Abstract
Thyrotropin-releasing hormone (TRH) is an important extracellular signal substance that acts as a hypothalamic-releasing factor, which stimulates the release of adenohypophyseal hormones and functions as a neurotransmitter/neuromodulator in the central and peripheral nervous system. The inactivation of TRH after its release is catalyzed by an ectoenzyme localized preferentially on neuronal cells in the brain and on lactotrophic pituitary cells. This enzyme exhibits a very high degree of substrate specificity as well as other unusual properties. The activity of the adenohypophyseal enzyme is stringently controlled by estradiol and thyroid hormones, indicating that this enzyme itself may serve regulatory functions. Fragments of the enzyme isolated from rat or pig brain were generated by enzymatic digestion or cyanogen bromide cleavage, purified by reverse-phase HPLC, and sequenced. PCR amplification and screening of cDNA libraries from rat brain and pituitary led to the identification and isolation of a cDNA that encodes a protein of 1025 amino acids. The analysis of the deduced amino acid sequence was consistent with the identification of the enzyme as a glycosylated, membrane-anchored Zn metallopeptidase. Furthermore, Northern blot analysis demonstrated that the mRNA levels paralleled the tissue distribution of the enzyme and that in pituitary tissue the transcript levels rapidly increased when the animals were treated with triiodothyronine. Finally, transient transfection of COS-7 cells with this cDNA led to the expression of an active ectopeptidase that displayed the characteristics of the TRH-degrading ectoenzyme.
Full text
PDF
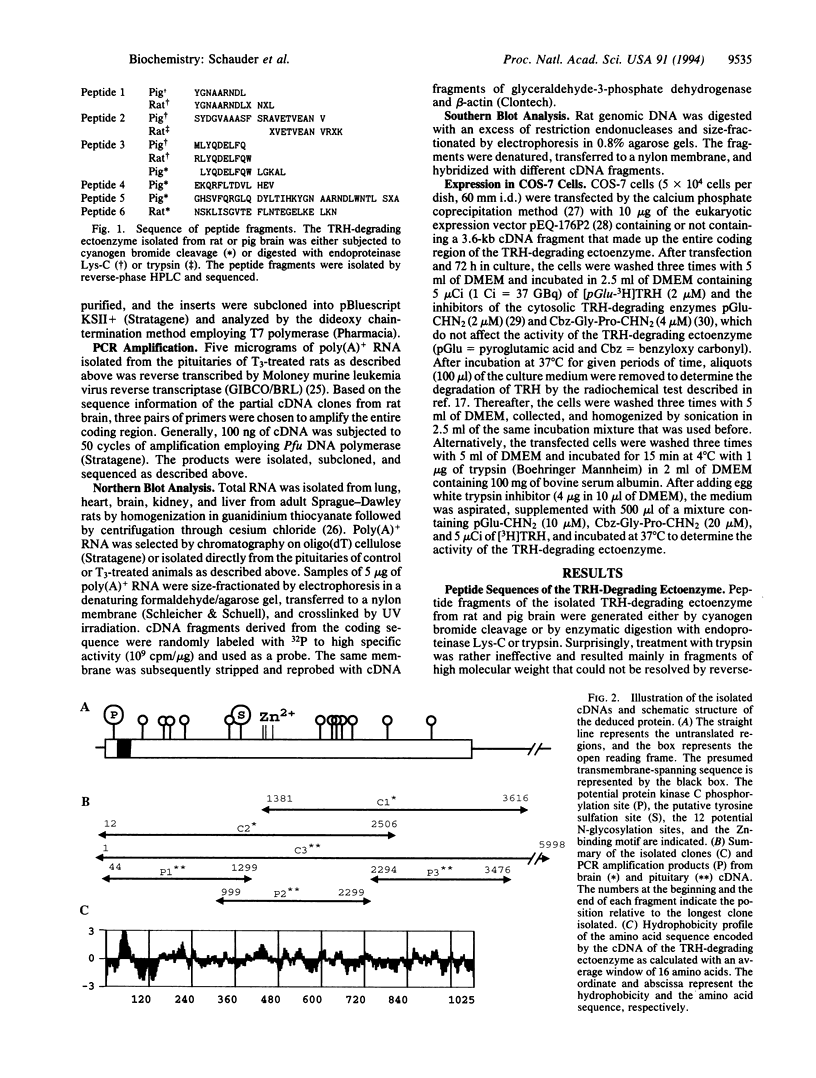

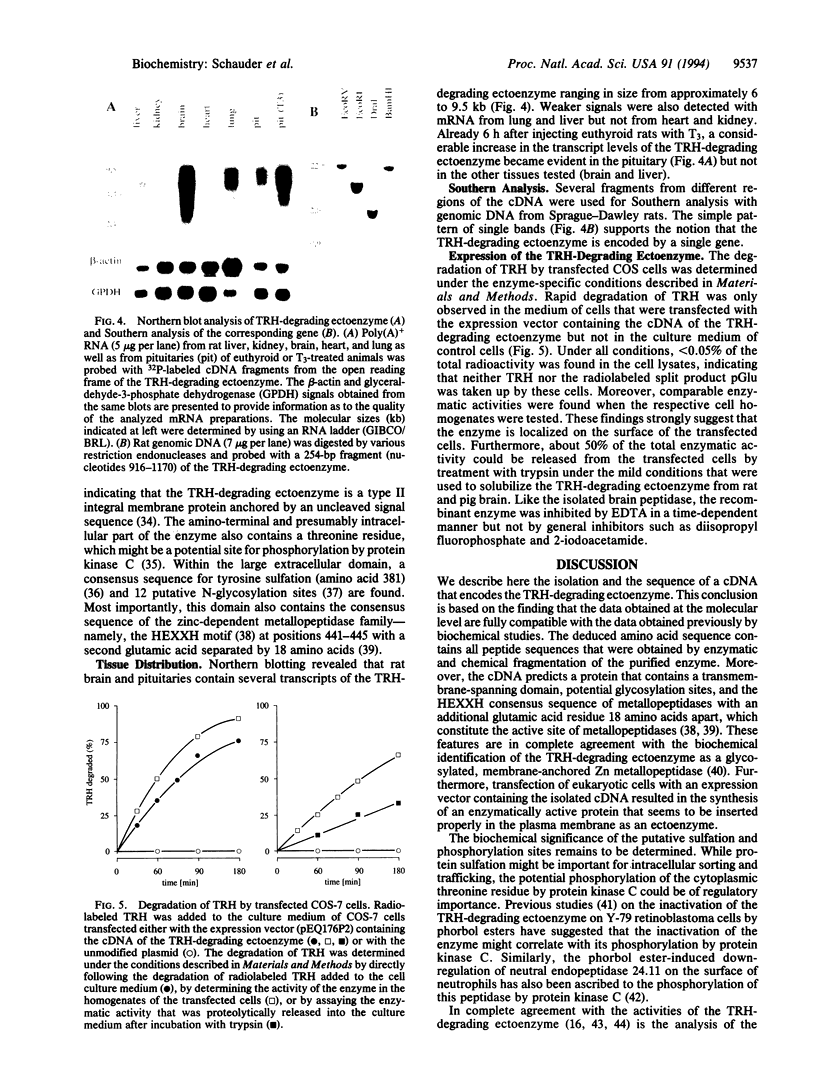

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bauer K. Adenohypophyseal degradation of thyrotropin releasing hormone regulated by thyroid hormones. 1987 Nov 26-Dec 2Nature. 330(6146):375–377. doi: 10.1038/330375a0. [DOI] [PubMed] [Google Scholar]
- Bauer K., Carmeliet P., Schulz M., Baes M., Denef C. Regulation and cellular localization of the membrane-bound thyrotropin-releasing hormone-degrading enzyme in primary cultures of neuronal, glial and adenohypophyseal cells. Endocrinology. 1990 Sep;127(3):1224–1233. doi: 10.1210/endo-127-3-1224. [DOI] [PubMed] [Google Scholar]
- Bauer K. Degradation and biological inactivation of thyrotropin releasing hormone (TRH): regulation of the membrane-bound TRH-degrading enzyme from rat anterior pituitary by estrogens and thyroid hormones. Biochimie. 1988 Jan;70(1):69–74. doi: 10.1016/0300-9084(88)90160-5. [DOI] [PubMed] [Google Scholar]
- Bauer K., Nowak P., Kleinkauf H. Specificity of a serum peptidase hydrolyzing thyroliberin at pyroglutamyl-histidine bone. Eur J Biochem. 1981 Aug;118(1):173–176. doi: 10.1111/j.1432-1033.1981.tb05501.x. [DOI] [PubMed] [Google Scholar]
- Chirgwin J. M., Przybyla A. E., MacDonald R. J., Rutter W. J. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry. 1979 Nov 27;18(24):5294–5299. doi: 10.1021/bi00591a005. [DOI] [PubMed] [Google Scholar]
- Cruz C., Charli J. L., Vargas M. A., Joseph-Bravo P. Neuronal localization of pyroglutamate aminopeptidase II in primary cultures of fetal mouse brain. J Neurochem. 1991 May;56(5):1594–1601. doi: 10.1111/j.1471-4159.1991.tb02056.x. [DOI] [PubMed] [Google Scholar]
- Czekay G., Bauer K. Identification of the thyrotropin-releasing-hormone-degrading ectoenzyme as a metallopeptidase. Biochem J. 1993 Mar 15;290(Pt 3):921–926. doi: 10.1042/bj2900921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erdös E. G., Wagner B., Harbury C. B., Painter R. G., Skidgel R. A., Fa X. G. Down-regulation and inactivation of neutral endopeptidase 24.11 (enkephalinase) in human neutrophils. J Biol Chem. 1989 Aug 25;264(24):14519–14523. [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity. Anal Biochem. 1983 Jul 1;132(1):6–13. doi: 10.1016/0003-2697(83)90418-9. [DOI] [PubMed] [Google Scholar]
- Firzlaff J. M., Lüscher B., Eisenman R. N. Negative charge at the casein kinase II phosphorylation site is important for transformation but not for Rb protein binding by the E7 protein of human papillomavirus type 16. Proc Natl Acad Sci U S A. 1991 Jun 15;88(12):5187–5191. doi: 10.1073/pnas.88.12.5187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman T. C., Wilk S. Delineation of a particulate thyrotropin-releasing hormone-degrading enzyme in rat brain by the use of specific inhibitors of prolyl endopeptidase and pyroglutamyl peptide hydrolase. J Neurochem. 1986 Apr;46(4):1231–1239. doi: 10.1111/j.1471-4159.1986.tb00643.x. [DOI] [PubMed] [Google Scholar]
- Griffiths E. C. Thyrotrophin releasing hormone: endocrine and central effects. Psychoneuroendocrinology. 1985;10(3):225–235. doi: 10.1016/0306-4530(85)90001-0. [DOI] [PubMed] [Google Scholar]
- Gubler U., Hoffman B. J. A simple and very efficient method for generating cDNA libraries. Gene. 1983 Nov;25(2-3):263–269. doi: 10.1016/0378-1119(83)90230-5. [DOI] [PubMed] [Google Scholar]
- Guillemin R. Peptides in the brain: the new endocrinology of the neuron. Science. 1978 Oct 27;202(4366):390–402. doi: 10.1126/science.212832. [DOI] [PubMed] [Google Scholar]
- Hinkle P. M. Pituitary TRH receptors. Ann N Y Acad Sci. 1989;553:176–187. doi: 10.1111/j.1749-6632.1989.tb46639.x. [DOI] [PubMed] [Google Scholar]
- Horita A., Carino M. A., Lai H. Pharmacology of thyrotropin-releasing hormone. Annu Rev Pharmacol Toxicol. 1986;26:311–332. doi: 10.1146/annurev.pa.26.040186.001523. [DOI] [PubMed] [Google Scholar]
- Horsthemke B., Leblanc P., Kordon C., Wattiaux-De Coninck S., Wattiaux R., Bauer K. Subcellular distribution of particle-bound neutral peptidases capable of hydrolyzing gonadoliberin, thyroliberin, enkephalin and substance P. Eur J Biochem. 1984 Mar 1;139(2):315–320. doi: 10.1111/j.1432-1033.1984.tb08009.x. [DOI] [PubMed] [Google Scholar]
- Hortin G., Folz R., Gordon J. I., Strauss A. W. Characterization of sites of tyrosine sulfation in proteins and criteria for predicting their occurrence. Biochem Biophys Res Commun. 1986 Nov 26;141(1):326–333. doi: 10.1016/s0006-291x(86)80372-2. [DOI] [PubMed] [Google Scholar]
- Hubbard S. C., Ivatt R. J. Synthesis and processing of asparagine-linked oligosaccharides. Annu Rev Biochem. 1981;50:555–583. doi: 10.1146/annurev.bi.50.070181.003011. [DOI] [PubMed] [Google Scholar]
- Hökfelt T., Tsuruo Y., Ulfhake B., Cullheim S., Arvidsson U., Foster G. A., Schultzberg M., Schalling M., Arborelius L., Freedman J. Distribution of TRH-like immunoreactivity with special reference to coexistence with other neuroactive compounds. Ann N Y Acad Sci. 1989;553:76–105. doi: 10.1111/j.1749-6632.1989.tb46633.x. [DOI] [PubMed] [Google Scholar]
- Jackson I. M. Thyrotropin-releasing hormone. N Engl J Med. 1982 Jan 21;306(3):145–155. doi: 10.1056/NEJM198201213060305. [DOI] [PubMed] [Google Scholar]
- Jongeneel C. V., Bouvier J., Bairoch A. A unique signature identifies a family of zinc-dependent metallopeptidases. FEBS Lett. 1989 Jan 2;242(2):211–214. doi: 10.1016/0014-5793(89)80471-5. [DOI] [PubMed] [Google Scholar]
- Knisatschek H., Bauer K. Specific inhibition of post proline cleaving enzyme by benzyloxycarbonyl-Gly-Pro-diazomethyl ketone. Biochem Biophys Res Commun. 1986 Jan 29;134(2):888–894. doi: 10.1016/s0006-291x(86)80503-4. [DOI] [PubMed] [Google Scholar]
- Kozak M. Structural features in eukaryotic mRNAs that modulate the initiation of translation. J Biol Chem. 1991 Oct 25;266(30):19867–19870. [PubMed] [Google Scholar]
- Kyte J., Doolittle R. F. A simple method for displaying the hydropathic character of a protein. J Mol Biol. 1982 May 5;157(1):105–132. doi: 10.1016/0022-2836(82)90515-0. [DOI] [PubMed] [Google Scholar]
- Lechan R. M., Segerson T. P. Pro-TRH gene expression and precursor peptides in rat brain. Observations by hybridization analysis and immunocytochemistry. Ann N Y Acad Sci. 1989;553:29–59. doi: 10.1111/j.1749-6632.1989.tb46630.x. [DOI] [PubMed] [Google Scholar]
- Malfroy B., Kado-Fong H., Gros C., Giros B., Schwartz J. C., Hellmiss R. Molecular cloning and amino acid sequence of rat kidney aminopeptidase M: a member of a super family of zinc-metallohydrolases. Biochem Biophys Res Commun. 1989 May 30;161(1):236–241. doi: 10.1016/0006-291x(89)91586-6. [DOI] [PubMed] [Google Scholar]
- O'Connor B., O'Cuinn G. Localization of a narrow-specificity thyroliberin hydrolyzing pyroglutamate aminopeptidase in synaptosomal membranes of guinea-pig brain. Eur J Biochem. 1984 Oct 15;144(2):271–278. doi: 10.1111/j.1432-1033.1984.tb08460.x. [DOI] [PubMed] [Google Scholar]
- O'Connor B., O'Cuinn G. Purification of and kinetic studies on a narrow specificity synaptosomal membrane pyroglutamate aminopeptidase from guinea-pig brain. Eur J Biochem. 1985 Jul 1;150(1):47–52. doi: 10.1111/j.1432-1033.1985.tb08986.x. [DOI] [PubMed] [Google Scholar]
- O'Cuinn G., O'Connor B., Elmore M. Degradation of thyrotropin-releasing hormone and luteinising hormone-releasing hormone by enzymes of brain tissue. J Neurochem. 1990 Jan;54(1):1–13. doi: 10.1111/j.1471-4159.1990.tb13276.x. [DOI] [PubMed] [Google Scholar]
- Olsen J., Cowell G. M., Kønigshøfer E., Danielsen E. M., Møller J., Laustsen L., Hansen O. C., Welinder K. G., Engberg J., Hunziker W. Complete amino acid sequence of human intestinal aminopeptidase N as deduced from cloned cDNA. FEBS Lett. 1988 Oct 10;238(2):307–314. doi: 10.1016/0014-5793(88)80502-7. [DOI] [PubMed] [Google Scholar]
- Ponce G., Charli J. L., Pasten J. A., Aceves C., Joseph-Bravo P. Tissue-specific regulation of pyroglutamate aminopeptidase II activity by thyroid hormones. Neuroendocrinology. 1988 Aug;48(2):211–213. doi: 10.1159/000125011. [DOI] [PubMed] [Google Scholar]
- Schally A. V. Aspects of hypothalamic regulation of the pituitary gland. Science. 1978 Oct 6;202(4363):18–28. doi: 10.1126/science.99816. [DOI] [PubMed] [Google Scholar]
- Sharif N. A. Quantitative autoradiography of TRH receptors in discrete brain regions of different mammalian species. Ann N Y Acad Sci. 1989;553:147–175. doi: 10.1111/j.1749-6632.1989.tb46638.x. [DOI] [PubMed] [Google Scholar]
- Singer S. J., Maher P. A., Yaffe M. P. On the transfer of integral proteins into membranes. Proc Natl Acad Sci U S A. 1987 Apr;84(7):1960–1964. doi: 10.1073/pnas.84.7.1960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suen C. S., Wilk S. Rapid inactivation and phosphorylation of pyroglutamyl peptidase II in Y-79 human retinoblastoma cells after exposure to phorbol ester. Endocrinology. 1990 Dec;127(6):3038–3046. doi: 10.1210/endo-127-6-3038. [DOI] [PubMed] [Google Scholar]
- Suen C. S., Wilk S. Regulation of thyrotropin releasing hormone degrading enzymes in rat brain and pituitary by L-3,5,3'-triiodothyronine. J Neurochem. 1989 Mar;52(3):884–888. doi: 10.1111/j.1471-4159.1989.tb02537.x. [DOI] [PubMed] [Google Scholar]
- Vallee B. L., Auld D. S. Zinc coordination, function, and structure of zinc enzymes and other proteins. Biochemistry. 1990 Jun 19;29(24):5647–5659. doi: 10.1021/bi00476a001. [DOI] [PubMed] [Google Scholar]
- Vargas M. A., Cisneros M., Herrera J., Joseph-Bravo P., Charli J. L. Regional distribution of pyroglutamyl peptidase II in rabbit brain, spinal cord, and organs. Peptides. 1992 Mar-Apr;13(2):255–260. doi: 10.1016/0196-9781(92)90105-c. [DOI] [PubMed] [Google Scholar]
- Watt V. M., Yip C. C. Amino acid sequence deduced from a rat kidney cDNA suggests it encodes the Zn-peptidase aminopeptidase N. J Biol Chem. 1989 Apr 5;264(10):5480–5487. [PubMed] [Google Scholar]
- Wigler M., Silverstein S., Lee L. S., Pellicer A., Cheng Y. c., Axel R. Transfer of purified herpes virus thymidine kinase gene to cultured mouse cells. Cell. 1977 May;11(1):223–232. doi: 10.1016/0092-8674(77)90333-6. [DOI] [PubMed] [Google Scholar]
- Wilk S., Friedman T. C., Kline T. B. Pyroglutamyl diazomethyl ketone: potent inhibitor of mammalian pyroglutamyl peptide hydrolase. Biochem Biophys Res Commun. 1985 Jul 31;130(2):662–668. doi: 10.1016/0006-291x(85)90468-1. [DOI] [PubMed] [Google Scholar]
- Woodgett J. R., Gould K. L., Hunter T. Substrate specificity of protein kinase C. Use of synthetic peptides corresponding to physiological sites as probes for substrate recognition requirements. Eur J Biochem. 1986 Nov 17;161(1):177–184. doi: 10.1111/j.1432-1033.1986.tb10139.x. [DOI] [PubMed] [Google Scholar]
- Wu Q., Lahti J. M., Air G. M., Burrows P. D., Cooper M. D. Molecular cloning of the murine BP-1/6C3 antigen: a member of the zinc-dependent metallopeptidase family. Proc Natl Acad Sci U S A. 1990 Feb;87(3):993–997. doi: 10.1073/pnas.87.3.993. [DOI] [PMC free article] [PubMed] [Google Scholar]