Abstract
The objectives of this study were to determine whether thermal quantitative sensory testing (QST) can be performed in client-owned dogs, is repeatable and whether QST differs between normal dogs and dogs with hind limb osteoarthritis (OA). This clinical, prospective, observational study used clinically normal dogs (n = 23) and dogs with OA-associated hind limb pain (n = 9). Thermal QST was performed in standing dogs using a high-powered light source delivered by a previously validated system. Dogs were tested on two occasions, 2 weeks apart. Five tests were performed on each hind limb at each time point. Repeated measures analysis of variance was used to evaluate the effects of leg, time point and OA/normal status on thermal threshold latencies (TTL). Additionally, paired t tests were used to compare the TTL of left and right limbs within groups and between time points.
Thermal thresholds were successfully measured in 32 client-owned dogs without prior training. TTL were significantly different between normal and OA dogs (P = 0.012). There was no difference between limbs (P = 0.744) or time periods (P = 0.572), when analyzed by repeated measures analysis of variance, and no interactions between group and limb, visit and limb, or visit and group. In conclusion, thermal thresholds can be measured in client owned dogs with no prior training and are repeatable from week to week. Further data are required to determine if OA results in thermal hypoalgesia as measured at the distal hind limb and whether this is an indication of central sensitization.
Keywords: Canine, Quantitative sensory testing, Osteoarthritis, Thermal threshold
Introduction
Osteoarthritis (OA) of synovial joints is the most common form of degenerative joint disease (DJD) in all mammals (Wieland et al., 2005), affects approximately 20% of adult dogs (Johnston, 1997; Johnston et al., 2008; Davies, 2012; Walton et al., 2013), is a chronic disease and can be associated with pain.
Pain is not only a result of an afferent noxious input, but is also dependent on neuroplasticity of the nociceptive transmission system (Woolf, 2004, 2011). As a result of nociceptive afferent input, such as that associated with chronic pain (Kuner, 2010), central sensitization (CS) results due to neuroplasticity, which in turn leads to altered sensory processing and a state of facilitated nociceptive transmission (Kuner, 2010; Woolf, 2011). This is thought to be the mechanism underlying secondary hyperalgesia and allodynia (Kuner, 2010; Woolf, 2011).
Quantitative sensory testing (QST) uses reactions to applied stimuli (such as heat) to test for altered sensory processing, and therefore, by inference, central changes (Wylde et al., 2011b; Lascelles, 2013). There is an increasingly large body of work in the literature showing altered sensory processing in conjunction with chronically painful musculoskeletal diseases in humans (Bajaj et al., 2001; Imamura et al., 2008; Arendt-Nielsen et al., 2010; Wylde et al., 2011a; Graven-Nielsen et al., 2012; Suokas et al., 2012). Interestingly, clinical data in humans show that pain-induced neuroplasticity can result in both facilitated and decreased sensory processing (hyperalgesia and hypoalgesia, respectively) depending on the type of stimulus being investigated, on where it is applied, and on what pain condition is being investigated (Wylde et al., 2011a).
Recently, investigators have used mechanical and cold QST as a putative measure of CS in dogs with cranial cruciate ligament rupture (Brydges et al., 2012), and mechanical QST as a measure of sensory function in dogs with acute spinal cord injury (Moore et al., 2013), and as a measure of CS in dogs undergoing total hip replacement (Tomas et al., in press). Another form of QST is thermal testing, a semi-quantitative measure of the thermal stimulus intensity or latency of a fixed intensity, required to activate nociceptors in a particular tissue and to initiate a response.
Wegner et al. (2008) described a canine nociceptive thermal escape model (Canine Thermal Escape System or CTES). They tested thermal thresholds in laboratory dogs that were trained and acclimated to the device for 2 weeks, and evaluated changes in response to systemically administered analgesics (Wegner et al., 2008). The dogs were restrained in a fabric sling over the thermal stimulus (halogen light) and the anterior center of the metatarsal pad was tested. These investigators presented their acute nociceptive model as a technique to facilitate quantification of the effects of parenterally and neuraxially administered analgesics in dogs. Repeatability of the testing apparatus was not evaluated and measurements were not attempted in client-owned untrained dogs. To date, no studies have evaluated thermal (hot) QST in client-owned normal dogs or dogs with a naturally occurring disease, such as OA.
We hypothesized that the CTES could be used to collect QST data (thermal threshold latencies, TTL) in client-owned dogs and that these data would be repeatable (test–re-test reliability). Further, we hypothesized that TTL would be significantly different in client-owned dogs with hind limb OA-associated pain compared to normal dogs.
Materials and methods
This study was approved by the Institutional Animal Care and Use Committee (IACUC) of North Carolina State University (protocol 11-073-O). Owners were required to review and sign an informed consent form prior to the procedures being undertaken. This process involved review of a video of the testing procedure being performed on a dog. The IACUC approved the use of dogs with OA-associated pain. All owners were asked to let study personnel know if they felt their dog needed treatment at any time during the study, and all owners were offered a consultation regarding treatment and non-steroidal anti-inflammatory drug (NSAID) therapy (or another appropriate treatment) at the end of the study period (visit 2).
Animals
Thirty-two client-owned dogs, older than 2 years and weighing between 15 and 52 kg were used. Dogs were recruited into two groups, clinically normal dogs (n = 23) and those with hind limb OA (n = 9). Dogs were recruited via e-mail and flyers within the veterinary college community.
Screening
Dogs were screened using an orthopedic examination, a clinical metrology instrument designed to test for OA-associated pain and mobility impairment, and review of their medical history. Inclusion criteria for normal dogs were that they showed no evidence of abnormalities on orthopedic examination (no pain, no decreased muscle mass, no neurological deficits and no joint instability or other pathology), no history of impairment recognized by owner and were not receiving anti-inflammatory medications. Inclusion criteria for dogs with hind limb OA were that they had OA-associated pain in at least one hind limb joint detected on orthopedic examination, no other orthopedic or neurological abnormalities, a positive medical history of OA in the painful joint, radiographic evidence of OA in the painful joint, owner-recognized impairment in mobility and had not received any antiinflammatory medications for 1 month. Additionally, all dogs were required to be free of concomitant pathology that might impair mobility, for example, hypothyroidism, blindness, neoplasia, skin disease of the feet, or any other chronically painful disease.
The Canine Brief Pain Inventory (CBPI) was used to assess OA-related mobility impairment as assessed by their owners. This assessment was completed at both appointments to ensure that no significant changes occurred between appointments. Inclusion criteria for normal dogs were mean CBPI scores <0.75 per question for pain intensity and pain interference (Brown et al., 2009) and with quality of life scores ≥4 (very good). Inclusion criteria for OA dogs were mean CBPI scores >0.75 per question for pain intensity and pain interference (Brown et al., 2013).
Experimental design
All dogs that met the inclusion criteria visited the clinic on two occasions, 2 weeks apart. At each appointment, dogs underwent thermal QST. This required them to stand on the CTES device (Wegner et al., 2008) with the hind feet on the glass insert (see below).
Equipment
Thermal thresholds were measured in minimally restrained standing dogs using the purpose built thermal stimulus delivering device, the CTES (Fig. 1) purchased from the laboratory that originally described the system (Wegner et al., 2008). Briefly, this device was composed of: a 12 V power supply for a halogen stimulus bulb; a 5 V power supply for electronic circuits; logic circuit boards controlling the timer and movement sensors; bulb and motion sensors contained in a carriage head; digital timer displaying latency in seconds; digital multimeter displaying amps, and a glass plate above the bulb and motion sensors. The glass used was 3.175 mm (0.125 inch) thick standard (i.e. not safety) glass. The device incorporated an automatic cut-off after 40 s. All components were housed in a purpose made wooden box. This was incorporated into a custom-made heavy duty shelving system with the metal struts covered in protective foam. Prior to use, we evaluated the apparatus and found the glass temperature to be a mean of 54.2 °C and 58.9 °C after 20 and 30 s, respectively, very similar to the original report describing this apparatus (Wegner et al., 2008).
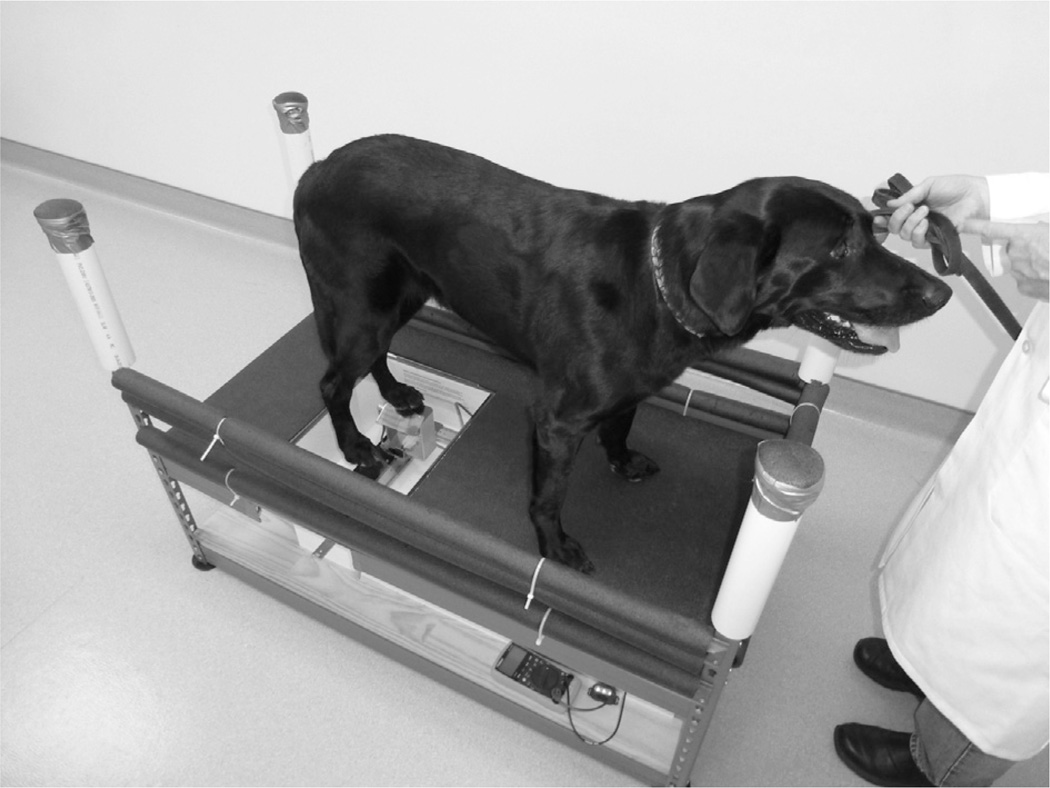
Fig. 1.
Dog shown standing on the Canine Thermal Escape System (CTES). This was a client-owned dog that had not seen the CTES previously. The CTES used for thermal quantitative sensory testing (QST) is housed in a purpose made wooden box. This is incorporated into a heavy-duty metal shelving system with the metal struts covered in protective foam. Dogs stand on the top surface, which is covered in rubber matting except for the area of glass that covers the area containing the halogen bulb. During testing, one individual is positioned in front of the dog or to the left side, gently dissuading the dog from moving. The other individual is kneelt on the floor, operating the arm connected to the carriage head with the halogen light source in it and positioning it under the paw. Positioning was facilitated by observing the reflection in the mirror attached to the carriage head.
Testing procedure
During preliminary testing, the anterior one-third of the metatarsal pad was used as in previous studies (Wegner et al., 2008). However, in preliminary testing, we found that the middle of digital pad III of the hind appeared to have more consistent contact with the glass plate and was therefore used for stimulation in the present study.
Dogs were acclimated to the laboratory for 10 min. During this time, dogs were free to roam and investigate surroundings. Dogs were lead up to and onto the CTES. Dogs either jumped up onto the CTES or were gently assisted up onto the apparatus. Dogs were minimally restrained by one investigator (AK) standing on the left side of the dog with one hand gently placed under the inguinal region, but not supporting any weight, to encourage the dog to stay standing still. Dogs were required to stand approximately ‘square’ during the testing procedure (Fig. 1). Gentle repositioning was necessary to ensure that the appropriate hind limb foot was on the glass plate and in a position where it could be stimulated. The light source was positioned below the center of digital pad III by the same evaluator (MW) on each occasion, and the stimulus initiated. Termination of the stimulus occurred automatically when the foot was lifted or moved off the glass plate. The stimulus was applied for a maximum of 30 s. The time between stimulus initiation and automatic termination was called the thermal threshold latency (TTL) and was recorded. This was repeated five times per hind foot, with 2 min between each test on each hind limb. In between tests, dogs were free to sit or stay standing on device. The testing protocol was repeated for each dog 2 weeks later. At each time point, the initial limb tested was randomly selected using a coin-toss. Tested areas were carefully examined for any evidence of tissue injury at the second testing time point and owners asked to report any signs following the second testing.
Outcome measures
The primary outcome measure was TTL (in s). A feasibility score was also recorded for each dog at the first testing time point to assess the ease with which data were collected. The feasibility scores assigned were based on a 6-point scale (0–5) as described in Table 1.
Table 1.
Feasibility scoring rubric for evaluation of the ease with which thermal quantitative sensory testing (QST) data could be collected from an individual dog.
| Feasibility score | Description | |
|---|---|---|
| 0 | No problem | Minimum restraint needed; excellent cooperation; clear reaction to stimuli |
| 1 | Mild difficulty | Mild restraint needed; good cooperation; clear reaction to stimuli |
| 2 | Moderate difficulty | Moderate restraint needed; good cooperation >50% of the time; mild sensitivity of feet being touched; mild variation in reaction to stimuli |
| 3 | Significant difficulty | Significant restraint needed and resisted lateral recumbency; good cooperation <25% of the time; moderate sensitivity to feet being touched; moderate variation in reaction to stimuli |
| 4 | Extreme difficulty | Constant restraint required; not cooperative; unclear reaction to stimuli, not confident in data collected |
| 5 | Impossible | Could not collect data due to the dogs disposition and/or lack of confidence in the reactions seen being due to the stimulus |
Statistical analysis
All data were entered in Microsoft Excel and data analysis was performed using statistical software (JMP 10 for the Mac; SAS 9.0). Data were tested for normality. Descriptive statistics were used to describe the normal dogs (control group) and hind limb OA dogs; appropriate tests (t test, Wilcoxon, chi) were used to compare group characteristics. Feasibility scores were described and scores in the two groups were compared using a Fisher’s exact test after grouping the scores into dichotomized variables (easy, difficult).
Significant relationships between potential covariates (age, bodyweight) were explored using linear regression. Repeated measures analysis of variance was used to evaluate the effects of leg, time point (appointment 1 or 2), OA/normal status and significant covariates on TTL. Additionally, as a further test of repeatability, TTL of the left and right limbs were compared within both groups, for each time point, using a paired t test, and TTL of the left and right limbs were compared within groups between time points using a paired t test. TTL data were also averaged across all tests, both legs, for each dog in the normal group and the agreement was visualized using a Bland–Altman plot. In all tests, P < 0.05 was considered statistically significant.
Results
The normal dogs (n = 23) were a mean (±SD) of 5.8 (2.5) years old and had a mean bodyweight of 28.3 (6.0) kg. The OA dogs (n = 9) were a mean 10.7 (3.4) years old, and had a mean bodyweight of 35.4 (9.0) kg. The median (range) CBPI scores for the normal dogs were 0 (0–0.5) (pain intensity), 0 (0–0.5) (pain interference) at visit 1; and 0 (0–0.5) (pain intensity), 0 (0–0.5) (pain interference) at visit 2. The median (range) CBPI scores for the OA dogs were 2.0 (0.5–5.5) (pain intensity), 1.0 (0.5–7.5) (pain interference) at visit 1; and 2.0 (0.5–3.5) (pain intensity), 2.5 (1.0– 7.5) (pain interference) at visit 2. The OA dogs were significantly older (P = 0.001) and heavier (P = 0.014). All OA dogs had bilateral hip OA. Three dogs also had bilateral stifle OA. Pain scores were not different between the left and right hind limbs in the OA dogs (P = 0.195).
Feasibility
The overall distribution of feasibility scores (i.e. how easy data were collected) across all dogs was: score 0, n = 9/32; score 1, n = 11/32; score 2, n = 6/32; score 3, n = 2/32; score 4, n = 4/32; and score 5, n = 0/32. The distribution of scores within normal and hind limb OA dogs is shown in Table 2. Scores were grouped as easy (0, 1, 2) or difficult (3, 4, 5) and no difference between normal and OA groups was detected (P = 0.314).
Table 2.
The distribution of thermal quantitative sensory testing (QST) feasibility scores assigned to normal dogs and dogs with hind limb (HL) osteoarthritis (OA) undergoing thermal QST using the Canine Thermal Escape System (CTES).
| Feasibility score | 0 | 1 | 2 | 3 | 4 | 5 |
|---|---|---|---|---|---|---|
| Normal (n = 23) | 7 | 8 | 5 | 1 | 2 | 0 |
| HL OA (n = 9) | 2 | 3 | 1 | 1 | 2 | 0 |
Overall effects
Thermal threshold latency was not significantly correlated with bodyweight (r2 = 0.056; P = 0.194) or age (r2 = 0.087; P = 0.109). In the repeated measures analysis of variance, TTL was significantly different between normal and OA dogs (P = 0.012), with TTL being longer in dogs with hind limb OA. There was no difference between limbs (P = 0.744) or time periods (P = 0.572), and no interactions between group and limb, visit and limb, or visit and group.
Repeatability (test–re-test reliability)
In the normal dogs, there was no difference between the right and left hind limbs at the first visit (P = 0.403) or second visit (P = 0.093), or between the first and second visit for the right hind (P = 0.989 or left hind (P = 0.878). Average hind limb TTL for the first and second visits was not significantly different (P = 0.935). Thermal threshold latency values are shown in Tables 3 and 4.
Table 3.
Thermal threshold latencies (in s, ±SD) for the left and right hind limb for normal and osteoarthritis (OA) groups for each appointment.
| Group | Right hind limb | Left hind limb | ||||
|---|---|---|---|---|---|---|
| Appointment 1 | Appointment 2 | Appointment 1 vs. 2 | Appointment 1 | Appointment 2 | Appointment 1 vs. 2 | |
| Normal (n = 23) | 16.09 (±3.69) | 16.08 (±2.64) | P = 0.867 | 15.36 (±2.81) | 15.46 (±2.45) | P = 0.878 |
| OA (n = 9) | 17.91 (±5.81) | 18.07 (±5.00) | P = 0.878 | 19.22 (±4.80) | 18.10 (±5.43) | P = 0.047 |
Table 4.
Comparison of right (R) and left (L) hind limb (HL) thermal threshold latencies (in s, ±SD) at each testing time point (appointment).
| Group | Appointment 1 | Appointment 2 | ||||
|---|---|---|---|---|---|---|
| R HL | L HL | R vs. L HL | R HL | L HL | R vs. L HL | |
| Normal (n = 23) | 16.09 (±3.69) | 15.36 (±2.81) | P = 0.403 | 16.08 (±2.64) | 15.46 (±2.45) | P = 0.093 |
| OA (n = 9) | 17.91 (±5.81) | 19.22 (±4.80) | P = 0.213 | 18.07 (±5.00) | 18.10 (±5.43) | P = 0.948 |
OA, osteoarthritis.
In the OA dogs, there was no difference between the right and left hind limbs at the first visit (P = 0.213) or second visit (P = 0.975), or between the first and second visit for the right hind (P = 0.870). However, the TTL were significantly different for the left hind limb between the first and second visit (P = 0.0473). Average hind limb TTL for the first and second visits was not significantly different (P = 0.428). TTL values are shown in Tables 3 and 4. Contemporaneous notes indicated that the dogs with OA appeared to be less willing to stand on the device for the duration of the testing; however, this was not scored. All dogs were carefully examined for any evidence of tissue injury at the second visit and none was seen. No owners reported any problems following the second visit and testing.
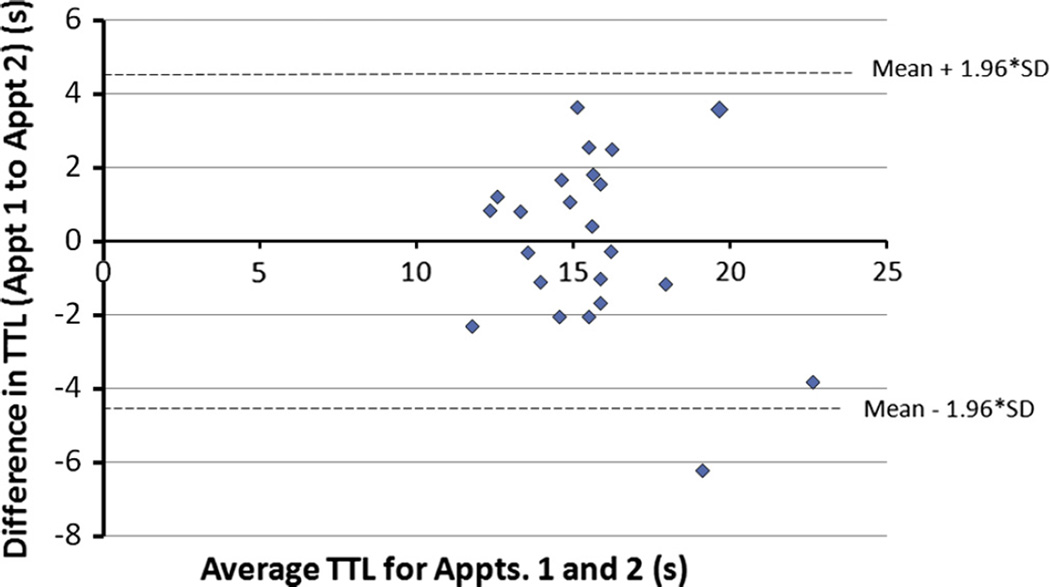
TL was averaged between both hind limbs in the normal dogs, and a Bland–Altman plot of the difference in values at the two time points against the mean of the two time points was constructed (Fig. 2). This plot shows that the difference in the TTL between the two testing time points lay within the 95% confidence limits for each dog except one, showing overall good agreement. Additionally, this plot suggests that as TTL increases, agreement between time points appears to be slightly less good (spread of points increases as TTL increases).
Fig. 2.
Bland–Altman plot of difference between thermal threshold latency (TTL) between appointments (Appt.) 1 and 2 (2 weeks apart) and the average of the TTL for appointments 1 and 2, for normal dogs.
Discussion
Based on the data collected from these 32 dogs, use of the CTES was feasible in client-owned normal dogs and dogs with hind limb OA, without prior training. The QST and TTL measured with this device were repeatable (from week to week) and reliable (right and left hind limbs comparisons) in the normal and OA dogs. Significant differences were seen between normal and OA dogs, with TTL in the OA dogs being higher, suggesting thermal hypoalgesia as measured by a noxious hot thermal stimulus.
Although not directly supported by the data, it seemed to be more difficult to gather data from dogs with hind limb OA compared to the normal dogs. We found that the dogs with OA appeared to be less willing to stand on the device for the duration of the testing. This may be due to several factors, including joint pain or decreased muscle mass that may contribute to a decreased willingness to stand on the device for the duration required. Although this did not affect feasibility scores, we caution that this may limit the utility of the CTES when testing dogs with painful OA. A further observation was that many of the dogs did not seem to like the slickness of the glass and tried to avoid standing with their feet on the glass.
In contrast to previous reports, the third digital pad was used instead of the metatarsal pad, as the third digital pad seemed to have better contact with the glass surface. This may result in variation in TTL between studies if different areas of the foot are used as stimulation point.
Our findings of good test–re-test repeatability mirrors work in healthy adults and those with OA (Geber et al., 2011; Moloney et al., 2011). However, a recent study (Wylde et al., 2011b) highlighted the variability in thermal QST in comparison to mechanical QST, and a systematic review clearly shows considerable variability in the reliability of thermal QST parameters across studies due to methodology, equipment and testing site (Moloney et al., 2012). Such work emphasizes the importance of careful evaluation of test–re-test repeatability, and careful documentation of the methods, as a basis for work aimed at evaluating changes due to disease or treatment. It should be noted that although we found good test– re-test repeatability, it was only over a 2-week period and further work should evaluate repeatability over longer time periods. Thermal threshold testing of both the left and right limbs is rarely performed in studies with humans and comparisons are also not reported (Sarlani et al., 2003; Wylde et al., 2011b). The most comprehensive work in this respect has been done with mechanical stimuli, and this work showed no significant right–left differences in normal subjects (Rolke et al., 2005). However, Sarlani et al. (2003) did find thermal sensitivity differences between the dominant and subdominant hand, and it is known that dogs appear to have ‘dominant limbs’ (Colborne et al., 2011), suggesting that further work should evaluate left–right differences in more detail.
Thermal hypoalgesia was detected in this cohort of dogs with hind limb OA. The rationale for performing these studies was that it is a method to detect altered sensory processing (central sensitization) in dogs suffering from chronic pain (Woolf, 2011; Lascelles, 2013). As such, one might assume that thermal hyperalgesia would be seen (decreased latency to reaction compared to normal dogs). In humans with OA, contradictory findings of hypoanesthesia alongside hyperalgesia have been seen across various QST modalities (Wylde et al., 2011a). In our current study, we evaluated thermal (hot) QST and our assumption is that this relates to ‘hot pain thresholds’ measured in humans (Kosek and Ordeberg, 2000; Wylde et al., 2011a,b). However, our testing paradigm may also be more akin to ‘heat detection’ thresholds in people. Additionally, we tested an area of the body remote from the painful joint, but on the same limb. Studies in humans vary from testing the local area, or area of most intense pain around a joint, to remote areas (but often on a different limb, such as the forearm in patients with knee OA) (Wylde et al., 2011a). All these variations in methodology affect thermal thresholds and make direct comparison between results in dogs and humans difficult.
Thermal detection hypoalgesia was found in OA patients tested at the index joint (knee), but not at the forearm in one study (Wylde et al., 2011a). However, in the same study there was no difference in hot pain thresholds between OA patients and healthy participants at either the knee or forearm (Wylde et al., 2011a). This is in contrast to another study, using patients with hip OA, that found thermal hyperalgesia (to innocuous warmth) in the hip area bilaterally, and a tendency towards bilateral thermal hyperalgesia to hot pain thresholds in the hip area (Kosek and Ordeberg, 2000).
We recruited dogs with OA that were not currently receiving analgesics and this may have biased our recruitment towards less severely affected OA cases, as shown when comparing the CBPI scores against those of other studies (Brown et al., 2008, 2013). This may have influenced thermal QST values and further work should evaluate the relationship between QST values and severity of disease.
Further work is required to determine if thermal QST values are different in dogs with OA compared to normal dogs. Despite the lack of correlation of thermal thresholds with bodyweight or age, an age- and weight-matched control group and increased numbers of dogs with hind limb OA are needed to before strong conclusions can be made. Evaluating additional QST modalities would help establish which stimuli, if any, are more sensitive in detecting central sensitization. However, such work needs to be performed together with ‘gold standard’ methods for detecting central sensitization. Short of removing the spinal cord and performing neurobiological analysis, the best method may be using nociceptive withdrawal reflexes measured using electromyographic apparatus (Bergadano et al., 2006; Courtney et al., 2009).
Conclusions
Thermal QST can be measured in client-owned dogs with no training, and values are repeatable and reliable from week to week over a 2-week period in both hind limbs. Further data are required to determine if OA results in remote thermal hypoalgesia as suggested in this study.
Acknowledgements
The authors would like to thank all the Comparative Pain Research Laboratory staff for their assistance and support and to Dr. Kwan for her assistance with the orthopedic evaluations in the dogs. This study was funded by the Comparative Pain Research Laboratory, at the College of Veterinary Medicine (North Carolina State University).
B.D.X. Lascelles was a member of the Morris Animal Foundation Small Animal Advisory Board. M. Williams and A. Kirkpatrick received Merck-Merial/Ms. Debora Resnick Scholars Summer Research Program stipends. J. Benito was receiving salary support from the Morris Animal Foundation as part of other projects.
Footnotes
Conflict of interest statement
No other potential conflicts of interest were identified.
Preliminary results were presented as poster presentations at the Merial-NIH Symposium in Orlando, Florida, USA, 4–7 August 2011; at the AVA Scientific Spring Meeting, Davos, Switzerland, 21–23 March 2012; at the International Association for the Study of Pain, Non-Human Species Special Interest Group Satellite Meeting, 26 August 2012; and as an oral presentation at the North Carolina State University College of Veterinary Medicine Annual Research Forum in Raleigh, North Carolina, USA, 20 January 2011.
References
- Arendt-Nielsen L, Nie H, Laursen MB, Laursen BS, Madeleine P, Simonsen OH, Graven-Nielsen T. Sensitization in patients with painful knee osteoarthritis. Pain. 2010;149:573–581. doi: 10.1016/j.pain.2010.04.003. [DOI] [PubMed] [Google Scholar]
- Bajaj P, Graven-Nielsen T, Arendt-Nielsen L. Osteoarthritis and its association with muscle hyperalgesia: An experimental controlled study. Pain. 2001;93:107–114. doi: 10.1016/S0304-3959(01)00300-1. [DOI] [PubMed] [Google Scholar]
- Bergadano A, Andersen OK, Arendt-Nielsen L, Schatzmann U, Spadavecchia C. Quantitative assessment of nociceptive processes in conscious dogs by use of the nociceptive withdrawal reflex. American Journal of Veterinary Research. 2006;67:882–889. doi: 10.2460/ajvr.67.5.882. [DOI] [PubMed] [Google Scholar]
- Brown DC, Boston RC, Coyne JC, Farrar JT. Ability of the canine brief pain inventory to detect a response to treatment in dogs with osteoarthritis. Journal of the American Veterinary Medical Association. 2008;233:1278–1283. doi: 10.2460/javma.233.8.1278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown DC, Boston RC, Coyne JC, Farrar JT. A novel approach to the use of animals in studies of pain: Validation of the canine brief pain inventory in canine bone cancer. Pain Medicine. 2009;10:133–142. doi: 10.1111/j.1526-4637.2008.00513.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown DC, Boston RC, Farrar JT. Comparison of force plate gait analysis and owner assessment of pain using the canine brief pain inventory in dogs with osteoarthritis. Journal of Veterinary Internal Medicine. 2013;27:22–30. doi: 10.1111/jvim.12004. [DOI] [PubMed] [Google Scholar]
- Brydges NM, Argyle DJ, Mosley JR, Duncan JC, Fleetwood-Walker S, Clements DN. Clinical assessments of increased sensory sensitivity in dogs with cranial cruciate ligament rupture. The Veterinary Journal. 2012;193:545–550. doi: 10.1016/j.tvjl.2012.01.019. [DOI] [PubMed] [Google Scholar]
- Colborne GR, Good L, Cozens LE, Kirk LS. Symmetry of hind limb mechanics in orthopedically normal trotting Labrador Retrievers. American Journal of Veterinary Research. 2011;72:336–344. doi: 10.2460/ajvr.72.3.336. [DOI] [PubMed] [Google Scholar]
- Courtney CA, Lewek MD, Witte PO, Chmell SJ, Hornby TG. Heightened flexor withdrawal responses in subjects with knee osteoarthritis. Journal of Pain. 2009;10:1242–1249. doi: 10.1016/j.jpain.2009.05.004. [DOI] [PubMed] [Google Scholar]
- Davies M. Geriatric screening in first opinion practice – Results from 45 dogs. Journal of Small Animal Practice. 2012;53:507–513. doi: 10.1111/j.1748-5827.2012.01247.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geber C, Klein T, Azad S, Birklein F, Gierthmuhlen J, Huge V, Lauchart M, Nitzsche D, Stengel M, Valet M, et al. Test–retest and interobserver reliability of quantitative sensory testing according to the protocol of the German Research Network on Neuropathic Pain (DFNS): A multi-centre study. Pain. 2011;152:548–556. doi: 10.1016/j.pain.2010.11.013. [DOI] [PubMed] [Google Scholar]
- Graven-Nielsen T, Wodehouse T, Langford RM, Arendt-Nielsen L, Kidd BL. Normalization of widespread hyperesthesia and facilitated spatial summation of deep-tissue pain in knee osteoarthritis patients after knee replacement. Arthritis and Rheumatism. 2012;64:2907–2916. doi: 10.1002/art.34466. [DOI] [PubMed] [Google Scholar]
- Imamura M, Imamura ST, Kaziyama HH, Targino RA, Hsing WT, de Souza LP, Cutait MM, Fregni F, Camanho GL. Impact of nervous system hyperalgesia on pain, disability, and quality of life in patients with knee osteoarthritis: A controlled analysis. Arthritis and Rheumatism. 2008;59:1424–1431. doi: 10.1002/art.24120. [DOI] [PubMed] [Google Scholar]
- Johnston SA. Osteoarthritis. Joint anatomy, physiology, and pathobiology. Veterinary Clinics of North America (Small Animal Practice) 1997;27:699–723. doi: 10.1016/s0195-5616(97)50076-3. [DOI] [PubMed] [Google Scholar]
- Johnston SA, McLaughlin RM, Budsberg SC. Nonsurgical management of osteoarthritis in dogs. Veterinary Clinics of North America (Small Animal Practice) 2008;38:1449–1470. doi: 10.1016/j.cvsm.2008.08.001. [DOI] [PubMed] [Google Scholar]
- Kosek E, Ordeberg G. Abnormalities of somatosensory perception in patients with painful osteoarthritis normalize following successful treatment. European Journal of Pain. 2000;4:229–238. doi: 10.1053/eujp.2000.0175. [DOI] [PubMed] [Google Scholar]
- Kuner R. Central mechanisms of pathological pain. Nature Medicine. 2010;16:1258–1266. doi: 10.1038/nm.2231. [DOI] [PubMed] [Google Scholar]
- Lascelles BD. Getting a sense of sensations. The Veterinary Journal. 2013;197:115–117. doi: 10.1016/j.tvjl.2013.02.025. [DOI] [PubMed] [Google Scholar]
- Moloney NA, Hall TM, Doody CM. Reliability of thermal quantitative sensory testing: A systematic review. Journal of Rehabilitation Research and Development. 2012;49:191–207. doi: 10.1682/jrrd.2011.03.0044. [DOI] [PubMed] [Google Scholar]
- Moloney NA, Hall TM, O’Sullivan TC, Doody CM. Reliability of thermal quantitative sensory testing of the hand in a cohort of young, healthy adults. Muscle and Nerve. 2011;44:547–552. doi: 10.1002/mus.22121. [DOI] [PubMed] [Google Scholar]
- Moore SA, Hettlich BF, Waln A. The use of an electronic von Frey device for evaluation of sensory threshold in neurologically normal dogs and those with acute spinal cord injury. The Veterinary Journal. 2013;197:216–219. doi: 10.1016/j.tvjl.2012.11.003. [DOI] [PubMed] [Google Scholar]
- Rolke R, Andrews Campbell K, Magerl W, Treede RD. Deep pain thresholds in the distal limbs of healthy human subjects. European Journal of Pain. 2005;9:39–48. doi: 10.1016/j.ejpain.2004.04.001. [DOI] [PubMed] [Google Scholar]
- Sarlani E, Farooq N, Greenspan JD. Gender and laterality differences in thermosensation throughout the perceptible range. Pain. 2003;106:9–18. doi: 10.1016/s0304-3959(03)00211-2. [DOI] [PubMed] [Google Scholar]
- Suokas AK, Walsh DA, McWilliams DF, Condon L, Moreton B, Wylde V, Arendt-Nielsen L, Zhang W. Quantitative sensory testing in painful osteoarthritis: A systematic review and meta-analysis. Osteoarthritis Cartilage. 2012;20:1075–1085. doi: 10.1016/j.joca.2012.06.009. [DOI] [PubMed] [Google Scholar]
- Tomas A, Marcellin-Little D, Roe S, Motsinger-Reif A, Lascelles BD. Relationship between mechanical thresholds and limb use in dogs with coxofemoral joint OA-associated pain and the modulating effects of pain alleviation from total hip replacement on mechanical thresholds. Veterinary Surgery. doi: 10.1111/j.1532-950X.2014.12160.x. in press. [DOI] [PubMed] [Google Scholar]
- Walton MB, Cowderoy E, Lascelles D, Innes JF. Evaluation of construct and criterion validity for the ‘Liverpool Osteoarthritis in Dogs’ (LOAD) clinical metrology instrument and comparison to two other instruments. PLoS ONE. 2013;8:e58125. doi: 10.1371/journal.pone.0058125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wegner K, Horais KA, Tozier NA, Rathbun ML, Shtaerman Y, Yaksh TL. Development of a canine nociceptive thermal escape model. Journal of Neuroscience Methods. 2008;168:88–97. doi: 10.1016/j.jneumeth.2007.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wieland HA, Michaelis M, Kirschbaum BJ, Rudolphi KA. Osteoarthritis – An untreatable disease? Nature Reviews Drug Discovery. 2005;4:331–344. doi: 10.1038/nrd1693. [DOI] [PubMed] [Google Scholar]
- Woolf CJ. Pain: Moving from symptom control toward mechanism-specific pharmacologic management. Annals of Internal Medicine. 2004;140:441–451. doi: 10.7326/0003-4819-140-8-200404200-00010. [DOI] [PubMed] [Google Scholar]
- Woolf CJ. Central sensitization: Implications for the diagnosis and treatment of pain. Pain. 2011;152:S2–S15. doi: 10.1016/j.pain.2010.09.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wylde V, Palmer S, Learmonth ID, Dieppe P. Somatosensory abnormalities in knee OA. Rheumatology. 2011a;51:535–543. doi: 10.1093/rheumatology/ker343. [DOI] [PubMed] [Google Scholar]
- Wylde V, Palmer S, Learmonth ID, Dieppe P. Test–retest reliability of Quantitative Sensory Testing in knee osteoarthritis and healthy participants. Osteoarthritis Cartilage. 2011b;19:655–658. doi: 10.1016/j.joca.2011.02.009. [DOI] [PubMed] [Google Scholar]