi: 10.1093/schbul/sbq059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreyenbuhl J, Buchanan RW, Dickerson FB, Dixon LB Schizophrenia Patient Outcomes Research Team (PORT) The schizophrenia patient outcomes research team (PORT): updated treatment recommendations 2009. Schizophr Bull. 2009;36(1):94–103. doi: 10.1093/schbul/sbp130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurtz MM, Moberg PJ, Ragland JD, Gur RC, Gur RE. Symptoms versus neurocognitive test performance as predictors of psychosocial status in schizophrenia: A 1- and 4-year prospective study. Schizophr Bull. 2005;31(1):167–74. doi: 10.1093/schbul/sbi004. [DOI] [PubMed] [Google Scholar]
- Liberman RP, Glynn SM, Blair KE, Ross D, Marder SR. In vivo amplified skills training: promoting generalization of independent living skills for clients with schizophrenia. Psychiatry. 2002;65(2):137–55. doi: 10.1521/psyc.65.2.137.19931. [DOI] [PubMed] [Google Scholar]
- Maples NJ, Velligan DI. Cognitive adaptation training: Establishing environmental supports to bypass cognitive deficits and improve functional outcomes. Am J Psychiatr Rehabil. 2008;11(2):164–80. [Google Scholar]
- Milev P, Ho B, Arndt S, Andreasen N. Predictive values of neurocognition and negative symptoms on functional outcome in schizophrenia: A longitudinal first-episode study with 7 year follow-up. Am J Psychiatry. 2005;162(3):495–506. doi: 10.1176/appi.ajp.162.3.495. [DOI] [PubMed] [Google Scholar]
- Mueser KT, Bellack AS, Morrison RL, Wixted JT. Social Competence in schizophrenia: Premorbid adjustment, social skill, and domains of functioning. J Psychiatr Res. 1990;24:51–63. doi: 10.1016/0022-3956(90)90024-k. [DOI] [PubMed] [Google Scholar]
- Nuechterlein KH, Green MF, Kern RS, et al. The MATRICS Consensus Cognitive Battery: part 1. Test selection, reliability, and validity. Am J Psychiatry. 2008;165:203–213. doi: 10.1176/appi.ajp.2007.07010042. [DOI] [PubMed] [Google Scholar]
- Riggs SE, Grant PM, Perivoliotis D, Beck AT. Assessment of cognitive insight: a qualitative review. Schizophr Bull. 2012;38(2):338–350. doi: 10.1093/schbul/sbq085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts DL, Penn DL. Social cognition and interaction training (SCIT) for outpatients with schizophrenia: a preliminary study. Psychiatry Res. 2009;166(23):141–147. doi: 10.1016/j.psychres.2008.02.007. [DOI] [PubMed] [Google Scholar]
- Strauss GP, Waltz JA, Gold JM. A review of reward processing and motivational impairment in schizophrenia. Schizophr Bull. 2014;40(S2):107–116. doi: 10.1093/schbul/sbt197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Umbricht D, Alberati D, Martin-Facklam M, Borroni E, et al. Effect of bitopertin, a glycine reuptake inhibitor, on negative symptoms of schizophrenia: a randomized, double-blind, proof of concept study. JAMA Psychiatry. 2014;71(6):637–646. doi: 10.1001/jamapsychiatry.2014.163. [DOI] [PubMed] [Google Scholar]
- Velligan DI. Cognitive adaptation training: Targeted use of automatic and controlled processes to improve functional outcomes 2012. Schizophrenia Research. 136(S!):S375. [Google Scholar]
- Velligan DI, Diamond PM, Mueller J, Xueying L, Maples NJ, Wang M, Miller AL. The short term impact of generic versus individualized environmental supports on functional outcomes and target behaviors in schizophrenia. Psychiatry Res. 2009b;168(2):94–101. doi: 10.1016/j.psychres.2008.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Velligan DI, Diamond PM, Maples NJ, Mintz J, Li X, Glahn DC, Miller AL. Comparing the efficacy of interventions that use environmental supports to improve outcomes in patients with schizophrenia. Schizophr Res. 2008a;102:312–19. doi: 10.1016/j.schres.2008.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Velligan DI, Diamond PM, Mintz J, Maples N, Li X, Zeber J, Ereshefsky L, Lam YW, Castillo D, Miller AL, Maples NJ. The use of individually tailored environmental supports to improve medication adherence and outcomes in schizophrenia. Schizophr Bull. 2008b;34(3):483–93. doi: 10.1093/schbul/sbm111. http://schizophreniabulletin.oxfordjournals.org/cgi/reprint/34/3/483 http://www.ncbi.nlm.nih.gov/pubmed/17932089?dopt=Abstract. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Velligan DI, Bow-Thomas CC, Huntzinger CD, Ritch J, Ledbetter N, Prihoda TJ, Miller AL. Randomized controlled trial of the use of compensatory strategies to enhance adaptive functioning in outpatients with schizophrenia. Am J Psychiatry. 2000a;157(8):1317–23. doi: 10.1176/appi.ajp.157.8.1317. http://www.ncbi.nlm.nih.gov/pubmed/10910797?dopt=Abstract. [DOI] [PubMed] [Google Scholar]
- Velligan DI, Bow-Thomas CC, Mahurin RK, Miller AL, Halgunseth LC. Do specific neurocognitive deficits predict specific domains of community function in schizophrenia? J Nerv Ment Dis. 2000b;188(8):518–24. doi: 10.1097/00005053-200008000-00007. [DOI] [PubMed] [Google Scholar]
- Velligan DI, Mahurin RK, Giesecke SL, Miller AL. The functional significance of symptomatology and cognitive dysfunction in schizophrenia. Schizophr Res. 1997;25:21–31. doi: 10.1016/S0920-9964(97)00010-8. [DOI] [PubMed] [Google Scholar]
- Velligan DI, Maples N, Roberts DL, Medellin EM. Integrated psychosocial treatment for negative symptoms. Am J Psychiatr Rehabil. 2014;17(1):1–19. [Google Scholar]
- Ventura J, Green MF, Shaner A, Liberman RP. Training and quality assurance with the Brief Psychiatric Rating Scale: ‘The drift busters’. Int J Meth Psychiatr Res. 1993;3(4):221–244. [Google Scholar]
- Wise R. Neuroleptics and operant behavior: The anhedonia hypothesis. Behav Brain Sci. 1982;5(1):39–87. [Google Scholar]