Abstract
Cyclopia is characterized by the presence of a single eye, with varying degrees of doubling of the intrinsic ocular structures, located in the middle of the face. It is the severest facial expression of the holoprosencephaly (HPE) spectrum. This study describes the prevalence, associated malformations, and maternal characteristics among cases with cyclopia. Data originated in 20 Clearinghouse (ICBDSR) affiliated birth defect surveillance systems, reported according to a single pre-established protocol. A total of 257 infants with cyclopia were identified. Overall prevalence was 1 in 100,000 births (95%CI: 0.89–1.14), with only one program being out of range. Across sites, there was no correlation between cyclopia prevalence and number of births (r = 0.08; P=0.75) or proportion of elective termination of pregnancy (r= −0.01; P=0.97). The higher prevalence of cyclopia among older mothers (older than 34) was not statistically significant. The majority of cases were liveborn (122/200; 61%) and females predominated (male/total: 42%). A substantial proportion of cyclopias (31%) were caused by chromosomal anomalies, mainly trisomy 13. Another 31% of the cases of cyclopias were associated with defects not typically related to HPE, with more hydrocephalus, heterotaxia defects, neural tube defects, and preaxial reduction defects than the chromosomal group, suggesting the presence of ciliopathies or other unrecognized syndromes. Cyclopia is a very rare defect without much variability in prevalence by geographic location. The heterogeneous etiology with a high prevalence of chromosomal abnormalities, and female predominance in HPE, were confirmed, but no effect of increased maternal age or association with twinning was observed.
Keywords: cyclopia, holoprosencephaly, trisomy 13, prevalence, global, world prevalence, epidemiology, clinical
INTRODUCTION
“Cyclopia is a congenital malformation characterized by the presence of a single eye, which usually manifests various degrees of doubling of intrinsic structures, located in the middle of the face in the place normally occupied by the root of the nose.”
With this elegant definition Sedano and Gorlin began their 1963 article about the oral manifestation of cyclopia, reporting two cyclopia patients and a literature review about the specific manifestations of cyclopia that deserves an actual reading because of its completeness. Holoprosencephaly (HPE) was also reviewed in extenso in a special issue of Part C in the American Journal of Medical Genetics[Muenke et al., 2010]. Thus only new pertinent information will be included here.
Usually considered as the severest gradation of facial malformation associated with HPE, cyclopia rarely is presented separately from other HPE types. Cyclopia by itself appeared in the epidemiological work of Källén et al. [1992], in the chapter in a more general work about HPE [Cohen and Sulik, 1992], and among few median anomalies in the interpretative work of O’Railly and Muüller [1989]. However, there are hundreds of case reports of cyclopia in humans and in other vertebrates, besides the experimental studies in animal models causing cyclopia. This vast amount of case reports in the literature on cyclopia allows us to have an exact idea about the phenotypic variation and possible etiologies of this condition. The rarity of the condition, however, does not allow epidemiological studies to demonstrate the risk factors and the contribution of each one to the onset of cyclopia. Using material registered by the Clearinghouse [ICBDSR, 2009] from millions of births surveyed by 20 surveillance programs worldwide, our aim here is to analyze the prevalence and possible risk factors of cyclopia.
Historical Aspects
Recent reviews of teratology and mythology by Cohen [2010b], and by Stahl and Tourame [2010] agreed with previous reviewers that real newborns with those defects existed in the origin of the mythological creatures and fantastic beings. Although there is no way to be sure of the population number at the year 800 BC in all the world, an educated guess suggested 66,000,000[Mc Evedy and Jones, 1978], and another guess suggested a crude birth rate of 80 per 1,000 for this period (http://www.prb.org/Articles/2002/HowManyPeopleHaveEverLivedonEarth.aspx)opia w. If so, around the time Odyssey was being composed, approximately 53 cases of cyclopia were born by year, in the world population. We can speculate on how this small number of cases could have caused such an impressive impact on the people’s imagination. One possibility is that in those earlier times, the prevalence of cyclopia was higher than it is now. There are many other possibilities as there are scholarly theories of the myths. The study of the origin of the myths probably requires tools from other fields such as anthropology, psychology, sociology, or semiology.
Normal and Abnormal Development
As part of the HPE spectrum, the prosencephalon in cyclopia cases fails to develop into two hemispheres [Cohen and Sulik, 1992]. Although HPE is usually divided into alobar, semilobar, and lobar types according to severity, to the presence or not of the interhemispheric fissure and the extent of separation of both hemispheres [DeMyer and Zeman, 1963], cyclopia presents almost always as the alobar type. Only few instances of semilobar HPE were found in the literature [Orioli and Castilla, 2007; Dane et al., 2009]. In the alobar type there is complete or near complete lack of interhemispheric separation, single midline forebrain ventricle, absent interhemispheric fissure, falx cerebri, olfactory bulbs, and corpus callosum; and nonseparation of deep gray nuclei, as summarized in the HPE flashcards produced by Solomon et al. [2010]. Also published were detailed aspects on early pathogenesis [Shiota and Yamada, 2010], neuropathology [Hahn and Barnes, 2010], and neuroimaging [Marcorelles and Laquerriere, 2010].
In 1963, Sedano and Gorlin presented the discussion between two apparently conflicting theories, among others, to explain the pathogenesis of cyclopia. In one theory the condition is said to be caused by abnormal differentiation of the prochordal mesoderm in the central part of the developing head region. Another hypothesis states that the brain malformation is the primary anomaly. Today, it is clear that both are involved but one of the key signaling centers for the pathogenesis of HPE is the most anterior extent of the midline mesoderm, called the prechordal plate. Several signals emanate from the prechordal plate and trigger a secondary patterning center in the ventral forebrain. Two complete reviews [Klingensmith et al., 2010; Roessler and Muenke, 2010] show that the requirement for delicate balancing of numerous key influences includes hedgehogs, fibroblast growth factors (Fgfs), bone morphogenic proteins (Bmps), retinoic acid, and canonical and noncanonical Wnt signaling.
England et al. [2006] labeled every cell nuclei of zebra-fish embryos with green fluorescent protein to visualize and track their movements and produced a dynamic fate map of the forebrain showing how the vertebrate eyes form. The authors also tested zebrafish embryos with two different mutations causing cyclopia showing that cyclopia in Cyclops (loss of Ndr2) results in corporation of eye tissue into an inappropriate location within the medial neural keel (an intermediate stage between the neural plate and neural rod during the early segmentation period in the morphogenesis of the central nervous system primordium); the much reduced convergent and forward movement of lateral-posterior eye-field cells fated to the optic stalk in Silberblick cyclopia mutants (loss of Wnt11) results in medial-posterior eye-field cells remaining medial. These two defects of forebrain morphogenesis are temporally and spatially distinct pointing to the recognized etiologic heterogeneity of cyclopia.
Genetics and Clinical Genetics
Cyclopia is an etiologically heterogeneous condition, which can result from chromosomal defects, genetic mutations, or environmental teratogenic factors. Several important reviews address the HPE etiology, mostly by M Michael Cohen Jr., but also by Maximilian Muenke, and by Sylvie Odent and Veronique David groups. In general there is little information about the etiology of cyclopia specifically in those reviews because cyclopia is considered to be the severest form of HPE [Cohen, 1989a; Muenke and Beachy, 2000; Dubourg et al., 2007].
Trisomy 13 is the most common chromosomal disorder associated with HPE. The trisomies 18 and 21 have also been described, as well as triploidy. The structural abnormalities described in the literature on 11 different chromosomes allowed the identification of 12 loci for HPE [Roessler and Muenke, 1998]. These loci are called HPE1 to HPE12 and are located in regions 21q33.3, 2p21 (SIX3), 7q36 (SHH), 18p11.3 (TGIF), 13q32 (ZIC2), 2q371–q37.3, 9q22.3 (PTCH1), prox 14q, 20p13, 1q42-qter (DISP1), 5pter, and 6q26-qter [Dubourg et al., 2007]. Only six genes (in parentheses) were assigned to the loci HPE2, HPE3, HPE4, HPE5, HPE7, and HPE10. There are no genes reported yet for the other six loci. Cohen [2006, 2010a] presented complete reviews including other genes associated with HPE; however, only the Smith–Lemli–Opitz syndrome gene, DHCR7 on 11q12–q13, was, in the literature, associated with cyclopia in one case.
Point mutations are found in syndromes presenting HPE. The OMIM database (http://www.ncbi.nlm.nih.-gov/omim/), visited on March 30th, 2011) presented 31 syndromes showing HPE (Table I). A careful review of them shows that only four, the dysgnathia complex (or agnathia–HPE or otocephaly) (OMIM 202650), the Pseudotrisomy 13 syndrome (OMIM 264480), the Steinfeld syndrome (OMIM 184705); and the Smith–Lemli–Opitz syndrome (OMIM 270400), had cyclopia [Atkin, 1988; Cohen and Gorlin, 1991; Nöthen et al., 1993; Rolland et al., 1991; Weaver et al., 2010]. Also, only these four syndromes plus osteopathia striata with cranial sclerosis (OMIM 300373) presented with the alobar type of HPE. There are descriptions of other syndromes presenting HPE in the literature, as Rubinstein–Taybi syndrome, Meckel syndrome [Hsia et al., 1971], and Martin syndrome [Martin et al., 1977], not disclosed in Table I, since they are not associated with HPE in the OMIM database (Table I).
TABLE I.
Syndromes That Could Present Holoprosencephaly (HPE) Among Their Clinical Features According to OMIM (Online Mendelian Inheritance in Man)
| MIM IDa | Syndrome | Chromosome region |
Gene | Alobar type |
Cyclopia | Notes |
|---|---|---|---|---|---|---|
| % 202650 | Dysgnathia complex | — | — | Yes | Yes | Ciliopathy |
| 264480 | Pseudo-trisomy 13 | — | — | Yes | Yes | AR? Microdeletion? |
| 184705 | Steinfeld | — | — | Yes | Yes | AD |
| # 276400 | Smith–Lemli–Opitz | 11q12–q13 | DHCR7 | Yes | Yes | AR |
| # 300373 | Osteopathia striata with cranial sclerosis |
Xq11.1 | WTX | Yes | No | LXD |
| # 176450 | Currarino | 7q36 | HLXB9 | No | No | Microdeletion? |
| # 147791 | Jacobsen, Chr. 11q deletion | 11q23 | — | No | No | Microdeletion |
| % 129900 | EEC 1 | 7q11.2–q21.3 | — | No | No | — |
| # 236680 | Hydrolethalus 1 | 11q24.2 | HYLS1 | ? | No | — |
| % 612776 | Hypoglossia with situs inversus |
— | — | No | No | Mild form of agnathia-HPE? |
| + 187395 | Teratocarcinoma derived growth factor 1 |
3p23–p21 | TDGF1 | No | No | Only 1 paperb |
| % 605627 | Cerebrooculonasal | PTCH? | No | No | HPE7? | |
| # 192430 | Velocardiofacial | 22q11.2 | TBX1 | No | No | Only 1 paperb |
| 187100 | Supernumerary teeth, mesiodens |
— | — | No | No | — |
| # 147950 | Kallmann 2 | — | FGFR1 | No | No | — |
| 300706 | Mental retardation XL | Xp11.2 | HUWE1 | No | No | XL |
| 303073 | Fetal akinesia XL | — | — | No | No | XL |
| # 253800 | MDDGA4, Walker– Warburg |
9q31 | FKTN | No | No | AR, dystroglycanopathy |
| # 214800 | Charge | 8q12.1 | CHD7 | No | No | — |
| 7q21.11 | SEMA3E | |||||
| # 206900 | Microphtalmia and esophageal atresia |
3q26.3–q27 | SOX2 | No | No | Only one paperb |
| + 180200 | Retinoblastoma | 13q14.1–q14.2 | RB1 | No | No | Del 13q14? |
| 156810 | Microgastria-limb reduction |
— | — | No | No | — |
| 300571 | Hartsfield | — | — | No | No | — |
| 601370 | Genoa | — | — | No | No | AR? |
| 306990 | HPE with fetal akinesia | — | — | No | No | XL? |
| 610680 | HPE, recurrent infection, monocytosis |
— | — | No | No | AD? |
| 245552 | Lambotte | — | — | No | No | — |
| 146510 | Pallister–Hall | 7p13 | GLI3 | No | No | — |
| 601357 | Amelia, forebrain defects, and clefts |
— | — | No | No | — |
| 612651 | Endocrine cerebroosteodysplasia |
6p12.3 | ICK | No | No | — |
| % 600674 | Microtia anotia | — | — | No | No | — |
(+) gene with known sequence and phenotype;(#)phenotype description, molecular basis known; (%) mendelian phenotypeor molecular basis unknown; (none) other, mainly phenotype with suspected mendelian basis. MIM: Online Mendelian Inheritance in Man number
Only one paper described HPE in the condition.
Some cyclopia patients present with one or more unrelated congenital anomalies that are not part of the non-chromosomal syndromes cited above. Concurrence of cyclopia and sirenomelia in the same patient was reported by Martínez-Frías et al. [1998], while associations of both defects with similar epidemiological risk factors were found by Källén et al. [1992]; involvement in the same clusters was reported by Castilla et al. [2008], and sharing of a similar pathogenetic mechanism was noted by O’Railly and Mu¨ller [1989].
Classification and Nomenclature
A classical paper, whose title humorously and intelligently, two conditions rarely found in medical literature, proposed that “The face predicts the brain” was published by DeMyer et al. [1964]. However, as science has no room for poetic licenses, this publication was criticized based on reported patients which did not fit into this axiom [Olsen et al., 1997; Plawner et al., 2002], while Cohen [1989b] quantified the exceptions to the rule, concluding that the proportion of patients where the face did not predict the brain comprised from 10 to 39% of all HPE patients [Levey et al., 2010].
From the anatomo-pathological point of view, three types of HPE were described by DeMyer and Zeman [1963], in decreasing severity: alobar, semilobar, and lobar; while clinically the following types were proposed with certain degree of correspondence with the brain anatomy [DeMyer et al., 1963]: (1) Medial monophtalmia with arrhinia and proboscis (cyclopia), (2) ethmocephaly with supra-orbital proboscis, (3) hypotelorism, inter- or infra-orbital proboscis with single nostril (cebocephaly), (4) median cleft of the upper lip with agenesis of premaxilla (with HPE obviously).
Proboscis refers to a blind-ending tube-like structure at or near the midline of the face, and can be supra or infra orbital, synophthalmia refers to merged ocular globes with variable degrees of fused ocular structures. Synophtalmia is sometimes used as cyclopia synonym as pointed out by Cohen and Sulik [1992] or to mean fused eyes in one orbit, as used by Solomon et al. [2010]. Since this is not a real fusion but rather a defect in the patterning of the eye fields, synophtalmia could be a misleading term. The origin of the word cyclopia is also controversial and it might not even mean one-eyed people.
Cyclopia represents between 10% [Orioli and Castilla, 2007] and 20% [Mastroiacovo et al., 1992] of all HPE as reported by the two largest published series, the difference being probably due to variation in phenotypic documentation.
Epidemiology (Includes Prevalence, and Risk Factors, Known or Hypothetical)
In a recent review of HPE epidemiology [Orioli and Castilla, 2010], that included prevalence and risk factors, 24 HPE published series around the world were reviewed. Two years before, HPE data from 24 of the 46 Birth Defects Registry Members of the International Clearinghouse for Birth Defects Surveillance and Research (ICBDSR)[Leoncini et al., 2008] were also analyzed. Thirteen members of the ICBDSR also participated in the unique epidemiology study dealing only with cyclopias [Källén et al., 1992]. From these three studies, we concluded that there are several factors to explain the observed epidemiologic differences in maternal age, twinning rate and sex among the studied populations. Operational factors as the different proportions of embryos, fetuses, still-borns, and liveborns in each studied population will result in different proportions of HPE caused by chromosomal abnormalities. The younger the patients the higher the prevalence of chromosomal abnormalities. Then, variables such as maternal age and other associated with it will change accordingly.
In regard to specific environmental risk factors, Cohen and Shiota [2002] reviewed several factors, including ethyl alcohol, diabetic embryopathy, retinoic acid, and several anecdotal suggestions of teratogenic factors for HPE, including viruses, and salicylates. Orioli and Castilla [2007] confirmed in a South American series maternal diabetes and maternal flu as more prevalent in HPE than in controls. Miller et al. [2010] analyzed case patients and controls from the National Birth Defects Prevention Study and found HPE to be associated with pre-existing diabetes, aspirin use, lower education level, and use of assisted reproductive technologies. In the same issue, Johnson and Rasmussen [2010] provided a summary of nongenetic risk factors for HPE that have been investigated in case reports and case series, animal studies, and epidemiologic studies, including maternal illnesses, therapeutic and nontherapeutic exposures, nutritional factors, and sociodemographic factors.
METHODS
Birth defects surveillance programs that are part of ICBDSR were asked to provide de-identified case records following a common protocol, with information on phenotype, genetic testing, and selected demographic and prenatal information. Further details on the methodologies can be found in Castilla and Mastroiacovo [2011] in this issue. As part of the Very Rare Defect study of the ICBDSR, 20 surveillance programs in 25 countries (10 countries represented in Estudo Colaborativo Latino Americano de Malformações Congênitas: ECLAMC), from North and South America, Europe, Israel, China and Australia provided data on cyclopia from an underlying cohort of 25.6 million births. The years represented were 1968–2006, depending on the reporting site.
Clinical and demographic data were reviewed centrally by two authors with experience in dysmorphology (IO and PM). Additional information for inclusion or exclusion of cases was also requested in a second step by one author (IO). After the identification of all chromosomal and nonchromosomal syndromes, the remaining cases with multiple congenital anomalies (MCA) were classified according to the number of unrelated defects to the HPE spectrum [Orioli and Castilla, 2007], and according to the presence of postaxial polydactyly All cases were reported by verbatim description, and centrally classified without coding. Nevertheless in 222 of the 257 patients (86%), the defect was reported by a single word (i.e., cyclopia), therefore consisting of just a naming rather than of a real description. In 35 cases more details were provided on the HPE type, and/or the presence of proboscis, and/or the number of eyes inside the orbit.
Occurrence was expressed as total prevalence [number of live births, stillbirths and elective termination of pregnancy for fetal anomaly (ETOPFA) with cyclopia per 100,000 births] with its 95% confidence intervals (CI). For each program the expected number of cases was calculated under the hypothesis of a homogeneous prevalence among all programs. Using the expected values we calculated the exact Poisson probabilities of observing N or more cases [P(N ≥ x)] in each registry. Maternal age-specific prevalence ratios were calculated across several clinical subtypes (isolated, MCA, and chromosomal syndromes) with women <20 years of age serving as the referent group. Odds ratios and 95%CI were computed across clinical subtypes to examine the association of various characteristics using both isolated and MCA as a referent group. Pearson correlation was used as a measure of correlation between the prevalence of cyclopia and two variables: the number of births and the proportion of ETOPFA in each registry.The 95% CI were computed using the Poisson distribution. Statistical tests significance was set to P < 0.05. Statistical analyses were done with Stata software, version 10.0 [StataCorp., 2007].
RESULTS
Prevalence
The total number of births and of cyclopia cases is given in Table II for each one of the 20 surveillance programs members of the ICBDSR. A total of257 infants with cyclopia were identified among 25,580,661 births, giving a total prevalence of 1.0 per 100,000 births (95%CI: 0.89–1.14).
TABLE II.
Total Prevalence (Per 100,000 Births) of Cyclopia in 20 Surveillance Programs Members of the International Clearinghouse for Birth Defects Surveillance and Research (ICBDSR)
| Surveillance program | Period | Births | Total cases |
% of ETOPFA |
Prevalence (per 100.000 births) |
95%CI |
|---|---|---|---|---|---|---|
| Canada Alberta | 1980–2005 | 1,062,483 | 9 | 0 | 0.85 | 0.39–1.61 |
| USA Utah | 1997–2004 | 380,706 | 2 | 50.0 | 0.53 | 0.06–1.90 |
| USA Atlanta | 1968–2004 | 1,283,999 | 13 | 38.5 | 1.01 | 0.54–1.73 |
| USA Texas | 1996–2002 | 2,054,788 | 25 | 32.0 | 1.22 | 0.79–1.80 |
| Mexico RYVEMCE | 1978–2005 | 1,058,885 | 18 | NP | 1.70 | 1.01–2.69 |
| South America ECLAMC | 1982–2006 | 4,556,173 | 55 | NP | 1.21 | 0.91–1.57 |
| Finland | 1993–2004 | 713,494 | 8 | 50.0 | 1.12 | 0.48–2.21 |
| Wales | 1998–2004 | 222,309 | 5 | 40.0 | 2.25 | 0.73–5.25 |
| Northern Netherlands | 1981–2003 | 369,658 | 3 | 0 | 0.81 | 0.17–2.37 |
| Germany Saxony-Anhalt | 1980–2004 | 355,184 | 3 | 100 | 0.84 | 0.17–2.47 |
| Slovak Republic | 2000–2005 | 318,257 | 1 | 100 | 0.31 | 0.01–1.75 |
| Hungary | 1980–2005 | 3,022,194 | 8 | 0 | 0.26 | 0.11–0.52 |
| France Central East | 1979–2004 | 2,500,214 | 30 | 56.7 | 1.20 | 0.81–1.71 |
| Italy North East | 1981–2004 | 1,186,497 | 21 | 47.6 | 1.77 | 1.10–2.71 |
| Italy Tuscany | 1992–2004 | 336,744 | 2 | 50.0 | 0.59 | 0.07–2.15 |
| Italy Campania | 1992–2004 | 643,962 | 2 | 50.0 | 0.31 | 0.04–1.12 |
| Spain ECEMC | 1980–2004 | 2,045,751 | 14 | NR | 0.68 | 0.37–1.15 |
| Israel | 1975–2005 | 151,562 | 1 | 0 | 0.66 | 0.02–3.68 |
| China Beijing | 1992–2005 | 1,927,622 | 29 | NR | 1.50 | 1.01–2.16 |
| Australia Victoria | 1983–2004 | 1,390,179 | 8 | 50.0 | 0.58 | 0.25–1.13 |
| 25,580,661 | 257 | 40.4 | 1.00 | 0.89–1.14 |
RYVEMCE, Registro y Vigilancia Epidemiológica de Malformaciones Congénitas; ECLAMC, Estudo Colaborativo Latino Americano de Malformações Congênitas; ECEMC, Spanish Collaborative Study of Congenital Malformations; ETOPFA, elective termination of pregnancy for fetal anomaly; NP, not permitted; NR, not reported.
About half (54.0%) of the cases with cyclopia in this study were provided by four reporting surveillance programs: South America ECLAMC, France Central East, China Beijing, and USA Texas.
ETOPFA is not permitted for two surveillance programs (Mexico RYVEMCE: Registro y Vigilancia Epidemiológica de Malformaciones Congénitas, and South America ECLAMC). Furthermore, it was not recorded in two other surveillance programs (Spain ECEMC: Spanish Collaborative Study of Congenital Malformations, and China, Beijing), and was recorded at an unknown and probably variable ascertainment rate in the rest.
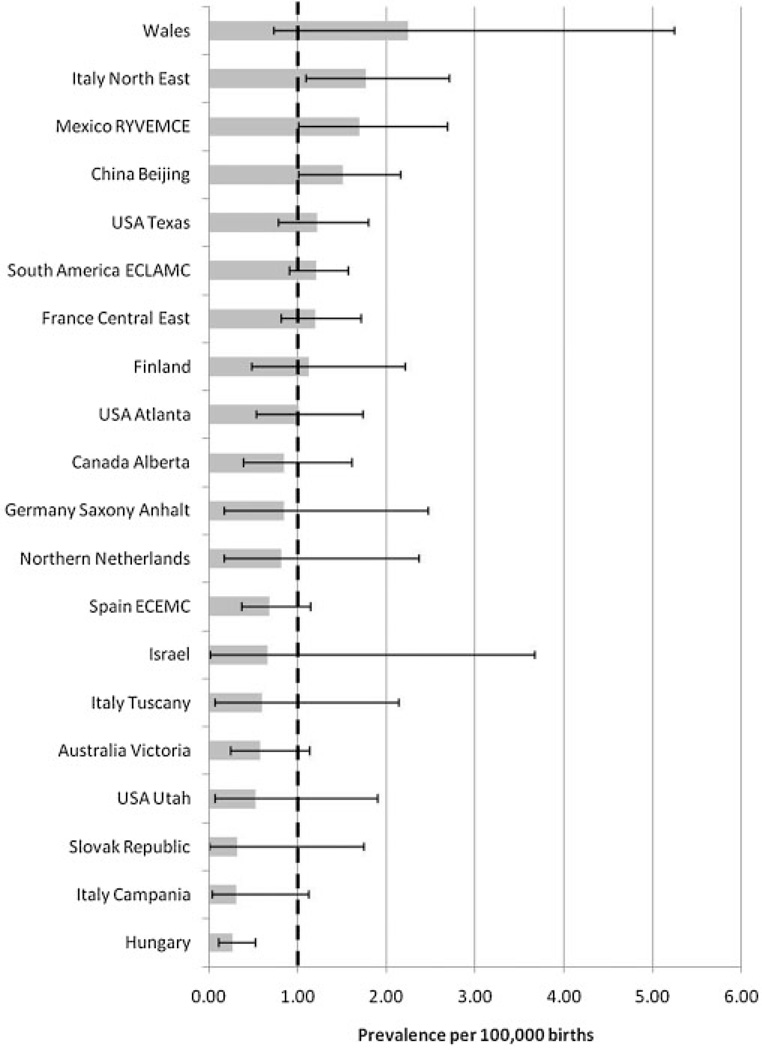
Figure 1 compares estimates of the cyclopia prevalences with their 95%CI among the different surveillance programs. Only Hungary’s prevalence’s upper confidence limit was below the total prevalence of 1.0 per 100,000 births suggesting under-registration (0,26 per 100,000; CI: 0.11–0.52, P< 0.0001). Excluding this program, the overall prevalence of 1.10 per 100,000 is estimated for all the remaining programs, with a marginal statistically significant higher prevalence estimated in Italy North-East (1.77, CI: 1.10–2.71). There was no correlation between the cyclopia prevalence and number of births (r = 0.08; P=0.75) or proportion of elective termination of pregnancy (r=−0.01; P=0.97) in each surveillance program.
Figure 1.
Total prevalence per 100,000 births (bar) and 95% confidence interval (line) by surveillance program and overall (dotted line) of cyclopia in 20 surveillance programs members of the International Clearinghouse for Birth Defects Surveillance and Research (ICBDSR).
Secular Variation and Clustering of Cases
The rarity of cyclopia induces great variation in the annual frequencies within each one of the 20 programs without evident secular trends in any program. None of the programs reported an evidence of a cluster of cases.
Maternal Age
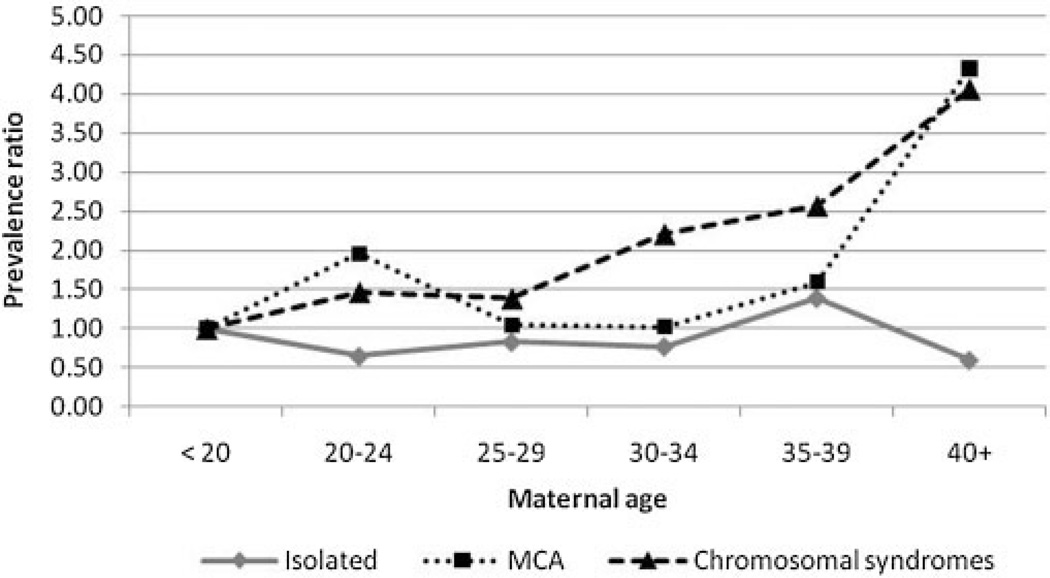
Maternal age was not specified in 9.8% of the total births and in 18.4% of the cases with cyclopia. Maternal age was analyzed by 5-year groups in 19 programs by clinical phenotypes: isolated, MCA, and chromosomal abnormalities. Figure 2 shows that cases with chromosomal abnormalities presented a statistically significant increasing trend (P=0.015), as expected. The MCA case group did not show any maternal age trend, but the oldest mothers (>40 years of age) had a prevalence that was over four times the prevalence among the referent group of youngest mothers (<20 years of age) (prevalence ratio 4.33, 95%CI: 1.16–16.12). Isolated cases did not present any maternal age effect, These results suggest that a number of undiagnosed cases of chromosomal trisomies could be present within the MCA group, but not within the isolated group.
Figure 2.
Prevalence ratios for maternal age groups relative to the reference age group of <20 years with corresponding 95%CI, for cyclopia in 20 surveillance programs members of the International Clearinghouse for Birth Defects Surveillance and Research (ICBDSR).
Cases’ Characteristics by Clinical Phenotype
Chromosomal syndromes
There were 79 cases with chromosomal syndromes, accounting for 31% of the cyclopias. Only 23% of total cases had an available karyotype, since karyotyping was not done or reported in all cases. Given the limited reporting on karyotypes, it is possible that the estimate of chromosomal syndromes may be higher than the 31% referred here. For example, two South American associated cases left out from the chromosomal syndromic group in the material presented here, were later on proved to have a chromosomal anomaly by multiplex ligation-dependent probe amplification (MLPA) analysis. Most of these 81 cases (79 + 2) were trisomy 13 (n = 68; 84%), followed by trisomy 18 (n = 6)or partial shortarm monosomy (n=3) (subtotal n=9; 10%). In addition there were two cases with triploidy, one with trisomy 21, and one with a partial deletion of 7q36.
The main characteristics of chromosomal syndromes are shown in Table III. The proportion of males (0.47) did not differ from the expected in the 78 specified cases. More than half of cases are stillborn or submitted to ETOPFA, and almost 80% have low birth weight.
TABLE III.
Characteristics of the Cases With Cyclopia
| Total cases (n = 257) |
Isolated cases (n = 97) |
Cases with associated malformations (n = 81) |
Chromosomal syndromes (n = 79) |
|||||
|---|---|---|---|---|---|---|---|---|
| n | % | n | % | n | % | n | % | |
| Sex | ||||||||
| Male | 103 | 40.1 | 36 | 37.1 | 30 | 37.0 | 37 | 46.8 |
| Female | 143 | 55.6 | 59 | 60.8 | 43 | 53.1 | 41 | 51.9 |
| Indeterminate | 6 | 2.3 | 0 | 0.0 | 6 | 7.4 | 0 | 0.0 |
| Missing data | 5 | 2.0 | 2 | 2.1 | 2 | 2.5 | 1 | 1.3 |
| Outcome | ||||||||
| Livebirths | 122 | 47.5 | 51 | 52.6 | 35 | 43.2 | 36 | 45.6 |
| Stillbirths | 78 | 30.4 | 35 | 36.1 | 27 | 33.3 | 16 | 20.3 |
| ETOPFA | 57 | 22.2 | 11 | 11.3 | 19 | 23.5 | 27 | 34.2 |
| Missing data | ||||||||
| Birth weight (g)a | ||||||||
| <1,500 | 32 | 26.2 | 11 | 21.6 | 14 | 40.0 | 7 | 19.4 |
| 1,500–2,500 | 59 | 48.4 | 25 | 49.0 | 13 | 37.1 | 21 | 58.3 |
| >2,500 | 26 | 21.3 | 14 | 27.5 | 7 | 20.0 | 5 | 13.9 |
| Missing data | 5 | 4.1 | 1 | 2.0 | 1 | 2.9 | 3 | 8.3 |
| Gestational age (week)a | ||||||||
| <32 | 21 | 17.2 | 9 | 17.7 | 8 | 22.9 | 4 | 11.1 |
| 32–36 | 52 | 42.6 | 19 | 37.3 | 13 | 37.1 | 20 | 55.6 |
| >37 | 41 | 33.6 | 20 | 39.2 | 11 | 31.4 | 10 | 27.8 |
| Missing data | 8 | 6.6 | 3 | 5.9 | 3 | 8.6 | 2 | 5.6 |
| Parity | ||||||||
| 0 | 35 | 13.6 | 18 | 18.6 | 12 | 14.8 | 5 | 6.3 |
| 1 | 93 | 36.2 | 40 | 41.2 | 29 | 35.8 | 24 | 30.4 |
| 2 or more | 39 | 15.2 | 15 | 15.5 | 11 | 13.6 | 13 | 16.5 |
| Missing data | 90 | 35.0 | 24 | 24.7 | 29 | 35.8 | 37 | 46.8 |
| Previous spontaneous abortio | ns | |||||||
| 0 | 81 | 31.5 | 29 | 29.9 | 27 | 33.3 | 25 | 31.7 |
| 1 | 14 | 5.5 | 5 | 5.2 | 5 | 6.2 | 4 | 5.1 |
| Missing data | 162 | 63.0 | 63 | 65.0 | 49 | 60.5 | 50 | 63.3 |
| Plurality | ||||||||
| Single | 225 | 87.6 | 87 | 89.7 | 70 | 86.4 | 68 | 86.1 |
| Twin | 6 | 2.3 | 2 | 2.1 | 2 | 2.5 | 2 | 2.5 |
| Missing data | 26 | 10.1 | 8 | 8.3 | 9 | 11.1 | 9 | 11.4 |
| Maternal age | ||||||||
| <20 | 18 | 7.0 | 9 | 9.3 | 5 | 6.2 | 4 | 5.1 |
| 20–24 | 63 | 24.5 | 20 | 20.6 | 28 | 34.6 | 15 | 19.0 |
| 25–29 | 63 | 24.5 | 29 | 29.9 | 17 | 21.0 | 17 | 21.5 |
| 30–34 | 47 | 18.3 | 16 | 16.5 | 12 | 14.8 | 19 | 24.1 |
| 35–39 | 28 | 10.9 | 11 | 11.3 | 8 | 9.9 | 9 | 11.4 |
| >40 | 8 | 3.1 | 1 | 1.0 | 4 | 4.9 | 3 | 3.8 |
| Missing data | 30 | 11.7 | 11 | 11.3 | 7 | 8.6 | 12 | 15.2 |
| Parental age difference | ||||||||
| Mother same age or older | 22 | 8.6 | 10 | 10.3 | 7 | 8.6 | 5 | 6.3 |
| Mother 1–2 years younger | 24 | 9.3 | 9 | 9.3 | 9 | 11.1 | 6 | 7.6 |
| Mother 3–5 years younger | 24 | 9.3 | 8 | 8.3 | 11 | 13.6 | 5 | 6.3 |
| Mother >5 years younger | 14 | 5.5 | 5 | 5.2 | 4 | 4.9 | 5 | 6.3 |
| Missing data | 173 | 67.3 | 65 | 67.0 | 50 | 61.7 | 58 | 73.4 |
| Maternal education (years) | ||||||||
| <9 | 20 | 7.8 | 7 | 7.2 | 12 | 14.8 | 1 | 1.3 |
| 9 or more | 52 | 20.2 | 26 | 26.8 | 15 | 18.5 | 11 | 13.9 |
| Missing data | 185 | 72.0 | 64 | 66.0 | 54 | 66.7 | 67 | 84.8 |
Birth weight, gestational age: the data are for live births only.
The comparison of the characteristics of chromosomal syndrome cases versus isolated and MCA cases are shown in Table IV, where the odds ratios of the possible “risk factors” (characteristics) with their 95%CI were computed using both isolated and MCA as a referent group. In this analysis only programs with less than 20% of unknown information were used. The occurrence of an elective termination (or ETOPFA) was approximately 3.5 times more likely among chromosomal cases than isolated cases (OR = 3.48, 95%CI: 1.53–7.90). No significant associations were found when chromosomal cases were compared with MCA cases.
TABLE IV.
Odds Ratios (OR) of the Association of the Various Characteristics of: (A) Multiple Congenital Anomalies (MCA) Cases Compared to Isolated, (B) Chromosomal Syndromes Compared to Isolated, and (C) Chromosomal Syndromes Compared to MCA Cases
| (A) MCA vs. isolated cases |
(B) Chromosomal syndromes vs. isolated cases |
(C) Chromosomal syndromes vs. MCA |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| Crude OR |
95%CI | Crude OR |
95%CI | Crude OR |
95%CI | ||||
| Sex | |||||||||
| Male | 1.00 | 1.00 | 1.00 | ||||||
| Female | 0.87 | 0.47 | 1.63 | 0.68 | 0.37 | 1.24 | 0.77 | 0.41 | 1.47 |
| Outcome | |||||||||
| Livebirths | 1.00 | 1.00 | 1.00 | ||||||
| Stillbirths | 1.12 | 0.58 | 2.18 | 0.65 | 0.31 | 1.34 | 0.58 | 0.26 | 1.25 |
| ETOPFA | 2.52 | 1.07 | 5.94 | 3.48 | 1.53 | 7.90 | 1.38 | 0.65 | 2.92 |
| Birth weight (g)a | |||||||||
| <1,500 | 2.54 | 0.76 | 8.47 | 1.78 | 0.44 | 7.18 | 0.70 | 0.16 | 3.02 |
| 1,500–2,500 | 1.04 | 0.34 | 3.21 | 2.35 | 0.73 | 7.61 | 2.26 | 0.59 | 8.64 |
| >2,500 | 1.00 | 1.00 | 1.00 | ||||||
| Gestational age (week)a | |||||||||
| <32 | 1.62 | 0.48 | 5.38 | 0.89 | 0.22 | 3.61 | 0.55 | 0.12 | 2.40 |
| 32–36 | 1.24 | 0.45 | 3.45 | 2.10 | 0.79 | 5.64 | 1.69 | 0.56 | 5.11 |
| ≥37 | 1.00 | 1.00 | 1.00 | ||||||
| Parity | |||||||||
| 0 | 1.00 | 1.00 | 1.00 | ||||||
| 1 | 0.30 | 0.08 | 1.10 | 0.50 | 0.12 | 2.08 | 1.65 | 0.48 | 5.68 |
| 2 or more | 0.28 | 0.07 | 1.21 | 0.63 | 0.13 | 2.91 | 2.20 | 0.54 | 8.96 |
| Previous spontaneous abortions | |||||||||
| 0 | 1.00 | 1.00 | 1.00 | ||||||
| 1 or more | 1.17 | 0.18 | 7.79 | 1.50 | 0.25 | 9.11 | 1.28 | 0.26 | 6.34 |
| Plurality | |||||||||
| Single | 1.00 | 1.00 | 1.00 | ||||||
| Twin | 1.24 | 0.17 | 9.05 | 1.28 | 0.17 | 9.32 | 1.03 | 0.14 | 7.52 |
| Maternal age | |||||||||
| <20 | 1.00 | 1.00 | 1.00 | ||||||
| 20–24 | 2.52 | 0.73 | 8.66 | 1.69 | 0.43 | 6.54 | 0.67 | 0.16 | 2.87 |
| 25–29 | 1.05 | 0.30 | 3.67 | 1.32 | 0.35 | 4.94 | 1.25 | 0.28 | 5.47 |
| 30–34 | 1.35 | 0.36 | 5.08 | 2.67 | 0.69 | 10.33 | 1.98 | 0.44 | 8.87 |
| 35–39 | 1.31 | 0.31 | 5.43 | 1.84 | 0.42 | 8.01 | 1.41 | 0.28 | 7.13 |
| ≥40 | 7.20 | 0.62 | 83.34 | 6.75 | 0.53 | 86.56 | 0.94 | 0.13 | 6.87 |
| Parental age difference | |||||||||
| Mother same age or older | 0.70 | 0.18 | 2.66 | 0.75 | 0.17 | 3.33 | 1.07 | 0.23 | 5.02 |
| Mother 1–2 years younger | 1.00 | 1.00 | 1.00 | ||||||
| Mother 3–5 years younger | 1.37 | 0.38 | 5.03 | 0.94 | 0.20 | 4.29 | 0.68 | 0.15 | 2.99 |
| Mother >5 years younger | 0.80 | 0.16 | 3.99 | 1.50 | 0.30 | 7.53 | 1.87 | 0.35 | 9.98 |
Surveillance programs where missing data were more than 20% were excluded from the analysis
Birth weight, gestational age: the data are for live births only.
Multiple congenital anomalies (MCA)
There were 81 cyclopia cases (31%) with associated defects not usually considered as part of the HPE spectrum. As mentioned before, we expected that with all cases fully analyzed for chromosomal abnormalities this proportion could be lower. When grouping these cases according the number of non-related HPE defects, 45 had only one associated defect (55%), 19 had two (24%), and 17 (21%) had three, four, or five associated defects. Most of these associated defects were similar to the ones found in the chromosomal syndromes, mainly omphalocele, anal atresia, cardiac, renal, and postaxial polydactyly. Postaxial polydactyly was present in 22/81 (27%) of the MCA cases. Different from the chromosomal syndromes, this group presented more cases with heterotaxia defects (6/81), neural tube defects (10/81), and preaxial limb reduction defects (9/81). Few nonchromosomal syndromes or associations could be suspected among the MCA cases: there were two cases with otocephaly—HPE, and two less typical examples of the dysgnathia complex, one case of prune belly, one case of VATER association with hydrocephalus, one chondrodystrophy not further specified, and one case of cyclopia and sirenomelia in the same case. This last case, according to the partial description, probably was a case with cyclopia, sirenomelia, and acardia-acephaly. The defects presented by some of these cases are displayed in Box I.
BOX 1. Defects Described in Six Cases With Cyclopia.
| ID | Defects | Karyotype | Diagnoses hypotheses |
|---|---|---|---|
| 1 | Cyclopia; alobar HPE; microcephaly; external hydrocephaly; arhinia; microstomia; prominent ears; anomalous mandible; esophageal atresia; thoracic hemivertebras; butterfly vertebras; anomalous pelvic bone; preaxial polydactyly; polyhydramnion |
46,XX | OMIM # 276950 VATER with hydrocephalus |
| 2 | Cyclopia; unspecified septal ventricular defect; polycystic kidneys adult type; anomalies of hand (lobster claw hand); Arthrogryposis multiplex congenital |
— | OMIM 200980 Acrorenal-mandibular with HPE |
| 3 | Cyclopia; microcephalus; jaw defect; microtia; preauricular appendage; microstomia; Meckel diverticulum; radius absent |
— | OMIM % 202650 Dysgnathia complex? Ciliopathy? |
| 4 | Cyclopia; proboscis above eye; otocephaly; micropene; bilateral criptorquidia; pilonidal pit |
— | OMIM % 202650 Dysgnathia complex |
| 5 | Cyclopia, partially fused eyes; proboscis above eyes; alobar HPE; arhinia; microstomia; mouth could not be open; microtia; missing first, second and thirds fingers bilaterally; feet syndactyly between second and third right toes and between third and fourth left toes; bilateral agenesis of radius; anal atresia; ambiguous genitalia; pulmonary isomerism; polisplenia; heart and abdominal organs in the midline (situs ambiguous); ovaries and uterus didelphus; one pelvic kidney with two short ureteres |
46,XX | OMIM % 202650 Dysgnathia complex? Ciliopathy? |
| 6 | Cyclopia; alobar HPE; mandible agenesis; microtia grade 1; melotia; preauricular fistula; absent mouth; absent tongue; pharyngeal stenosis; hypoplastic lungs; hypoplastic adrenal glands |
46,XX | OMIM % 202650 Dysgnathia complex |
The main characteristics of the MCA cases are shown in Table III. The proportion of male (M/T = 0.41) observed did not differ from the expected. More than half of cases were stillborn or submitted to ETOPFA.
Comparing these characteristics with the isolated cases (the comparison with chromosomal syndromes is given above) revealed only one marginal statistical association with ETOPFA (OR= 2.52; 95%CI: 1.07–5.94).
Isolated cases
The main characteristics of isolated cases are shown in Table III. The proportion of males (0.38) was statistically significant different from the expected (χ2 = 6.53; P < 0.05). More than half of cases were liveborn, and more than 50% have low birth weight.
DISCUSSION
Prevalence
The cyclopia prevalence 1.0 per 100,000 births (CI: 0.89–1.14) found in over 25 million births did not differ from 1.03 found previously by Källén et al. (1992) Although both series of data came from the Clearinghouse, there were data overlapping only for the Mexican, South American, Spanish, and French registries. The other 16 registries did not participate in the former work(Källén et al., 1992).
Cyclopia has been reported as between 10% and 18% of the HPE published series, as revised by Orioli and Castilla, (2010). There are two epidemiology works about HPE using the Kyoto Collection of Embryos [Matsunaga and Shiota, 1977; Yamada et al., 2004], however only 11 embryos at Carnegie stage 8–21 had facial anomalies described in the last work. Two embryos presented complete cyclopia and three presented partially fused eyes in a single eye fissure, elevating the proportion of cyclopias among HPE to 45% in embryos.
Few studies report on the proportion of cyclopias or HPE among trisomy 13 patients. Källén et al. [1992] found 8 cyclopias in 436 (1.8%), and Wyllie et al. [1994] found one HPE among 36 trisomy 13 patients (2.8%). Considering a recent estimate of trisomy 13 prevalence of 0.14/1,000 (0.12–0.17) [Irving et al., 2011] we would have expected 3,581 patients of trisomy 13 among the 25,580,661 births, and also expected 99 cyclopias with trisomy 13. However, we detected only 68 cyclopia cases (69%) with trisomy 13.
Clustering of Cases
None of the reporting programs, including South America, reported evidence of a cluster of cases. A significant cluster of sirenomelia and cyclopia in the city of Cali, Colombia [Castilla et al., 2008], was not reflected in the South American material presented in this present work since the four cases of cyclopia born in Cali in 2005 were diluted when merged together with another 243 cases from other South American cities and periods. This exemplifies well the need for active ongoing surveillance of the collected data, which allowed the ECLAMC program to detect the cluster within a few weeks after the fourth case of this epidemic was born. When active surveillance is routinely working, the cluster is first suspected as a rumor that arises by an “alert practitioner” who was part of an epidemiology system, capable of following up on the rumor.
Maternal Age
As an important proportion of cyclopias (29%) are associated with trisomy, with an expected increased maternal age among deliveries, we expected a higher proportion of older age mothers among the cyclopia patients. However, the increased rate of cyclopias seen in the older maternal age groups (above 29 years old) in the total sample was not statistically significant. Only mothers 40 years old or above in the MCA group were in excess with respect to the mothers in the range <20. This suggests two possible explanations: (1) there is a substantial number of trisomy cases under-diagnosed among the MCA nonchromosomal group; and (2) a maternal age effect in trisomy 13 is not as important as the maternal age effect reported in other trisomies, as trisomy 18, for example [Crider et al., 2008].
Twinning
Only 6 from 231 infants with cyclopia were twins (2.6%). This low frequency of twinning differs from the excess of twinning reported by Källén et al. [1992]. The greater size of the present sample (25.6 million births) compared with the sample size used by Källén et al. [1992] (10.1 million births) could be an explanation.
Sex
Mastroiacovo et al. [1992], Rasmussen et al. [1996], and Orioli and Castilla [2010] did not confirm the excess of females among HPE patients as described in other series. The excess of female patients among cyclopias as seen in our work among the isolated cases or in Källén et al. [1992], or in other previous HPE series [Roach et al., 1975; Croen et al., 1996, 2000; Forrester and Merz, 2000; Chen et al., 2005] could be attributed to the excess loss of male embryos through spontaneous abortion [Rasmussen et al., 1996]. This idea was founded on studies of HPE in embryos [Matsunaga and Shiota, 1977], who showed a much higher rate of HPE than in newborns, and also on studies of fetuses with HPE, where an equal sex ratio or even a male excess could be observed [Blaas et al., 2002]. The lack of sex difference in the MCA, chromosomal syndromes and ETOPFA samples in the present work is consistent with this hypothesis, as well as the already mentioned presence of undetected chromosome syndrome patients in the MCA group.
Nonchromosomal Syndromes or Associations
In Table I are presented 31 syndromes that, with three possible exceptions, are nonchromosomal syndromes. The exceptions are pseudo-trisomy 13 (HPE-polydactyly), Currarino syndrome, and Jacobsen syndrome that could be caused by microdeletions on chromosome 13, 7q36, and 11q chromosomal regions, respectively. We scrutinized the 81 patients in the MCA group looking for examples of these syndromes without much exit. Several cases could be suspected of trisomy 13 or of pseudo-trisomy 13, mainly those with postaxial polydactyly. Few were suspected of other nonchromosomal syndromes has can be viewed in Box I.
It is out of the scope of this work to confirm the suggested diagnoses in Box I. However, the two otocephaly or agnathia-HPE patients have clear diagnoses. A recent otocephaly review [Faye-Petersen et al., 2006] shows an otocephaly prevalence around 1:70,000 births and reported that half of them present HPE. Since a conservative estimate of cyclopia among HPE is 10%, we must expect 18 patients with cyclopia-otocephaly association in our material {[(25,580,661/70,000)/2)/0.10}. The poor description observed in 86% of our cases with cyclopia could explain why we identify only 10% of the expected number of this association.
There is another possible reason to explain the few examples of syndromes we found in our MCA material. A careful review of the type of HPE associated with each one of those 31 syndromes in Table I shows that only the first four were ever associated with alobar HPE and with cyclopia: dysgnathia complex (OMIM 202650), pseu-do-trisomy 13 (OMIM 264480), Steinfeld syndrome (OMIM 184705), and Smith–Lemli–Opitz syndrome (OMIM 276400).
The interesting case with cyclopia, sirenomelia, and acardia-acephaly was not found previously described in the literature. However, several patients reviewed by Siebert [2007] presented cerebral defects as cyclopia, aprosencephaly, or atelencephaly with acardiac twinning. Hypoxia-ischemia due to twin reversed arterial perfusion (TRAP) is a common explanation for these defects and probably can explain the presence of sirenomelia in our present case. Acardia-acephaly with sirenomelia is also a combination of two very rare defects already published [Martínez-Frías, 2009; Orioli et al., 2011, this issue).
Ultimately, a new type of pathogenesis, the ciliopathies, have been proposed to explain a large number of diseases, mainly heterotaxia defects, hydrocephaly, neural tube defects, and other defects related to twining [Hildebrandt et al., 2011]. Six patients within the MCA group of cyclopias presented with these kind of heterotaxic defects as accessory spleen, situs inversus, situs ambiguous, and lung isomerism; 6 presented with hydrocephalus, and 10 presented with NTD. With the exception of hydrocephalus, these defects were not found in excess among a South American HPE series [Orioli and Castilla, 2007]. We cannot test the statistical significance of this excess in our cyclopia sample; however, only 2 cases with bilobar lung, and no cases with hydrocephalus or NTD occurred in the chromosomal anomaly group of 79 patients. Also, only one patient with preaxial reduction defect was seen in the chromosomal group. There are several phenotypes associated with cilia dysfunction in mammals including randomization of the left–right body axis, abnormalities in neural tube closure and patterning, skeletal defects such as poly-dactyly, etc. A new locus for Meckel syndrome (MK8), a diagnosis that can be confounded with trisomy 13, was described [Shaheen et al., 2011], and map to TCTN2 a paralog for Tectonic 1, which was involved in Sonic Hedgehog (SHH) signaling. SHH has been described as one of the most important genes causing HPE what reinforces the possible causal role of ciliopathies in the cyclopia causation.
Are Cyclopias Different From HPE?
Since cyclopias are rare, there are difficulties in collecting enough patients to compare epidemiologically with HPE in general. In this work a sample of 257 cyclopias could be analyzed and no important differences were demonstrated with respect to HPE [Mastroiacovo et al., 1992; Orioli and Castilla, 2007; Orioli and Castilla, 2010]. Although the analyses of environmental factors was limited by missing data, the available data show one patient of mother with diabetes, no patients of alcoholic mothers, two patients born after threatened abortion, one using misoprostol and one not further specified, and a half dozen patients born after maternal flu or fever, among a few other gestational exposures. In general, these limited findings agree with previous HPE epidemiological data reviewed by Orioli and Castilla [2010]. There are several possible causes of HPE, but we could not highlight any of them as more important or more specific to cause cyclopia. Only the pattern of associated defects in the group MCA seems to indicate a possible role of ciliopathy disorders to explain some cases of cyclopia.
CONCLUSION
The cyclopia prevalence of 1 per 100,000 (0.89–1.14) did not differ from the previously published in the literature and was similar among most of the registries around the world. Neither the proportion of cyclopias submitted to ETOPFA, nor the numberof births in each surveillance program were correlated with the cyclopia prevalence.
An important proportion of cyclopias (31%) was associated with chromosomal anomalies, mainly trisomy 13. Another 31% presented with defects that are not related to HPE. This last group also had more occurrences of other defects, namely hydrocephalus, heterotaxic defects, NTDs, and preaxial reduction defects than the chromosomal group, suggesting the presence of ciliopathies or other unrecognized syndromes. The proportion of isolated cases (38%) seems inflated, since in 86% of these cases the defect was reported by a single word (i.e., cyclopia), suggesting the practice of naming rather than providing a real description. Few non-chromosomal syndromes or associations could be suspected among the MCA cases, probably because of the paucity of the clinical descriptions.
The prevalence of all cyclopias by 5-year maternal age groups was higher among mothers in the two oldest age groups (35–39 and 40 years old or above), although this finding was not statistically significant. There was an expected increased prevalence with maternal age in the chromosomal anomaly case group. The prevalence ratio for the older maternal age group, relative to the reference age group, was higher and statistically significant in the MCA group of cyclopias, suggesting a possible contribution in this group with non-recognized cases of trisomies.
The already described excess of females in HPE was seen for the cyclopia casess, in livebirths, stillbirths, and in the total sample, without sex differences in the ETOPFA sample, MCA, and chromosomal syndrome groups.
Cyclopia differ from other very rare defects by the large contribution of chromosomal anomalies to its etiology, underlying the importance of the chromosomal examination, direct or through molecular techniques, in isolated or in associated patients. Also etiologically important are the nonchromosomal syndromes, making the accurate description of the phenotype, including cerebral imaging, and careful collection of familial history essential requirements. When possible, molecular studies should be performed since so many genes are already associated to this defect. Congenital defects registries around the world must be aware of the difficulty of gather this precious material if the verbatim description are the result of de-codification. The very rare defects deserve, inside those registries, a special treatment, with detailed phenotype descriptions and collection of all possible familial information, in order to improve future epidemiological studies.
ACKNOWLEDGMENTS
The authors are grateful to each surveillance program’s staff and members for their work in collecting case data and submission to the ICBDSR Centre. This work was supported by grant from MCT/CNPq, Brazil (573993/2008-4, 476978/2008-4, 554755/ 2009-2, 306750/2009-0, 402045/ 2010-6); FAPERJ, Brazil (E-26/ 102.748/2008, E-26/170.007/2008); CAPES, Brazil (1957/2009, 2799/ 2010). Work conducted at the Centre of the ICBDSR was supported by the Center for Disease Control and Prevention (1U50DD000524-02). This work was in part supported by Instituto de Salud Carlos III (ISCIII, Ministry of Science and Innovation) of Spain, and the Fundacio´n 1000 Sobre Defectos Conge´nitos of Spain. CIBERER is an initiative of ISCIII. CIBERER is an initiative of ISCIII. Components of ECEMC’s Peripheral Group are gratefully acknowledged.
The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention.
REFERENCES
- Atkin JF. A new syndrome with cyclopia and trisomy 13 features. Am J Hum Genet. 1988;43:36. [Google Scholar]
- Blaas HG, Eriksson AG, Salvesen KA, Isaksen CV, Christensen B, Møllerløkken G, Eik-Nes SH. Brains and faces in holoprosencephaly: Pre- and postnatal description of 30 cases. Ultrasound Obstet Gynecol. 2002;19:24–38. doi: 10.1046/j.0960-7692.2001.00154.x. [DOI] [PubMed] [Google Scholar]
- Castilla EE, Mastroiacovo PP. Introduction paper. Am J Med Genet Part C. 2011 (this issue) [Google Scholar]
- Castilla EE, Mastroiacovo P, López-Camelo JS, Saldarriaga W, Isaza C, Orioli IM. Sirenomelia and cyclopia cluster in Cali, Colombia. Am J Med Genet Part A. 2008;146A:2626–2636. doi: 10.1002/ajmg.a.32506. [DOI] [PubMed] [Google Scholar]
- Chen CP, Chern SR, Lin CJ, Lee CC, Wang W, Tzen CY. A comparison of maternal age, sex ratio and associated anomalies among numerically aneuploid, structurally aneuploid and euploid holoprosencephaly. Genet Couns. 2005;16:49–57. [PubMed] [Google Scholar]
- Cohen MM., Jr Perspectives on holoprosencephaly: Part I. Epidemiology, genetics, and syndromology. Teratology. 1989a;40:211–235. doi: 10.1002/tera.1420400304. [DOI] [PubMed] [Google Scholar]
- Cohen MM., Jr Perspectives on holoprosencephaly: Part III. Spectra, distinctions, continuities, and discontinuities. Am J Med Genet. 1989b;34:271–288. doi: 10.1002/ajmg.1320340232. [DOI] [PubMed] [Google Scholar]
- Cohen MM., Jr Holoprosencephaly: Clinical, anatomic, and molecular dimensions. Birth Defects Res A Clin Mol Teratol. 2006;76:658–673. doi: 10.1002/bdra.20295. [DOI] [PubMed] [Google Scholar]
- Cohen MM., Jr Hedgehog signaling update. Am J Med Genet Part A. 2010a;152A:1875–1914. doi: 10.1002/ajmg.a.32909. [DOI] [PubMed] [Google Scholar]
- Cohen MM., Jr Holoprosencephaly: A mythologic and teratologic distillate. Am J Med Genet Part C. 2010b;154C:8–12. doi: 10.1002/ajmg.c.30252. [DOI] [PubMed] [Google Scholar]
- Cohen MM, Jr, Gorlin RJ. Pseudo-trisomy 13 syndrome. Am J Med Genet. 1991;39:332–335. doi: 10.1002/ajmg.1320390316. [DOI] [PubMed] [Google Scholar]
- Cohen MM, Jr, Shiota K. Teratogenesis of holoprosencephaly. Am J Med Genet. 2002;109:1–15. doi: 10.1002/ajmg.10258. [DOI] [PubMed] [Google Scholar]
- Cohen MM, Jr, Sulik KK. Perspectives on holoprosencephaly. Part II. Central nervous system, craniofacial anatomy, syndrome commentary, diagnostic approach, and experimental studies. J Craniofacial Genet Dev Biol. 1992;12:196–244. [PubMed] [Google Scholar]
- Crider KS, Olney RS, Cragan JD. Trisomies 13 and 18: Population prevalences, characteristics, and prenatal diagnosis, metropolitan Atlanta, 1994–2003. AmJ Med Genet Part A. 2008;146A:820–826. doi: 10.1002/ajmg.a.32200. [DOI] [PubMed] [Google Scholar]
- Croen LA, Shaw GM, Lammer EJ. Holoprosencephaly: Epidemiologic and clinical characteristics of a California population. Am J Med Genet. 1996;64:465–472. doi: 10.1002/(SICI)1096-8628(19960823)64:3<465::AID-AJMG4>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- Croen LA, Shaw GM, Lammer EJ. Risk factors for cytogenetically normal holoprosencephaly in California: A populationbased case-control study. Am J Med Genet. 2000;90:320–325. [PubMed] [Google Scholar]
- Dane B, Dane C, Aksoy F, Yayla M. Semilobar holoprosencephaly with associated cyclopia and radial aplasia: First trimester diagnosis by means of integrating 2D-3D ultrasound. Arch Gynecol Obstet. 2009;280:647–651. doi: 10.1007/s00404-009-0975-6. [DOI] [PubMed] [Google Scholar]
- DeMyer W, Zeman W. Alobar holoprosencephaly (arhinencephaly) with median cleft lip and palate: Clinical, electroence-phalographic and nosologic considerations. Confin Neurol. 1963;23:1–36. doi: 10.1159/000104278. [DOI] [PubMed] [Google Scholar]
- DeMyer WE, Zeman W, Palmer CG. Familial alobar holoprosencephaly (arhinencephaly) with median cleft lip and palate: Report of patient with 46 chromosomes. Neurology. 1963;13:913–918. doi: 10.1212/wnl.13.11.913. [DOI] [PubMed] [Google Scholar]
- DeMyer W, Zeman W, Palmar CG. The face depicts the brain: Diagnostic significance of median facial anomalies for holoprosencephaly (arhinencephaly) with median cleft lip and palate. Pediatrics. 1964;34:256–263. [PubMed] [Google Scholar]
- Dubourg C, Bendavid C, Pasquier L, Henry C, Odent S, David V. Holoprosencephaly. Orphanet J Rare Dis. 2007;2:8. doi: 10.1186/1750-1172-2-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- England SJ, Blanchard GB, Mahadevan L, Adams RJ. A dynamic fate map of the forebrain shows how vertebrate eyes form and explains two causes of cyclopia. Development. 2006;133:4613–4617. doi: 10.1242/dev.02678. [DOI] [PubMed] [Google Scholar]
- Faye-Petersen O, David E, Rangwala N, Seaman JP, Hua Z, Heller DS. Otocephaly: Report of five new cases and a literature review. Fetal Pediatr Pathol. 2006;25:277–296. doi: 10.1080/15513810601123417. [DOI] [PubMed] [Google Scholar]
- Forrester MB, Merz RD. Epidemiology of holoprosencephaly in Hawaii, 1986–97. Paediatr Perinat Epidemiol. 2000;14:61–63. doi: 10.1046/j.1365-3016.2000.00211.x. [DOI] [PubMed] [Google Scholar]
- Hahn JS, Barnes PD. Neuroimaging advances in holoprosencephaly: Refining the spectrum of the midline malformation. Am J Med Genet Part C Semin Med Genet. 2010;154C:120–132. doi: 10.1002/ajmg.c.30238. [DOI] [PubMed] [Google Scholar]
- Hildebrandt F, Benzing T, Katsanis N. Ciliopathies. N Engl J Med. 2011;364:1533–1543. doi: 10.1056/NEJMra1010172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsia YE, Bratu M, Herbordt A. Genetics of the Meckel syndrome (dysencephalia splanchnocystica) Pediatrics. 1971;48:237–247. [PubMed] [Google Scholar]
- ICBDSR International Clearinghouse for Birth Defects Surveillance and Research. [November 27, 2009];Annual Report 2007. 2009 Roma and website: http://www.icbdsr.org, [Google Scholar]
- Irving C, Richmond S, Wren C, Longster C, Embleton ND. Changes in fetal prevalence and outcome for trisomies 13 and 18: A population-based study over 23 years. J Matern Fetal Neonatal Med. 2011;24:137–141. doi: 10.3109/14767051003758879. [DOI] [PubMed] [Google Scholar]
- Johnson CY, Rasmussen SA. Non-genetic risk factors for holoprosencephaly. Am J Med Genet Part C. 2010;154C:73–85. doi: 10.1002/ajmg.c.30242. [DOI] [PubMed] [Google Scholar]
- Källén B, Castilla EE, Lancaster PA, Mutchinick O, Knudsen LB, Martínez-Frías ML, Mastroiacovo P, Robert E. The cyclops and the mermaid: An epidemiological study of two types of rare malformation. J Med Genet. 1992;29:30–35. doi: 10.1136/jmg.29.1.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klingensmith J, Matsui M, Yang Y-P, Anderson RM. Roles of bone morphogenetic protein signaling and its antagonism in holoprosencephaly. Am J Med Genet Part C. 2010;154C:43–51. doi: 10.1002/ajmg.c.30256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leoncini E, Baranello G, Orioli IM, Annerén G, Bakker M, Bianchi F, Bower C, Canfield MA, Castilla EE, Cocchi G, Correa A, De Vigan C, Doray B, Feldkamp ML, Gatt M, Irgens LM, Lowry RB, Maraschini A, McDonnell R, Morgan M, Mutchinick O, Poetzsch S, Riley M, Ritvanen A, Gnansia ER, Scarano G, Sipek A, Tenconi R, Mastroiacovo P. Frequency of holoprosencephaly in the International Clearinghouse Birth Defects Surveillance Systems: Searching for population variations. Birth Defects Res A Clin Mol Teratol. 2008;82:585–591. doi: 10.1002/bdra.20479. [DOI] [PubMed] [Google Scholar]
- Levey EB, Stashinko E, Clegg NJ, Delgado MR. Management of children with holoprosencephaly. Am J Med Genet Part C Semin Med Genet. 2010;154C:183–190. doi: 10.1002/ajmg.c.30254. [DOI] [PubMed] [Google Scholar]
- Marcorelles P, Laquerriere A. Neuropathology of holoprosencephaly. Am J Med Genet Part C. 2010;154C:109–119. doi: 10.1002/ajmg.c.30249. [DOI] [PubMed] [Google Scholar]
- Martin AO, Perrin JC, Muir WA, Ruch E, Schafer IA. An autosomal dominant midline cleft syndrome resembling familial holoprosencephaly. Clin Genet. 1977;12:65–72. doi: 10.1111/j.1399-0004.1977.tb00903.x. [DOI] [PubMed] [Google Scholar]
- Martínez-Frías ML. Epidemiology of acephalus/acardius monozygotic twins: New insights into an epigenetic causal hypothesis. Am J Med Genet Part A. 2009;149A:640–649. doi: 10.1002/ajmg.a.32741. [DOI] [PubMed] [Google Scholar]
- Martínez-Frías ML, Garcia A, Bermejo E. Cyclopia and sirenomelia in a liveborn infant(letter) J Med Genet. 1998;35:263–264. doi: 10.1136/jmg.35.3.263-a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mastroiacovo P, Botto LD, Cavalcanti DP, Zam-pino G, Serafini MA. Epidemiological and genetic study of holoprosencephaly in 106 cases observed in the Itralian Multicentric Registry; Paper presented at Proceedings of the 1st International Meeting of the Genetic and Reproductive Epidemiology Research Society (GRERS); Roma. 1992. pp. 1978–1989. [Google Scholar]
- Matsunaga E, Shiota K. Holoprosencephaly in human embryos: Epidemiologic studies of 150 cases. Teratology. 1977;16:261–272. doi: 10.1002/tera.1420160304. [DOI] [PubMed] [Google Scholar]
- Mc Evedy C, Jones R. Atlas of world population history. Middlesex Penguin. 1978 [Google Scholar]
- Miller EA, Rasmussen SA, Siega-Riz AM, Frias JL, Honein MA. The National Birth Defects Prevention Study. Risk factors for non-syndromic holoprosencephaly in the National Birth Defects Prevention Study. Am J Med Genet Part C. 2010;154C:62–72. doi: 10.1002/ajmg.c.30244. [DOI] [PubMed] [Google Scholar]
- Muenke M, Beachy PA. Genetics of ventral forebrain development and holoprosencephaly. Curr Opin Genet Dev. 2000;10:262–269. doi: 10.1016/s0959-437x(00)00084-8. [DOI] [PubMed] [Google Scholar]
- Muenke M, Solomon BD, Odent S. Introduction to the American Journal of Medical Genetics Part C on holoprosencephaly. Am J Med Genet Part C. 2010;154C:1–2. doi: 10.1002/ajmg.c.30255. [DOI] [PubMed] [Google Scholar]
- Nöthen MM, Knöpfle G, Födisch HJ, Zerres K. Steinfeld syndrome: Report of a second family and further delineation of a rare autosomal dominant disorder. Am J Med Genet. 1993;46:467–470. doi: 10.1002/ajmg.1320460426. [DOI] [PubMed] [Google Scholar]
- O’Railly R, M¨ller F. Interpretation of some median anomalies as illustrated by cyclopia and symmelia. Teratology. 1989;40:409–421. doi: 10.1002/tera.1420400502. [DOI] [PubMed] [Google Scholar]
- Olsen C, Hughes J, Youngblood L, Sharpe-Stimac M. Epidemiology of holoprosencephaly and phenotypic characteristics of affected children: New York State, 1984-1989. Am J Med Genet. 1997;73:217–226. doi: 10.1002/(sici)1096-8628(19971212)73:2<217::aid-ajmg20>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- Orioli IM, Castilla EE. Clinical epidemiologic study of holoprosencephaly in South America. Am J Med Genet Part A. 2007;143A::3088–3099. doi: 10.1002/ajmg.a.32104. [DOI] [PubMed] [Google Scholar]
- Orioli IM, Castilla EE. Epidemiology of holoprosencephaly: Prevalence and risk factors. Am J Med Genet Part C. 2010;154C:13–21. doi: 10.1002/ajmg.c.30233. [DOI] [PubMed] [Google Scholar]
- Orioli IM, Amar E, Arteaga-Vazquez J, Bakker MK, Bianca S, Botto LD, Clementi M, Correa A, Csaky-Szunyogh M, Leoncini E, López-Camelo JS, Li RB, Marengo ZLowry L, Martínez-Fríaz ML, Mastrioia-covo P, Morgan M, Pierini A, Ritvanen A, Scarano G, Szabova E, Castilla EE. Sirenomelia: An epidemiologic study in a large dataset from the International Clearinghouse of Birth Defects Surveillance and Research, and literature review. Am J Med Genet Part C. 2011 doi: 10.1002/ajmg.c.30324. (this issue) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plawner LL, Delgado M, Miller V, Levey E, Kinsman S, Barkovich AJ, Simon E, Clegg N, Sweet V, Stashinko E, Hahn JS. Neuroanatomy of holoprosencephaly as predictor of function: Beyond the face predicting the brain. Neurology. 2002;59:1058–1066. doi: 10.1212/wnl.59.7.1058. [DOI] [PubMed] [Google Scholar]
- Rasmussen SA, Moore CA, Khoury MJ, Cordero JF. Descriptive epidemiology of holoprosencephaly and arhinencephaly in metropolitan Atlanta, 1968–1992. Am J Med Genet. 1996;66:320–333. doi: 10.1002/(SICI)1096-8628(19961218)66:3<320::AID-AJMG16>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- Roach E, Demyer W, Conneally PM, Palmer C, Merritt AD. Holoprosencephaly: Birth data, benetic and demographic analyses of 30 families. Birth Defects Orig Artic Ser. 1975;11:294–313. [PubMed] [Google Scholar]
- Roessler E, Muenke M. Holoprosencephaly: A paradigm for the complex genetics of brain development. J Inherit Metab Dis. 1998;21:481–497. doi: 10.1023/a:1005406719292. [DOI] [PubMed] [Google Scholar]
- Roessler E, Muenke M. The molecular genetics of holoprosencephaly. Am J Med Genet Part C. 2010;154C:52–61. doi: 10.1002/ajmg.c.30236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rolland M, Sarramon MF, Bloom MC. Astomia-agnathia-holoprosencephaly association. Prenatal diagnosis of a new case. Prenat Diagn. 1991;11:199–203. doi: 10.1002/pd.1970110310. [DOI] [PubMed] [Google Scholar]
- Sedano HO, Gorlin RJ. The oral manifestations of cyclopia. Review of the literature and report of two cases. Oral Surg Oral Med Oral Pathol. 1963;16:823–838. doi: 10.1016/0030-4220(63)90321-9. [DOI] [PubMed] [Google Scholar]
- Shaheen R, Faqeih E, Seidahmed MZ, Sunker A, Alali FE, Khadijah A, Alkuraya FS. A TCTN2 mutation defines a novel Meckel Gruber syndrome locus. Hum Mutat. 2011;32:573–578. doi: 10.1002/humu.21507. [DOI] [PubMed] [Google Scholar]
- Shiota K, Yamada S. Early pathogenesis of holoprosencephaly. Am J Med Genet Part C. 2010;154C:22–28. doi: 10.1002/ajmg.c.30248. [DOI] [PubMed] [Google Scholar]
- Siebert JR. Cyclopia, aprosencephaly, and acardiac twinning: Is hypoxia-ischemia a unifying mechanism? Am J Med Genet Part A. 2007;143A:3100–3106. doi: 10.1002/ajmg.a.32027. [DOI] [PubMed] [Google Scholar]
- Solomon BD, Pineda-Alvarez DE, Mercier S, Raam MS, Odent S, Muenke M. Holoprosencephaly flashcards: A summary for the clinician. Am J Med Genet Part C. 2010;154C:3–7. doi: 10.1002/ajmg.c.30245. [DOI] [PubMed] [Google Scholar]
- Stahl A, Tourame P. De la tératologie aux monstres de la mythologie et des le gendes antiques (From teratology to mythology: Ancient legends) Arch Pédiatr. 2010;17:1716–1724. doi: 10.1016/j.arcped.2010.09.004. (French.) [DOI] [PubMed] [Google Scholar]
- StataCorp. Stata Statistical Software: Release 10. College Station, TX: StataCorp LP; 2007. [Google Scholar]
- Weaver DD, Solomon BD, Akin-Samson K, Kelley RI, Muenke M. Cyclopia (synophthalmia) in Smith-Lemli-Opitz syndrome: First reported case and consideration of mechanism. Am J Med Genet Part C. 2010;154C(1):142–145. doi: 10.1002/ajmg.c.30241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wyllie JP, Wright MJ, Burn J, Hunter S. Natural history of trisomy 13. Arch Dis Child. 1994;71:343–345. doi: 10.1136/adc.71.4.343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada S, Uwabe C, Fujii S, Shiota K. Phenotypic variability in human embryonic holoprosencephaly in the Kyoto Collection. Birth Defects Res A Clin Mol Teratol. 2004;70:495–508. doi: 10.1002/bdra.20048. [DOI] [PubMed] [Google Scholar]