Abstract
Background
Some studies have indicated alcohol abuse as one of the contributors to the development of cardiovascular disease, particularly coronary heart disease. However, this relationship is controversial.
Objective
To investigate the relationship between post-acute coronary syndrome (ACS) alcohol abuse in the Acute Coronary Syndrome Registry Strategy (ERICO Study).
Methods
146 participants from the ERICO Study answered structured questionnaires and underwent laboratory evaluations at baseline, 30 days and 180 days after ACS. The Alcohol Use Disorders Identification Test (AUDIT) was applied to assess harmful alcohol consumption in the 12 months preceding ACS (30 day-interview) and six months after that.
Results
The frequencies of alcohol abuse were 24.7% and 21.1% in the 12 months preceding ACS and six months after that, respectively. The most significant cardiovascular risk factors associated with high-risk for alcohol abuse 30 days after the acute event were: male sex (88.9%), current smoking (52.8%) and hypertension (58.3%). Six months after the acute event, the most significant results were replicated in our logistic regression, for the association between alcohol abuse among younger individuals [35-44 year-old multivariate OR: 38.30 (95% CI: 1.44-1012.56) and 45-54 year-old multivariate OR: 10.10 (95% CI: 1.06-96.46)] and for smokers [current smokers multivariate OR: 51.09 (95% CI: 3.49-748.01) and past smokers multivariate OR: 40.29 (95% CI: 2.37-685.93)].
Conclusion
Individuals younger than 54 years and smokers showed a significant relation with harmful alcohol consumption, regardless of the ACS subtype.
Keywords: Alcoholism, Acute Coronary Syndrome, Myocardial Infarction, Alcohol Drinking, Questionnaires
Introduction
According to recent data from the World Health Organization (WHO), the prevalence of alcohol dependence can reach 12% of the adult population. The probability of alcohol dependence of subjects with any mental disorder can be at least two times greater than that of individuals without the disorder1. The burden is not equally distributed among the countries. Alcohol consumption is the leading risk factor for the burden of disease in developing countries and the third largest risk factor in developed countries1. It should be noted that drinking patterns have not only been linked to acute health outcomes, such as injuries2,3, but also to chronic diseases, such as coronary heart disease (CHD)4-7. In fact, some studies even indicate alcohol abuse as one of the contributors to the development of cardiovascular diseases (CVD), particularly CHD4-9. However, this relationship is controversial8. Although some beneficial effects of moderate alcohol intake have been described, it can become a risk factor for CHD if the alcohol consumption pattern is characterized as “binge drinking or heavy episodic drinking”, which is defined as more than five drinks for men and four drinks for women in just one occasion1,6-9.
In developing countries, such as Brazil, alcohol is also considered a risk factor that contributes most to the burden of diseases, such as cirrhosis of the liver and several types of cancer1.
Therefore, we aimed to evaluate the hazardous and harmful uses of alcohol and its dependence symptoms 30 days and 180 days after an acute coronary event among participants from the ERICO study (Acute Coronary Syndrome Registry Strategy)10.
Materials and Methods
Study design and population
This sub-study assessed harmful alcohol consumption, hazardous alcohol consumption and dependence symptoms in a subsample of the ERICO study, a prospective cohort study, ongoing since 2009, which included potential participants with ACS admitted to the São Paulo University-affiliated hospital (HU-USP) in the city of São Paulo, Brazil10.
After signing the Informed Consent Form, patients with confirmed medical diagnosis of ACS [ST-elevation myocardial infarction (STEMI), non-ST-elevation acute myocardial infarction (NSTEMI) or unstable angina (UA)] were invited to participate in our sub-study 30 days after the acute event. The eligibility criteria were: age ≥ 18 years, confirmed diagnosis of ACS, and ability to understand and speak Portuguese. The usual treatment for ACS has not changed and the procedures followed were in accordance with the ethical standards approved by the HU-USP Institutional Review Board.
Exclusion criteria were based on a Psychological Screening Questionnaire built on Structured Clinical Interview for DSM Disorders (SCID - I)11 to identify individuals with psychotic, schizophrenia or bipolar disorders, and on a test based on the Mini-Mental State Exam (MMSE)12 to exclude those with cognitive impairment or dementia 30 days after the acute event.
30- and 180-day follow-up
All participants who fulfilled the eligibility criteria answered structured questionnaires and underwent clinical and laboratory evaluations, including depression evaluation using the Brazilian-Portuguese version of the Patient Health Questionnaire (PHQ-9), which is composed of nine questions that assess depressive mood and anhedonia based on the Diagnostic and Statistical Manual of Mental Disorders fourth edition (DSM-IV). The PHQ-9 scores each of the nine DSM-IV criteria as “0” (not at all) to “3” (nearly every day), the total score ranging from 0 to 27, and, in this study, PHQ-9 was applied at baseline, and 30 and 180 days after ACS13,14.
In addition, hazardous and harmful alcohol consumption and dependence symptoms were assessed by using the Alcohol Use Disorders Identification Test (AUDIT) in a personal and in a telephone interview, 30 and 180 days after ACS, respectively15. Of note, 30 days after ACS, participants were asked about alcohol abuse during the last 12 months, and, during the 180-day interview, they were asked about changes in their alcohol behavior six months after ACS.
Acute coronary syndrome definition
Myocardial infarction (MI) was defined as the presence of symptoms consistent with cardiac ischemia in the 24 hours preceding hospital presentation and troponin I levels above the 99th percentile with a test-specific coefficient of variation <10%16,17. STEMI was defined as the presence of MI criteria plus one of the following: (a) persistent ST-segment elevation ≥1 mm in two contiguous electrocardiographic leads or (b) the presence of a new or presumably new left bundle branch block. NSTEMI was defined as the presence of MI criteria, but not of STEMI. The UA diagnosis required the presence of symptoms consistent with cardiac ischemia 24 hours prior to hospital admission, absence of MI criteria and at least one of the following: (a) history of CHD; (b) positive coronary disease stratification test (invasive or non‑invasive); (c) transient ST‑segment changes ≥ 0.5 mm in two contiguous leads, new T-wave inversion ≥1 mm, and/or pseudonormalization of previously inverted T waves; (d) troponin I >0.4 ng/mL (which guarantees a troponin I level above the 99th percentile regardless of the kit used); or (e) diagnostic concordance between two independent doctors.
Alcohol abuse definition
Based on the AUDIT score that ranges from 1 to 40, the following cutoff points were considered for main analyses: ≤ 7, low-risk drinking; and ≥ 8, high-risk alcohol abuse18-20.
In secondary analysis, the following three AUDIT domains were also evaluated:
Hazardous alcohol use (1-7 points) - characterized as a pattern that increases the risk of harmful consequences for the user and/or others. These patterns are of public health significance despite the absence of any current disorder in the user;
Harmful alcohol use (8-19 points) - refers to alcohol intake that might result in consequences for physical and mental health. Some would also consider the social consequences of the harm caused by alcohol;
Alcohol dependence symptoms (≥ 20 points) - characterized by a cluster of behavioral, cognitive and physiological phenomena, which may develop after repeated alcohol use. Typically, these phenomena include a strong desire to consume alcohol, impaired control of its use, persistent drinking, despite harmful consequences, a higher priority given to consumption than to other activities and obligations, increased substance tolerance and physical withdrawal reaction when the substance use is discontinued18-20.
Two trained psychologists administered all questionnaires during the follow-up.
Statistical analysis
The participants’ baseline characteristics, including ACS subtypes, were described according to the alcohol abuse symptoms assessed by using the AUDIT questionnaire, with the following cutoff points suggested in the literature19-21: ≤ 7, low-risk drinking; and ≥ 8, high-risk alcohol abuse18-20. In addition, the baseline characteristics were classified according to the AUDIT domains (hazardous alcohol use, harmful alcohol use and alcohol dependence symptoms).
Categorical variables were analyzed by using chi‑square test, and continuous variables, by using Student t or Mann‑Whitney test, according to continuous variables distribution. Additionally, we performed multivariate logistic regression adjusted to potential confounders (or those with a p-value < 0.2 on univariate analysis) identified at 30 days to evaluate the odds ratios (OR) with 95% confidence intervals (CI) for the possible association of some classical cardiovascular risk factors (CVRF) with alcohol abuse 180 days after the acute event. All analyses with a p-value < 0.05 were considered statistically significant. The SPSS software, version 19.0, was used to perform all statistical analyses.
Results
Case series
Of 225 patients with a confirmed diagnosis of ACS (STEMI, NSTEMI or UA) and included in the main study, 146 (64.9%) were enrolled in the present sub-study. At 180 days, 142 (63%) were evaluated, because four died during the period.
The reasons for no inclusion in this study were early death (16/225, 7.1%) within 30 days and exceeded time limit for the interview (63/209, 30.1%).
Evaluation of alcohol abuse during follow-up
30-day follow-up
The baseline characteristics of all 146 participants in the main study were described according to alcohol abuse symptoms assessed by using the information obtained from AUDIT (Table 1) in a personal interview 30 days after ACS. The frequency of alcohol abuse was 24.7% in the first period, reflecting the alcohol abuse in the 12 months preceding ACS.
Table 1.
Baseline characteristics of the 146 participants in the sub-study of alcohol abuse/dependence in the ERICO study, according to the presence of harmful alcohol use 30 days after an acute coronary event
| Sociodemographic characteristics | Low-risk | High-risk * | Total | p value | |
|---|---|---|---|---|---|
| n = 110 (75.3%) | n = 36 (24.7%) | n = 146 (100%) | |||
| Age range (%) | |||||
| 35-44 years | 4 (3.6) | 3 (8.3) | 7 (4.8) | 0.046 | |
| 45-54 years | 19 (17.3) | 13 (36.1) | 32 (21.9) | ||
| 55-64 years | 39 (35.5) | 12 (33.3) | 51 (34.9) | ||
| 65-74 years | 28 (25.5) | 3 (8.3) | 31 (21.2) | ||
| ≥ 75 years | 20 (18.2) | 5 (13.9) | 25 (17.1) | ||
| Gender (%) | |||||
| Male | 63 (57.3) | 32 (88.9) | 95 (65.1) | 0.001 | |
| Female | 47 (42.7) | 4 (11.1) | 51 (34.9) | ||
| Educational level (%) | |||||
| Illiterate | 9 (8.2) | 2 (5.6) | 11 (7.5) | 0.47 | |
| 1-7 years of education | 42 (38.2) | 18 (50.0) | 60 (41.1) | ||
| ≥ 8 years of education | 59 (53.6) | 16 (44.4) | 75 (51.4) | ||
| Marital Status (%) | |||||
| Single | 8 (7.3) | 4 (11.1) | 12 (8.2) | 0.20 | |
| Married | 73 (66.4) | 28 (77.8) | 101 (69.2) | ||
| Separated | 8 (7.3) | 3 (8.3) | 11 (7.5) | ||
| Widow(er) | 21 (19.1) | 1 (2.8) | 22(15.1) | ||
| Self-reported ethnicity (%) | |||||
| White | 75 (68.2) | 19 (54.3) | 94 (64.8) | 0.25 | |
| Brown | 31 (28.2) | 14 (40.0) | 45 (31.0) | ||
| Black | 2 (1.8) | 2 (5.7) | 4 (2.8) | ||
| Yellow | 2 (1.8) | - | 2 (1.4) | ||
| Clinical comorbidities (%) | |||||
| Smoking | |||||
| Current | 30 (27.3) | 19 (52.8) | 49 (33.6) | 0.004 | |
| Past | 45 (40.9) | 14 (38.9) | 59 (40.4) | ||
| Never | 35 (31.8) | 3 (8.3) | 38 (26.0) | ||
| Hypertension | 83 (76.9) | 21(58.3) | 104 (72.2) | 0.03 | |
| Diabetes mellitus | 37 (33.9) | 7 (20.6) | 44 (30.8) | 0.14 | |
| Dyslipidemia | 57 (56.4) | 13(39.4) | 70 (52.2) | 0.09 | |
| Sedentary lifestyle | 73 (68.9) | 18 (51.4) | 91 (64.5) | 0.06 | |
| Major depression † | 37 (33.6) | 15 (41.7) | 52 (35.6) | 0.38 | |
| Acute Coronary Syndrome subtype (%) | |||||
| Unstable angina | 28 (25.5) | 4 (11.1) | 32 (21.9) | 0.16 | |
| NSTEMI | 46 (41.8) | 16 (44.4) | 62 (42.5) | ||
| STEMI | 36 (32.7) | 16 (44.4) | 52 (35.6) | ||
STEMI: ST-elevation myocardial infarction; NSTEMI: non-ST-elevation myocardial infarction. Some proportions might not add up to 100% due to rounding or missing values.
Individuals who scored 8 points or more on the AUDIT were considered with tendency to alcohol abuse.
The PHQ-9 score ≥ 10 points suggested major depression. P-values are derived from the Chi-square test.
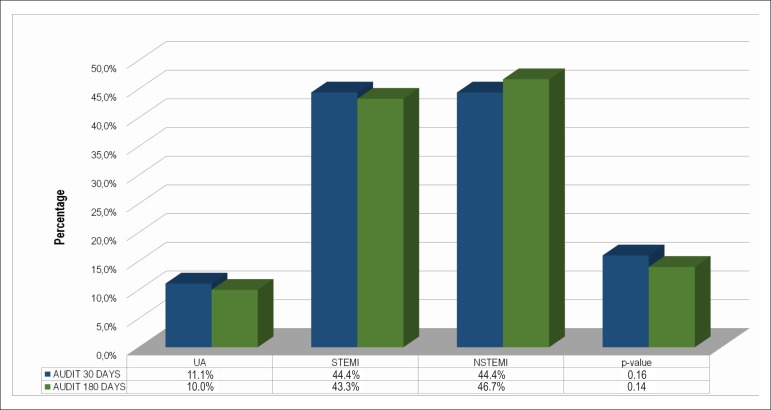
The overall mean AUDIT score was 4.8 points, being higher in men as compared to women (6.3 vs. 2.0, p ≤ 0.001). In addition, the frequency of alcohol abuse (score ≥ 8) was higher among men as compared to women (88.9% vs. 11.1%, p = 0.001) and among current smokers as compared to past or non-smokers (52.8% vs. 38.9% vs. 8.3%, p = 0.004) and patients with hypertension (58.3%, p = 0.03) (Table 1). Interestingly, the sample with alcohol abuse suggested by AUDIT showed a statistically lower frequency of classical CVRF, such as dyslipidemia, diabetes and sedentary lifestyle, as compared to individuals without alcohol abuse, but with no statistical significance (Table 1). The frequency of alcohol abuse did not differ in the ACS subtypes during follow-up (Figure 1).
Figure 1.
Alcohol consumption suggestive of alcohol abuse detected by AUDIT in the subsample from the ERICO study. UA: Unstable Angina; NSTEMI: non-ST-elevation acute myocardial infarction; STEMI: ST-elevation myocardial infarction.
At 30 days, we also found statistically significant associations between some sociodemographic and cardiovascular risk factors and each item of the AUDIT domains, except for questions 7 (“Guilt after drinking”) and 8 (“Blackouts”) in the “Harmful alcohol use” domain. In general, high frequencies of positive answers were found among men and smokers (Table 2). Interestingly, we found higher frequencies of positive answers for question 5 (“Increased salience of drinking”) from the “Dependence symptoms” domain among those who had STEMI as compared to the other ACS subtypes (Table 2). Further, we found higher frequencies of positive answers among participants who had hypertension, diabetes, dyslipidemia and a sedentary lifestyle as compared to those without these comorbidities in the three domains, particularly in the “Dependence symptoms” and the “Harmful alcohol use” domains (Table 2).
Table 2.
Distribution of positive answers according to baseline characteristics by AUDIT domains 30 days after an acute event in the sub‑sample of 146 participants from the ERICO study
| Domains | ||
|---|---|---|
| Subgroup of risk | Hazardous alcohol consumption | p-value |
| Frequency of drinking (question 1) | ||
| Male gender (%) | 46 (79.3) | 0.003 |
| Marital status (%) | ||
| Single | 6 (10.3) | 0.04 |
| Married | 43 (74.1) | |
| Separated | 6 (10.3) | |
| Widow(er) | 3 (5.2) | |
| Smoking (%) | ||
| Current | 28 (48.3) | <0.001 |
| Past | 24 (41.4) | |
| Never | 6 (10.3) | |
| Typical quantity (question 2) | ||
| Male gender (%) | 21 (84,0) | 0.003 |
| Smoking (%) | ||
| Current | 17(68,0) | < 0.001 |
| Past | 7 (28,0) | |
| Never | 1(4,0) | |
| Frequency of heavy drinking (question 3) | ||
| Male gender (%) | 36 (87.8) | < 0.001 |
| Smoking (%) | ||
| Current | 20 (48.8) | 0.02 |
| Past | 16 (39.0) | |
| Never | 5 (12.2) | |
| Dependence Symptoms | ||
| Impaired control over drinking (question 4) | ||
| Acute coronary syndrome subtype (%) | ||
| Unstable angina | 0 (0) | 0.053 |
| NSTEMI | 5 (38.5) | |
| STEMI | 8 (61.5) | |
| Acute coronary syndrome subtype (%) | Increased salience of drinking (question 5) | |
| Unstable angina | 0 (0) | 0.046 |
| NSTEMI | 2 (25.0) | |
| STEMI | 6 (75.0) | |
| Morning drinking (question 6) | ||
| Male gender (%) | 18 (85.7) | 0.03 |
| Hypertension (%) | 11 (52.4) | 0.03 |
| Diabetes mellitus (%) | 2 (10.0) | 0.03 |
| Harmful alcohol consumption | ||
| Guilt after drinking(question 7) | ||
| None | ||
| Blackouts (question 8) | ||
| None | ||
| Alcohol-related injuries (question 9) | ||
| Male gender | 9 (100,0) | 0.02 |
| Dyslipidemia (%) | 1 (12,5) | 0.02 |
| Others concerned about drinking consumption (question 10) | ||
| Age range (years) (%) | ||
| 35-44 | 4 (9.8) | 0.02 |
| 45-54 | 14 (34.1) | |
| 55-64 | 14 (34.1) | |
| 65-74 | 3 (7.3) | |
| ≥ 75 | 6 (14.6) | |
| Male gender (%) | 34 (82.9) | 0.005 |
| Smoking (%) | ||
| Current | 24 (58.5) | < 0.001 |
| Past | 15 (36.6) | |
| Never | 2 (4.9) | |
| Hypertension (%) | 23 (56.1) | 0.006 |
| Sedentary lifestyle (%) | 19 (47.5) | 0.008 |
P-values are derived from the Chi-square test. Some proportions may not round up to 100% because of missing data STEMI: ST-elevation myocardial infarction; NSTEMI: non-ST-elevation myocardial infarction.
180-day follow-up
Six months after the acute event, we observed a slight reduction in the alcohol abuse frequency, which was 21.1% among the 142 survivors by the end of the follow-up. In addition, the mean AUDIT score was almost the same (3.8 points) for the entire sample and by sex (men: 5.1 vs. women: 1.6). The most significant results observed at 30 days were replicated in our logistic regression after 180 days, for the association between alcohol abuse among younger individuals [35‑44 year‑old multivariate OR: 38.30 (95% CI: 1.44-1012.56) and 45‑54 year‑old multivariate OR: 10.10 (95% CI: 1.06‑96.46)] and for smokers [current smokers multivariate OR: 51.09 (95% CI: 3.49-748.01) and past smokers multivariate OR: 40.29 (95% CI: 2.37-685.93)] (Table 3).
Table 3.
Factors associated with alcohol abuse/dependence assessed on logistic regression by AUDIT on 142 participants of the ERICO study 180 days after an acute coronary event
| Acute Coronary Syndrome | Low-risk OR (95%CI) | High-risk * multivariate OR (95%CI) |
|---|---|---|
| UA | Reference (1.0) | Reference (1.0) |
| NSTEMI | Reference (1.0) | 1.27 (0.18-8.92) |
| STEMI | Reference (1.0) | 1.77 (0.24-12.99) |
| Gender | ||
| Female | Reference (1.0) | Reference (1.0) |
| Male | Reference (1.0) | 3.51 (0.78-15.82) |
| Age Range | ||
| 35-44 years | Reference (1.0) | 38.30 (1.44-1021.56) |
| 45-54 years | Reference (1.0) | 10.10 (1.06-96.46) |
| 55-64 years | Reference (1.0) | 0.71 (0.09-5.87) |
| 65-74 years | Reference (1.0) | 1.07 (0.11-10.81) |
| ≥ 75 years | Reference (1.0) | Reference (1.0) |
| Smoking | ||
| Current | Reference (1.0) | 51.09 (3.49-748.01) |
| Past | Reference (1.0) | 40.29 (2.37-685.93) |
| Never | Reference (1.0) | Reference (1.0) |
| Hypertension | Reference (1.0) | 1.43 (0.36-5.68) |
| Diabetes mellitus | Reference (1.0) | 0.90 (0.19-4.25) |
| Dyslipidemia | Reference (1.0) | 0.36 (0.10-1.30) |
| Sedentary lifestyle | Reference (1.0) | 0.29 (0.09-0.99) |
Subjects who scored 8 points or more on the AUDIT were considered with high-risk alcohol abuse.
STEMI: ST-elevation myocardial infarction; NSTEMI: non-ST-elevation myocardial infarction; UA: unstable angina. OR (odds ratio), 95% confidence interval (95%CI). Multivariate OR was adjusted for ACS subtype, gender, age, smoking, medical diagnosis or medication use for hypertension, diabetes mellitus and dyslipidemia, and sedentary lifestyle. Except itself.
Depression versus alcohol abuse during follow-up
In this sub-sample, no statistical association was found between depression and alcohol use after multivariate analysis during the follow-up.
Sensitivity analyses
In additional analyses, we compared ERICO participants who were excluded (79) or did not complete the entire follow‑up (four deaths between 30 days and 180 days after the acute event) with those who completed the 180-day follow‑up (142) in this sub-study. Individuals who were followed up for six months after an acute event in this sub‑study had a higher educational level (9-11 years of formal education: 59.9% vs. 56.1, p = 0.04), were mostly white or of mixed self-reported heritage (96.4% vs. 89%, p = 0.03) and had a more sedentary lifestyle (63.8% vs. 81.5%, p = 0.006) than those who were not followed‑up (Table 4). Moreover, comparing ERICO participants included in this sub-study of alcohol abuse (146) with the ERICO population (820), we found that the former had a higher educational level (≥ 11 years of formal education: 12.3% vs. 6.8%, p=0.02) and most were married (69.2% vs. 59.6%, p = 0.03). In addition, their frequencies of diabetes (30.8% vs. 41.2%, p=0.02) and of sedentary lifestyle (64.8% vs. 73.0%, p = 0.05) were lower than those found in the ERICO population. Finally, a high proportion of STEMI cases (36.3% vs. 26.5%, p = 0.02) was detected in this sub-study (Table 5).
Table 4.
Comparison of baseline characteristics of 142 individuals from the alcohol abuse sub-study and those of 83 individuals who were not followed-up 180 days post-ACS in the ERICO study
| Baseline characteristics | ERICO participants with 180-day follow-up in the alcohol abuse sub-study | p-value | |
|---|---|---|---|
| YES (n = 142) | NO (n = 83) | ||
| Age range (years) (%) | |||
| 35-44 | 7 (4.9) | 2 (2.4) | 0.21 |
| 45-54 | 34 (23.9) | 14 (16.9) | |
| 55-64 | 50 (35.2) | 24 (28.9) | |
| 65-74 | 28 (19.7) | 23 (27.7) | |
| ≥ 75 | 23 (16.2) | 20 (24.1) | |
| Gender (%) | |||
| Male | 91 (64.1) | 48 (57.8) | 0.35 |
| Female | 51 (35.9) | 35 (42.2) | |
| Educational level (years) (%) | 0.04 | ||
| Up to 8 | 28 (19.7) | 15 (18.3) | |
| 9-11 | 85 (59.9) | 46 (56.1) | |
| ≥ 11 | 29 (20.4) | 20 (24.4) | |
| Marital status (%) | 0.18 | ||
| Single | 12 (8.5) | 11 (13.4) | |
| Married | 99 (69.7) | 44 ( 53.7) | |
| Divorced | 9 (6.3) | 9 (11.0) | |
| Widow(er) | 22 (15.5) | 18 (22.0) | |
| Self-reported ethnicity (%) | 0.03 | ||
| White | 91 (64.5) | 57(69.5) | |
| Brown | 45 (31.9) | 16 (19.5) | |
| Black | 3 (2.1) | 8 (9.8) | |
| Asian | 2 (1.4) | 1 (1.2) | |
| Clinical comorbidities (%) | |||
| Smoking | 0.34 | ||
| Current | 49 (34.5) | 25 (30.5) | |
| Past | 56 (39.4) | 28 (34.1) | |
| Never | 37 (26.1) | 29 (35.4) | |
| Hypertension | 100 (71.4) | 63 (75.9) | 0.47 |
| Diabetes mellitus | 41 (29.5) | 32 (38.6) | 0.17 |
| Dyslipidemia | 67 (51.5) | 41 (56.9) | 0.46 |
| Sedentary lifestyle | 88 (63.8) | 66 (81.5) | 0.006 |
| Acute coronary syndrome (%) | 0.09 | ||
| Unstable angina | 36 (25.4) | 30 (36.1) | |
| NSTEMI | 54 (38.0) | 34 ( 41.0) | |
| STEMI | 52 (36.6) | 19 (22.9) |
STEMI: ST-elevation myocardial infarction; NSTEMI: non-ST-elevation myocardial infarction Some proportions might not add up to 100% due to rounding or missing values.
P-values are derived from the Chi-square test.
Table 5.
Comparison of baseline characteristics of 146 participants from the alcohol abuse sub-study and those of the ERICO study population
| Baseline characteristics | Participants (n = 146) | Non participants (n = 820) | p-value |
|---|---|---|---|
| Mean age ( ± SD) | 62 (11.8) | 63 (13.6) | 0.26 |
| Gender (%) | |||
| Male | 95 (65.1) | 476 (58.0) | 0.11 |
| Female | 51 (34.9) | 344 (42.0) | |
| Educational level (years) (%) | |||
| Up to 8 | 98 (67.1) | 631 (77.1) | |
| 9-11 | 30 (20.5) | 131 (16.0) | |
| ≥ 11 | 18 (12.3) | 56 (6.8) | |
| Marital status (%) | 0.03 | ||
| Single | 45 (30.8) | 329 (40.4) | |
| Married | 101 (69.2) | 486(59.6) | |
| Self-reported ethnicity (%) | |||
| White | 94 (64.4) | 538(65.6) | |
| Brown | 45 (30.8) | 224 (27.3) | |
| Black | 4 (2.7) | 46(5.6) | |
| Asian | 3 (2.1) | 12 (1.5) | |
| Clinical comorbidities (%) | |||
| Smoking | 0.12 | ||
| Current | 49 (33.6) | 257 (28.8) | |
| Past | 59 (40.4) | 325 (36.5) | |
| Never | 38 (26.0) | 309 (34.7) | |
| Hypertension | 104 (72.2) | 624 (78.0) | 0.13 |
| Diabetes mellitus | 44(30.8) | 326 (41.2) | 0.02 |
| Dyslipidemia | 70 (52.2) | 386 (55.4) | 0.50 |
| Sedentary lifestyle | 92 (64.8) | 552 (73.0) | 0.05 |
| Acute coronary syndrome (%) | 0.02 | ||
| Unstable angina | 36 (24.7) | 282 (34.4) | |
| NSTEMI | 57 (39.0) | 321 (39.1) | |
| STEMI | 53 (36.3) | 217 (26.5) |
Some proportions might not add up to 100% due to rounding or missing values.
P-values are derived from the Chi-square test. STEMI: ST-elevation myocardial infarction; NSTEMI: non-ST-elevation myocardial infarction
Discussion
This sub-study showed that three of ten patients were at high risk for alcohol abuse. The alcohol abuse frequency was 24.7% 30 days after ACS, and decreased by 4% six months later. As expected, higher frequencies of alcohol abuse were observed in men, younger individuals and smokers.
Data from a Brazilian population-based study, the Megacity Mental Health Survey, performed with 5,037 individuals in the city of São Paulo, reported an overall lifetime prevalence of alcohol abuse of 9.8%. In a sex-stratified analysis, a higher prevalence of alcohol abuse was found among men (16.4% vs. 4.0%) based on the DSM-VI and WHO-Composite International Diagnostic Interview (WMH-CIDI)21.
Another Brazilian hospital-based study performed with 345 patients with ACS (206 with MI and 139 with UA) interviewed about sociodemographic data, smoking status, screening for depression (Prime-MD and Beck Depression Inventory - BDI) and anxiety (State-Trait Anxiety Inventory for Adults - STAI), and alcohol consumption (AUDIT) has reported lower AUDIT scores for both sexes as compared to those found in our study. Similar to our findings, that study has reported no association between alcohol intake and depression22.
Regarding CVRF, participants at high risk for alcohol abuse had a higher frequency of smoking but lower frequencies of hypertension, dyslipidemia, diabetes and sedentary lifestyle as compared to low-risk participants. However, when analyzing each item separately, significant associations were observed with some binging behaviors in the AUDIT domains, such as “increased salience of drinking” and “morning drinking”, and smoking, sedentary lifestyle, hypertension, diabetes, and even ACS subtype. After six months, a high risk of alcohol abuse remained among younger individuals (≤ 54 years) and smokers.
The relationship between alcohol consumption and CVD, particularly CHD, is controversial4-9,23.
Some studies have suggested that a light-moderate alcohol consumption may have a favorable impact on morbidity and mortality from ischemic heart disease6-8. However, the cardioprotective effect of drinking disappears with heavy drinking (binge)6-8. Russel et al.6 have tested a linear dose-response model for the association between drinking patterns and MI6. A lower MI risk was associated with the consumption of less than 4.55 drinks per day for men (95% CI: 2.77 to 7.18) and less than 3.08 drinks per day for women (95% CI: 1.35 to 5.16), and that risk increased after these crossover points were exceeded. The MI risk increased as drinking dosage doubled, regardless of sex6.
The Prospective Epidemiological Study of Myocardial Infarction (PRIME) has investigated the effect of alcohol intake patterns on ischemic heart disease in Northern Ireland and France in 9,778 men aged 50-59 years, free of ischemic heart disease at baseline, during a 10-year follow-up7. After multivariate analysis for classic CVRF and center, the hazard ratio for hard coronary events (incident MI and coronary death) compared with regular drinkers was as follows: 1.97 (95% CI: 1.21 to 3.22) for binge drinkers; 2.03 (95% CI: 1.41 to 2.94) for never drinkers; and 1.57 (95% CI: 1.11 to 2.21) for former drinkers for the entire cohort. Only wine drinking was associated with a lower risk of hard coronary events, irrespective of the country7.
A systematic review that investigated the relationship between alcohol consumption and some CVD endpoints performed with more than 4,000 studies has described a dose-response effect, demonstrated by the lowest risk of CHD mortality occurring with one to two drinks per day23.
Another systematic review including 44 observational studies (case-control or cohort) has reported a relative risk of alcohol intake in relation to ischemic heart disease risk. The analyses included 957,684 participants and substantial heterogeneity across studies was found, making it difficult to confirm any cardioprotective effect of alcohol use on ischemic heart disease for all drinkers, even at low intake levels8.
The pathophysiology of the cardioprotective effects of most alcoholic beverages is probably due to a high-density lipoprotein elevation and the ability of alcohol to prevent platelet aggregation and increase fibrinolysis; particularly an increased favorable effect from red wine24.
Limitations
We did not evaluate the alcohol intake with a specific food questionnaire. However, we used a very reliable instrument to screen alcohol abuse/dependence16. In the present study, two well-trained and experienced psychologists interviewed our patients; however the presence of a psychiatrist during the study could have contributed further to the detection of new cases of alcohol abuse or even depression.
We found some significant differences in the frequency of some sociodemographic factors, such as educational level, self-reported ethnicity and marital status, as well as, some CVRF, such as diabetes and sedentary lifestyle, comparing participants from the alcohol abuse study with those who did not participate in this sub-study. Of note, all these characteristics are very subject to recall bias and had no interference on the alcohol abuse pattern in our main analyses.
Additionally, the extent to which the findings can be generalized is limited due to the small sample size from one single center. Thus, we cannot rule out the possibility of a selection bias.
Conclusions
We found high frequency of alcohol abuse, which remained during the six-month follow-up, regardless of the ACS subtype. Hazardous alcohol consumption was strongly evident among younger individuals aged 35-54 years and smokers. In the present study, the binge drinking pattern was observed among smokers, individuals with a sedentary lifestyle, hypertension, diabetes and STEMI.
Footnotes
Author contributions
Conception and design of the research: Morilha A, Karagulian S, Goulart AC. Acquisition of data: Morilha A, Karagulian S, Goulart AC. Analysis and interpretation of the data: Lotufo PA, Santos IS, Goulart AC. Statistical analysis: Morilha A, Lotufo PA, Santos IS, Benseñor IM, Goulart AC. Obtaining financing: Goulart AC. Writing of the manuscript: Morilha A, Karagulian S, Lotufo PA, Santos IS, Benseñor IM, Goulart AC. Critical revision of the manuscript for intellectual content: Morilha A, Karagulian S, Lotufo PA, Santos IS, Benseñor IM, Goulart AC. Supervision / as the major investigador: Morilha A, Goulart AC.
Potential Conflict of Interest
No potential conflict of interest relevant to this article was reported.
Sources of Funding
This study was funded by FAPESP.
Study Association
This article is part of the thesis of master submitted by Abner Morilha, from University of São Paulo (USP).
References
- 1.World Health Organization (WHO) International guide for monitoring alcohol consumption and related harm. 2000. [cited 2013 August 21]. Available from: http://whqlibdoc.who.int/hq/2000/who_msd_msb_00.4.pdf.
- 2.Greenfield TK. Individual risk of alcohol-related disease and problems. In: Heather N, Peters TJ, Stockwell T, editors. International handbook of alcohol dependence and problems - Part IV: drinking patterns and types of alcohol problem. Chichester: John Wiley & Sons Ltd; 2001. pp. 413–438. [Google Scholar]
- 3.Rossow I, Amundsen A. Alcohol abuse and suicide: a 40-year prospective study of Norwegian conscripts. Addiction. 1995;90(5):685–691. doi: 10.1046/j.1360-0443.1995.9056859.x. [DOI] [PubMed] [Google Scholar]
- 4.Britton A, McKee M. The relation between alcohol and cardiovascular disease in Eastern Europe: explaining the paradox. J Epidemiol Commun Health. 2000;54(5):328–332. doi: 10.1136/jech.54.5.328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Puddey IB, Rakic V, Dimmitt SB, Beilin LJ. Influence of pattern of drinking on cardiovascular disease and cardiovascular risk factors: a review. Addiction. 1999;94(5):649–663. doi: 10.1046/j.1360-0443.1999.9456493.x. [DOI] [PubMed] [Google Scholar]
- 6.Russell M, Chul Chu B, Banerjee A, Fan AZ, Trevisan M, Dorn JM, et al. Drinking Patterns and Myocardial Infarction: A Linear Dose-Response Model. Alcohol Clin Exp Res. 2009;33(2):324–331. doi: 10.1111/j.1530-0277.2008.00836.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ruidavets JB, Ducimetière P, Evans A, Montaye M, Haas B, Bingham A, et al. Patterns of alcohol consumption and ischaemic heart disease in culturally divergent countries: the Prospective Epidemiological Study of Myocardial Infarction (PRIME) BMJ. 2010;341:c6077–c6077. doi: 10.1136/bmj.c6077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Roerecke M, Rehm J. The cardioprotective association of average alcohol consumption and ischaemic heart disease: a systematic review and meta-analysis. Addiction. 2012;107(7):1246–1260. doi: 10.1111/j.1360-0443.2012.03780.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Corrao G, Rubbiati L, Bagnardi V, Zambon A, Poikolainen K. Alcohol and coronary heart disease: a meta-analysis. Addiction. 2000;95(10):1505–1523. doi: 10.1046/j.1360-0443.2000.951015056.x. [DOI] [PubMed] [Google Scholar]
- 10.Goulart AC, Santos IS, Sitnik D, Staniak HL, Fedeli LM, Pastore CA, et al. Design and baseline characteristics of a coronary heart disease prospective cohort: two-year experience from the strategy of registry of acute coronary syndrome study (ERICO study) Clinics (Sao Paulo) 2013;68(3):431–434. doi: 10.6061/clinics/2013(03)RC02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Spitzer MB, Robert L, Gibbon M, Gibbons W, Janet BW. Structured clinical interview for DSM-IV-TR Axis I Disorders, Research Version, Non-patient Edition. (SCID-I/NP) New York: Biometrics Research; New York State Psychiatric Institute; 2002. [cited 2013 August 21]. Available from: http://www.scid4.org/index.html. [Google Scholar]
- 12.Lourenço RA, Veras RP. Mini mental State Examination: psychometric characteristics in elderly outpatients. Rev Saude Publica. 2006;40(4):712–719. doi: 10.1590/s0034-89102006000500023. [DOI] [PubMed] [Google Scholar]
- 13.Kroenke K, Spitzer RL, Williams JBW. The PHQ-9 Validity of a brief depression severity measure. J Gen Intern Med. 2001;16(9):606–613. doi: 10.1046/j.1525-1497.2001.016009606.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Osório FL, Mendes AV, Crippa A, Loureiro SR. Study of the discriminative validity of the PHQ-9 and PHQ-2 in a sample of Brazilian women in the context of primary health care. Perspect Psychiatr Care. 2009;45(3):216–227. doi: 10.1111/j.1744-6163.2009.00224.x. [DOI] [PubMed] [Google Scholar]
- 15.Lima CT, Freire ACC, Silva AP, Teixeira RM, Farrell M, Prince M. Concurrent and construct validity of the AUDIT in an urban Brazilian sample. Alcohol. 2005;40(6):584–589. doi: 10.1093/alcalc/agh202. [DOI] [PubMed] [Google Scholar]
- 16.Luepker RV, Apple FS, Christenson RH, Crow RS, Fortmann SP, Goff D, et al. Case definitions for acute coronary heart disease in epidemiology and clinical research studies: a statement from the AHA Council on Epidemiology and Prevention; AHA Statistics Committee; World Heart Federation Council on Epidemiology and Prevention; the European Society of Cardiology Working Group on Epidemiology and Prevention; Centers for Disease Control and Prevention; and the National Heart, Lung, and Blood Institute. Circulation. 2003;108(20):2543–2549. doi: 10.1161/01.CIR.0000100560.46946.EA. [DOI] [PubMed] [Google Scholar]
- 17.Thygesen K, Alpert JS, White HD, Joint ESC/ACCF/AHA/WHF Task Force for the Redefinition of Myocardial Infarction Universal definition of myocardial infarction. Eur Heart J. 2007;28(20):2525–2538. doi: 10.1093/eurheartj/ehm355. [DOI] [PubMed] [Google Scholar]
- 18.Lykouras L, Rontos I, Rontos K, Katsaras A, Markoulis T, Papasteriades E, et al. Detecting alcohol-related problems among general hospital patients with heart disease. Psychother Psychosom. 2001;70(1):25–29. doi: 10.1159/000056221. [DOI] [PubMed] [Google Scholar]
- 19.Mendoza-Sassi RA, Béria JU. Prevalence of alcohol use disorders and associated factors: A population-based study using AUDIT in southern Brazil. Addiction. 2003;98(6):799–804. doi: 10.1046/j.1360-0443.2003.00411.x. [DOI] [PubMed] [Google Scholar]
- 20.Barbor FT, Higgins-Biddle JC, Saunders JB, Monteiro MG. AUDIT: The Alcohol use disorder identification test. Guidelines for use in primary care. nd. Washington (DC): World Health Organization, Department of Mental Health and Substance Dependence; 2001. [cited 2013 August 21]. Available from: http://whqlibdoc.who.int/hq/2001/who_msd_msb_01.6a.pdf. [Google Scholar]
- 21.Viana CM, Andrade HL. Lifetime prevalence, age and gender distribution and age-of-onset of psychiatric disorders in the São Paulo Metropolitan Area, Brazi: lresults from the São Paulo megacity mental health survey. Rev Bras Psiquiatr. 2012;34(3):249–260. doi: 10.1016/j.rbp.2012.03.001. [DOI] [PubMed] [Google Scholar]
- 22.Perez GH, Nicolau JC, Romano BW, Laranjeira R. Depression and acute coronary syndromes: gender-related differences. Arq Bras Cardiol. 2005;85:319–326. doi: 10.1590/s0066-782x2005001800004. [DOI] [PubMed] [Google Scholar]
- 23.Ronksley PE, Brien SE, Turner BJ, Mukamal KJ, Ghali WA. Association of alcohol consumption with selected cardiovascular disease outcomes: a systematic review and meta-analysis. BMJ. 2011;342:d671–d671. doi: 10.1136/bmj.d671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Constant J. Alcohol, ischemic heart disease, and the French paradox. Coron Artery Dis. 1997;8(10):645–649. doi: 10.1097/00019501-199710000-00007. [DOI] [PubMed] [Google Scholar]