Abstract
Background
Few epidemiologic studies have investigated the use of venlafaxine (Effexor®), an antidepressant used to treat major depression and anxiety disorders in adults, during pregnancy. Our objective was to determine whether use of venlafaxine during pregnancy is associated with specific birth defects.
Methods
We used data from the National Birth Defects Prevention Study (NBDPS), a population-based, case-control study in the United States. Our analysis included mothers with pregnancies affected by one of 30 selected birth defects (cases) and babies without birth defects (controls) with estimated dates of delivery between 1997–2007. Exposure was any reported use of venlafaxine from one month preconception through the third month of pregnancy. We calculated adjusted odds ratios (aORs) and 95% Fisher’s Exact confidence intervals (CIs) for 24 birth defect groups for which at least 400 case mothers were interviewed. Our adjusted analyses controlled for maternal age and race-ethnicity.
Results
Among the 27,045 NBDPS participants who met inclusion criteria, 0.17% (14/8,002) of control mothers and 0.40% (77/19,043) of case mothers reported any use of venlafaxine from one month preconception through the third month of pregnancy. Statistically significant associations were found for anencephaly, atrial septal defect (ASD) secundum or ASD not otherwise specified, coarctation of the aorta, cleft palate, and gastroschisis.
Conclusions
Our data suggest associations between periconceptional use of venlafaxine and some birth defects. However, sample sizes were small, confidence intervals were wide, and additional studies are needed to confirm these results.
Keywords: Venlafaxine, Birth Defects, Pregnancy, Antidepressants, Epidemiology
Introduction
Venlafaxine (Effexor®) is a medication that is used to treat major depressive, generalized anxiety and social anxiety disorders in adults. The prevalence of depression during pregnancy is about 18% and the prevalence of generalized anxiety disorders is estimated to be 8.5% among pregnant women (Ross and McLean, 2006; Yonkers and others, 2009). Venlafaxine is a serotonin-norepinephrine reuptake inhibitor (SNRI), which functions to block the reuptake of both serotonin and norepinephrine, and at high doses, also may affect reuptake of dopamine (Sussman, 2003). The mechanism of action for SNRIs is similar to another common class of antidepressants, selective-serotonin reuptake inhibitors (SSRIs), which act to block the reuptake of serotonin alone (Sussman, 2003).
Few epidemiologic studies have investigated the use of venlafaxine during pregnancy and birth defects. Because of small numbers, some studies have grouped exposure to venlafaxine with exposure to SSRIs (citalopram, fluoxetine, paroxetine, and sertraline) or other newer antidepressants, which does not allow for examination of specific effects of venlafaxine exposure separate from these other antidepressant medications (Einarson and others, 2009; Ferreira and others, 2007; Kallen, 2004b; Lennestal and Kallen, 2007; Reis and Kallen, 2010; Wichman and others, 2009; Yaris and others, 2004). In general, these studies found no associations between major malformations and SNRI medications as a group. Of those who assessed venlafaxine separately, no significant associations were observed. For example, a study by Einarson and colleagues found no significant differences in the prevalence of birth defects between women who were exposed to venlafaxine and women exposed to SSRIs or medications believed to be non-teratogenic during pregnancy (n=150 per group) (Einarson and others, 2001; Oberlander and others, 2008). Similarly, Oberlander and colleagues used administrative health data to look at serotonin reuptake inhibitors including venlafaxine, individually and as a group, and found no increased risk for birth defects overall or specifically for cardiac defects when compared to women unexposed to SSRIs, SNRIs, and benzodiazepines (Oberlander and others, 2008).
Several studies have examined the associations between use of SSRIs during pregnancy and birth defects and have reported mixed results. Some suggest that SSRIs might be associated with an increased risk of heart defects, mainly septal defects and right ventricular outflow tract obstruction (RVOTO) defects (Bar-Oz and others, 2007; Berard and others, 2007; Cole and others, 2007; Diav-Citrin and others, 2008; Einarson and others, 2008; Ferreira and others, 2007; Kallen, 2004a; Kallen and Otterblad Olausson, 2007; Louik and others, 2007). A previous analysis of data from the National Birth Defects Prevention Study (NBDPS) showed associations between SSRIs and anencephaly, craniosynostosis, and omphalocele (Alwan and others, 2007). A similar study by Louik and colleagues found associations between individual SSRIs and certain birth defects, such as sertraline and septal heart defects, sertraline and omphalocele, and paroxetine and right ventricular outflow tract obstruction defects (Louik and others, 2007). Although SSRIs and SNRIs might function similarly to treat depressive symptoms, much less is known about the risk of SNRIs (such as venlafaxine) and their use during pregnancy.
We used data from the NBDPS to assess whether reported use of venlafaxine just before and during early pregnancy is associated with specific birth defects.
Methods
Study Population
The NBDPS is an ongoing, population-based, case-control study designed to investigate genetic and environmental risk factors for major structural birth defects (Rasmussen and others, 2003; Yoon and others, 2001). Birth defects data are collected by ten birth defects surveillance systems in the United States: Arkansas (statewide), California (region near Fresno), Georgia (metropolitan Atlanta), Iowa (statewide), Massachusetts (eastern counties), New Jersey (statewide, ending in 2002), New York (western NY and Hudson Valley), North Carolina (19 central counties, beginning in 2003), Texas (varying regions, currently Health Service Region 11), and Utah (statewide, beginning in 2003). The study was approved by institutional review boards of the Centers for Disease Control and Prevention and all study centers.
Cases include live births, stillbirths (≥20 weeks gestation), and elective terminations diagnosed with one of more than 30 selected major birth defects. To focus on birth defects of unknown etiology, infants with recognized or strongly suspected chromosomal abnormalities or single-gene disorders are excluded. Control infants are liveborn infants without birth defects randomly selected from hospital birth records or birth certificate records from the same source population and time period as the case infants. To confirm study eligibility, all case records are reviewed by clinical geneticists to determine if they meet the standard case definitions. Clinical information on infants with heart defects also are reviewed by clinicians with expertise in pediatric cardiology. All heart defect diagnoses are confirmed using reports from echocardiography, cardiac catheterization, surgery, or autopsy (Yoon and others, 2001).
Mothers of case and control infants are contacted within 6 weeks to 24 months after the estimated date of delivery (EDD). If the mother agrees to participate in the study, a trained interviewer administers a standardized computer-assisted telephone interview in English or Spanish. The interview ascertains specific exposures and behaviors throughout pregnancy and the three months preconception. During the interview, mothers are asked about maternal and paternal demographic characteristics, pregnancy history, medication and vitamin use, dietary details, drug and alcohol use, occupational exposures, and reproductive health information.
Venlafaxine Exposure
Exposure was defined as any reported use of venlafaxine from one month before conception through the third month of pregnancy (periconceptional). This exposure definition does include timing outside of organogenesis; however, because it is possible that women may not recall dates exactly correctly (of pregnancy or of timing of the medication use), we included a broader time frame. During the medication portion of the NBDPS interview, mothers are asked about prescription and non-prescription medications that they may have taken from 3 months before conception through delivery. Interviewers read a list of brand name medications (e.g., Prozac®, Zoloft®) to the mothers, and after each medication is read, mothers respond yes or no to report if they had taken the medication. At the end of the list, they are asked if they had taken any medications that were not mentioned. For births with an EDD during 1997–2005, venlafaxine (Effexor®) was not one of the specific medications listed. In 2005, the interview was revised, and Effexor® (the more commonly recognized brand name for venlafaxine) was added to the list of medications that were read. Thus, for births with an EDD during 2006–2007, mothers responded yes or no when they heard venlafaxine listed. In addition, during the maternal health portion of the NBDPS interview, mothers are asked open-ended questions about illnesses or diseases they may have had during pregnancy and about any medications used for this illness. Regardless of where in the interview the mother reported use of a specific medication, subsequent questions were asked regarding start date, stop date, and frequency of use. If a mother reported any brand (Effexor®) or generic (venlafaxine) name for venlafaxine and reported periconceptional use of the antidepressant, they were classified as exposed.
Statistical Analysis
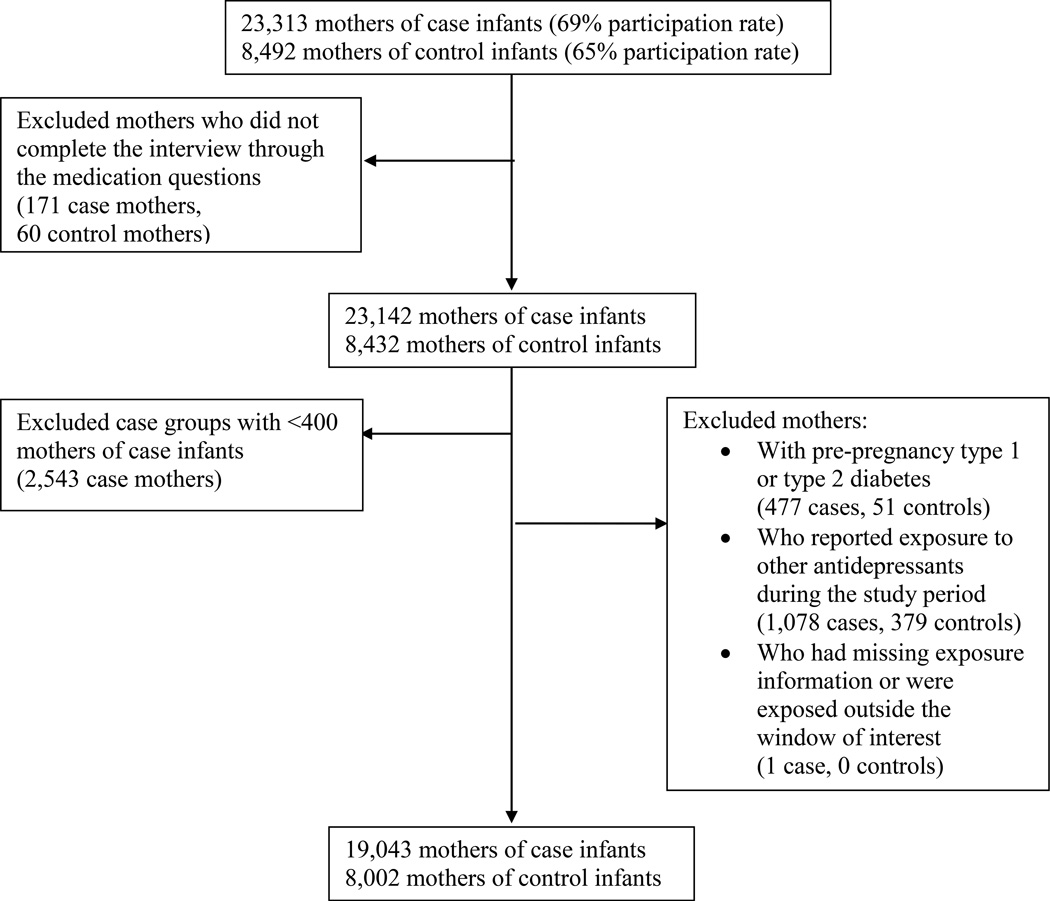
These analyses were limited to mothers who had an EDD after October 1, 1997 through December 31, 2007. We excluded mothers who did not complete the maternal interview through all of the medication questions and included only those birth defect groups for which there were at least 400 case mothers interviewed. Due to the strong association observed between pre-pregnancy diabetes and birth defects, we excluded mothers who reported pre-pregnancy type 1 or type 2 diabetes (Correa and others, 2008). To assess effects of venlafaxine separate from other antidepressants, mothers who reported other periconceptional antidepressant use were excluded. Lastly, we excluded mothers who had missing information on the timing of exposure during pregnancy or who were exposed to venlafaxine outside the periconceptional window of interest (Figure 1). Women were classified as unexposed if they did not report use of any antidepressant from three months before conception through the end of pregnancy.
Figure 1.
Flow diagram showing study participation rates and exclusion criteria for mothers of case and control infants in the analyses of venlafaxine and birth defects, National Birth Defects Prevention Study, 1997–2007
To examine the association between exposure to venlafaxine and the occurrence of birth defects, we estimated crude and adjusted odds ratios (ORs) and 95% Fisher’s Exact confidence intervals (CIs) if there were more than 2 exposed cases. Because of the small number of exposed mothers, we controlled for only two variables chosen a priori: maternal age (<30, ≥30 years) and race/ethnicity (non-Hispanic white, other). Because gastroschisis is associated with young maternal age, the association with gastroschisis was adjusted for maternal age (<20, ≥20 years) and race/ethnicity (non-Hispanic white, other). These factors were self-reported during the maternal interview. Birth defect groups are not mutually exclusive; case infants with more than one birth defect are included in more than one category. Therefore, we conducted a sub-analysis restricted to cases with isolated birth defects, estimating crude ORs (cORs) because of decreased sample size.
After reviewing preliminary results, post-hoc analyses were performed to further understand observed associations. Fewer case and control mothers with EDDs from 1997–2002 reported using venlafaxine. Therefore, we conducted sub-analyses limiting the data to the most recent 5-year time period (EDD: 2003–2007), estimating cORs and 95% Fisher’s Exact CIs. We also examined other medications reported and other illnesses or diseases reported by venlafaxine-exposed mothers. To explore the role of recall bias, we estimated cORs under the assumption that, among controls only, 30% of exposed mothers erroneously had not reported exposure. In this hypothetical scenario, we assumed that 20 control mothers were truly exposed; therefore, in addition to the 14 control mothers who reported exposure, six more mothers were actually exposed but failed to report it (6/20=30%). We calculated hypothetical cORs under these assumptions to assess the effect of potential under-reporting among control mothers.
All statistical analyses were performed using SAS 9.3 (SAS Institute Inc, Cary, NC).
Results
Among mothers who met our inclusion criteria, 0.17% (14/8,002) of control mothers and 0.40% (77/19,043) of case mothers reported any periconceptional use of venlafaxine. Duration of use ranged from 1–120 days, but the majority (54%) of mothers reported using venlafaxine for the entire period of one month before pregnancy through the end of the first trimester. Exposure was more likely among those with a higher education and of non-Hispanic white race-ethnicity. These mothers also more frequently reported any folic acid use from one month preconception through the first month of pregnancy (Table 1). Of the exposed mothers, only 24% had EDDs during 1997–2002; the remaining 76% of exposed mothers had EDDs from 2003–2007 (Table 1).
Table 1.
Demographic and Lifestyle Characteristics of Mothers, Exposed and Unexposed to Venlafaxine During Early Pregnancy, National Birth Defects Prevention Study, 1997–2007
| Used Venlafaxine (n=91) |
Unexposed (n=26,954) |
P-value | |||
|---|---|---|---|---|---|
| n | % | n | % | ||
| Age | |||||
| <30 years | 48 | 52.8 | 16344 | 60.6 | 0.12 |
| ≥ 30 years | 43 | 47.2 | 10610 | 39.4 | |
| Education | |||||
| ≤High School | 19 | 20.9 | 11594 | 43.0 | <0.01 |
| >High School | 72 | 79.1 | 15107 | 56.0 | |
| Missing | 253 | 0.9 | |||
| Race | |||||
| NH White | 83 | 91.2 | 15778 | 58.5 | <0.01 |
| Other | 8 | 8.8 | 11167 | 41.4 | |
| Missing | 9 | 0.0 | |||
| Obesity1 | |||||
| Yes | 23 | 25.3 | 4558 | 16.9 | 0.06 |
| No | 68 | 74.7 | 21171 | 78.5 | |
| Missing | 1225 | 4.5 | |||
| Smoking2 | |||||
| Any | 21 | 23.1 | 5150 | 19.1 | 0.35 |
| None | 70 | 76.9 | 21612 | 80.2 | |
| Missing | 192 | 0.7 | |||
| Alcohol2 | |||||
| Any | 39 | 42.9 | 9678 | 35.9 | 0.19 |
| None | 52 | 57.1 | 16986 | 63.0 | |
| Missing | 290 | 1.1 | |||
| Folic Acid Use3 | |||||
| Any | 67 | 73.6 | 13477 | 50.0 | <0.01 |
| None | 24 | 26.4 | 13477 | 50.0 | |
| Parity | |||||
| No previous live birth | 32 | 35.2 | 11338 | 42.1 | 0.18 |
| At least 1 previous live birth | 59 | 64.8 | 15585 | 57.8 | |
| Missing | 31 | 0.1 | |||
| Estimated Date of Delivery | |||||
| 1997 | 0 | 0 | 308 | 1.1 | <.01 |
| 1998 | 2 | 2.2 | 2359 | 8.8 | |
| 1999 | 1 | 1.1 | 2769 | 10.3 | |
| 2000 | 5 | 5.5 | 2946 | 10.9 | |
| 2001 | 3 | 3.3 | 2725 | 10.1 | |
| 2002 | 11 | 12.1 | 2385 | 8.9 | |
| 2003 | 6 | 6.6 | 2591 | 9.6 | |
| 2004 | 19 | 20.9 | 3020 | 11.2 | |
| 2005 | 17 | 18.7 | 2825 | 10.5 | |
| 2006 | 12 | 13.2 | 2509 | 9.3 | |
| 2007 | 15 | 16.5 | 2517 | 9.3 | |
| Study Centers | |||||
| Arkansas | 16 | 17.6 | 3515 | 13.0 | <.01 |
| California | 7 | 7.7 | 3505 | 13.0 | |
| Georgia/CDC | 7 | 7.7 | 3062 | 11.4 | |
| Iowa | 16 | 17.6 | 2601 | 9.7 | |
| Massachusetts | 9 | 9.9 | 3451 | 12.8 | |
| New Jersey | 1 | 1.1 | 1957 | 7.3 | |
| New York | 5 | 5.5 | 2048 | 7.6 | |
| North Carolina | 5 | 5.5 | 1561 | 5.8 | |
| Texas | 6 | 6.6 | 3232 | 12.0 | |
| Utah | 19 | 20.9 | 2022 | 7.5 | |
Obesity is defined as a body mass index greater than or equal to 30 and is based on self-reported height and weight.
1 month prior to pregnancy through third month of pregnancy
1 month prior to pregnancy through first month of pregnancy
We assessed 24 groups of birth defects that had 400 or more case infants. One group, esophageal atresia (n=499), had no mothers exposed to venlafaxine, and six groups had 2 or fewer mothers exposed: anotia/microtia, d-transposition of the great arteries, tetralogy of Fallot, hypoplastic left heart syndrome, anorectal atresia, and diaphragmatic hernia. ORs were not assessed for these seven defects (Table 2). We observed all effect estimates to be elevated, but select groups had statistically significantly elevated ORs: anencephaly; septal heart defects, likely driven by the association with the subcategory atrial septal defect secundum (ASD) or ASD not otherwise specified; left ventricular outflow tract obstruction (LVOTO) defects, likely driven by the association with the subcategory coarctation of the aorta; cleft palate; and gastroschisis (Table 2).
Table 2.
Association between use of venlafaxine just before and during early pregnancy and the occurrence of certain birth defects, National Birth Defects Prevention Study, 1997–2007
| Birth Defects | Total | Exposed | Crude Odds Ratio 95% Exact CI |
Adjusted Odds Ratio1 95% Exact CI |
|---|---|---|---|---|
| No Major Birth Defects (Control Infants) | 8002 | 14 | 1.0 (ref) | 1.0 (ref) |
| Anencephaly | 411 | 4 | 5.6 (1.3–18.0) | 6.3 (1.5–20.2) |
| SpinaBifida | 874 | 3 | 2.0 (0.4–7.1) | 2.1 (0.4–7.6) |
| Anotia or Microtia | 470 | 1 | § | § |
| Conotruncal Heart Defects | 1754 | 6 | 2.0 (0.6–5.4) | 1.9 (0.6–5.3) |
| D-Transposition of the Great Arteries | 545 | 2 | § | § |
| Tetralogy of Fallot | 799 | 1 | § | § |
| Septal Heart Defects | 3621 | 18 | 2.9 (1.3–6.2) | 3.0 (1.4–6.4) |
| Perimembranous Ventricular Septal Defect | 1410 | 6 | 2.4 (0.8–6.8) | 2.4 (0.8–6.7) |
| Atrial Septal Defect, type 2 or not otherwise specified | 2181 | 11 | 2.9 (1.2–6.9) | 3.1 (1.3–7.4) |
| Ventricular Septal Defect-Atrial Septal Defect Association | 576 | 3 | 3.0 (0.5–10.8) | 3.1 (0.6–11.3) |
| Right Ventricular Outflow Tract Obstruction Defects2 | 1250 | 5 | 2.3 (0.6–6.7) | 2.3 (0.6–6.6) |
| Pulmonary Valve Stenosis | 985 | 5 | 2.7 (0.8–8.1) | 2.7 (0.8–7.9) |
| Left Ventricular Outflow Tract Obstruction Defects | 1444 | 9 | 3.6 (1.4–8.9) | 3.3 (1.2–8.2) |
| Hypoplastic Left Heart Syndrome | 425 | 2 | § | § |
| Coarctation of the Aorta | 768 | 6 | 4.5 (1.4–12.5) | 4.1 (1.3–11.5) |
| Cleft Lip with or without Cleft Palate | 2134 | 6 | 1.6 (0.5–4.4) | 1.5 (0.5–4.3) |
| Cleft Palate alone | 1123 | 7 | 3.5 (1.2–9.3) | 3.3 (1.1–8.8) |
| AnorectalAtresia | 741 | 1 | § | § |
| Esophageal Atresia | 499 | 0 | § | § |
| Hypospadias, 2nd/3rd degree3 | 1571 | 7 | 2.6 (0.8–8.7) | 2.3 (0.7–7.9) |
| Any Limb Reduction Defect | 847 | 3 | 2.0 (0.4–7.3) | 2.1 (0.4–7.6) |
| Craniosynostosis | 982 | 3 | 1.7 (0.3–6.3) | 1.5 (0.3–5.4) |
| Diaphragmatic Hernia | 594 | 2 | § | § |
| Gastroschisis | 911 | 6 | 3.8 (1.2–10.5) | 5.7 (1.8–15.9)4 |
Two or fewer exposed cases; No odds ratios calculated.
Adjusted for age (<30, ≥30 years) and race (NH White, Other)
Excluding Ebstein’s Anomaly
Hypospadias among male infants only; 4063 control male infants
Association with gastroschisis adjusted for age (<20, ≥20 years) and race (NH White, Other)
When restricting analysis to isolated birth defects, the majority of associations remained statistically significant, although differences were observed for some groups. Effect estimates for some associations, including anencephaly, cleft palate alone, and gastroschisis, increased slightly (cOR: 6.2; 95% CI: 1.5–20.0; cOR: 4.4; 95% CI: 1.5–11.6; and cOR: 4.2; 95% CI: 1.3–11.7, respectively). The association with LVOTO defects decreased (cOR: 3.1; 95% CI: 1.1–8.3), likely because the association with subcategory coarctation of the aorta was no longer statistically significant (cOR: 3.5; 95% CI: 0.8–11.1).
In our post-hoc analyses restricting the data to EDDs in the most recent 5-year time period, effect estimates remained elevated; however, most were no longer statistically significant. The associations with anencephaly and LVOTO defects remained statistically significantly elevated, while the associations with cleft palate and gastroschisis were of borderline significance (Table 3).
Table 3.
Association between use of venlafaxine just before and during early pregnancy and the occurrence of certain birth defects, National Birth Defects Prevention Study, Restricted to 2003–2007
| Birth Defects | Total | Exposed | Crude Odds Ratio (Exact 95% CI) |
|---|---|---|---|
| No Major Birth Defects (Control Infants) | 4038 | 12 | 1.0 (ref) |
| Anencephaly | 210 | 4 | 6.5 (1.5–21.7) |
| SpinaBifida | 431 | 2 | § |
| Anotia or Microtia | 216 | 1 | § |
| Contruncal Heart Defects | 826 | 3 | 1.2 (0.2–4.5) |
| D-Transposition of the Great Arteries | 254 | 2 | § |
| Tetralogy of Fallot | 388 | 0 | § |
| Septal Heart Defects | 1795 | 11 | 2.1 (0.8–5.1) |
| Perimembranous Ventricular Septal Defect | 659 | 4 | 2.0 (0.5–6.8) |
| Atrial Septal Defect type 2 or not otherwise specified | 1221 | 6 | 1.7 (0.5–4.8) |
| Ventricular Septal Defect-Atrial Septal Defect Association | 308 | 1 | § |
| Right Ventricular Outflow Tract Obstruction Defects1 | 669 | 3 | 1.5 (0.3–5.6) |
| Pulmonary Valve Stenosis | 543 | 3 | 1.9 (0.3–6.9) |
| Left Ventricular Outflow Tract Obstruction Defects | 790 | 7 | 3.0 (1.0–8.3) |
| Hypoplastic Left Heart Syndrome | 220 | 2 | § |
| Coarctation of the Aorta | 427 | 4 | 3.2 (0.7–10.5) |
| Cleft Lip with or without Cleft Palate | 1036 | 5 | 1.6 (0.4–4.8) |
| Cleft Palate alone | 522 | 5 | 3.1 (0.9–9.6) |
| AnorectalAtresia | 344 | 1 | § |
| Esophageal Atresia | 215 | 0 | § |
| Hypospadias, 2nd/3rd degree2 | 777 | 6 | 3.2 (0.8–13.5) |
| Any Limb Reduction Defect | 385 | 3 | 2.6 (0.5–9.8) |
| Craniosynostosis | 574 | 2 | § |
| Diaphragmatic Hernia | 305 | 1 | § |
| Gastroschisis | 508 | 5 | 3.3 (0.9–10.2) |
Two or fewer exposed cases; No odds ratios calculated.
Excluding Ebstein’s Anomaly
Hypospadias among male infants only; 2089control male infants
In our post-hoc evaluation of other medications or illnesses during pregnancy, we did not observe any patterns among venlafaxine-exposed mothers. Two exposed case mothers (2.6%) were also exposed to high blood pressure medications, seven exposed case mothers (9.0%) were exposed to antiepileptic medications (not valproic acid), and two exposed case mothers (2.6%) reported opioid analgesic medications. Among these mothers exposed to multiple medications, no patterns of medications or of birth defects were observed. For example, three of the case mothers exposed to venlafaxine were also exposed to clonazepam, an antiepileptic medication, but each of their infants had a different condition: cleft palate, an association of heart defects—ASD and ventricular septal defect, and tetralogy of Fallot. In the open-ended questions about general illness during pregnancy, responses varied; however, no pattern of other illness was observed among venlafaxine-exposed mothers (data not shown).
In our post-hoc analysis exploring recall bias, we re-assessed the crude associations assuming under-reporting among control mothers. If we assumed 30% of exposed control mothers did not report exposure, all associations remained elevated, although closer to the null. For example, the association with anencephaly decreased to 3.9 (95% CI: 1.1–11.8). In this hypothetical scenario, the association with coarctation of the aorta decreased to 3.1 (95% CI: 1.0–8.1), and the association with cleft palate alone decreased to 2.5 (95% CI: 0.9–6.1).
Discussion
Using data from the NBDPS, a multi-site, population-based case-control study in the United States, we observed that periconceptional venlafaxine exposure might be associated with certain birth defects, specifically anencephaly, cleft palate, gastroschisis, and some heart defects, such as ASD secundum or ASD not otherwise specified and coarctation of the aorta. However, because these analyses were based on a small number of exposed cases, many of the estimates were imprecise with wide confidence intervals.
Reported exposure to venlafaxine during early pregnancy increased among all mothers over time. This corroborates previous studies that have shown an increase in antidepressant use in the United States over time (Harman and others, 2009; Marcus and Olfson, 2010; Olfson and Marcus, 2009), including use among pregnant women (Alwan and others, 2011; Andrade and others, 2008; Cooper and others, 2007).
Our findings differed from earlier studies. One prospective cohort study by Einarson and colleagues enrolled pregnant women via seven pregnancy counseling centers. The authors compared venlafaxine-exposed women to women exposed to SSRIs and women exposed to medications believed to be non-teratogenic during pregnancy. Two of the exposed babies had a major malformation; however, no significant differences were found between the three groups. This study consisted of a small number of subjects, which limited its ability to examine associations with rare outcomes (Einarson et al., 2001). Oberlander and colleagues used linked administrative health data and prescription information to determine venlafaxine exposure during the first trimester. They found no increased risk of birth defects overall or for cardiovascular defects compared to women unexposed to SSRIs, other SNRIs, and benzodiazepines (Oberlander et al., 2008). Of the limited information from animal studies, one study reported that venlafaxine use during pregnancy had adverse effects on rat fetuses (da-Silva and others, 1999), but studies on rats and rabbits performed by the manufacturer found no teratogenic effects (2007).
Biologically, venlafaxine functions in the nervous system by blocking the reuptake of two neurotransmitters, serotonin and norepinephrine, at the synaptic junction (2007). During embryogenesis, these neurotransmitters are expressed in early phases, potentially playing a role as early as gastrulation (Lauder, 1988). It is believed that these neurotransmitters may act as morphogens, signaling molecules that have a dose-dependent function on receptive cells (Buznikov and others, 2001; Lauder, 1988). Several animal studies have provided specific evidence for serotonin acting as a morphogen in craniofacial and cardiac development (Choi and others, 1997; Cote and others, 2007; Moiseiwitsch, 2000; Moiseiwitsch and Lauder, 1995; Nebigil and others, 2000; Nebigil and Maroteaux, 2001). Studies have also shown that norepinephrine influences neural crest cell formation and differentiation during embryogenesis (Hu and others, 2009; Ren and others, 2001; Ren and others, 2003; Sieber-Blum and Ren, 2000; Zhang and others, 1997). Thus, if venlafaxine is taken during early pregnancy and interferes with these embryologic signaling pathways, it is plausible that venlafaxine could affect craniofacial and cardiac development. This is consistent with our findings of associations with septal heart defects, LVOTO defects and cleft palate.
Another potential explanation for the associations observed is that women who use venlafaxine have underlying differences compared to non-users. Some differences in demographic and lifestyle factors between exposed and exposed mothers were noted. For example, exposure was more common among non-Hispanic white mothers and folic acid users. However, no differences were observed between venlafaxine-exposed and unexposed mothers in terms of potential risk factors such as exposure to cigarette smoking or alcohol consumption during early pregnancy. We also looked at exposure to other medications that might increase the risk for birth defects; however, no patterns were observed.
Because venlafaxine is a second-line treatment that is prescribed to individuals that do not respond well to first-line treatments like SSRIs (Schueler and others, 2011), it is possible that women treated with venlafaxine have more severe disease or underlying differences in their depression, which might account for the associations we observed. Depression, itself, has been shown to be associated with a number of adverse pregnancy outcomes, such as spontaneous abortion, fetal death, low birth weight, and preterm birth (Bonari and others, 2004; Grote and others, 2010). In our study, over 50% of venlafaxine-exposed mothers reported using the medication during the entire early pregnancy period, which suggests long-term treatment. Although we ask questions in the maternal interview about maternal disease, we do not have specific questions about depression, so we are unable to assess confounding by indication.
Given that the maternal interview took place after the birth outcome is known and that we found all effect estimates to be elevated, we were concerned about recall bias. In our post-hoc analysis examining the potential role of under-reporting among control mothers, effect estimates remained elevated, although were closer to the null, suggesting that our observed results were unlikely the result of significant recall bias. Our assumption that 30% of exposed control mothers did not report exposure is likely too high; the magnitude of under-reporting is probably smaller. Previous studies have shown that recall bias might not have as considerable an effect in practice as one might expect (Drews and others, 1990; Khoury and others, 1994; Mackenzie and Lippman, 1989; Zierler and Rothman, 1985). In the NBDPS, we study a number of different medications, including antidepressant exposures, and previous NBDPS studies have not observed a broad increase in the number of elevated associations with birth defects. Thus, it is not likely that recall bias would play a major role for venlafaxine, but not other NBDPS exposures (Alwan and others, 2010; Alwan and others, 2007). Additionally, it has been shown that asking about medications in relation to treating illness as well as asking directly about specific medications can improve postnatal recall (Mitchell and others, 1986). Both techniques are utilized in the NBDPS interview, but venlafaxine was not included in the list of specific medications listed until 2006; however, first line medications for depression (e.g., paroxetine, fluoxetine, sertraline) have always been included, probably prodding the report of other antidepressants as well. Differential reporting of exposure between case and control mothers might occur because of differences in the timing of the interview after birth. However, average time to interview is relatively similar for both case and control mothers—about 11 months for case mothers and about 9 months for control mothers.
Our analysis should be interpreted in light of several additional limitations. In the maternal interview, mothers are asked about the frequency of medication use but not the dose, which could affect dose-response relationships. Since the mothers are asked to recall pregnancy exposures and the information collected during the interview was not validated, misclassification of exposures is possible. Since venlafaxine was not specifically asked about in the maternal interview until 2006, it is possible the medication was under-reported during the earlier time period. Because we conducted a large number of tests, some of the associations may be due to chance. We examined associations with 24 groups of birth defects at the 0.05 significance level, so we would expect at least 1 positive result to occur by chance. It is possible that by selecting groups of birth defects with at least 400 cases, we inadvertently selected for those with elevated risk point estimates. Another potential limitation to consider is that NBDPS study centers may have under-ascertained cases that were prenatally diagnosed and terminated. This would most likely affect birth defects with higher proportions of terminations (e.g., anencephaly, spina bifida, or omphalocele), and this potential under-ascertainment might have biased the results for these birth defect outcomes (Waller and others, 2000). Lastly, the NBDPS had a non-participation rate of about 30%; however, it is unlikely that participation would differ between cases and controls based on whether or not the woman used venlafaxine. A previous analysis that investigated selection bias in the NBDPS determined that, although there were some small statistically significant differences in some demographic characteristics, the NBDPS control population is generally representative of the base population (Cogswell and others, 2009).
Strengths of our study include the fact that our data come from a large, population-based, case-control study, which allowed us to look at a large number of birth defect groups and the ability to examine venlafaxine exposure separate from other antidepressant medications. All the defects included have consistent case definitions, and the cases in the study are classified by clinical experts in genetics and pediatric cardiology.
In conclusion, our data suggest that maternal periconceptional use of venlafaxine might be associated with certain birth defects, specifically anencephaly, cleft palate, gastroschisis, and some heart defects, such as ASD secundum or ASD not otherwise specified and coarctation of the aorta. However, our analyses are based on a small number of mothers exposed to venlafaxine during early pregnancy, which led to imprecise estimates with wide confidence intervals. Additional studies are needed to confirm these results.
Acknowledgments
Coding of drug information in NBDPS used the Slone Drug Dictionary, under license from the Slone Epidemiology Center at Boston University, Boston, MA. We thank all collaborators from the Centers for Birth Defects Research and Prevention in Arkansas, California, Georgia, Iowa, Massachusetts, New Jersey, New York, North Carolina, Texas, and Utah for their significant contributions. We also thank the families that participated in the NBDPS.
This work was supported through cooperative agreements under PA 96043, PA02081 and FOA DD09-001 from the Centers for Disease Control and Prevention to the Centers for Birth Defects Research and Prevention participating in the National Birth Defects Prevention Study.
Footnotes
CDC DISCLAIMER
The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention.
Presented at the 23rd Annual Meeting of the Society for Pediatric and Perinatal Epidemiologic Research, Seattle, WA, June 23–24, 2010, the 43rd Annual Meeting of the Society for Epidemiologic Research, Seattle, WA, June 21–24, 2010, the 50th Annual Meeting of the Teratology Society, Louisville, KY, June 26–30, 2010, the 31st Annual David W. Smith Workshop on Malformations and Morphogenesis, Union, WA, August 27-September 1, 2010, and the 27th International Conference on Pharmacoepidemiology and Therapeutic Risk Management, Chicago, IL, August 14–17, 2011.
References
- Effexor XR Capsules Product Monograph. Wyeth Canada: 2007. [Google Scholar]
- Alwan S, Reefhuis J, Botto LD, Rasmussen SA, Correa A, Friedman JM. Maternal use of bupropion and risk for congenital heart defects. Am J Obstet Gynecol. 2010;203(1):52 e51–52 e56. doi: 10.1016/j.ajog.2010.02.015. [DOI] [PubMed] [Google Scholar]
- Alwan S, Reefhuis J, Rasmussen SA, Friedman JM. Patterns of antidepressant medication use among pregnant women in a United States population. J Clin Pharmacol. 2011;51(2):264–270. doi: 10.1177/0091270010373928. [DOI] [PubMed] [Google Scholar]
- Alwan S, Reefhuis J, Rasmussen SA, Olney RS, Friedman JM. Use of selective serotonin-reuptake inhibitors in pregnancy and the risk of birth defects. N Engl J Med. 2007;356(26):2684–2692. doi: 10.1056/NEJMoa066584. [DOI] [PubMed] [Google Scholar]
- Andrade SE, Raebel MA, Brown J, Lane K, Livingston J, Boudreau D, Rolnick SJ, Roblin D, Smith DH, Willy ME, Staffa JA, Platt R. Use of antidepressant medications during pregnancy: a multisite study. Am J Obstet Gynecol. 2008;198(2):194 e191–194 e195. doi: 10.1016/j.ajog.2007.07.036. [DOI] [PubMed] [Google Scholar]
- Bar-Oz B, Einarson T, Einarson A, Boskovic R, O'Brien L, Malm H, Berard A, Koren G. Paroxetine and congenital malformations: meta-Analysis and consideration of potential confounding factors. Clin Ther. 2007;29(5):918–926. doi: 10.1016/j.clinthera.2007.05.003. [DOI] [PubMed] [Google Scholar]
- Berard A, Ramos E, Rey E, Blais L, St-Andre M, Oraichi D. First trimester exposure to paroxetine and risk of cardiac malformations in infants: the importance of dosage. Birth Defects Res (Part B) 2007;80(1):18–27. doi: 10.1002/bdrb.20099. [DOI] [PubMed] [Google Scholar]
- Bonari L, Pinto N, Ahn E, Einarson A, Steiner M, Koren G. Perinatal risks of untreated depression during pregnancy. Can J Psychiat. 2004;49(11):726–735. doi: 10.1177/070674370404901103. [DOI] [PubMed] [Google Scholar]
- Buznikov GA, Lambert HW, Lauder JM. Serotonin and serotonin-like substances as regulators of early embryogenesis and morphogenesis. Cell Tissue Res. 2001;305(2):177–186. doi: 10.1007/s004410100408. [DOI] [PubMed] [Google Scholar]
- Choi DS, Ward SJ, Messaddeq N, Launay JM, Maroteaux L. 5-HT2B receptor-mediated serotonin morphogenetic functions in mouse cranial neural crest and myocardiac cells. Development. 1997;124(9):1745–1755. doi: 10.1242/dev.124.9.1745. [DOI] [PubMed] [Google Scholar]
- Cogswell ME, Bitsko RH, Anderka M, Caton AR, Feldkamp ML, Hockett Sherlock SM, et al. Control selection and participation in an ongoing, population-based, case-control study of birth defects: the National Birth Defects Prevention Study. Am J Epidemiol. 2009;170:975–985. doi: 10.1093/aje/kwp226. [DOI] [PubMed] [Google Scholar]
- Cole JA, Ephross SA, Cosmatos IS, Walker AM. Paroxetine in the first trimester and the prevalence of congenital malformations. Pharmacoepidemiol Drug Saf. 2007;16(10):1075–1085. doi: 10.1002/pds.1463. [DOI] [PubMed] [Google Scholar]
- Cooper WO, Willy ME, Pont SJ, Ray WA. Increasing use of antidepressants in pregnancy. Am J Obstet Gynecol. 2007;196(6):544 e541–545 e541. doi: 10.1016/j.ajog.2007.01.033. [DOI] [PubMed] [Google Scholar]
- Correa A, Gilboa SM, Besser LM, Botto LD, Moore CA, Hobbs CA, Cleves MA, Riehle-Colarusso TJ, Waller DK, Reece EA. Diabetes mellitus and birth defects. Am J Obstet Gynecol. 2008;199(3):237 e231–239 e231. doi: 10.1016/j.ajog.2008.06.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cote F, Fligny C, Bayard E, Launay JM, Gershon MD, Mallet J, Vodjdani G. Maternal serotonin is crucial for murine embryonic development. Proc Natl Acad Sci U S A. 2007;104(1):329–334. doi: 10.1073/pnas.0606722104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- da-Silva VA, Altenburg SP, Malheiros LR, Thomaz TG, Lindsey CJ. Postnatal development of rats exposed to fluoxetine or venlafaxine during the third week of pregnancy. Braz J Med Biol Res. 1999;32(1):93–98. doi: 10.1590/s0100-879x1999000100014. [DOI] [PubMed] [Google Scholar]
- Diav-Citrin O, Shechtman S, Weinbaum D, Wajnberg R, Avgil M, Di Gianantonio E, Clementi M, Weber-Schoendorfer C, Schaefer C, Ornoy A. Paroxetine and fluoxetine in pregnancy: a prospective, multicentre, controlled, observational study. Brit J Clin Pharmaco. 2008;66(5):695–705. doi: 10.1111/j.1365-2125.2008.03261.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drews CD, Kraus JF, Greenland S. Recall bias in a case-control study of sudden infant death syndrome. Int J Epidemiol. 1990;19(2):405–411. doi: 10.1093/ije/19.2.405. [DOI] [PubMed] [Google Scholar]
- Einarson A, Choi J, Einarson TR, Koren G. Incidence of major malformations in infants following antidepressant exposure in pregnancy: results of a large prospective cohort study. Can J Psychiat. 2009;54(4):242–246. doi: 10.1177/070674370905400405. [DOI] [PubMed] [Google Scholar]
- Einarson A, Fatoye B, Sarkar M, Lavigne SV, Brochu J, Chambers C, Mastroiacovo P, Addis A, Matsui D, Schuler L, Einarson TR, Koren G. Pregnancy outcome following gestational exposure to venlafaxine: a multicenter prospective controlled study. Am J Psychiatry. 2001;158(10):1728–1730. doi: 10.1176/appi.ajp.158.10.1728. [DOI] [PubMed] [Google Scholar]
- Einarson A, Pistelli A, DeSantis M, Malm H, Paulus WD, Panchaud A, Kennedy D, Einarson TR, Koren G. Evaluation of the risk of congenital cardiovascular defects associated with use of paroxetine during pregnancy. Am J Psychiatry. 2008;165(6):749–752. doi: 10.1176/appi.ajp.2007.07060879. [DOI] [PubMed] [Google Scholar]
- Ferreira E, Carceller AM, Agogue C, Martin BZ, St-Andre M, Francoeur D, Berard A. Effects of selective serotonin reuptake inhibitors and venlafaxine during pregnancy in term and preterm neonates. Pediatr. 2007;119(1):52–59. doi: 10.1542/peds.2006-2133. [DOI] [PubMed] [Google Scholar]
- Grote NK, Bridge JA, Gavin AR, Melville JL, Iyengar S, Katon WJ. A meta-analysis of depression during pregnancy and the risk of preterm birth, low birth weight, and intrauterine growth restriction. Arch Gen Psychiatry. 2010;67(10):1012–1024. doi: 10.1001/archgenpsychiatry.2010.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harman JS, Edlund MJ, Fortney JC. Trends in antidepressant utilization from 2001 to 2004. Psychiatr Serv. 2009;60(5):611–616. doi: 10.1176/ps.2009.60.5.611. [DOI] [PubMed] [Google Scholar]
- Hu YF, Caron MG, Sieber-Blum M. Norepinephrine transport-mediated gene expression in noradrenergic neurogenesis. BMC Genomics. 2009;10:151. doi: 10.1186/1471-2164-10-151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kallen B. Fluoxetine use in early pregnancy. Birth Defects Res (Part B) 2004a;71(6):395–396. doi: 10.1002/bdrb.20025. [DOI] [PubMed] [Google Scholar]
- Kallen B. Neonate characteristics after maternal use of antidepressants in late pregnancy. Arch Pediatr Adolesc Med. 2004b;158(4):312–316. doi: 10.1001/archpedi.158.4.312. [DOI] [PubMed] [Google Scholar]
- Kallen BA, Otterblad Olausson P. Maternal use of selective serotonin re-uptake inhibitors in early pregnancy and infant congenital malformations. Birth Defects Res A Clin Mol Teratol. 2007;79(4):301–308. doi: 10.1002/bdra.20327. [DOI] [PubMed] [Google Scholar]
- Khoury MJ, James LM, Erickson JD. On the Use of Affected Controls to Address Recall Bias in Case-Control Studies of Birth-Defects. Teratology. 1994;49(4):273–281. doi: 10.1002/tera.1420490407. [DOI] [PubMed] [Google Scholar]
- Lauder JM. Neurotransmitters as morphogens. In: Boer GJ, Feenstra MGP, Mimiran D, Swaab DF, Van Haaren F, editors. Progress in Brain Research: Elsevier Science Publishers B.V. 1988. pp. 365–387. [DOI] [PubMed] [Google Scholar]
- Lennestal R, Kallen B. Delivery outcome in relation to maternal use of some recently introduced antidepressants. J Clin Psychopharmacol. 2007;27(6):607–613. doi: 10.1097/jcp.0b013e31815ac4d2. [DOI] [PubMed] [Google Scholar]
- Louik C, Lin AE, Werler MM, Hernandez-Diaz S, Mitchell AA. First-trimester use of selective serotonin-reuptake inhibitors and the risk of birth defects. N Engl J Med. 2007;356(26):2675–2683. doi: 10.1056/NEJMoa067407. [DOI] [PubMed] [Google Scholar]
- Mackenzie SG, Lippman A. An investigation of report bias in a case-control study of pregnancy outcome. Am J Epidemiol. 1989;129(1):65–75. doi: 10.1093/oxfordjournals.aje.a115125. [DOI] [PubMed] [Google Scholar]
- Marcus SC, Olfson M. National trends in the treatment for depression from 1998 to 2007. Arch Gen Psychiatry. 2010;67(12):1265–1273. doi: 10.1001/archgenpsychiatry.2010.151. [DOI] [PubMed] [Google Scholar]
- Mitchell AA, Cottler LB, Shapiro S. Effect of questionnaire design on recall of drug exposure in pregnancy. Am J Epidemiol. 1986;123(4):670–676. doi: 10.1093/oxfordjournals.aje.a114286. [DOI] [PubMed] [Google Scholar]
- Moiseiwitsch JR. The role of serotonin and neurotransmitters during craniofacial development. Crit Rev Oral Biol Med. 2000;11(2):230–239. doi: 10.1177/10454411000110020601. [DOI] [PubMed] [Google Scholar]
- Moiseiwitsch JR, Lauder JM. Serotonin regulates mouse cranial neural crest migration. Proc Natl Acad Sci U S A. 1995;92(16):7182–7186. doi: 10.1073/pnas.92.16.7182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nebigil CG, Choi DS, Dierich A, Hickel P, Le Meur M, Messaddeq N, Launay JM, Maroteaux L. Serotonin 2B receptor is required for heart development. Proc Natl Acad Sci U S A. 2000;97(17):9508–9513. doi: 10.1073/pnas.97.17.9508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nebigil CG, Maroteaux L. A novel role for serotonin in heart. Trends Cardiovasc Med. 2001;11(8):329–335. doi: 10.1016/s1050-1738(01)00135-9. [DOI] [PubMed] [Google Scholar]
- Oberlander TF, Warburton W, Misri S, Riggs W, Aghajanian J, Hertzman C. Major congenital malformations following prenatal exposure to serotonin reuptake inhibitors and benzodiazepines using population-based health data. Birth Defects Res (Part B) 2008;83(1):68–76. doi: 10.1002/bdrb.20144. [DOI] [PubMed] [Google Scholar]
- Olfson M, Marcus SC. National patterns in antidepressant medication treatment. Arch Gen Psychiatry. 2009;66(8):848–856. doi: 10.1001/archgenpsychiatry.2009.81. [DOI] [PubMed] [Google Scholar]
- Rasmussen SA, Olney RS, Holmes LB, Lin AE, Keppler-Noreuil KM, Moore CA. Guidelines for case classification for the National Birth Defects Prevention Study. Birth Defects Res A Clin Mol Teratol. 2003;67(3):193–201. doi: 10.1002/bdra.10012. [DOI] [PubMed] [Google Scholar]
- Reis M, Kallen B. Delivery outcome after maternal use of antidepressant drugs in pregnancy: an update using Swedish data. Psychological Medicine. 2010;40(10):1723–1733. doi: 10.1017/S0033291709992194. [DOI] [PubMed] [Google Scholar]
- Ren ZG, Porzgen P, Zhang JM, Chen XR, Amara SG, Blakely RD, Sieber-Blum M. Autocrine regulation of norepinephrine transporter expression. Mol Cell Neurosci. 2001;17(3):539–550. doi: 10.1006/mcne.2000.0946. [DOI] [PubMed] [Google Scholar]
- Ren ZG, Porzgen PP, Youn YH, Sieber-Blum M. Ubiquitous embryonic expression of the norepinephrine transporter. Dev Neurosci. 2003;25(1):1–13. doi: 10.1159/000071462. [DOI] [PubMed] [Google Scholar]
- Ross LE, McLean LM. Anxiety disorders during pregnancy and the postpartum period: A systematic review. J Clin Psychiatry. 2006;67(8):1285–1298. doi: 10.4088/jcp.v67n0818. [DOI] [PubMed] [Google Scholar]
- Schueler YB, Koesters M, Wieseler B, Grouven U, Kromp M, Kerekes MF, Kreis J, Kaiser T, Becker T, Weinmann S. A systematic review of duloxetine and venlafaxine in major depression, including unpublished data. Acta Psychiatr Scand. 2011;123(4):247–265. doi: 10.1111/j.1600-0447.2010.01599.x. [DOI] [PubMed] [Google Scholar]
- Sieber-Blum M, Ren Z. Norepinephrine transporter expression and function in noradrenergic cell differentiation. Mol Cell Biochem. 2000;212(1–2):61–70. [PubMed] [Google Scholar]
- Sussman N. SNRIs Versus SSRIs: Mechanisms of Action in Treating Depression and Painful Physical Symptoms. Primary Care Companion J Clin Psychiatry. 2003;5(Suppl 7):19–26. [Google Scholar]
- Waller DK, Pujazon MA, Canfield MA, Scheuerle AE, Byrne JL. Frequency of prenatal diagnosis of birth defects in Houston, Galveston and the Lower Rio Grande Valley, Texas 1995. Fetal Diagn Ther. 2000;15(6):348–354. doi: 10.1159/000021035. [DOI] [PubMed] [Google Scholar]
- Wichman CL, Moore KM, Lang TR, St Sauver JL, Heise RH, Jr, Watson WJ. Congenital heart disease associated with selective serotonin reuptake inhibitor use during pregnancy. Mayo Clin Proc. 2009;84(1):23–27. doi: 10.4065/84.1.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yaris F, Kadioglu M, Kesim M, Ulku C, Yaris E, Kalyoncu NI, Unsal M. Newer antidepressants in pregnancy: prospective outcome of a case series. Reprod Toxicol. 2004;19(2):235–238. doi: 10.1016/j.reprotox.2004.07.004. [DOI] [PubMed] [Google Scholar]
- Yonkers KA, Wisner KL, Stewart DE, Oberlander TF, Dell DL, Stotland N, Ramin S, Chaudron L, Lockwood C. The management of depression during pregnancy: a report from the American Psychiatric Association and the American College of Obstetricians and Gynecologists. Obstet Gynecol. 2009;114(3):703–713. doi: 10.1097/AOG.0b013e3181ba0632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoon PW, Rasmussen SA, Lynberg MC, Moore CA, Anderka M, Carmichael SL, Costa P, Druschel C, Hobbs CA, Romitti PA, Langlois PH, Edmonds LD. The National Birth Defects Prevention Study. Public Health Rep. 2001;116(Suppl 1):32–40. doi: 10.1093/phr/116.S1.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang JM, Dix J, Langtimm-Sedlak CJ, Trusk T, Schroeder B, Hoffmann R, Strosberg AD, Winslow JW, Sieber-Blum M. Neurotrophin-3- and norepinephrine-mediated adrenergic differentiation and the inhibitory action of desipramine and cocaine. J Neurobiol. 1997;32(3):262–280. [PubMed] [Google Scholar]
- Zierler S, Rothman KJ. Congenital heart disease in relation to maternal use of Bendectin and other drugs in early pregnancy. N Engl J Med. 1985;313(6):347–352. doi: 10.1056/NEJM198508083130603. [DOI] [PubMed] [Google Scholar]