Abstract
Purpose
To report our experience with bilateral placement of dexamethasone 0.7 mg (DEX) sustained-release intravitreal implant in the management of noninfectious posterior uveitis or macular edema secondary to retinal vein occlusion.
Methods
A retrospective chart review of patients with bilateral noninfectious posterior uveitis and macular edema secondary to retinal vein occlusion who were treated with DEX intravitreal implant was performed. Ocular side effects such as intraocular pressure (IOP), cataract, and tolerability of bilateral injections was reviewed.
Results
Twenty-two eyes of eleven patients treated with a total of 32 DEX implants were included. Ten of eleven patients received bilateral implants due to active noninfectious uveitis while the other demonstrated macular edema in both eyes following separate central retinal vein occlusions. Among the patients with bilateral uveitis, the mean interval between DEX implant in the initial eye and the subsequent DEX in the fellow eye was 15.6 days (range 2–71 days). Seven of the ten patients received the second implant in the fellow eye within 8 days of the initial implantation. None of the patients had bilateral implantations on the same day. Seven eyes required reimplantation for recurrence of inflammation (mean interval between first and repeat implantation was 6.00±2.39 months). Following single or, in the case of the aforementioned seven eyes, repeat DEX implantation, all 20 uveitic eyes demonstrated clinical and/or angiographic evidence of decreased inflammation in the form of reduction in vitreous cells on slit lamp ophthalmoscopy, macular edema on ophthalmoscopy, or optical coherence tomography and/or disc and vascular leakage on fluorescein angiography. The mean follow-up for all eyes after initial implantation was 23.57 months (range 1–48 months). IOP was significantly higher (P=0.028) at 6 months (16.62 mmHg ±5.97) but not (P=0.82) at most recent follow-up (14.9±3.37 mmHg) when compared with baseline (14.68±3.02 mmHg). Four eyes (18.2%) required initiation of IOP-lowering medications. During the follow-up period, no eyes underwent filtration or cataract extraction. No serious ocular adverse effects were noted during the follow-up period.
Conclusion
In patients with bilateral noninfectious posterior uveitis and macular edema secondary to vein occlusion, bilateral injection of DEX intravitreal implant was well tolerated and had an acceptable safety profile.
Keywords: bilateral uveitis, dexamethasone implant, Ozurdex
Introduction
Corticosteroids have a necessary role in the therapeutic approach to vitreoretinal disease. The various routes include oral, intravenous, topical, periocular, and intravitreal, with each route demonstrating discernible flaws.1 Systemic side effects limit intravenous and oral steroid use while topical steroids often do not provide adequate posterior segment penetration. While more localized and safer than periocular steroids, intravitreal steroids still carry notable risks such as ocular hypertension and cataract progression.
There are currently three main commercially available slow-release intravitreal corticosteroid implants licensed by the US Food and Drug Administration (FDA): Retisert, Iluvien, and Ozurdex.2 Retisert (Bausch & Lomb Incorporated, Bridgewater, NJ, USA) is a sterile, nonbiodegradable intravitreal implant deposited surgically through a scleral opening in the pars plana, containing 0.59 mg fluocinolone acetonide and designed to release approximately 0.5 mg/day for 1,000 days in the treatment of chronic noninfectious posterior uveitis.3 Iluvien (Alimera Sciences, Alpharetta, GA, USA) is an injectable intravitreal insert, delivered using a 25 G injector system and designed to release fluocinolone acetonide for up to 3 years, recently approved for the treatment of diabetic macular edema.4 Ozurdex (Allergan Inc., Irvine, CA, USA) is a biodegradable dexamethasone 0.7 mg (DEX) sustained-release intravitreal implant composed of a copolymer of lactic acid and glycolic acid, utilizing Novadur drug-delivery technology to progressively dissolve in the vitreous gel, delivering 0.7 mg of potent preservative-free DEX directly within the vitreous cavity, translating to robust anti-inflammatory properties with a favorable side-effect profile.5–7 The FDA has approved the use of DEX implant in the treatment of macular edema associated with central retinal vein occlusion (CRVO) and branch retinal vein occlusion, noninfectious posterior uveitis, and, most recently, diabetic macular edema.8
Lowder et al showed that DEX intravitreal implant was effective in controlling ocular inflammation and had an advantageous safety profile, with less than 10% of treated eyes having an intraocular pressure (IOP) of 25 mmHg or greater and no significant increased risk of cataract.9 Others have shown a similar safety profile from repeated implantations.7,10 Bilateral use has been reported in cases of Vogt–Koyanagi–Harada and retinitis pigmentosa-related macular edema.11,12 Sharma et al suggest a fellow eye effect for unilateral DEX implantation, yet these steroids do not achieve significant systemic serum levels when applied topically or injected intravitreally.13–15 With the recent expansion of indications for use of DEX into the realm of diabetic edema, a notoriously bilateral disease, data on bilateral DEX implants is justified. We sought to better evaluate the efficacy and tolerability of bilateral DEX for the treatment of noninfectious posterior uveitis and macular edema secondary to retinal vein occlusion.
Methods and materials
This is a retrospective chart review of patients treated with DEX bilaterally between 2010 and 2014 at Weill Cornell Eye Associates. The study was approved by the institutional review board of Weill Cornell Medical College. Data collection included demographics, details of comprehensive eye exam, imaging studies, past ocular history or surgeries, and prior local or systemic treatment for uveitis or vein occlusion.
Imaging studies included Heidelberg SPECTRALIS® spectral-domain optical coherence tomography (OCT), wide-field fluorescein angiography (FA), and fundus photography. A systemic work-up included laboratory and radiographic imaging for infectious and noninfectious etiologies. Noninfectious posterior uveitis was determined by clinical exam and imaging findings, including vitreous cells on slit lamp ophthalmoscopy, macular edema on ophthalmoscopy or OCT, and disc and vascular leakage on FA. Ozurdex was administered in accordance with the manufacturer’s instructions using the 22-gauge applicator device (http://www.allergan.com/assets/pdf/ozurdex_pi.pdf).
Best-corrected visual acuity (BCVA), IOP, cataract status, and central macular thickness (CMT) on spectral-domain OCT were compared at the time of each implantation, at post-implantation follow-up visits including 6 months following implantation, and at the most recent visit using the paired Student’s t-test in Microsoft Excel software. The accepted level of significance for all tests was 0.05. P-values were not corrected for multiple testing and so should be viewed as nominal. Continuous data are presented as mean ± standard error of the mean.
Results
Twenty-two eyes of eleven patients (nine female) treated with a total of 32 DEX implants were included (Table 1).
Table 1.
Patient demographics and findings
| Patients | Age, years | Sex | Diagnosis | Eye | Duration between DEX implants, days | CME at implantation | BCVA at implantation | Lens status at implantation | BCVA at 6 months | Duration of follow-up, months | Duration to repeat DEX implants months | BCVA at most recent visit | Lens status at most recent visit |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 41 | F | Idiopathic post uveitis | OD | 2 | N | 20/25+2 | Clear | N/A | 5 | 20/20−1 | Clear | |
| Idiopathic post uveitis | OS | N | 20/25 | Clear | N/A | 4 | 20/20 | Clear | |||||
| 2 | 29 | M | Idiopathic post uveitis | OD | N | 20/40−1 | Clear | 20/30 | 9 | 4 | 20/20+2 | Clear | |
| Idiopathic post uveitis | OS | 3 | N | 20/50 | Clear | 20/20−2 | 9 | 4 | 20/20+1 | Clear | |||
| 3 | 63 | F | Idiopathic post uveitis | OD | Y | 20/25−3 | PCIOL | 20/20 | 22 | 20/20 | PCIOL | ||
| Idiopathic post uveitis | OS | 5 | Y | 20/30−2 | PCIOL | 20/300 | 22 | 5 | 20/30 | PCIOL | |||
| 4 | 41 | F | Idiopathic post uveitis | OD | Y | 20/25−2 | Trace NS | 20/25+1 | 10 | 7 | 20/30+2 | 1+ NS | |
| Idiopathic post uveitis | OS | 7 | Y | 20/25−2 | Trace NS | 20/30−2 | 10 | 7 | 20/30−1 | 1+ NS | |||
| 5 | 70 | F | Idiopathic post uveitis | OD | 7 | Y | 20/150 | PCIOL | 20/300 | 36 | 20/80−1 | PCIOL | |
| Idiopathic post uveitis | OS | N | 20/80−2 | PCIOL | 20/300 | 36 | 20/100−1 | PCIOL | |||||
| 6 | 71 | F | Idiopathic post uveitis | OD | 7 | N | 20/20 | PCIOL | 20/20 | 29 | 20/25 | PCIOL | |
| Idiopathic post uveitis | OS | N | 20/40 | ACIOL | 20/60−2 | 29 | 11 | 20/60+2 | ACIOL | ||||
| 7 | 50 | F | Idiopathic post uveitis | OD | 25 | N | 20/20−1 | Trace NS | 20/20 | 24 | 20/20+2 | Trace NS | |
| Idiopathic post uveitis | OS | N | 20/20 | Trace NS | 20/20 | 24 | 20/15 | Trace NS | |||||
| 8 | 52 | F | Polyarteritis nodosa post uveitis | OD | N | 20/20 | 1+ NS | 20/20 | 28 | 20/30−2 | 1+ NS | ||
| Polyarteritis nodosa post uveitis | OS | 8 | N | 20/30−2 | 1+ NS | 20/20 | 28 | 20/40−2 | 2+ NS | ||||
| 9 | 30 | F | Sarcoid panuveitis | OD | N | 20/20−2 | Clear | 20/15 | 47 | 20/20−1 | Trace NS | ||
| Sarcoid panuveitis | OS | 21 | N | 20/20 | Clear | 20/15 | 48 | 20/20−1 | Trace NS | ||||
| 10 | 45 | M | Vogt–Koyanagi–Harada | OD | Y | 20/50−1 | Clear | 20/40 | 36 | 20/25 | Trace NS | ||
| Vogt–Koyanagi–Harada | OS | 71 | Y | 20/250 | Clear | 20/40 | 39 | 4 | 20/25−3 | 1–2+ NS | |||
| 11 | 63 | F | CRVO | OD | Y | 20/40 | PCIOL | 20/25+1 | 41 | 4, 4, 31 | 20/40−1 | PCIOL | |
| CRVO | OS | 1,225 | N | CF 3′ | 3+ NS | N/A | 1 | CF 3′ | 3+ NS |
Abbreviations: ACIOL, anterior chamber intraocular lens; BCVA, best-corrected visual acuity; CF, count fingers; CF3′, count fingers at 3 feet; CME, cystoid macular edema; CRVO, central retinal vein occlusion; DEX, dexamethasone; F, female; M, male; post, posterior; N/A, not applicable; NS, nuclear sclerosis; PCIOL, posterior chamber intraocular lens; OD (oculus dexter), right eye; OS (oculus sinister), left eye.
The mean follow-up for all eyes after initial implantation was 23.57 months (range 1–48 months). Seven eyes received two and one eye received four implants during the follow-up period. Ten of eleven patients received bilateral DEX implants due to active noninfectious uveitis: seven patients had bilateral idiopathic posterior uveitis (Figures 1 and 2), one patient had Polyarteritis nodosa-associated posterior inflammation, one had sarcoidosis-associated bilateral panuveitis, and one patient had active Vogt–Koyanagi–Harada syndrome.
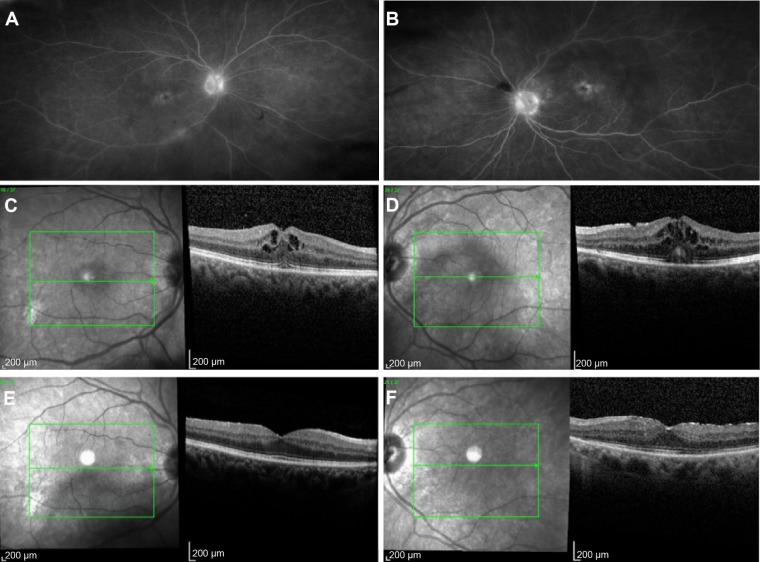
Figure 1.
Resolution of cystoid macular edema following bilateral implantation of sustained-release dexamethasone intravitreal implants in patient with idiopathicnoninfectious posterior uveitis.
Notes: Late-phase fluorescein angiography in (A) right and (B) left eye showing leakage at disc and fovea in patient 3. Spectral-domain optical coherence tomography showing bilateral cystoid macular edema preimplantation of dexamethasone 0.7 mg in (C) right and (D) left eye, and bilateral resolution 6 months following implantation in (E) right and (F) left eye in the same patient.
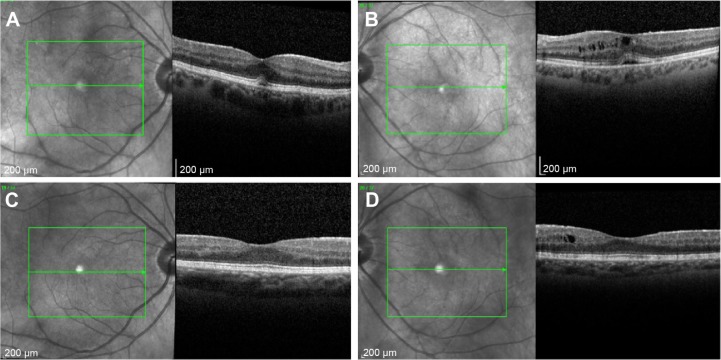
Figure 2.
Spectral-domain optical coherence tomography showing improvement in asymmetric cystoid macular edema, left more than right, following bilateral implantation of sustained-release dexamethasone intravitreal implants in patient with idiopathic noninfectious posterior uveitis.
Notes: Spectral-domain optical coherence tomography at preimplantation of dexamethasone 0.7 mg in (A) right and (B) left eye, and bilateral improvement 3 months following implantation in (C) right and (D) left eye in patient 4.
Among the patients with bilateral active noninfectious uveitis, the mean interval between the DEX implant in the initial eye and the subsequent DEX in the fellow eye was 15.6 days (range 2–71 days). Seven of the ten patients received the second implant in the fellow eye within 8 days of the initial implantation. None of the patients had bilateral implantations on the same day.
At baseline, eight of 22 eyes demonstrated cystoid macular edema (CME) on OCT with an average CMT of 563.00±468.02 μm.
In the case of our Vogt–Koyanagi–Harada syndrome patient, the level of intraretinal and subretinal fluid was such that a central retinal thickness could not be estimated (Figure 3). We utilized the topographic localization of the fovea on other scans to approximate the location of the fovea and, thus, provide an estimate of CMT.
Figure 3.
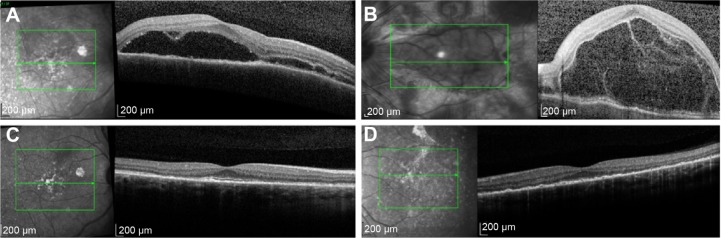
Spectral-domain optical coherence tomography at preimplantation and 5 months following bilateral implantation of sustained-release dexamethasone intravitreal implants in patient diagnosed with Vogt–Koyanagi–Harada syndrome.
Notes: Spectral-domain optical coherence tomography at Preimplantation of dexamethasone 0.7 mg in (A) right and (B) left eye, showing massive subretinal fluid, left more than right, with cystic change in both eyes, and bilateral improvement 5 months following implantation in (C) right and (D) left eye of patient 10.
The eleventh patient in the series presented with bilateral CRVO (Figure 4). The interval between the DEX implant in the initial eye and the subsequent DEX in the fellow eye was 40.76 months (reflecting the interval between the CRVOs).
Figure 4.
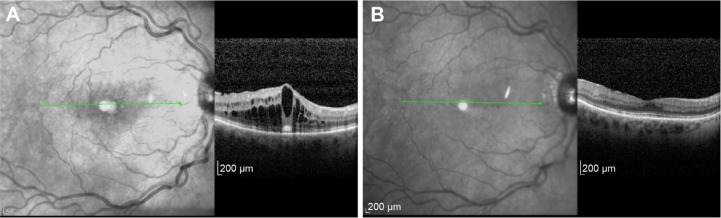
Spectral-domain optical coherence tomography showing improvement in cystoid macular edema following implantation of sustained-release dexamethasone intravitreal implants in patient with central retinal vein occlusion in right eye.
Notes: Spectral-domain optical coherence tomography at pre-implantation of dexamethasone 0.7 mg in right eye (A) and (B) 6 months after in patient 11.
Seven of 22 eyes (31.8%) were pseudophakic at the time of initial DEX implantation with six posterior chamber intraocular lenses and one anterior chamber intraocular lens (ACIOL). Besides the ACIOL which was placed prior to arrival at our clinic, no eyes had a ruptured posterior capsule either surgically or via Nd:YAG laser at the time of implantation.
Patient 5 had a diagnosis of moderate primary open-angle glaucoma at the time of DEX implantation, having undergone prior bilateral trabeculectomies and a tube shunt in the right eye. Of the 22 eyes, only these two eyes (9.1%) were on topical IOP-lowering agents. Of the remaining 20 eyes, there were no known steroid responders.
Six patients (patients 2, 5, 7, 8, 9, and 10) were on oral prednisone at the time of implantation. One patient (patient 7) was on methotrexate (10 mg by mouth weekly) and adalimumab (40 mg every other week). All regimens of oral immunosuppressive medications, including oral prednisone, were continued during the follow-up period. Three eyes (left eye [OS] of patient 3, OS of patient 9, and OS of patient 10) had received prior sub-Tenon’s triamci-nolone injections. One eye (right eye [OD] of patient 3) had received a prior intravitreal triamcinolone injection. Eight eyes were on topical corticosteroids at the time of implantation, including four (OD and OS of patient 8, OD and OS of patient 10) on prednisolone acetate, two (OD and OS of patient 1) on difluprednate, and two (OD and OS of patient 3) on loteprednol. All topical corticosteroids were stopped after the implantation of DEX implant.
Seven eyes required reimplantation for recurrence of inflammation (mean interval between first and repeat implantation was 6.00±2.39 months). Following single or, in the case of the aforementioned seven eyes, repeat DEX implantation, all 20 uveitic eyes demonstrated clinical and/or angiographic evidence of decreased inflammation in the form of reduction in vitreous cells on slit lamp ophthalmoscopy, macular edema on ophthalmoscopy or OCT, and/or disc and vascular leakage on FA. During the follow-up period, no eyes underwent cataract extraction. No serious ocular or systemic adverse effects were noted during the follow-up period.
At 6 months’ follow-up, there was no statistically significant difference (P=0.95) in BCVA at 6 months (LogMAR 0.32±0.54) compared to BCVA prior to treatment (LogMAR 0.34±0.46). IOP was significantly higher (P=0.028) at 6 months (16.62±5.97 mmHg) when compared to baseline (14.68±3.02 mmHg) (Table 2). During the 6-month period following the initial implantation of DEX, the average maximum IOP was found to be 19.41±4.81 mmHg (range 10–32 mmHg) at an average of 2.95±2.01 months (range 1–6 months). When compared with IOP at the time of implantation, this was found to be statistically significant (P=0.000003). Of the eyes with baseline CME, the 6-month OCT revealed CMT was reduced to an average of 313.75±52.40 μm (−245.25 μm) which was not statistically significant (P=0.21). The follow-up OCT was taken on average 5.7±0.78 months after the initial OCT.
Table 2.
Baseline (at initial implantation) and follow-up BCVA and IOP values (all 22 eyes), and CMT values (eight CME eyes)
| At initial implantation | At 6 months | At most recent visit | |
|---|---|---|---|
| All (n=22 eyes) | |||
| BCVA (logMAR) | 0.34±0.46 | 0.32±0.54 (P=0.95)* | 0.29±0.46 (P=0.52)* |
| IOP (mmHg) | 14.68±3.02 | 16.62±5.97 (P=0.028)* | 14.9±3.37 (P=0.82)* |
| CME (n=8 eyes) | |||
| CMT (μm) | 563.0±468.02 | 313.75±52.40 (P=0.21)* | 302.13±36.24 (P=0.18)* |
Note:
Compared to baseline.
Abbreviations: BCVA, best-corrected visual acuity; CME, cystoid macular edema; CMT, central macular thickness; IOP, intraocular pressure.
On average, the BCVA of the 22 eyes improved from 20/43.4 (LogMAR 0.34±0.46) at the time of the initial implantation to 20/38.9 (LogMAR 0.29±0.46) at the most recent follow-up (P=0.52). During the follow-up period, the mean IOP increased from 14.7±3.02 mmHg to 14.9±3.37 mmHg (P=0.82). Four eyes (18.2%) required initiation of IOP-lowering medications, for an average of 0.68±1.18 drops per patient as compared with 0.27±0.86 drops at the time of initial implantation (P=0.059).
No patients required glaucoma laser or filtering procedure during the follow-up period. Of the eyes with baseline CME, on the most recent OCT, CMT was reduced to an average of 302.13±36.24 μm (−260.88 μm) which was not statistically significant (P=0.18). Six of the eight eyes (75%) showed resolution of CME on the latest OCT, while the fellow CRVO eye demonstrated new CME at its latest follow-up, 3 months following DEX implantation.
Discussion
In this study, we show that the DEX implant is effective in controlling ocular inflammation and, when used bilaterally, has a good safety profile. While our data did not support a statistically significant improvement in visual acuity, this is not surprising given that our data’s baseline visual acuity (slightly worse than 20/40) was much better than baseline visual acuity (both near 20/80) seen in the GENEVA and HURON studies.7,9
When looking at available data on intravitreal steroids, the fluocinolone acetonide implant has significant ocular adverse outcomes requiring surgery for the management of cataracts and glaucoma after 30 months.3,16 These findings are in contrast to the DEX implant data where few patients required additional ocular surgery for the management of adverse effects after 6 months.6,7 Certainly, longer duration of exposure of ocular structures to steroids may play a role in adverse effects as well as molecular composition. Nevertheless, it must be noted that chronic ocular inflammation may require repeated DEX implantation, previously shown to be well-tolerated for macular edema related to posterior uveitis and vein occlusions.10,17 In our study, while no patients underwent cataract surgery in the follow-up period following DEX implantation, there was a statistically significant elevation in IOP within and until 6 months, but not at the most recent follow-up, following baseline implantation. This IOP increase was most pronounced between months 2 and 3 and was manageable with medication only. This may correspond with the peak level of activity as the duration of action for biodegradable DEX sustained-release intravitreal implant has been reported to be between 90 and 180 days.7 Although one patient with an ACIOL was included, there were no instances of anterior chamber migration, known to be a devastating complication.18
While seven of the ten patients received the second implant in the fellow eye within 8 days of the initial implantation, none received same-day implantations. Bakri et al showed that bilateral, same-day intravitreal injections in the outpatient setting were well tolerated.19 Should a physician pursue bilateral, simultaneous implantations in an effort to achieve a safe and perhaps more efficient method for bilateral DEX administration, we would recommend frequent tonometry given the possibility of elevated IOP within the first few months.
In addition to the new FDA-approved indication for the treatment of diabetic macular edema, there may be a role for DEX in the treatment of persistent uveitic macular edema in the absence of active inflammation, another potentially bilateral condition.20,21 Bilateral DEX implants appear to be well tolerated and a safe treatment option in the setting of posterior noninfectious uveitis and retinal vein occlusion. The safety profile of bilateral implants appears to be similar to that seen in the pivotal DEX clinical trials, in which only one eye of each patient was included.5–7,9 More data on bilateral use of intravitreal DEX will prove useful and beneficial.
Footnotes
Disclosure
Szilárd Kiss serves on the Physician Speakers Bureau for Allergan, Inc, Irvine, CA, USA. Swetangi Bhaleeya, Danilo Iannetta, and Steven J Ryder report no conflicts of interest in this work.
References
- 1.Kapoor KG, Wagner MG, Wagner AL. The Sustained-Release Dexamethasone Implant: Expanding Indications in Vitreoretinal Disease. Semin Ophthalmol. 2014 Mar 21; doi: 10.3109/08820538.2014.889179. Epub. [DOI] [PubMed] [Google Scholar]
- 2.Comyn O, Lightman SL, Hykin PG. Corticosteroid intravitreal implants vs ranibizumab for the treatment of vitreoretinal disease. Curr Opin Ophthalmol. 2013;24:248–254. doi: 10.1097/ICU.0b013e32835fab27. [DOI] [PubMed] [Google Scholar]
- 3.Callanan DG, Jaffe GJ, Martin DF, Pearson PA, Comstock TL. Treatment of posterior uveitis with a fluocinolone acetonide implant: three-year clinical trial results. Arch Ophthalmol. 2008;126:1191–1201. doi: 10.1001/archopht.126.9.1191. [DOI] [PubMed] [Google Scholar]
- 4.Kane FE, Burdan J, Cutino A, Green KE. Iluvien: a new sustained delivery technology for posterior eye disease. Expert Opin Drug Deliv. 2008;5:1039–1046. doi: 10.1517/17425247.5.9.1039. [DOI] [PubMed] [Google Scholar]
- 5.Williams GA, Haller JA, Kupperman BD, et al. Dexamethasone posterior-segment drug delivery system in the treatment of macular edema resulting from uveitis or Irvine-Gass syndrome. Am J Ophthalmol. 2009;147:1048–1054. doi: 10.1016/j.ajo.2008.12.033. [DOI] [PubMed] [Google Scholar]
- 6.Haller JA, Kupperman BD, Blumenkranz MS, et al. Randomized controlled trial of intravitreous dexamethasone drug delivery system in patients with diabetic macular edema. Arch Ophthalmol. 2010;128:289–296. doi: 10.1001/archophthalmol.2010.21. [DOI] [PubMed] [Google Scholar]
- 7.Haller JA, Bandello F, Belfort R, Jr, et al. OZURDEX GENEVA Study Group Randomized, sham-controlled trial of dexamethasone intravitreal implant in patients with macular edema due to retinal vein occlusion. Ophthalmology. 2010;117:1134–1146. doi: 10.1016/j.ophtha.2010.03.032. [DOI] [PubMed] [Google Scholar]
- 8.US Food and Drug Administration . Ozurdex®: Highlights of Prescribing Information. Silver Spring: US Food and Drug Administration; 2014. [Accessed January 17, 2015]. Available from: http://www.accessdata.fda.gov/drugsatfda_docs/label/2014/022315s010lbl.pdf. [Google Scholar]
- 9.Lowder C, Belfort R, Jr, Lightman S, et al. Ozurdex HURON Study Group Dexamethasone intravitreal implant for noninfectious intermediate or posterior uveitis. Arch Ophthalmol. 2011;129(5):545–553. doi: 10.1001/archophthalmol.2010.339. [DOI] [PubMed] [Google Scholar]
- 10.Tomkins-Netzer O, Taylor SR, Bar A, et al. Treatment with repeat dexamethasone implants results in long-term disease control in eyes with noninfectious uveitis. Ophthalmology. 2014;121(8):1649–1654. doi: 10.1016/j.ophtha.2014.02.003. [DOI] [PubMed] [Google Scholar]
- 11.Latronico ME, Rigante D, Caso F, et al. Bilateral dexamethasone intravitreal implant in a young patient with Vogt-Koyanagi-Harada disease and refractory uveitis. Clin Rheumatol. 2014 Apr 25; doi: 10.1007/s10067-014-2623-1. Epub. [DOI] [PubMed] [Google Scholar]
- 12.Saatci AO, Selver OB, Seymenoglu G, Yaman A. Bilateral intravitreal dexamethasone implant for retinitis pigmentosa-related macular edema. Case Rep Ophthalmol. 2013;4(1):53–58. doi: 10.1159/000350544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sharma A, Sheth J, Madhusudan RJ, Sundaramoorthy SK. Effect of intravitreal dexamethasone implant on the contralateral eye: a case report. Retin Cases Brief Rep. 2013;7(3):217–219. doi: 10.1097/ICB.0b013e31828993a1. [DOI] [PubMed] [Google Scholar]
- 14.Kiernan DF, Mieler WF. The use of intraocular corticosteroids. Expert Opin Pharmacother. 2009;10(15):2511–2525. doi: 10.1517/14656560903160671. [DOI] [PubMed] [Google Scholar]
- 15.Driot JY, Novack GD, Rittenhouse KD, Milazzo C, Pearson PA. Ocular pharmacokinetics of fluocinolone acetonide after Retisert intravitreal implantation in rabbits over a 1-year period. J Ocul Pharmacol Ther. 2004;20:269–275. doi: 10.1089/1080768041223611. [DOI] [PubMed] [Google Scholar]
- 16.Goldstein DA, Godfrey DG, Hall A, et al. Intraocular pressure in patients with uveitis treated with fluocinolone acetonide implants. Arch Ophthalmol. 2007;125:1478–1485. doi: 10.1001/archopht.125.11.ecs70063. [DOI] [PubMed] [Google Scholar]
- 17.Haller JA, Bandello F, Belfort R, Jr, et al. Dexamethasone intravitreal implant in patients with macular edema related to branch or central retinal vein occlusion twelve-month study results. Ophthalmology. 2011;118(12):2453–2460. doi: 10.1016/j.ophtha.2011.05.014. [DOI] [PubMed] [Google Scholar]
- 18.Khurana RN, Appa SN, McCannel CA, et al. Dexamethasone implant anterior chamber migration: risk factors, complications, and management strategies. Ophthalmology. 2014;121(1):67–71. doi: 10.1016/j.ophtha.2013.06.033. [DOI] [PubMed] [Google Scholar]
- 19.Bakri SJ, Risco M, Edwards AO, Pulido JS. Bilateral simultaneous intravitreal injections in the office setting. Am J Ophthalmol. 2009;148(1):66–69. doi: 10.1016/j.ajo.2009.02.013. [DOI] [PubMed] [Google Scholar]
- 20.Montero JA, Ruiz-Moreno JM. Intravitreal inserts of steroids to treat diabetic macular edema. Curr Diabetes Rev. 2009;5(1):26–32. doi: 10.2174/157339909787314211. [DOI] [PubMed] [Google Scholar]
- 21.Cao JH, Mulvahill M, Zhang L, Joondeph BC, Dacey MS. Dexamethasone intravitreal implant in the treatment of persistent uveitic macular edema in the absence of active inflammation. Ophthalmology. 2014;121:1871–1876. doi: 10.1016/j.ophtha.2014.04.012. [DOI] [PubMed] [Google Scholar]