Abstract
Background
Elevated intracranial pressure is one of the most common problems in patients with diverse intracranial disorders, leading to increased morbidity and mortality. Effective management for increased intracranial pressure is based mainly on surgical and medical techniques with hyperosmolar therapy as one of the core medical treatments. The study aimed to explore the effects of continuous micro-pump infusions of 3% hypertonic saline combined with furosemide on intracranial pressure control.
Material/Methods
We analyzed data on 56 eligible participants with intracranial pressure >20 mmHg from March 2013 to July 2014. The target was to increase and maintain plasma sodium to a level between 145 and 155 mmol/L and osmolarity to a level of 310 to 320 mOsmol/kg.
Results
Plasma sodium levels significantly increased from 138±5 mmol/L at admission to 151±3 mmol/L at 24 h (P<0.01). Osmolarity increased from 282±11 mOsmol/kg at baseline to 311±8 mOsmol/kg at 24 h (P<0.01). Intracranial pressure significantly decreased from 32±7 mmHg to 15±6 mmHg at 24 h (P<0.01). There was a significant improvement in CPP (P<0.01). Moreover, central venous pressure, mean arterial pressure, and Glasgow Coma Scale slightly increased. However, these changes were not statistically significant.
Conclusions
Continuous infusion of 3% hypertonic saline + furosemide is effective and safe for intracranial pressure control.
MeSH Keywords: Furosemide; Intracranial Pressure; Mannitol; Saline Solution, Hypertonic
Background
Intracranial hypertension is a common complication in many neurological diseases. Causes of intracranial hypertension include intracranial factors such as cerebral ischemia/hemorrhage, trauma, brain tumor, cerebral edema, and hydrocephalus, while extracranial factors include hypoxia, hypercarbia, hyperpyrexia, seizures, and drugs or their metabolites [1]. A series of measures are needed to control raised intracranial pressure (ICP) above 20 to 25 mmHg. The most commonly used approach to the treatment of ICP is medical management, including techniques such as sedation, hyperventilation, hypothermia, and hyperosmolar therapy, which is based on the generation of an osmotic concentration gradient between the blood and brain tissue, being the first-line treatment. In general, hypertonic saline, mannitol, and furosemide are the 3 most commonly used agents in the treatment of raised ICP. However, emergent surgical management should be considered in cases that are refractory to medical management.
Over the last 3 decades, mannitol has been the most widely used hyperosmolar dehydrating agent for the treatment of elevated ICP [2,3] due to its rheologic and osmotic effects. However, adverse effects such as the rebound effect, serum electrolyte imbalance, acute tubular necrosis, and renal failure, lead to certain restrictions in clinical practice [4]. In addition, previous studies have indicated that, following excessive infusion, mannitol may pass from the blood vessels into the brain tissue, causing further brain swelling and increased ICP.
In recent years, hypertonic saline (HS) concentrations ranging from 3% to 23.4% have been used with increasing frequency in the treatment of intracranial hypertension caused by various causes. Studies have shown that, HS, not mannitol, should be considered the gold-standard medical therapy for intracranial hypertension.[3,5–9]. Furthermore, administration of mannitol can further reduce body perfusion and blood pressure (BP) due to its diuretic effect; thus, in such circumstances HS has a clear advantage over mannitol. HS solutions can effectively lower ICP while preserving normal BP and cerebral perfusion pressure (CPP). Potential adverse effects associated with HS infusion include myelinolysis, acute heart or renal failure, acute pulmonary edema, and serum electrolyte imbalance [10].
Furosemide, a traditional diuretic agent, is also commonly used to reduce elevated ICP by reducing the water content and thus the brain bulk in neurological patients. Furosemide is usually used in combination with mannitol; however, to the best of our knowledge, few studies have explored the effects of micro-pump-infused HS combined with furosemide on ICP control. We hypothesized that co-administration of furosemide would enhance the ICP-lowering properties of HS infusion through a marked increase in serum osmolarity and a decrease in brain water content. The aim of the study was to retrospectively investigate the efficacy and safety of 3% HS + furosemide delivered via continuous micro-pump infusion for controlling elevated ICP.
Material and Methods
We performed a single-center retrospective study from March 2013 to July 2014 in the Neurological Intensive Care Unit (NICU) of the Fourth Military Medical University Tangdu Hospital, China. All procedures were approved by the Institutional Investigational Review board at the Fourth Military Medical University.
Patients
A total of 56 neurosurgical patients (26 males, 30 females) were enrolled in the study. All patients with ICP >20 mmHg were treated with continuous 3% HS + furosemide infusion adapted to a defined target of plasma sodium and osmolarity. All ICP values were acquired by ventricular ICP monitors (the ventricular catheter connected to an external strain gauge). The inclusion and exclusion criteria are summarized in Table 1.
Table 1.
Inclusion and exclusion criteria in the study.
| Inclusion criteria |
| 1. Patients with ventricular ICP monitors |
| 2. The value of ICP remained constantly >20 mmHg |
| 3. Age 18 years or older and less than 90 years |
| 4. Patients with central venous line |
| Exclusion criteria |
| 1. Hyponatremia or hypopotassium |
| 2. Clinical and radiological signs of brain herniation |
| 3. Patients with coagulopathy |
| 4. Initial plasma sodium >150 mmol/L and serum osmolarity >320 mosm/kg |
| 5. Heart failure, renal failure, or pulmonary edema |
| 6. Pregnancy, lactation, or parturition |
ICP – intracranial pressure.
General care
All patients received the standard intensive care management. Critically ill patients were kept in a semirecumbent position and patients with GCS <9 scores or a rapid deterioration in neurological status were mechanically ventilated. As agitation and pain may significantly increase BP and ICP, all patients were treated with midazolam (starting dose of 0.2 mg/kg/h, increased as needed) for sedation and fentanyl (starting dose of 2 μg/kg/h, increased as needed) for analgesia. If necessary, patients were treated with the neuromuscular blocking agent, vecuronium (0.1 mg/kg). We ensured normoglycemia and kept the body temperature between 36.0°C and 37.0°C. All patients were subjected to blood gas analysis at least once a day and expiratory end-tidal (Et) CO2 was continuously monitored to avoid hypoxemia, hypoventilation, or hyperventilation. All patients used invasive monitoring devices to measure arterial pressure. During the period of infusion, mean arterial pressure (MAP) was maintained at ≥70 mmHg and the CPP at ≥60 mmHg, as CPP=MAP-ICP. Systolic BP was maintained between 120 and 160 mmHg. To avoid hypervolemia or hypovolemia, a central venous catheter was needed to help evaluate volume status and maintain central venous pressure (CVP) of 4–12 cm H2O.
Study protocol
Continuous micro-pump administration of 3% HS solution and furosemide was performed when ICP value exceeded the 20 mmHg threshold. Infusion of 3% HS solution and furosemide was started at rates of 25 mL/h and 2 mg/h, respectively, with both agents administered simultaneously. Plasma sodium and serum osmolarity levels were tested at least every 4 h. The target was to increase and maintain plasma sodium to a level between 145 and 155 mmol/L and osmolarity to a level of 310 to 320 mOsmol/kg. Furthermore, plasma sodium levels were to be elevated by a maximum of 15 mmol/L in the first 24 h of administration. Infusion was continued until: (a) the level of plasma sodium was greater than 155 mmol/L; (b) serum osmolarity was greater than 320 mOsmol/kg; (c) the value of ICP remained constant at <15 mmHg for at least 24 h; (d) pulmonary edema, acute heart or renal failure and other side-effects associated with the infusion of both drugs limited their use. Once the ICP fell below 15 mmHg for at least 24 h, the target of plasma sodium was gradually decreased by <5 mmol/L daily to reach the normal range over 2–3 days. In the process, if the ICP increased obviously with the decrease in plasma sodium levels, the plasma sodium values were returned to an appropriate level. When control of ICP was poor, as the continuous osmotherapy failed with the ICP remained >20 mmHg 30 min, the patients were treated in accordance with guidelines [11], including repeated boluses of 125 to 250 mL of 20% mannitol, pentobarbital (loading dose of 3–10 mg/kg, by infusing at a rate of 1 mg/kg per min) adapted to the ICP evolution, and continuous cerebrospinal fluid (CSF) drainage or decompressive craniectomy should be discussed.
Data collection
Data collected from each eligible patient included plasma sodium and osmolarity, serum potassium, and potential adverse effects associated with HS + furosemide administration, such as acute heart failure, acute renal injury (defined by a increase in serum creatinine concentration at least 200% compared with that at admission) or failure, pulmonary edema, plasma electrolyte imbalance, coagulopathy disorder, phlebitis, and central pontine myelinolysis. Therefore, serum creatinine and blood urea nitrogen (BUN), troponin, activated partial thromboplastin time (APTT), international normalized ratio, neurological consciousness assessment (Glasgow Coma Scale, GCS), temperature, glucose, and blood gas analysis were tested every 4 h. Furthermore, brain and chest CT scans and electrocardiography (ECG) were performed daily as a part of routine intensive care unit management. CVP, MAP, ICP and CPP were monitored continuously throughout the infusion.
Statistical analysis
Statistical tests were performed with the SPSS 16.0 software. Continuous variables were tested for normality by the Kolmogorov-Smirnov test. Normally distributed data are reported as mean and mean percentage and compared by one-way ANOVA. Non-parametric data are reported as median and interquartile range (IQR) values and were analyzed using the Kruskal-Wallis test. P<0.05 was considered statistically significant.
Results
Patient characteristics
A total of 56 neurosurgical patients (26 males, 30 females) with standard intensive care management were recruited according to the inclusion criteria in the study.
As shown in Table 2, baseline characteristics include age, sex, body weight, initial heart rate (HR), ICP, MAP, CPP, CVP, GCS score, plasma sodium, and osmolarity levels.
Table 2.
Baseline characteristics.
| Parameters | |
|---|---|
| Female, n (%) | 30 (54) |
| Age (years), (IQR) | 66 (58–78) |
| Weight (kg), (IQR) | 67 (51–85) |
| GCS (scores), (IQR) | 7 (4–12) |
| CPP (mmHg), (SD) | 64±7 |
| MAP (mmHg), (SD) | 85±16 |
| HR (bpm), (IQR) | 82 (51–107) |
| CVP (cmH2O), (IQR) | 8 (6–9) |
| ICP (mmHg), (SD) | 23±7 |
| Plasma sodium (mmol/L), (SD) | 139±5 |
| Osmolarity (mOsmol/kg), (SD) | 282±11 |
IQR – interquartile range; HR – heart rate; GCS – Glasgow Coma Scale; MAP – mean arterial pressure; CPP – cerebral perfusion pressure; ICP – intracerebral pressure; CVP – central venous pressure.
Effect on plasma sodium and osmolarity
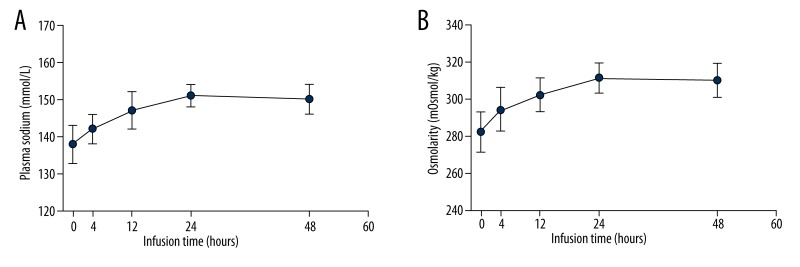
Evolution of plasma sodium and osmolarity over time are shown in Figure 1. Plasma sodium levels significantly increased from 138±5 mmol/L at admission to 142±4 mmol/L at 4 h (P<0.05; Figure 1A), and to 147±5 mmol/L at 12 h (P<0.05 vs. 4 h). Plasma sodium continuously increased to 151±3 mmol/L at 24 h (P<0.01 vs. 0 h). Subsequently, the HS infusion rate was decreased from 25 mL/h to 15 mL/h, while the furosemide infusion rate was unchanged, and the plasma sodium value remained stable at that level. Osmolarity increased from 282±11 mOsmol/kg at baseline to 294±12 mOsmol/kg at 4 h (P<0.05; Figure 1B), and to 302±9 mOsmol/kg at 12 h (P<0.05 vs. 4 h), then remaining stable at 311±8 mOsmol/kg until 24 h (P<0.01 vs. 0 h).
Figure 1.
(A) Plasma sodium and (B) osmolarity recordings over time during the continuous 3% HS and furosemide infusion. Results are given for the first 48 h of infusion.
Effect on CVP, MAP, ICP, CPP and GCS
CVP slightly increased from 7 (4–9) cmH2O at admission to 9 (5–11) cmH2O (P=0.512) at 4 h. Similarly, MAP increased from 82±16 mmHg to 85±11 mmHg (P=0.331) at 4 h and remained stable over the observation period. However, these changes were not statistically significant.
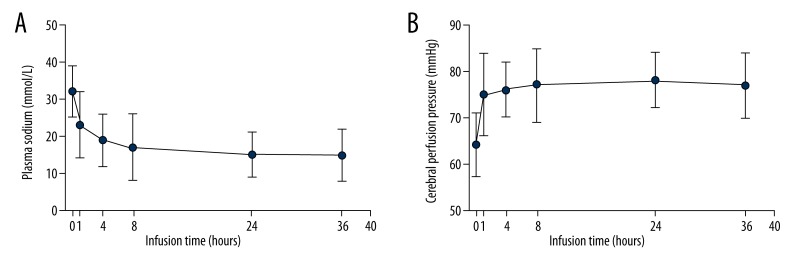
ICP decreased to <20 mmHg in 76.8% (43 of 56) of all patients, while decreased ICP was not achieved in 23.2% (13 of 56) of patients who received additional treatment such as 20% mannitol, deep sedation, and decompressive craniectomy for ICP control. As shown in Figure 2A, maximal changes in ICP were detected at 1 h. ICP significantly decreased from 32±7 mmHg to 23±9 mmHg at 1 h (P<0.01 vs. 0 h) after infusion, and from 19±7 mmHg at 4 h to 17±9 mmHg at 8 h (P<0.05 vs. 4 h), and to 15±6 mmHg at 24 h (P<0.01 vs. 0 h). Subsequently, ICP remained stable during the period of infusion. Rebound ICP was observed in 2 of 43 (4.7%) patients who succeeded in achieving the target. There was a significant improvement in CPP, from 64±7 mmHg to 75±9 mmHg at 1 h (P<0.01 vs. 0 h; Figure 2B). Subsequently, the improvement was stably maintained during the whole study period. Moreover, the mean GCS scores increased from 7 (4–12) before infusion, up to 9 (5–13) (P=0.421) at 24 h.
Figure 2.
(A) Intracranial pressure (ICP) and (B) cerebral perfusion pressure (CPP) recordings over time during the continuous 3% HS and furosemide infusion. Results are provided for the first 36 h of infusion.
Adverse effects
Complications associated with HS + furosemide infusion were as follows. Overall, no instances of hypernatremia (plasma sodium >155 mmol/L) were recorded, but hypopotassium occurred in 6 (10.7%) patients. Acute renal injury, but not renal failure, occurred in 2 (3.6%) patients. The drug infusion produced no clinically relevant changes in troponin, international normalized ratio, or APTT and the ECG results remained unchanged. No cases of acute heart failure, pulmonary edema, hypercarbia or hypoxemia, phlebitis, and central pontine myelinolysis were recorded.
Discussion
Hypo-sodium and hypo-osmolarity occurs frequently in neurological patients, especially in patients using excessive diuretics [12]. Hyperosmolar therapy with either mannitol or HS is recommended for treating raised ICP [13–15], with mannitol replacing other osmotic agents as the mainstay of this therapy.
More recently, HS at various concentrations (3%, 7.2%, 10%, and 23.4%) has become prevalent for the clinical treatment of raised ICP without diuresis; however, the optimal administration and concentration, as well as timing and application schedule, remains unclear. Recently, Muizelaar et al. [16] reported that no major adverse effects were observed in critically ill patients with severe stroke treated with continuous infusion of 3% HS. In the NICU, we found that continuous micro-pump administration of 3% HS + furosemide effectively reduced ICP. Experimental and clinical evidence has demonstrated HS reduces ICP and improves CPP [17], which makes it an interesting alternative to mannitol. In accordance with these studies, we demonstrated that HS + furosemide infusion decreased ICP and improved CPP without severe hypernatremia. Interestingly, the mechanism of ICP reduction may be associated with the Na+ osmotic concentration gradient between the brain tissue and the blood. HS administration increases body perfusion, while HS itself has no diuretic effect, and this may weaken the ICP-lowering effects. Combining HS with furosemide increases intravascular osmolarity, causing the transfer of fluid from the brain tissue compartment to the intravascular compartment along the osmolarity gradient [18–20], and leading to a reduction in the water content without causing a volume overload, which may lead to various systemic adverse effects [21]. However, trials exploring the ICP-lowering effects of 3% HS + furosemide infusion are scarce. In the present retrospective study, we investigated the safety and efficacy of continuous 3% HS + furosemide infusion to increase serum sodium and osmolarity to the levels required to reduce ICP to <15 mmHg.
The target in the present study was to increase and maintain plasma sodium to a level of 145 to 155 mmol/L and osmolarity of 310 to 320 mOsmol/kg. The selection of these targets was based on previous studies in which these ranges effectively decreased ICP without major adverse effects [22]. Of note, a decline in ICP correlating with an increase in plasma sodium and osmolarity was observed. The target values for plasma sodium and osmolarity levels were reached at 24 h.
In the study, 23.2% (13 of 56) patients failed to achieve the target ICP level, mainly because 3% HS had no an immediate lowing-ICP effect like mannitol. Furthermore, our team analyzed the data from these patients, and found that all these ICP values had been declining over time. However, the rate of lowing ICP was slow. As a result, 3% HS was not an appropriate choice in treating acute intracranial hypertension. Despite the small sample size, we recommend that 3% HS + furosemide infusion could be beneficial with ICP less than 30 mmHg. However, more comprehensive and rigorous studies are needed to further explore the best applicable scope of 3% HS + furosemide in the treatment of intracranial hypertension.
A previous study reported that a bolus of osmotherapy increases cerebrospinal fluid osmolarity, which may increase the risk of ICP rebound [23]. Mannitol (20%) produced a rapid lowering of ICP, and the osmotic effect is delayed for 15–30 min after administering an intravenous bolus. The effect on ICP persisted for a variable period of 90 min to 6 h or more, depending on the clinical condition. In the present trial, it can be speculated that the low rate of rebound ICP observed (2 of 43 patients; 4.7%) is due to the stable maintenance of hyperosmolarity by continuous HS infusion. Although the immediate ICP-lowing effects of 3% HS + furosemide were not observed, continuous infusion produced a prolonged reduction in the ICP.
Potential adverse effects of 3% HS + furosemide administration include central pontine myelinolysis, phlebitis, hypernatremia, hyperosmolarity, hypokalemia, pulmonary edema, heart failure, renal failure, and coagulopathy [5,18]. Hypokalemia (plasma hypokalemia <3.5 mmol/L) were observed in 6 (10.7%) patients and this may be attributed to the continuous furosemide infusion. However, due to frequent monitoring every 4 h, serious outcomes were avoided by timely potassium supplementation. Acute renal injury, but not renal failure, was observed in 2 (3.6%) patients; however, it is not clear whether these effects were caused by HS infusion, since various drugs were included in the infusion; more in-depth studies are required to further explore this issue. Cases of pulmonary edema and heart failure did not occur, which may be because of the diuretic effect of furosemide. With the decline in ICP, the mean GCS scores increased from 7 (4–12) to 9 (5–13). HS administration carries the risk of central pontine myelinolysis if there is a rapid transition from hyponatremia to hypernatremia [24]. In the present study, hyponatremia was excluded before administration of HS; therefore, no instances of central pontine myelinolysis were recorded.
Specifically, no patients suffered from severe hypernatremia during the whole period of infusion. According to previous studies, continuous infusion of 3% HS in traumatic brain injury patients decreased ICP but induced severe hypernatremia, which reached 180 mmol/L, resulting in neurological complications and renal failure [25,26]. Continuous furosemide infusion leads to a marked decrease in Na+ reabsorption from the thick ascending limb of the renal medulla, resulting in the low incidence of hypernatremia observed in our study.
Some limitations of our study should be noted. First, ours was a retrospective study with no control groups; thus, a prospective randomized study is required to confirm our findings. Second, although the thresholds for plasma sodium and osmolarity were chosen based on previous studies, confirmation that these values represent the optimal range is required. Third, the inclusion and exclusion criteria in the study may not provide a complete and accurate assessment of the therapeutic response of both agents after infusion. Finally, the long-term neurological outcome and mortality was not investigated and the sample size (56 patients) was small. A larger and more complete study is required to elucidate the full effects of 3% HS + furosemide infusion.
Conclusions
Continuous infusion of 3% HS + furosemide is feasible and safe in maintaining ICP within goal in neurological patients with raised ICP (>20 mmHg) and appears to be a promising ICP-lowering therapy. Further prospective, random, and double-blind studies should be performed to confirm the full effects of the combination of 3% HS and furosemide infusion in the treatment of elevated ICP before widespread use is advocated.
Acknowledgements
The authors thank professor Qian Yang from the Department of Neurosurgery, Tangdu Hospital, The Fourth Military Medical University, China, for the critical reading and modification of the manuscript.
Footnotes
Competing interests
The authors declare that no competing interests exist.
Source of support: Departmental sources
References
- 1.El Ahmadieh TY, Adel JG, El Tecle NE, et al. Surgical treatment of elevated intracranial pressure: decompressive craniectomy and intracranial pressure monitoring. Neurosurg Clin N Am. 2013;24(3):375–91. doi: 10.1016/j.nec.2013.03.003. [DOI] [PubMed] [Google Scholar]
- 2.Strandvik GF. Hypertonic saline in critical care: a review of the literature and guidelines for use in hypotensive states and raised intracranial pressure. Anaesthesia. 2009;64(9):990–1003. doi: 10.1111/j.1365-2044.2009.05986.x. [DOI] [PubMed] [Google Scholar]
- 3.Kerwin AJ, Schinco MA, Tepas JJ, III, et al. The use of 23.4% hypertonic saline for the management of elevated intracranial pressure in patients with severe traumatic brain injury: a pilot study. J Trauma. 2009;67(2):277–82. doi: 10.1097/TA.0b013e3181acc726. [DOI] [PubMed] [Google Scholar]
- 4.Infanti JL. Challenging the gold standard: should mannitol remain our first-line defense against intracranial hypertension? J Neurosci Nurs. 2008;40(6):362–68. [PubMed] [Google Scholar]
- 5.Ware ML, Nemani VM, Meeker M, et al. Effects of 23.4% sodium chloride solution in reducing intracranial pressure in patients with traumatic brain injury: a preliminary study. Neurosurgery. 2005;57(4):727–36. discussion 736. [PubMed] [Google Scholar]
- 6.Battison C, Andrews PJ, Graham C, Petty T. Randomized, controlled trial on the effect of a 20% mannitol solution and a 7.5% saline/6% dextran solution on increased intracranial pressure after brain injury. Crit Care Med. 2005;33(1):196–202. doi: 10.1097/01.ccm.0000150269.65485.a6. discussion 257–58. [DOI] [PubMed] [Google Scholar]
- 7.da Silva JC, de Lima Fde M, Valenca MM, de Azevedo Filho HR. Hypertonic saline more efficacious than mannitol in lethal intracranial hypertension model. Neurol Res. 2010;32(2):139–43. doi: 10.1179/174313209X405119. [DOI] [PubMed] [Google Scholar]
- 8.Harutjunyan L, Holz C, Rieger A, et al. Efficiency of 7.2% hypertonic saline hydroxyethyl starch 200/0.5 versus mannitol 15% in the treatment of increased intracranial pressure in neurosurgical patients – a randomized clinical trial [ISRCTN62699180] Crit Care. 2005;9(5):R530–40. doi: 10.1186/cc3767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Marko NF. Hypertonic saline, not mannitol, should be considered gold-standard medical therapy for intracranial hypertension. Crit Care. 2012;16(1):113. doi: 10.1186/cc11182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Grande PO, Romner B. Osmotherapy in brain edema: a questionable therapy. J Neurosurg Anesthesiol. 2012;24(4):407–12. doi: 10.1097/01.ana.0000419730.29492.8b. [DOI] [PubMed] [Google Scholar]
- 11.Quinn TJ, Paolucci S, Sunnerhagen KS, et al. Evidence-based stroke r-ehabilitation: an expanded guidance document from the european stroke organisation (ESO) guidelines for management of ischaemic stroke and transient ischaemic attack 2008. J Rehabil Med. 2009;41(2):99–111. doi: 10.2340/16501977-0301. [DOI] [PubMed] [Google Scholar]
- 12.Ke C, Poon WS, Ng HK, et al. Impact of experimental acute hyponatremia on severe traumatic brain injury in rats: influences on injuries, permeability of blood-brain barrier, ultrastructural features, and aquaporin-4 expression. Exp Neurol. 2002;178(2):194–206. doi: 10.1006/exnr.2002.8037. [DOI] [PubMed] [Google Scholar]
- 13.Ichai C, Armando G, Orban JC, et al. Sodium lactate versus mannitol in the treatment of intracranial hypertensive episodes in severe traumatic brain-injured patients. Intensive Care Med. 2009;35(3):471–79. doi: 10.1007/s00134-008-1283-5. [DOI] [PubMed] [Google Scholar]
- 14.Kamel H, Navi BB, Nakagawa K, et al. Hypertonic saline versus mannitol for the treatment of elevated intracranial pressure: a meta-analysis of randomized clinical trials. Crit Care Med. 2011;39(3):554–59. doi: 10.1097/CCM.0b013e318206b9be. [DOI] [PubMed] [Google Scholar]
- 15.Francony G, Fauvage B, Falcon D, et al. Equimolar doses of mannitol and hypertonic saline in the treatment of increased intracranial pressure. Crit Care Med. 2008;36(3):795–800. doi: 10.1097/CCM.0B013E3181643B41. [DOI] [PubMed] [Google Scholar]
- 16.Muizelaar JP, Shahlaie K. Hypertonic saline in neurocritical care: Is continuous infusion appropriate? Crit Care Med. 2009;37(4):1521–23. doi: 10.1097/CCM.0b013e31819d3ea0. [DOI] [PubMed] [Google Scholar]
- 17.Walsh JC, Zhuang J, Shackford SR. A comparison of hypertonic to isotonic fluid in the resuscitation of brain injury and hemorrhagic shock. J Surg Res. 1991;50(3):284–92. doi: 10.1016/0022-4804(91)90192-o. [DOI] [PubMed] [Google Scholar]
- 18.Qureshi AI, Suarez JI. Use of hypertonic saline solutions in treatment of cerebral edema and intracranial hypertension. Crit Care Med. 2000;28(9):3301–13. doi: 10.1097/00003246-200009000-00032. [DOI] [PubMed] [Google Scholar]
- 19.White H, Cook D, Venkatesh B. The use of hypertonic saline for treating intracranial hypertension after traumatic brain injury. Anesth Analg. 2006;102(6):1836–46. doi: 10.1213/01.ane.0000217208.51017.56. [DOI] [PubMed] [Google Scholar]
- 20.Tyagi R, Donaldson K, Loftus CM, Jallo J. Hypertonic saline: a clinical review. Neurosurg Rev. 2007;30(4):277–89. doi: 10.1007/s10143-007-0091-7. discussion 289–90. [DOI] [PubMed] [Google Scholar]
- 21.Qureshi AI, Suarez JI, Bhardwaj A, et al. Use of hypertonic (3%) saline/acetate infusion in the treatment of cerebral edema: Effect on intracranial pressure and lateral displacement of the brain. Crit Care Med. 1998;26(3):440–46. doi: 10.1097/00003246-199803000-00011. [DOI] [PubMed] [Google Scholar]
- 22.Murphy N, Auzinger G, Bernel W, Wendon J. The effect of hypertonic sodium chloride on intracranial pressure in patients with acute liver failure. Hepatology. 2004;39(2):464–70. doi: 10.1002/hep.20056. [DOI] [PubMed] [Google Scholar]
- 23.Rudehill A, Gordon E, Ohman G, et al. Pharmacokinetics and effects of mannitol on hemodynamics, blood and cerebrospinal fluid electrolytes, and osmolality during intracranial surgery. J Neurosurg Anesthesiol. 1993;5(1):4–12. doi: 10.1097/00008506-199301000-00002. [DOI] [PubMed] [Google Scholar]
- 24.Bhardwaj A, Ulatowski JA. Hypertonic saline solutions in brain injury. Curr Opin Crit Care. 2004;10(2):126–31. doi: 10.1097/00075198-200404000-00009. [DOI] [PubMed] [Google Scholar]
- 25.Khanna S, Davis D, Peterson B, et al. Use of hypertonic saline in the treatment of severe refractory posttraumatic intracranial hypertension in pediatric traumatic brain injury. Crit Care Med. 2000;28(4):1144–51. doi: 10.1097/00003246-200004000-00038. [DOI] [PubMed] [Google Scholar]
- 26.Froelich M, Ni Q, Wess C, et al. Continuous hypertonic saline therapy and the occurrence of complications in neurocritically ill patients. Crit Care Med. 2009;37(4):1433–41. doi: 10.1097/CCM.0b013e31819c1933. [DOI] [PubMed] [Google Scholar]