Abstract
Objective:
It remains unclear whether antipsychotic polypharmacy, a common clinical practice, is related to an increased risk of corrected time between start of Q wave and end of T wave (QTc) interval prolongation. We conducted a systematic review of the literature to address this important issue.
Method:
A systematic literature search was conducted in October 2014, using MEDLINE, Embase, and PsycINFO. Studies and case reports were included if they reported QTc intervals or QTc interval changes before and after antipsychotic polypharmacy or QTc intervals in both antipsychotic polypharmacy and monotherapy groups.
Results:
A total of 21 articles (10 clinical trials, 4 observational studies, and 7 case reports) met inclusion criteria. The clinical trials have shown that a combination treatment with risperidone or pimozide is not obviously related to an increase in QTc interval, whereas ziprasidone or sertindole combined with clozapine may prolong QTc interval. Among the 4 observational studies, antipsychotic polypharmacy was not clearly associated with QTc prolongation in 3 studies, each cross-sectional. In contrast, one prospective study showed a significant increase in QTc interval following antipsychotic coadministration. The case reports indicated an increased risk of QTc prolongation in at least some patients receiving antipsychotic polypharmacy.
Conclusions:
Currently available evidence fails to confirm that antipsychotic polypharmacy worsens QTc prolongation in general, although the evidence is scarce and inconsistent. Clinicians are advised to remain conservative in resorting to antipsychotic polypharmacy, as a combination of some QTc-prolongation liable antipsychotics may further prolong QTc interval, and efficacy supporting the clinical benefits of antipsychotic polypharmacy is equivocal, at best.
Keywords: antipsychotic, augmentation, combination, polypharmacy, corrected QT interval, systematic review, cardiac sudden death
Abstract
Objectif :
Il reste à déterminer si la polypharmacie antipsychotique, une pratique clinique courante, est liée à un risque accru de temps corrigé dans l’allongement de l’intervalle entre le début de l’onde Q et la fin de l’onde T (QTc). Nous avons mené une revue systématique de la littérature pour aborder ce problème important.
Méthode :
Une recherche systématique de la littérature a été menée en octobre 2014, dans les bases de données MEDLINE, Embase et PsycINFO. Les études et les études de cas étaient incluses si elles rendaient compte d’intervalles QTc ou de changements d’intervalles QTc avant et après la polypharmacie antipsychotique ou d’intervalles QTc dans les groupes de polypharmacie antipsychotique et de monothérapie.
Résultats :
Un total de 21 articles (10 essais cliniques, 4 études par observation, et 7 études de cas) satisfaisaient aux critères d’inclusion. Les essais cliniques ont montré qu’un traitement combiné par rispéridone ou pimozide n’est pas clairement lié à une augmentation de l’intervalle QTc, alors que la ziprasidone ou le sertindole combiné à la clozapine peuvent prolonger l’intervalle QTc. Dans les 4 études par observation, la polypharmacie antipsychotique n’était pas clairement associée à l’allongement du QTc dans 3 études, toutes transversales. Par contre, une étude prospective montrait une augmentation significative de l’intervalle QTc suivant une co-administration antipsychotique. Les études de cas indiquaient un risque accru d’allongement du QTc chez au moins certains patients recevant une polypharmacie antipsychotique.
Conclusions :
Les données probantes actuellement disponibles ne confirment pas que la polypharmacie antipsychotique aggrave l’allongement du QTc en général, car les données probantes sont rares et non consistantes. Il est recommandé que les cliniciens demeurent prudents lorsqu’ils ont recours à la polypharmacie antipsychotique, étant donné qu’une combinaison de certains antipsychotiques est susceptible de prolonger davantage l’intervalle QTc, et que l’efficacité à l’appui des avantages cliniques de la polypharmacie antipsychotique est au mieux équivoque.
Most typical and atypical antipsychotics have a potential to prolong the QTc interval (that is, QTc-prolonging drugs),1–3 at least in part by inhibiting the hERG- (also known as KCNH2) encoded potassium channels.4,5 The QT interval is the time between the beginning of the Q wave and the end of the T wave on the electrocardiogram, representing ventricular repolarization. Because QT interval shortens with increasing heart rate, it is usually corrected for heart rate (that is, QTc interval), with the 2 main formulae: Bazett’s formula (QTcBZT = QT/RR1/2), which is most widely used, and Fridericia’s formula (QTcFRD = QT/RR1/3). The QTcBZT is less accurate than the QTcFRD when a heart rate is altered, as it over-corrects at an elevated heart rate and under-corrects at a heart rate below 60 beats per minute.6 QTc interval prolongation is considered a risk factor in fatal polymorphic ventricular tachycardia, namely, TdP,7 which can result in sudden cardiac death. In fact, prolonged QTc interval is related to an increased risk of total, cardiovascular, coronary, and sudden cardiac death,8 which is in line with the observation that use of hERG channel blockers is associated with a risk of sudden cardiac death in the general population.9
While the risk of QTc prolongation caused by each individual antipsychotic has been the focus of extensive research, potential additive or synergistic effects of antipsychotic polypharmacy (that is, 2 or more antipsychotics concurrently prescribed) on QTc interval have rarely been reported in the literature, to date. This issue, critically important from a safety perspective, is particularly pertinent in light of the widespread use of antipsychotic polypharmacy; prevalence rates range from 12.9% to 35.0%,10 despite equivocal efficacy of antipsychotic combinations.11–13 To address this clinically relevant question, we conducted a systematic review of the literature on antipsychotic polypharmacy and QTc interval.
Clinical Implications
Existing evidence regarding antipsychotic polypharmacy and QTc interval is scarce and inconsistent.
Currently available evidence fails to confirm that antipsychotic polypharmacy worsens QTc prolongation in general.
A combination of some high-risk, QTc-prolonging antipsychotics may prolong QTc interval.
Limitations
The literature search was limited to the English language.
Most of the articles depend on Bazett’s formula rather than Fridericia’s or other formulae in calculating QTc intervals.
Too few data are available to evaluate possible additive or synergistic effects of specific antipsychotic combinations on QTc interval.
Method
A systematic literature search was conducted in accordance with the PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-analyses) Statement,14 using MEDLINE (1946–present), Embase (1947–present), and PsycINFO (1806–present) on October 15, 2014. The following key words were used: (qt OR qtc) AND antipsychotic* AND (combin* OR polypharmacy OR polytherapy OR augment* OR adjunct* OR adjuvant* OR add* OR concomitant* OR concurrent* OR comedication* OR cotreatment* OR coadministrat* OR enhance* OR simultaneous* OR supplement*) NOT (addict* OR address*). The key words related to antipsychotic polypharmacy were determined in reference to the search terms used in a previous meta-analysis comparing antipsychotic polypharmacy with monotherapy,15 with some modifications. The literature search was limited to the English language, and a cross-referencing of the identified references was also conducted.
Clinical trials (that is, intervention studies), observational studies, and case reports that met the following 2 criteria were included in this review: reports in which 2 or more antipsychotics were concurrently used; and reports in which QTc intervals or QTc interval changes before and after antipsychotic polypharmacy were recorded, or reports in which QTc intervals in antipsychotic polypharmacy and monotherapy groups were compared. As our focus was on antipsychotic polypharmacy and QTc intervals within usual clinical circumstances, studies on antipsychotic overdose were excluded.
The following information was collected from the reports included in the present review: study design (only for clinical trials and observational studies); study duration (only for clinical trials and prospective observational studies); the number of patients (only for clinical trials and observational studies); each patient’s diagnosis, age, and sex; QTc intervals or QTc interval changes before and after antipsychotic polypharmacy or QTc intervals in both antipsychotic polypharmacy and monotherapy groups; and, types and doses of antipsychotics (if available).
Results
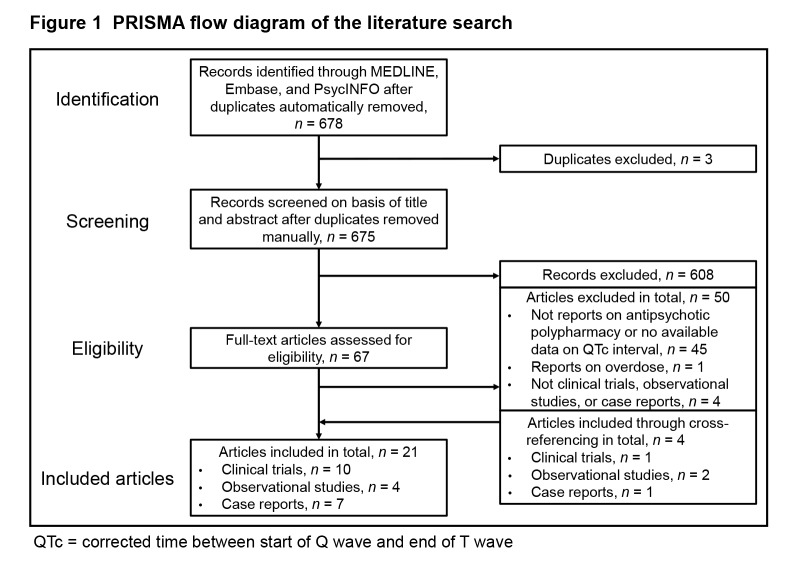
A total of 21 articles (10 clinical trials,16–25 4 observational studies,26–29 and 7 case reports30–36) were identified through our literature search (Figure 1). QTc interval was the primary focus reported in 9 of these articles (all 4 observational studies26–29 and 5 case reports30–33,36).
Figure 1.
PRISMA flow diagram of the literature search
QTc = corrected time between start of Q wave and end of T wave
Clinical Trials
Among the clinical trials,16–25 8 were RCTs16,18,19,21–25 and the remaining 2 were single-arm, prospective trials.17,20 Seven RCTs compared a combination of 2 antipsychotics with 1 of the 2 antipsychotics (plus placebo),16,18,21–25 while 1 RCT compared 2 different types of antipsychotic polypharmacy.19 All patients were diagnosed with schizophrenia or schizoaffective disorder. All but 1 trial examined augmentation of clozapine with another antipsychotic (note: in 1 study,20 clozapine or olanzapine plus another antipsychotic was examined).16–20,22–25 The results of QTc intervals at baseline and at end point, or changes in QTc interval from baseline to end point, are summarized in Table 1.
Table 1.
Clinical trials on antipsychotic polypharmacy and corrected QT (QTc) interval
| Study | Year | Design | Diagnosis | Duration | Antipsychotic polypharmacy | Antipsychotic monotherapy | Group difference | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
||||||||||||
| Antipsychotics (mean dose, mg/day) | n (male; female) | Mean age, years | Mean QTc baseline to end point difference, ms (within-group difference) | Antipsychotics (mean dose, mg/day) | n (male; female) | Mean age, years | Mean QTc baseline to end point difference, ms (within-group difference) | ||||||
| Anil Yağcioğlu et al16 | 2005 | DB-RCT | Sz and SAD | 6 weeks | CLZ (516) + RIS (6) | 16 (9; 7) | 35.3 | 441 to 430 (n/a) | CLZ (414) + PLB | 14 (11; 3) | 31.2 | 437 to 450 (n/a) | ns |
| Ziegenbein et al17 | 2005 | OL | Sz | 6 months | CLZ (570) + ZIP (147) | 9 (5; 4) | 37.3 | n/a | n/a | n/a | n/a | n/a | n/a |
| Chang et al18 | 2008 | DB-RCT | Sz | 8 weeks | CLZ (304) + APZ (15.5) | 29 (22; 7) | 33.2 | 439 to 443 (ns) | CLZ (291) + PLB | 32 (26; 6) | 31.7 | 440 to 441 (ns) | ns |
| Zink et al19 | 2009 | OL-RCT | Sz and SAD | 6 weeks | CLZ (361) + ZIP (134) | 12 (7; 5) | 31.8 | 388c to 403c (s) | n/a | n/a | n/a | n/a | s |
| CLZ (407) + RIS (3.8) | 12 (7; 5) | 37.3 | 391c to 381c (ns) | n/a | n/a | n/a | n/a | ||||||
| Henderson et al20 | 2009 | OL | Sz and SAD | 6 weeks | CLZ (n/a) or OLZ (n/a) + ZIP (160) | 21 (17; 4) | 49 | 417 to 420 (ns) | n/a | n/a | n/a | n/a | n/a |
| Lin et al21 | 2010 | DB-RCT | Sz | 6 weeks | RIS (2) + HPD (2) | 46a (n/a; n/a) | 38b | 410 to 407 (n/a) | RIS (4) | 42a (n/a; n/a) | 38b | 413 to 405 (n/a) | ns |
| Friedman et al22 | 2011 | DB-RCT | Sz and SAD | 12 weeks | CLZ (519) + PMZ (6.5) | 25 (21; 4) | 45.5 | +9.0 (n/a) | CLZ (478) + PLB | 28 (20; 8) | 44.4 | −1.5 (n/a) | ns |
| Nielsen et al23 | 2012 | DB-RCT | Sz | 12 weeks | CLZ (394) + SER (16) | 25 (15; 10) | 41.8 | 440 to 452 and 405c to 412c (n/a and ns) | CLZ (435) + PLB | 25 (15; 10) | 42.7 | 450 to 450 and 409c to 420c (n/a and n/a) | s and ns |
| Gunduz-Bruce et al24 | 2013 | DB-RCT | Sz and SAD | 12 weeks | CLZ (n/a) + PMZ (4) | 14 (10; 4) | 44.3 | 412 to 420 (n/a) | CLZ (n/a) + PLB | 14 (10; 4) | 41.5 | 409 to 413 (n/a) | ns |
| Muscatello et al25 | 2014 | DB-RCT | Sz | 16 weeks | CLZ (429) + ZIP (80) | 20 (5; 15) | 36.5 | 403 to 408 (s) | CLZ (463) + PLB | 20 (8; 12) | 33.5 | 408 to 405 (n/a) | ns |
QTc intervals were measured in 29 patients.
For whole patients
Calculated with Fridericia’s formula
CLZ = clozapine; DB = double-blind; HPD = haloperidol; n/a = not available or not applicable; ns = nonsignificant; OL = open-label; PLB = placebo; PMZ = pimozide; RCT = randomized controlled trial; RIS = risperidone; s = significant; SAD = schizoaffective disorder; SER = sertindole; Sz = schizophrenia; ZIP = ziprasidone
Risperidone was examined in 3 trials,16,19,21 with all showing that combination treatment with risperidone did not significantly increase QTc interval. Two RCTs found no significant difference between clozapine plus risperidone and clozapine plus placebo,16 or risperidone plus haloperidol and risperidone alone, although the dose of risperidone in the former was one-half of that in the latter (2 and 4 mg/ day).21 One RCT, comparing clozapine plus risperidone with clozapine plus ziprasidone, indicated that QTc changes were not significant in the clozapine plus risperidone group.19 Ziprasidone was tested in 4 trials.17,19,20,25 One RCT showed that clozapine plus ziprasidone significantly prolonged QTc interval, compared with clozapine plus risperdione; however, there was no monotherapy arm in this study.19 Conversely, no significant difference in QTc intervals was observed between clozapine plus ziprasidone and clozapine alone in the other RCT.25 Similarly, 2 single-arm trials17,20 found that ziprasidone augmentation of clozapine did not significantly increase QTc interval. Aripiprazole was investigated in one trial,18 revealing no significant difference in QTc changes between clozapine plus aripiprazole and clozapine plus placebo. Sertindole was examined in one study23; sertindole plus clozapine significantly increased QTcBZT interval, compared with clozapine alone, which was not the case with QTcFRD interval. Pimozide was examined in 2 studies,22,24 with both investigations demonstrating no significant effect of pimozide plus clozapine on QTc prolongation, compared with clozapine monotherapy.
Observational Studies
Four observational studies26–29 (3 cross-sectional studies26–28 and 1 prospective study29) were identified (Table 2). All 3 cross-sectional studies26–28 failed to demonstrate any significant effect of antipsychotic polypharmacy on the QTc interval: 2 studies26,27 compared QTc intervals between antipsychotic polypharmacy and monotherapy, failing to show any significant differences between the 2 groups; another study28 found that QTc interval was not significantly influenced by dose, class, or number of antipsychotics. In contrast to these cross-sectional studies, one prospective study29 indicated that a significant increase in QTc interval was found in patients who received another antipsychotic in addition to an ongoing antipsychotic (that is, monotherapy to polypharmacy), while no significant change in QTc interval was observed in patients who had their antipsychotics switched to another (that is, monotherapy to a different type of monotherapy).
Table 2.
Observational studies on antipsychotic polypharmacy and corrected QT (QTc) interval
| Study | Year | Design | Diagnosis | Antipsychotic polypharmacy | Antipsychotic monotherapy | Group difference | ||||
|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
|||||||||
| n (male; female) | Mean age, years | Mean QTc, ms at mg/day | n (male; female) | Mean age, years | Mean QTc, ms at mg/day | |||||
| Mackin and Young26 | 2005 | Cross-sectional | n/a | 12 (n/a; n/a) | 45.3a | 403 | 53 (n/a; n/a) | 45.3a | 416 | ns |
| Correll et al27 | 2009 | Cross-sectional | n/a | 38 (25; 13) | 40.9 | 403 at 525b,c | 73 (44; 29) | 44.5 | 408 at 245b,c | ns |
| Ramos-Ríos et al28 | 2010 | Cross-sectional | Sz | 137 (n/a; n/a) | 55.8a | n/a | 34 (n/a; n/a) | 55.8a | n/a | nsd |
| Di Sciascio et al29 | 2011 | Prospective | Sz and BD | 42 (30; 12) | 36.0 | 369 at 477b to 387 at 845b s |
33 (25; 8) | 39.2 | 365 at 398b to 363 at 449b ns |
n/a |
For whole patients
Mean chlorpromazine-equivalent dose
All patients were treated with atypical antipsychotics
The number of antipsychotics did not significantly predict QTc interval.
BD = bipolar disorder; n/a = not available or not applicable; ns = nonsignificant; s = significant; Sz = schizophrenia
Case Reports
A total of 11 cases in 7 case reports30–36 were identified (Table 3). In 2 cases,30,31 QTc prolongations were improved after transitioning 2 antipsychotics to 1 single, different antipsychotic; of note, this improvement could have been due to a difference in types of antipsychotics rather than to the switch itself, from antipsychotic polypharmacy to monotherapy. In 2 cases,32,36 the addition of haloperidol or clozapine to aripiprazole resulted in QTc prolongation, and discontinuation of these adjunctive antipsychotics resolved the issue. In 5 cases,34,35 adding quetiapine to sertindole or paliperidone to clozapine did not significantly prolong QTc intervals. In 2 case reports,33 a switch to risperidone from amisulpride or discontinuation of amisulpride, which was used in combination with LAI antipsychotics, normalized QTc prolongation.
Table 3.
Cases of corrected QT (QTc) interval prolongation with antipsychotic polypharmacy
| Study | Year | Patient, age, years, and sex | Diagnosis | QTc change, ms, and antipsychotic polypharmacy (dose, mg/day) |
|---|---|---|---|---|
| Gurovich et al30 | 2003 | 66, F | SAD | 450 at QTP (n/a) + CPZ (n/a), then 416 after changing them to OLZ (40) |
| Nandagopal et al31 | 2003 | 46, M | Sz | 504 at RIS (2) + HPD (5), then 400 after changing them to QTP (150) |
| Leo et al32 | 2008 | 43, F | Sz | 415 at APZ (30), 492 after adding HPD (5), then 428 after discontinuing HPD |
| Lin et al33 | 2009 | 37, F | Sz | 510 at ASP (1400) + FPX-LAI (20), then 430 after changing ASP to RIS (n/a) |
| Lin et al33 | 2009 | 38, F | Sz | 507 at ASP (1400) + HPD-LAI (50), then normalized after discontinuing ASP |
| Hanisch et al34 | 2010 | 46, M | Sz | Not increased after adding QTP (300) to SER (20) |
| Esslinger et al35 | 2010 | 25, M | Sz | Not significantly changed after adding PAL (12) to CLZ (700) |
| Esslinger et al35 | 2010 | 28, F | MS | Not significantly changed after adding PAL (9) to CLZ (700) |
| Esslinger et al35 | 2010 | 38, F | Sz | Not significantly changed after adding PAL (12) to CLZ (350) |
| Esslinger et al35 | 2010 | 27, M | SAD | Not significantly changed after adding PAL (6) to CLZ (550) |
| Dhillon et al36 | 2011 | 61, F | SAD | 434–453 at APZ (30), 488–505 after adding CLZ (175), then 446–470 after discontinuing CLZ |
APZ = aripiprazole; ASP = amisulpride; CLZ = clozapine; CPZ = chlorpromazine; F = female; FPX = flupentixol; HPD = haloperidol; LAI = long-acting injectable; M = male; MS = multiple sclerosis; n/a = not available; OLZ = olanzapine; PAL = paliperidone; QTP = quetiapine; RIS = risperidone; SAD = schizoaffective disorder; SER = sertindole; Sz = schizophrenia
Discussion
Given the high prevalence of antipsychotic polypharmacy in real-world clinical practice, we conducted a systematic literature search to examine its relation with QTc interval. Notably, there is a paucity of evidence specific to this topic, which is a serious concern, given how frequently antipsychotic polypharmacy is employed. To our knowledge, there has been one systematic review on safety and tolerability issues of antipsychotic polypharmacy that included QT prolongation.37 The authors of that review searched 2 electronic sources (PubMed and Google scholar) in October 2011, identifying 4 relevant studies, and concluded that the evidence on antipsychotic polypharmacy and QTc prolongation is mixed. Here, we used 3 electric sources (MEDLINE, Embase, and PsycINFO) in October 2014, and adopted broader key words and inclusion criteria. This resulted in more articles identified in our current systematic review (21 articles); however, it is important to note that the main conclusions from both reviews are not substantially different. Moreover, evidence is still not substantive enough to draw firm conclusions.
The findings from our current systematic review can be summarized as follows. First, the paucity of data addressing QTc interval and antipsychotic polypharmacy is worrisome in light of the frequent use of antipsychotic polypharmacy. Second, clinical trials have shown that while a combination of clozapine with risperidone, aripiprazole, or pimozide is not obviously related to an increase in QTc interval, the addition of ziprasidone or sertindole to clozapine may have the potential to prolong QTc interval. Third, among observational studies, cross-sectional investigations have demonstrated that antipsychotic polypharmacy is not clearly associated with QTc prolongation, whereas one prospective study has shown a significant increase in QTc interval following antipsychotic augmentation. Fourth, case reports do suggest a risk of QTc prolongation, at least in some patients receiving antipsychotic polypharmacy. It is possible, though, that case reports represent unusual or dramatic cases, possibly introducing bias, and do not accurately reflect true event rates and their consequences.
Beyond the limited evidence addressing antipsychotic polypharmacy and QTc interval, how the interval is calculated also warrants comment. First, most of the clinical trials and observational studies included in this review depended on QTcBZT intervals; only 2 studies19,23 reported both QTcBZT and QTcFRD intervals. It may be ideal to report both QTc intervals, especially when the data are somewhat equivocal. Second, a QTc interval of more than 450 ms in men and more than 470 ms in women is regarded to represent clinically significant QTc prolongation.38 In addition, a QTc interval of more than 500 ms in both men and women is related to risk of cardiac events, such as syncope, cardiac arrest, and sudden cardiac death.39 Together with the threshold values, it is also important to consider absolute change from baseline QTc interval (that is, an increase of more than 60 ms40). However, these indices were not documented in numerous studies; for example, only 1 out of 10 clinical trials16 referred to these parameters. Further, use of QTc-prolonging drugs is only one of various risk factors in QTc prolongation that also includes advanced age, female sex, history of QTc prolongation, bradykinesia, cardiac diseases, congenital long QT syndrome, hypokalemia, and hypomagnesemia.41 As sex difference in QTc intervals is an established finding in the literature,38,42 it would be more clinically relevant to analyze QTc intervals separately for men and women. Along similar lines, some of the studies included in our review (3 clinical trials20,24,25 and 1 observational study29) excluded patients who had a history of a QTc interval of more than 450 ms or cardiac disease, limiting generalizability of results. In their comprehensive review of QTc prolongation and TdP associated with second-generation antipsychotics and antidepressants, Hasnain and Vieweg2 pointed out these same issues as limitations.
Also note, potentially synergistic effects of antipsychotic polypharmacy on QTc intervals cannot be addressed in our systematic review. More specifically, it cannot be ruled out that a combination of lower-risk, QTc-prolonging antipsychotics can prolong QTc interval. All the clinical trials indicating that antipsychotic polypharmacy prolonged QTc interval examined augmentation of a higher-risk, QTc-prolonging antipsychotic (that is, ziprasidone or sertindole)2,3 with a moderate-to-high-risk, QTc-prolonging antipsychotic (that is, clozapine).2,3 Once again, the limited data available clearly underscores a need for more work on this important topic.
Limitations of our review warrant comment. Our literature search was confined to English and, as mentioned, despite a systematic literature search, only a small number of clinical trials and observational studies were identified. Further, sample sizes were small in most of the reports. As no clinical trials have examined the effects of LAI plus oral or LAI antipsychotics on QTc intervals, the current findings cannot be generalized to this formulation. Our focus here was on antipsychotic polypharmacy, but, in clinical practice, many high-risk, QTc-prolonging psychotropics (for example, some antidepressants) are used in combination with antipsychotics. This form of psychotropic polypharmacy is also common but beyond the scope of our review. Finally, and importantly, all changes in QTc intervals may not directly translate to clinical consequences; for example, it has been shown that a so-called higher-risk medication (that is, ziprasidone) was not associated with an elevated risk of either cardiovascular mortality or sudden cardiac death relative to olanzapine in real-world use.43 People vulnerable to life-threatening consequences of QTc prolongation are likely to exhibit decompensated repolarization reserve.44 Importantly, clinical studies frequently exclude such frail patients with cardiac conditions, whereas this is not so with case reports. Accordingly, we need to remain somewhat cautious regarding the conclusion that we were unable to find unequivocal evidence of QTc prolongation associated with antipsychotic polypharmcy.
Conclusions
In summary, antipsychotic polypharmacy is frequently used in real-world clinical practice in the absence of solid evidence. Concurrently, the body of evidence regarding antipsychotic polypharmacy and QTc intervals is scant and inconsistent, with further studies needed. Currently available evidence fails to confirm that antipsychotic polypharmacy worsens QTc prolongation in general, although a combination of some higher-risk, QTc-prolonging antipsychotics (for example, clozapine plus ziprasidone or sertindole) may lengthen QTc intervals. A further argument for caution is the lack of robust evidence regarding efficacy with antipsychotic polypharmacy, as well as increased liability regarding numerous other unwanted side effects.37,45 From the standpoint of QTc prolongation, special attention is warranted, particularly when antipsychotic polypharmacy is employed in patients who have other risk factors of QTc prolongation.
Acknowledgments
Dr Takeuchi is supported by a Canadian Institutes of Health Research Fellowship. This funding source had no role in this study’s design, statistical analysis, interpretation of findings, manuscript preparation, or submission. Dr Takeuchi has received fellowship grants from the Centre for Addiction and Mental Health Foundation, the Japanese Society of Clinical Neuropsychopharmacology, and Astellas Foundation for Research on Metabolic Disorders, and manuscript fees from Dainippon Sumitomo Pharma.
Dr Suzuki has received speaker or manuscript fees from Astellas, Dainippon Sumitomo, Eli Lilly, Elsevier Japan, Janssen, Novartis, Meiji Seika, Otsuka, and Weily Japan.
Dr Remington has received research support from Novartis, Medicure, and Neurocrine Bioscience, consultant fees from Laboratorios Farmacéuticos Rovi, Synchroneuron, Novartis, and Roche, and speaker’s fees from Novartis.
Dr Uchida has received grants from Astellas Pharmaceutical, Eisai, Otsuka Pharmaceutical, GlaxoSmithKline, Shionogi, Dainippon-Sumitomo Pharma, Eli Lilly, Mochida Pharmaceutical, Meiji-Seika Pharma, and Yoshitomi Yakuhin, and speaker’s honoraria from Otsuka Pharmaceutical, Eli Lilly, Shionogi, GlaxoSmithKline, Yoshitomi Yakuhin, Dainippon-Sumitomo Pharma, Meiji-Seika Pharma, Abbvie, MSD, and Janssen Pharmaceutical.
The Canadian Psychiatric Association proudly supports the In Review series by providing an honorarium to the authors.
Abbreviations
- hERG
human ether-à-go-go-related gene
- LAI
long-acting injectable
- QT
time between start of Q wave and end of T wave
- QTc
corrected QT
- QTcBZT
QTc calculated with Bazett’s formula
- QTcFRD
QTc calculated with Fridericia’s formula
- RCT
randomized controlled trial
- RR
time between 2 consecutive R waves
- TdP
torsade de pointes
References
- 1.Leucht S, Cipriani A, Spineli L, et al. Comparative efficacy and tolerability of 15 antipsychotic drugs in schizophrenia: a multiple-treatments meta-analysis. Lancet. 2013;382(9896):951–962. doi: 10.1016/S0140-6736(13)60733-3. [DOI] [PubMed] [Google Scholar]
- 2.Hasnain M, Vieweg W. QTc interval prolongation and torsade de pointes associated with second-generation antipsychotics and antidepressants: a comprehensive review. CNS Drugs. 2014;28(10):887–920. doi: 10.1007/s40263-014-0196-9. [DOI] [PubMed] [Google Scholar]
- 3.CredibleMeds Worldwide. Oro Valley (AZ): CredibleMeds Worldwide; 2014. QTdrugs list. [cited 2014 Dec 28]. Available from: https://www.crediblemeds.org/new-drug-list. [Google Scholar]
- 4.Kongsamut S, Kang J, Chen XL, et al. A comparison of the receptor binding and hERG channel affinities for a series of antipsychotic drugs. Eur J Pharmacol. 2002;450(1):37–41. doi: 10.1016/s0014-2999(02)02074-5. [DOI] [PubMed] [Google Scholar]
- 5.Titier K, Canal M, Déridet E, et al. Determination of myocardium to plasma concentration ratios of five antipsychotic drugs: comparison with their ability to induce arrhythmia and sudden death in clinical practice. Toxicol Appl Pharmacol. 2004;199(1):52–60. doi: 10.1016/j.taap.2004.03.016. [DOI] [PubMed] [Google Scholar]
- 6.Malik M, Färbom P, Batchvarov V, et al. Relation between QT and RR intervals is highly individual among healthy subjects: implications for heart rate correction of the QT interval. Heart. 2002;87(3):220–228. doi: 10.1136/heart.87.3.220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Moss AJ. Measurement of the QT interval and the risk associated with QTc interval prolongation: a review. Am J Cardiol. 1993;72(6):23B–25B. doi: 10.1016/0002-9149(93)90036-c. [DOI] [PubMed] [Google Scholar]
- 8.Zhang Y, Post WS, Blasco-Colmenares E, et al. Electrocardiographic QT interval and mortality: a meta-analysis. Epidemiology. 2011;22(5):660–670. doi: 10.1097/EDE.0b013e318225768b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Van Noord C, Sturkenboom MC, Straus SM, et al. Non-cardiovascular drugs that inhibit hERG-encoded potassium channels and risk of sudden cardiac death. Heart. 2011;97(3):215–220. doi: 10.1136/hrt.2009.188367. [DOI] [PubMed] [Google Scholar]
- 10.Gallego JA, Bonetti J, Zhang J, et al. Prevalence and correlates of antipsychotic polypharmacy: a systematic review and meta-regression of global and regional trends from the 1970s to 2009. Schizophr Res. 2012;138(1):18–28. doi: 10.1016/j.schres.2012.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sommer IE, Begemann MJ, Temmerman A, et al. Pharmacological augmentation strategies for schizophrenia patients with insufficient response to clozapine: a quantitative literature review. Schizophr Bull. 2012;38(5):1003–1011. doi: 10.1093/schbul/sbr004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Taylor DM, Smith L, Gee SH, et al. Augmentation of clozapine with a second antipsychotic—a meta-analysis. Acta Psychiatr Scand. 2012;125(1):15–24. doi: 10.1111/j.1600-0447.2011.01792.x. [DOI] [PubMed] [Google Scholar]
- 13.Veerman SR, Schulte PF, Begemann MJ, et al. Non-glutamatergic clozapine augmentation strategies: a review and meta-analysis. Pharmacopsychiatry. 2014;47(7):231–238. doi: 10.1055/s-0034-1385930. [DOI] [PubMed] [Google Scholar]
- 14.Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ. 2009;339:b2535. doi: 10.1136/bmj.b2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Correll CU, Rummel-Kluge C, Corves C, et al. Antipsychotic combinations vs monotherapy in schizophrenia: a meta-analysis of randomized controlled trials. Schizophr Bull. 2009;35(2):443–457. doi: 10.1093/schbul/sbn018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Anil Yağcioğlu AE, Kivircik Akdede BB, Turgut TI, et al. A double-blind controlled study of adjunctive treatment with risperidone in schizophrenic patients partially responsive to clozapine: efficacy and safety. J Clin Psychiatry. 2005;66(1):63–72. doi: 10.4088/jcp.v66n0109. [DOI] [PubMed] [Google Scholar]
- 17.Ziegenbein M, Kropp S, Kuenzel HE. Combination of clozapine and ziprasidone in treatment-resistant schizophrenia: an open clinical study. Clin Neuropharmacol. 2005;28(5):220–224. doi: 10.1097/01.wnf.0000183446.58529.30. [DOI] [PubMed] [Google Scholar]
- 18.Chang JS, Ahn YM, Park HJ, et al. Aripiprazole augmentation in clozapine-treated patients with refractory schizophrenia: an 8-week, randomized, double-blind, placebo-controlled trial. J Clin Psychiatry. 2008;69(5):720–731. doi: 10.4088/jcp.v69n0505. [DOI] [PubMed] [Google Scholar]
- 19.Zink M, Kuwilsky A, Krumm B, et al. Efficacy and tolerability of ziprasidone versus risperidone as augmentation in patients partially responsive to clozapine: a randomised controlled clinical trial. J Psychopharmacol. 2009;23(3):305–314. doi: 10.1177/0269881108089593. [DOI] [PubMed] [Google Scholar]
- 20.Henderson DC, Fan X, Copeland PM, et al. Ziprasidone as an adjuvant for clozapine- or olanzapine-associated medical morbidity in chronic schizophrenia. Hum Psychopharmacol. 2009;24(3):225–232. doi: 10.1002/hup.1012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lin CH, Kuo CC, Chou LS, et al. A randomized, double-blind comparison of risperidone versus low-dose risperidone plus low-dose haloperidol in treating schizophrenia. J Clin Psychopharmacol. 2010;30(5):518–525. doi: 10.1097/JCP.0b013e3181f28dff. [DOI] [PubMed] [Google Scholar]
- 22.Friedman JI, Lindenmayer JP, Alcantara F, et al. Pimozide augmentation of clozapine inpatients with schizophrenia and schizoaffective disorder unresponsive to clozapine monotherapy. Neuropsychopharmacology. 2011;36(6):1289–1295. doi: 10.1038/npp.2011.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nielsen J, Emborg C, Gydesen S, et al. Augmenting clozapine with sertindole: a double-blind, randomized, placebo-controlled study. J Clin Psychopharmacol. 2012;32(2):173–178. doi: 10.1097/JCP.0b013e318248dfb8. [DOI] [PubMed] [Google Scholar]
- 24.Gunduz-Bruce H, Oliver S, Gueorguieva R, et al. Efficacy of pimozide augmentation for clozapine partial responders with schizophrenia. Schizophr Res. 2013;143(2–3):344–347. doi: 10.1016/j.schres.2012.11.008. [DOI] [PubMed] [Google Scholar]
- 25.Muscatello MR, Pandolfo G, Micò U, et al. Augmentation of clozapine with ziprasidone in refractory schizophrenia: a double-blind, placebo-controlled study. J Clin Psychopharmacol. 2014;34(1):129–133. doi: 10.1097/JCP.0000000000000042. [DOI] [PubMed] [Google Scholar]
- 26.Mackin P, Young AH. QTc interval measurement and metabolic parameters in psychiatric patients taking typical or atypical antipsychotic drugs: a preliminary study. J Clin Psychiatry. 2005;66(11):1386–1391. doi: 10.4088/jcp.v66n1107. [DOI] [PubMed] [Google Scholar]
- 27.Correll CU, Frederickson AM, Figen V, et al. The QTc interval and its dispersion in patients receiving two atypical antipsychotics. Eur Arch Psychiatry Clin Neurosci. 2009;259(1):23–27. doi: 10.1007/s00406-008-0829-4. [DOI] [PubMed] [Google Scholar]
- 28.Ramos-Ríos R, Arrojo-Romero M, Paz-Silva E, et al. QTc interval in a sample of long-term schizophrenia inpatients. Schizophr Res. 2010;116(1):35–43. doi: 10.1016/j.schres.2009.09.041. [DOI] [PubMed] [Google Scholar]
- 29.Di Sciascio G, Calo S, Amodio G, et al. The use of first generation versus second generation antipsychotics as add-on or as switch treatment and its effect on QTC interval: the Italian experience in a real-world setting. Int J Immunopathol Pharmacol. 2011;24(1):225–230. doi: 10.1177/039463201102400127. [DOI] [PubMed] [Google Scholar]
- 30.Gurovich I, Vempaty A, Lippmann S. QTc prolongation: chlorpromazine and high-dosage olanzapine. Can J Psychiatry. 2003;48(5):348. doi: 10.1177/070674370304800513. [DOI] [PubMed] [Google Scholar]
- 31.Nandagopal JJ, Craig JM, Lippmann S. QTc prolongation: possible association with risperidone and/or haloperidol. Psychosomatics. 2003;44(6):521. doi: 10.1176/appi.psy.44.6.521. [DOI] [PubMed] [Google Scholar]
- 32.Leo R, Razzini C, Di Lorenzo G, et al. Asymptomatic QTc prolongation during coadministration of aripiprazole and haloperidol. J Clin Psychiatry. 2008;69(2):327–328. doi: 10.4088/jcp.v69n0221d. [DOI] [PubMed] [Google Scholar]
- 33.Lin Y, Sun IW, Liu SI, et al. QTc prolongation during concurrent treatment with depot antipsychotics and high-dose amisulpride: a report of 2 cases. J Intern Med Taiwan. 2009;20(6):544–549. [Google Scholar]
- 34.Hanisch F, Friedemann J, Pillmann F. Combined treatment with quetiapine and sertindole in therapy refractory insomnia after clozapine discontinuation. J Psychopharmacol. 2010;24(11):1725–1726. doi: 10.1177/0269881109348159. [DOI] [PubMed] [Google Scholar]
- 35.Esslinger C, Inta D, Englisch S, et al. Clozapine combined with paliperidone observations in schizophrenic patients with insufficient responses to clozapine monotherapy. German J Psychiatry. 2010;13:37–40. [Google Scholar]
- 36.Dhillon R, Bastiampillai T, Tee K, et al. Clozapine and associated QTc prolongation. Aust N Z J Psychiatry. 2011;45(12):1098–1099. doi: 10.3109/00048674.2011.619163. [DOI] [PubMed] [Google Scholar]
- 37.Gallego JA, Nielsen J, De Hert M, et al. Safety and tolerability of antipsychotic polypharmacy. Expert Opin Drug Saf. 2012;11(4):527–542. doi: 10.1517/14740338.2012.683523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Goldenberg I, Moss AJ, Zareba W. QT interval: how to measure it and what is “normal.”. J Cardiovasc Electrophysiol. 2006;17(3):333–336. doi: 10.1111/j.1540-8167.2006.00408.x. [DOI] [PubMed] [Google Scholar]
- 39.Priori SG, Schwartz PJ, Napolitano C, et al. Risk stratification in the long-QT syndrome. N Engl J Med. 2003;348(19):1866–1874. doi: 10.1056/NEJMoa022147. [DOI] [PubMed] [Google Scholar]
- 40.Clinical evaluation of QT/QTc interval prolongation and proarrhythmic potential for non-antiarrhythmic drugs. International Conference on Harmonisation (ICH) guidelines, E14.; Geneva (CH): ICH Expert Working Group; 2005. [cited 2014 Dec 28]. Available from: http://www.ich.org/fileadmin/Public_Web_Site/ICH_Products/Guidelines/Efficacy/E14/E14_Guideline.pdf. [PubMed] [Google Scholar]
- 41.Trinkley KE, Page RL, Lien H, et al. QT interval prolongation and the risk of torsades de pointes: essentials for clinicians. Curr Med Res Opin. 2013;29(12):1719–1726. doi: 10.1185/03007995.2013.840568. [DOI] [PubMed] [Google Scholar]
- 42.De Yang F, Wang XQ, Liu XP, et al. Sex difference in QTc prolongation in chronic institutionalized patients with schizophrenia on long-term treatment with typical and atypical antipsychotics. Psychopharmacology (Berl) 2011;216(1):9–16. doi: 10.1007/s00213-011-2188-5. [DOI] [PubMed] [Google Scholar]
- 43.Strom BL, Eng SM, Faich G, et al. Comparative mortality associated with ziprasidone and olanzapine in real-world use among 18,154 patients with schizophrenia: the Ziprasidone Observational Study of Cardiac Outcomes (ZODIAC) Am J Psychiatry. 2011;168(2):193–201. doi: 10.1176/appi.ajp.2010.08040484. [DOI] [PubMed] [Google Scholar]
- 44.Sugiyama A. Sensitive and reliable proarrhythmia in vivo animal models for predicting drug-induced torsades de pointes in patients with remodelled hearts. Br J Pharmacol. 2008;154(7):1528–1537. doi: 10.1038/bjp.2008.240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Fleischhacker WW, Uchida H. Critical review of antipsychotic polypharmacy in the treatment of schizophrenia. Int J Neuropsychopharmacol. 2014;17(7):1083–1093. doi: 10.1017/S1461145712000399. [DOI] [PubMed] [Google Scholar]