Abstract
Down syndrome (DS) is one of the most common aneuploidy. In general population, its prevalence is 1:600–1:800 live births. It is caused by a trisomy of chromosome 21. DS is phenotypically manifested by premature aging, upward slant to the eyes, epicanthus, flattened face, and poor muscle tone. In addition to physical changes, this syndrome is characterized by early onset of diseases specific to old age, such as Alzheimer's disease, vision and hearing problems, and precocious menopause. Since DS symptoms include premature aging, the shortening of telomeres might be one of the markers of cellular aging. Consequently, the aim of the study was to determine the length of the telomeres in leukocytes from the blood of juvenile patients with DS (n=68) compared to an age-matched control group (n=56) and also to determine the diagnostic or predictive value for this parameter. We show that, for the first time, in juveniles, the average relative telomere length in studied subjects is significantly longer than in the control group (50.46 vs. 40.56, respectively arbitrary units [AU]; p=0.0026). The results provide interesting basis for further research to determine the causes and consequences of telomere maintaining and the dynamics of this process in patients with DS.
Introduction
Among numerous functions of telomeres, they limit the number of cell divisions, thus preventing the accumulation of mutations that might lead to a malignant transformation. Telomere loss (also observed in Down syndrome [DS]) could provide the signal for cells to enter senescence, leading to the formation of dicentric chromosomes and classical breakage–fusion–bridge cycles, followed by genome rearrangements and aneuploidy. Consequently, this might lead to further impairments and faster dementia, observed in DS and in the course of many aging-associated disorders (Honig et al., 2012). A key concern to aging adults with DS is the increased risk of Alzheimer's disease (AD). The profile and sequence of cognitive impairments in adults with DS are similar to those seen with AD in the general population. Memory processes are affected early in the course of DS dementia (Krinsky-McHale and Silverman, 2013). Severe cognitive deterioration, such as acquired apraxia and agnosia, has been reported in 28% of individuals with DS at the age of 30 years, with a higher prevalence of these impairments in the subsequent years (Head et al., 2012). The earliest manifestations of dementia in DS may involve changes in personality and behavior. The diagnosis of this mental disorder in DS can be challenging against the background of preexisting intellectual impairment. Standardized criteria for the diagnosis of dementia in DS include both informant-based and direct measures (O'Caoimh and Clune, 2013). The severity of preexisting cognitive impairment may also be a predictor of the rate of cognitive deterioration in DS (Temple et al., 2001). However, determining whether it is a cause or a consequence is not clear yet. We still do not know what factors, when and how, induce increased aging and telomere loss in DS patients. This might be an individual issue resulting from the mother's metabolic profile (Révész et al., 2014), initial telomere at birth, or environmental factors (Nakamura et al., 2014).
Advanced maternal age is a well-documented risk factor for chromosome 21 nondisjunction in humans, but the understanding of this association at the genetic level is still limited. In particular, the state of maternal genetic age is unclear. Most studies focus on maternal and parental age and maternal telomere length (TL) in the context of DS birth risk (Ghosh et al., 2010; Eisenberg, 2014). Since replicative senescence could partially account for aging of the immune system in DS patients and in elderly individuals, TL assessment was proposed as a marker of aging and dementia status of those patients (Vaziri et al., 1993). However, even if telomere shortening is known to be associated with DS, the exact mechanism and association remain unclear. It is still not known which factors can trigger or accelerate this process and when they are turned on.
Materials and Methods
Studied patients and controls
The study group comprised 68 DS children (mean age 4.5±3.1 years; age ranged from 2 to 21 years; 28 boys, 40 girls). All patients were recruited from the Centre for Orthodontic Mini-implants at the Department and Clinic of Maxillofacial Orthopedics and Orthodontics, Poznan University of Medical Sciences in Poznan, Poland. The control group comprised 56 healthy age-matched children (mean age 4.5±3.4 years; 32 boys, 24 girls; age ranged from 2 to 17 years). Both patients and control participants were Caucasians. The study protocol was approved by the ethics committee of the Poznan University of Medical Sciences (97/11), and written consent was provided by the parents of participating minor individuals.
DNA isolation
DNA was extracted from peripheral blood leukocytes using a minicolumn-based DNA isolation kit (A&A Biotechnology, Gdynia, Poland) and stored at −20°C, as previously described (Rubiś et al., 2012). One hundred microliters of peripheral blood were used for isolation of DNA. High-concentration samples of genomic DNA were prepared in decimal concentrations (quantitative polymerase chain reaction [qPCR] standards) to cover all possible measurements range. One hundred microliters of peripheral blood were used for isolation of DNA, and 10 ng of genomic DNA were taken for qPCR.
Real-time PCR
TL was assessed using two pairs of primers, that is, telomere specific and single-copy (albumin) gene specific (Cawthon, 2009; O'Callaghan and Fenech, 2011). The specificity of primers has been affirmed by O'Callaghan and Fenech (2011) in conditions providing efficiency close to 100% (2.5 mM MgCl2; 0.5 μM primers) (Table 1). Initial denaturation and polymerase activation (hot start) were performed at 95°C for 10 min, followed by two cycles of 94°C/15 s and 49°C/15 s without fluorescence acquisition. The signal was detected during another 40 cycles of 94°C/10 s, 66°C/10 s, and 72°C/10 s. Melting analysis (65–95°C range, 0.2°C resolution) at the end of the reaction was performed to verify the specificity of the product and indicated a Tm of 81.7. The efficiency of the reaction was no lower than 97.8% and not higher than 102%. Importantly, this result was repeatable for all the samples that were analyzed (in serial dilutions). Similarly, reaction conditions for albumin were as follows: denaturation at 95°C/10 min—hot start, followed by 45 cycles of 94°C/10 s, 61°C/10 s, and 72/10 s. The concentration of primers was 0.5 μM and magnesium chloride was 2.5 mM. The Tm of the product (analysis performed as above) was 80.7 and the efficiency was 99.6%. The TL was assessed using a qPCR system (LC 2.0; Roche) and SybrGreen kit (Roche Diagnostics). Blind quality control samples were included into each qPCR. All samples were run in triplicates (additionally, one sample was repeatedly included in all the studied tests). The results were verified by analysis of four samples using the Southern blot assay, which revealed that the qPCR method is reproducible.
Table 1.
The Composition of the qPCR Mixture for Amplification of Telomeric Repeats and Albumin Gene Fragments
| Reagents | Volume (μl) | Final concentration |
|---|---|---|
| Sybr Green reaction mix | 1 | 1× |
| Primers F/R | 1 | 0.5 μM each |
| MgCl2 | 0.8 | 2.5 mM |
| H2O | 6.2 | |
| DNA | 1 (10 ng) | 1 ng/μL |
| Final volume | 10 |
F, forward; qPCR, quantitative polymerase chain reaction; R, reverse.
Southern blot analysis
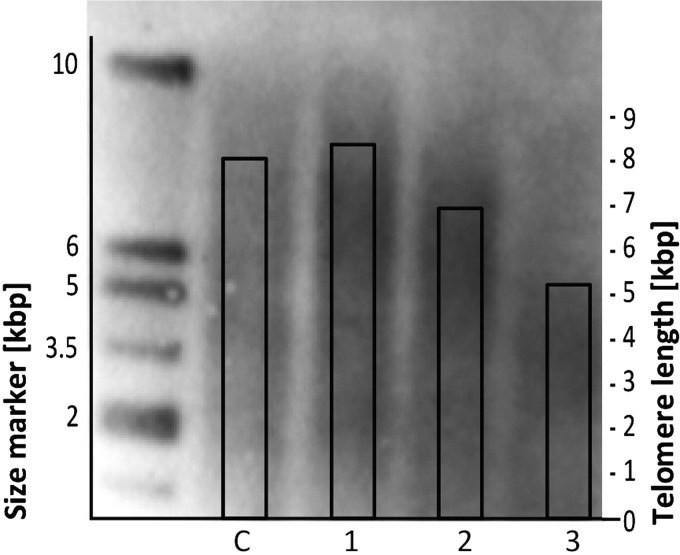
To verify the results obtained by TL real-time PCR, a part of the samples was analyzed by the reference method, Southern blot, as previously described (Kimura et al., 2010). We used a standard Southern blot (TeloTAGGG Telomere Length Assay Kit; Roche Diagnostics) to evaluate the TL assessing the terminal restriction fragment (TRF) according to the manufacturer's protocol. Briefly, 1.5 μg of DNA were digested with restriction enzymes (HinfI and RsaI), and then, DNA fragments were separated by gel electrophoresis and transferred to a nylon membrane. Subsequently, DIG-labeled probe specific for telomeric sequence was applied and detected using anti-DIG antibody conjugated with HRP. Chemiluminescent visualization of telomeric DNA was conducted with the ChemiDoc XRS system (BioRad) and analyzed using ImageJ to evaluate the mean length of TRF, which refers to the telomere length for each studied sample (Fig. 1). The difference between results obtained with the use of both methods might probably result from the fact that the subtelomeric region is also measured with the Southern blot.
FIG. 1.
Comparative analysis of telomere length (TL) assessment using qPCR [AU] and Southern blot analysis [kbp] in a subgroup of DS patients. C, control sample; 1–3, study samples. AU, arbitrary units; DS, down syndrome; qPCR, quantitative polymerase chain reaction.
Statistical analysis
Statistical analysis of the results was performed using the Shapiro–Wilk test, Mann–Whitney test, and Student's t-test calculated through GraphPad Prism 5. A p value of<0.05 was considered statistically significant, **p<0.01, *p<0.05. The error for TL assessment calculated on the basis of qPCR standard curves (serial decimal dilutions of studied samples) was 0.00144 and for albumin was 0.00451 (Roche LightCycler 2.0 software). Outliers were assessed based on the fit points method, which minimized the differences between replicates, and the range of Cp values for individual samples in different runs/replicates did not exceed 0.3.
Results
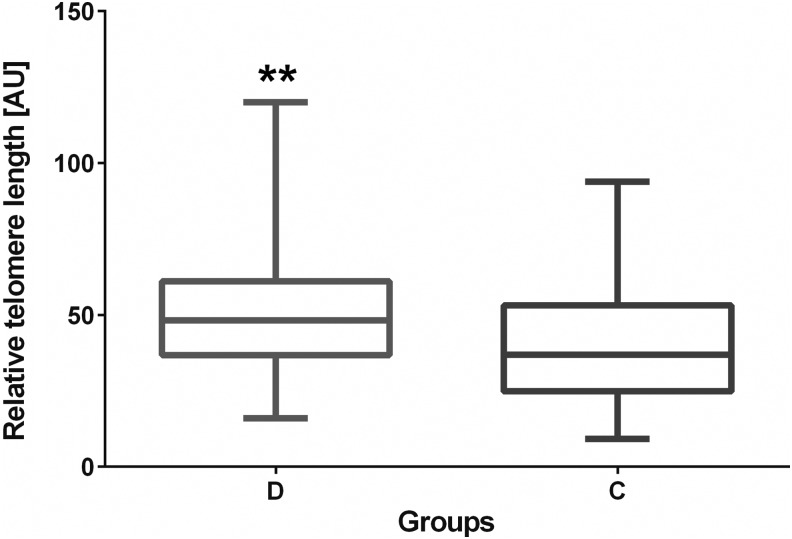
The relative telomere length (RTL) assessment, performed with the use of qPCR, revealed that the average TL in blood leukocytes from DS patients was significantly longer than in the control subjects (50.46 vs. 40.56, respectively [AU]; p=0.0026; Fig. 2). For normalization of quantitative expression data, a reference single-copy gene albumin was used.
FIG. 2.
Analysis of relative telomere length (RTL) [AU] in Down syndrome patients (D) and control subjects (C). The assessment was performed with the use of an optimized qPCR. **p=0.0026.
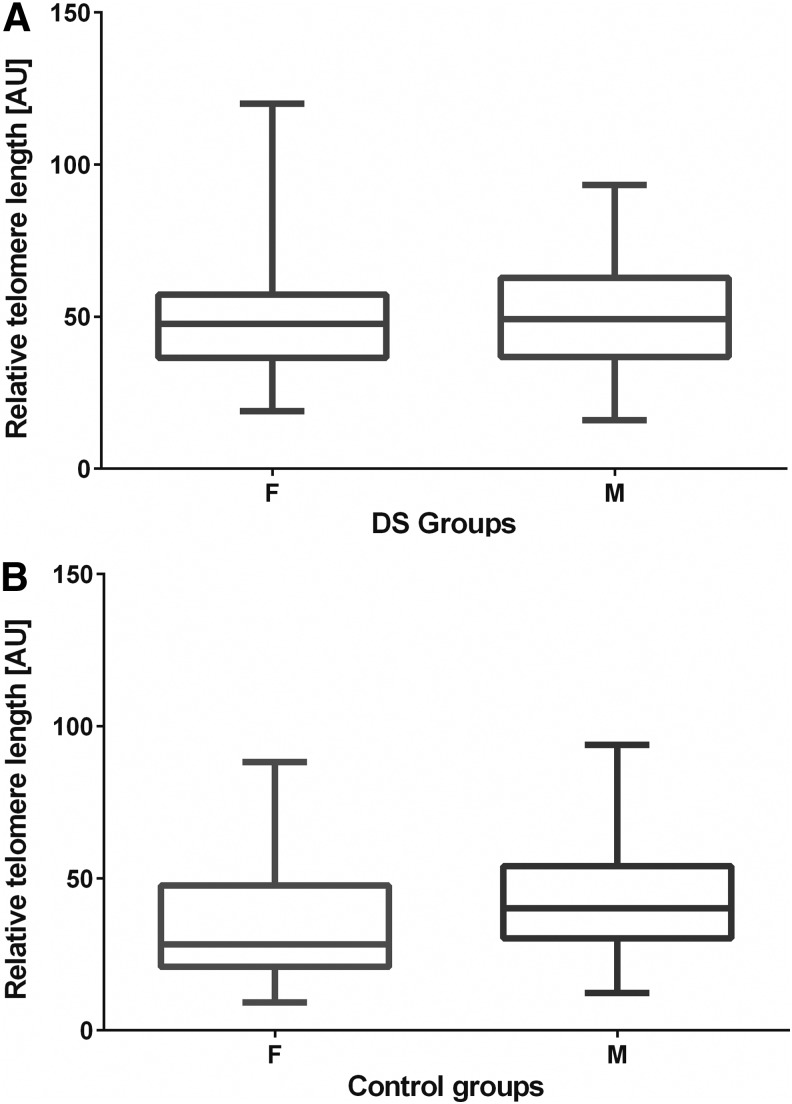
Further detailed analysis indicated that there was no significant difference between male and female subjects in the DS group (p=0.6492) (Fig. 3A). Similarly, in the control group, there was no significant difference (p=0.0641) in the TL between male (44.20 [AU]) and female subjects (35.71 [AU]) (Fig. 3B).
FIG. 3.
RTL [AU] according to gender in the DS (A) and control (B) groups. F, female; M, male.
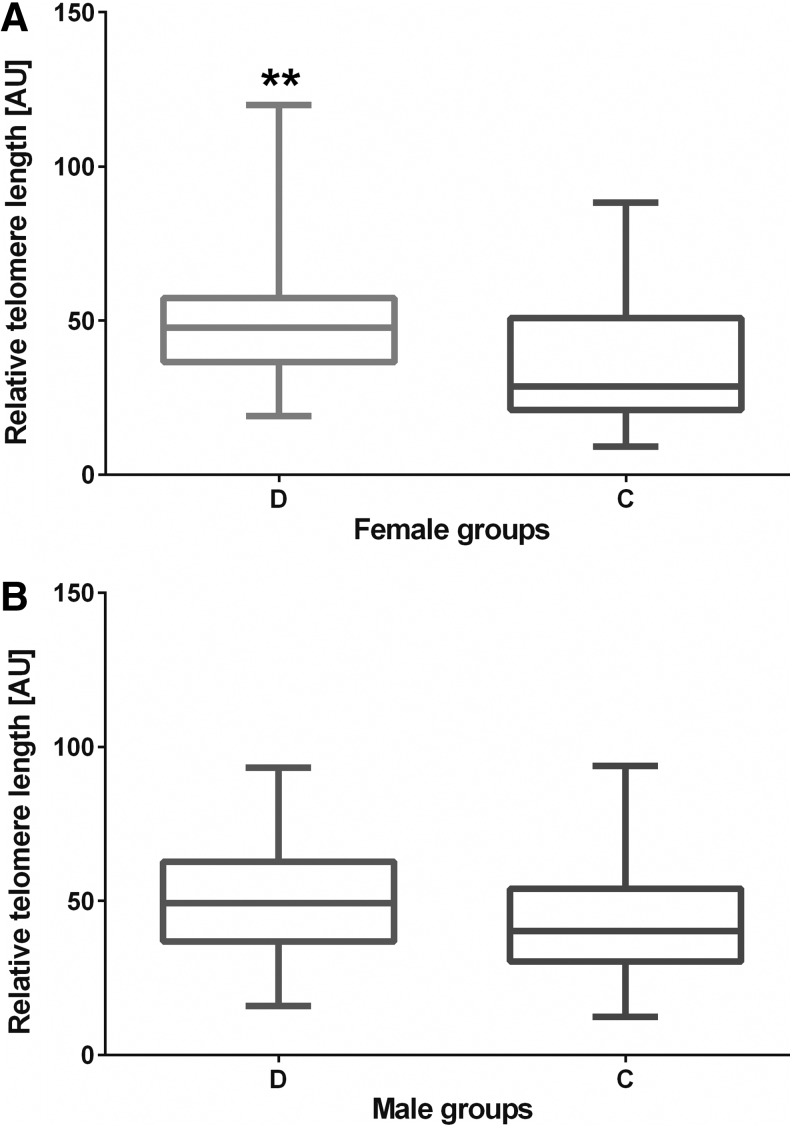
The comparative analysis of RTL in evaluated females from both groups revealed that in the studied group the telomere repeats number was 50.127 [AU], whereas in control subjects, it was 35.709 [AU]; p=0.0023 (Fig. 4A). In a similar comparison of TL between males from the DS and control groups, no significant differences were shown (p=0.1527) (Fig. 4B).
FIG. 4.
RTL [AU] in female (A) and male (B) subjects from the studied and control groups. **p=0.0023.
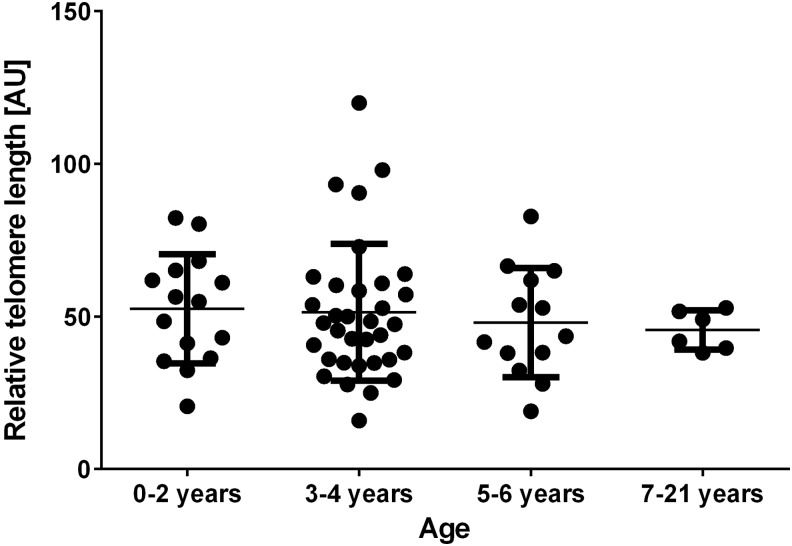
Comparative analysis of TL in DS patients relative to age revealed a trend demonstrating telomere shortening with age, but in the range of studied patients' age, no significant difference was revealed (Fig. 5).
FIG. 5.
Distribution of RTL [AU] in blood leukocytes according to the age in DS patients. The study group comprised 68 DS patients (mean age 4.5±3.1 years, age ranged from 2 to 21 years).
Discussion
Telomeres in DS
DS with its severe symptoms remains untreatable, and unfortunately, we still cannot prevent it or identify the molecular basis of the detailed mechanism of the disease. It is well known that the most common risk factor for DS is maternal age. Interestingly, it was reported that older paternal age at conception (PAC) is also associated with a longer leukocyte telomere length (LTL) in offsprings (Aviv and Susser, 2013). A variety of studies showed that LTL is on an average longer in the offspring of older fathers. It is well established that TL shortens with age in most proliferating tissues. As indicated by Kimura et al. (2008), the mean TL in sperm does not decrease. In fact, it increases with the donor's age. The most common explanation for longer TL is the high activity of telomerase in the testes. However, how it affects the TL in offsprings is unclear, especially since they get only half of their chromosomes from sperm (Eisenberg et al., 2012). In the studies performed by Lim et al. (2002), 143 mothers who were older than 30 years were enrolled. For this group, there was a statistically significant correlation between maternal age and mean TRF length of the newborns, with older mothers giving birth to newborns with longer telomeres. This might explain why DS children might initially (in childhood) have longer telomeres.
Telomeres in leukocytes
Recent studies have shown that longer LTL is associated with reduced atherosclerotic risk in adults and increased survival in the elderly (Aviv and Susser, 2013). In most studies, it was revealed that there was a difference in the average length of telomeres between DS patients (shorter) and control subjects (longer), but the subjects were usually adults. There is no doubt that, as indicated in numerous studies (Vaziri et al., 1993; Zigman, 2013), the rate of telomere loss is indeed greater in DS patients and is even three times faster than in controls.
As demonstrated by Brando et al. (2004), telomeres of DS patients shorten by 47% within 40 years. In addition, it was shown that the males lost telomeric DNA at a rate slightly faster than that of females (50±4.2 vs. 40±3.6 bp/year, respectively), but this difference did not reach statistical significance. Thus, it was suggested that telomere shortening might be a biological marker of DS patients' dementia status (Jenkins et al., 2010, 2012). Interestingly, telomeric length in fibroblasts from DS fetuses was shown to be significantly shorter when compared with their control counterparts (Gimeno et al., 2014). This is especially surprising since (i) telomerase should be still active in the fetus and should not let for a decrease in TL and (ii) it would implicate early and vast telomere attrition. This might also suggest that the TL is associated with additional factors.
As we show in juvenile patients, the TL was significantly longer in DS patients than in control subjects. We can hypothesize on several mechanisms that might cause such results. DS is directly related to slowing of cellular metabolism caused by hypothyroidism, which certainly decreases the rate of telomere loss, at least, in children (Purdy et al., 2014). DS patients also showed increased estradiol levels, which might induce the telomerase activity and also affect telomere shortening (Zhao et al., 2011; Zhou et al., 2013). Surprisingly, an increased telomerase activity was reported in young (average age 4.2) DS patients (Abdel-Salam et al., 2013), but not associated with increased TL as shown in older (age ranging from 42 to 80) DS patients (Jenkins et al., 2012). Observation of a high telomerase activity accompanied by telomere loss is regarded as one of the cytogenetic parameters that represent a state of genetic instability (Jenkins et al., 2008). Longer telomeres in our study could be the result of better physical condition of young DS patients compared to adults, which is closely related to the genetic stability. Moreover, DS patients were shown to reveal a higher cortisol level known to inhibit the telomerase activity (Choi et al., 2008). Hormonal disorders in DS are complex, and it seems that the changes in TL are the results of these dysfunctions. The hormonal profile might also be responsible for the differences in TL in a gender-dependent manner. Our study demonstrated that female DS patients had longer telomeres than control subjects (p=0.0026).
DS patients often reveal immunological system disorders and immunodeficiency, which are associated with heavy infections and autoimmunological diseases (e.g., celiac) or acute leukemia (Kusters et al., 2009). It results in a lowered proliferation of lymphocytes, which might have a protective action on TL. Consequently, TL assessment in leukocytes might reflect dementia and malignancy status and help to reveal the pathomechanism of DS (Sukenik-Halevy et al., 2011).
Summary and conclusions
There are several limitations to the TL study that must be considered. Variability between individuals in TL measured in peripheral blood leukocytes, especially from DS patients, may be a consequence of major amount of factors that influence blood cell composition. This may be affected by age, sex, hormones, lifestyle, addictions, obesity, and many others. As an individual marker, it is then probably not strong enough. However, when combined with other markers, it might be a significant factor indicating the rate of aging or dementia status. Noteworthy, the TL assessment still requires a golden standard of measurement method since those used so far did not always give comparable results (Gardner et al., 2014). However, there is no doubt that further studies should be conducted to assess this factor that might be indeed recognized as an indicator of DS subjects aging or general condition assessment.
To summarize, there are still a lot of things we do not know about telomere metabolism in DS. However, since their shortening with age is a known fact, they might be used as a marker of senescence and symptoms severity predictive marker. Since there are contradictive data concerning the moment when telomere shortening starts, this mechanism should be carefully studied. It would be also advisable for patients with DS to avoid unhealthy lifestyle habits and characteristics, such as obesity, cigarette smoking, chronic stress, and alcohol intake, which lead to marked telomere shortening.
Acknowledgment
The present work was supported by 2011/03/B/NZ7/00512 research grant.
Disclosure Statement
No competing financial interests exist.
References
- Abdel-Salam E., Abdel-Meguid I., and Korraa S. (2013). Telomerase activity and apoptosis genes as parameters of lymphocyte aging in Down syndrome patients. Egypt J Med Hum Genet 14, 171–176 [Google Scholar]
- Aviv A., and Susser E. (2013). Leukocyte telomere length and the father's age enigma: implications for population health and for life course. Int J Epidemiol 42, 457–462 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brando B., Longo A., Beltrami B., Passoni D., Verna R., Licastro F., and Corsi M.M. (2004). Determination of telomere length by flow-fluorescence in situ hybridization in Down's syndrome patients. Int J Tissue React 26, 61–64 [PubMed] [Google Scholar]
- Cawthon R.M. (2009). Telomere length measurement by a novel monochrome multiplex quantitative PCR method. Nucleic Acids Res 37, e21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi J., Fauce S.R., and Effros R.B. (2008). Reduced telomerase activity in human T lymphocytes exposed to cortisol. Brain Behav Immun 22, 600–605 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenberg D.T.A. (2014). Inconsistent inheritance of telomere length (TL): is offspring TL more strongly correlated with maternal or paternal TL? Eur J Hum Genet 22, 8–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenberg D.T.A., Hayes M.G., and Kuzawa C.W. (2012). Delayed paternal age of reproduction in humans is associated with longer telomeres across two generations of descendants. Proc Natl Acad Sci U S A 109, 10251–10256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardner M., Bann D., Wiley L., Cooper R., Hardy R., Nitsch D., et al. (2014). Gender and telomere length: systematic review and meta-analysis. Exp Gerontol 51, 15–27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosh S., Feingold E., Chakraborty S., and Dey S.K. (2010). Telomere length is associated with types of chromosome 21 nondisjunction: a new insight into the maternal age effect on Down syndrome birth. Hum Genet 127, 403–409 [DOI] [PubMed] [Google Scholar]
- Gimeno A., García-Giménez J.L., Audí L., Toran N., Andaluz P., Dasí F., and Pallardó F.V. (2014). Decreased cell proliferation and higher oxidative stress in fibroblasts from Down Syndrome fetuses. Preliminary study. Biochim Biophys Acta 1842, 116–125 [DOI] [PubMed] [Google Scholar]
- Head E., Silverman W., Patterson D., and Lott I.T. (2012). Aging and down syndrome. Curr Gerontol Geriatr Res 2012, 412536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honig L.S., Kang M.S., Schupf N., Lee J.H., and Mayeux R. (2012). Association of shorter leukocyte telomere repeat length with dementia and mortality. Arch Neurol 69, 1332–1339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenkins E.C., Tassone F., Ye L., Gu H., Xi M., Velinov M., et al. (2008). Reduced telomere length in older men with premutation alleles of the fragile X mental retardation 1 gene. Am J Med Genet A 146A, 1543–1546 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenkins E.C., Ye L., Gu H., Ni S.A., Velinov M., Pang D., et al. (2010). Shorter telomeres may indicate dementia status in older individuals with Down syndrome. Neurobiol Aging 31, 765–771 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenkins E.C., Ye L., Velinov M., Krinsky-McHale S.J., Zigman W.B., Schupf N., and Silverman W.P. (2012). Mild cognitive impairment identified in older individuals with Down syndrome by reduced telomere signal numbers and shorter telomeres measured in microns. Am J Med Genet B Neuropsychiatr Genet 159B, 598–604 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimura M., Cherkas L.F., Kato B.S., Demissie S., Hjelmborg J.B., Brimacombe M., et al. (2008). Offspring's leukocyte telomere length, paternal age, and telomere elongation in sperm. PLoS Genet 4, e37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimura M., Stone R.C., Hunt S.C., Skurnick J., Lu X., Cao X., Harley C.B., and Aviv A. (2010). Measurement of telomere length by the Southern blot analysis of terminal restriction fragment lengths. Nat Protoc 5, 1596–1607 [DOI] [PubMed] [Google Scholar]
- Krinsky-McHale S.J., and Silverman W. (2013). Dementia and mild cognitive impairment in adults with intellectual disability: issues of diagnosis. Dev Disabil Res Rev 18, 31–42 [DOI] [PubMed] [Google Scholar]
- Kusters M.A.A., Verstegen R.H.J., Gemen E.F.A., and de Vries E. (2009). Intrinsic defect of the immune system in children with Down syndrome: a review. Clin Exp Immunol 156, 189–193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim S.N., Yahya Z., Zeegers D., Moe T., Kyaw E.E.P, Yeo G.S., et al. (2002). Distribution of telomere length in the cord blood of Chinese newborns. Br J Med Med Res 3, 1004–1014 [Google Scholar]
- Nakamura K.I., Ishikawa N., Izumiyama N., Aida J., Kuroiwa M., Hiraishi N., et al. (2014). Telomere lengths at birth in trisomies 18 and 21 measured by Q-FISH. Gene 533, 199–207 [DOI] [PubMed] [Google Scholar]
- O'Callaghan N.J., and Fenech M. (2011). A quantitative PCR method for measuring absolute telomere length. Biol Proced Online 13, 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Caoimh R., and Clune Y. (2013). Screening for Alzheimer's disease in Downs syndrome. J Alzheimers Dis Parkinsonism 3, 1–6 [Google Scholar]
- Purdy I.B., Singh N., Brown W.L., Vangala S., and Devaskar U.P. (2014). Revisiting early hypothyroidism screening in infants with Down syndrome. J Perinatol 34, 936–940 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Révész D., Milaneschi Y., Verhoeven J.E., and Penninx B.W.J.H. (2014). Telomere length as a marker of cellular ageing is associated with prevalence and progression of metabolic syndrome. J Clin Endocrinol Metab 99, 4607–4615 [DOI] [PubMed] [Google Scholar]
- Rubiś B., Hołysz H., Barczak W., Gryczka R., Łaciński M., Jagielski P., et al. (2012). Study of ABCB1 polymorphism frequency in breast cancer patients from Poland. Pharmacol Rep 64, 1560–1566 [DOI] [PubMed] [Google Scholar]
- Sukenik-Halevy R., Biron-Shental T., Sharony R., Fejgin M.D., and Amiel A. (2011). Telomeres in trisomy 21 amniocytes. Cytogenet Genome Res 135, 12–18 [DOI] [PubMed] [Google Scholar]
- Temple V., Jozsvai E., Konstantareas M.M., and Hewitt T.A. (2001). Alzheimer dementia in Down's syndrome: the relevance of cognitive ability. J Intellect Disabil Res 45, 47–55 [DOI] [PubMed] [Google Scholar]
- Vaziri H., Schächter F., Uchida I., Wei L., Zhu X., Effros R., et al. (1993). Loss of telomeric DNA during aging of normal and trisomy 21 human lymphocytes. Am J Hum Genet 52, 661–667 [PMC free article] [PubMed] [Google Scholar]
- Zhao Q., Lee J.H., Pang D., Temkin A., Park N., Janicki S.C., et al. (2011). Estrogen receptor-Beta variants are associated with increased risk of Alzheimer's disease in women with down syndrome. Dement Geriatr Cogn Disord 32, 241–249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou C., Steplowski T.A., Dickens H.K., Malloy K.M., Gehrig P.A., Boggess J.F., et al. (2013). Estrogen induction of telomerase activity through regulation of the mitogen-activated protein kinase (MAPK) dependent pathway in human endometrial cancer cells. PLoS One 8, e55730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zigman W.B. (2013). Atypical aging in Down syndrome. Dev Disabil Res Rev 18, 51–67 [DOI] [PubMed] [Google Scholar]