Abstract
Central GABAA receptors mediate GABAergic phasic and tonic inhibition. While synaptic αβγ GABAA receptors primarily mediate phasic inhibition, extrasynaptic αβδ receptors play an important role in mediating tonic inhibition. Etomidate is a general anesthetic that produces its effects by enhancing GABAA receptor activity. We previously showed that etomidate modulates the gating of oocyte-expressed αβγ and αβδ receptors with similar overall allosteric impact, but different pharmacological patterns. In αβγ receptors, etomidate enhances apparent GABA sensitivity (reduces GABA EC50), modestly increases maximal GABA efficacy, and slows current deactivation without affecting desensitization (Zhong et al; Anesthesiology 2008; 108:103–12). In αβδ receptors characterized by low GABA efficacy, etomidate dramatically increases responses to both low and maximal GABA. The effects of etomidate on desensitization and deactivation of αβδ receptors are unknown. To investigate the kinetic effects of etomidate on α1β3δ receptors of defined subunit arrangement, we expressed concatenated trimer (β3-α1-δ) and dimer (β3-α1) GABAA receptor subunit assemblies in HEK293T cells and recorded whole-cell voltage-clamp currents during rapid external solution exchanges. As expected, etomidate substantially increased maximal GABA-induced currents and prolonged deactivation. Moreover, desensitization was significantly decreased by etomidate. During prolonged GABA applications, etomidate enhanced steady-state currents more than peak currents. Thus, etomidate enhances tonic GABAergic inhibition through extrasynaptic αβδ receptors by both augmenting gating and reducing desensitization.
Keywords: GABAA receptors, δ subunit, concatemers, etomidate, desensitization, deactivation
γ-Aminobutyric acid type A (GABAA) receptors are chloride-conducting pentameric ligand-gated ion channels and the major inhibitory receptors in the mammalian CNS (Olsen and Sieghart, 2008). To date, 16 GABAA receptor subunit subtypes have been identified: α1–α6, β1–β3, γ1–γ3, δ, ɛ, π and θ (Olsen and Sieghart, 2008). The αβγ receptors are mainly located in synapses, mediating GABAergic phasic inhibition in response to brief high concentrations of GABA. The αβδ receptors are extrasynaptic, and their activation by low concentrations of ambient GABA contributes to tonic inhibition (Mody and Pearce, 2004, Farrant and Nusser, 2005). Synaptic αβγ receptor kinetic properties (desensitization and deactivation) have been shown to play an important role in shaping GABAergic phasic responses (Jones and Westbrook, 1995, Bianchi et al., 2001). Compared with αβγ receptors, αβδ receptors are characterized by very low GABA efficacy as well as less and slower desensitization (Haas and Macdonald, 1999, Scheller and Forman, 2002, Feng, 2010).
Potent general anesthetics, including propofol, etomidate, barbiturates and the neuro-active steroid alphaxalone act by enhancing both tonic and phasic GABAA receptor activation. Etomidate, a potent and stereoselective anesthetic, may reveal similarities and differences between different GABAA receptor subtypes, because its mechanisms in αβγ receptors are well established. In αβγ receptors, etomidate acts as an allosteric agonist that significantly reduces GABA EC50 while modestly enhancing maximal GABA efficacy (Rusch et al., 2004, Feng et al., 2014). Etomidate and its derivatives also slow αβγ receptor deactivation without significantly altering desensitization rate or extent (Zhong et al., 2008). Etomidate binding sites have been identified at the β/α transmembrane interfaces of αβγ receptors (Li et al., 2006, Chiara et al., 2012). Etomidate also enhances currents mediated by α4β3δ receptors (Brown et al., 2002, Meera et al., 2009). In contrast to αβγ receptors, etomidate modestly reduces GABA EC50, but dramatically enhances maximal GABA-evoked currents mediated by α1β3δ receptors assembled from either free or concatenated subunits in Xenopus oocytes (Feng et al., 2014). Despite these differences, quantitative model-based analysis reveals that etomidate enhances channel gating similarly in both α1β3γ2 and α1β3δ receptors.
The effects of etomidate on desensitization and deactivation of α1β3δ GABAA receptors remain unknown. We hypothesize that etomidate will slow αβδ receptor deactivation, because this kinetic parameter reflects enhanced open-channel stability that also leads to increased GABA affinity in αβγ and increased GABA efficacy in αβδ receptors. Because anesthetics increase GABA efficacy, these drugs also increase the extent of αβδ desensitization (Feng, 2010), significantly influencing αβδ tonic activity in the presence of anesthetics. Slow solution exchange with Xenopus oocytes limits observation of current transition rates. Patch-clamp studies using small cells or excised patches and rapid solution exchange are required to assess fast kinetics such as fast desensitization and deactivation. Recently, Eaton et al (Eaton et al., 2014) reported that αβδ receptors assembled from free subunits may differ in oocytes and HEK293 cells, but that receptors assembled from concatenated subunits display similar properties in both expression systems. Thus, we used whole-cell voltage-clamp electrophysiology and rapid solution exchange to study etomidate effects on currents produced by concatenated α1β3δ receptors expressed in HEK293T cells. We find that etomidate substantially enhanced maximal GABA-evoked peak currents and prolonged deactivation, as expected. We also found that etomidate reduces the extent of GABA-induced desensitization of α1β3δ receptors, resulting in greater etomidate enhancement of steady-state than peak currents.
1. EXPERIMENTAL PROCEDURES
1.1. Cell culture and recombinant GABAA receptor expression
Human embryonic kidney (HEK293T) cells were cultured in Dulbecco’s modified Eagle’s medium, supplemented with 10% fetal bovine serum (Atlanta Biologicals, Flowery Branch, GA), 100 IU/ml penicillin, and 100 μg/ml streptomycin (Life Technologies, Grand Island, NY) in an incubator with 5% CO2 and 95% air at 37°C. Cells were grown on cover slips and transfected using lipofectamine (Invitrogen, Carlsbad, CA) with cDNAs encoding β3-α1-δ trimer and β3-α1 dimer GABAA receptor subunit assemblies (Desai et al., 2009, Feng et al., 2014). The total amount of cDNA per 3.5-cm diameter dish was 0.6, 2.0 or 6.0 μg with a 1:1 trimer:dimer molar ratio. With each transfection, 0.25 μg pmaxGFP (Amaxa, Gaithersburg, MD) was added for identification of transfected cells using fluorescence microscopy. Patch-clamp recordings were made 24 to 48 h after transfection.
1.2. Whole-cell patch-clamp recordings
Whole-cell macroscopic currents were obtained after lifting cells and positioning them near the tip of a perfusion pipette (Feng et al., 2004, Desai et al., 2009). External bath solution was composed of 142 mM NaCl, 1 mM CaCl2, 6 mM MgCl2, 8 mM KCl, 10 mM glucose, and 10 mM HEPES (pH 7.4 with osmolality between 325 and 329 mOsm). Recording electrodes were pulled from the thin-wall borosilicate glass tubing (i.d., 1.12 mm; o.d., 1.5 mm) (WPI, Sarasota, FL) on a P-87 Flaming Brown micropipette puller (Sutter Instrument Company, Rafael, CA). The electrode resistance was 1.0 to 2.0 MΩ with internal solution, consisting of 153 mM KCl, 1 mM MgCl2, 10 mM HEPES, and 5 mM EGTA (pH 7.3 and osmolality between 301 and 309 mOsm). MgATP (2 mM) was added to the internal solution on recording days. Experiments were performed at room temperature. Cells were voltage clamped at −50 mV with an Axopatch 200B amplifier (Molecular Devices, Sunnyvale, CA). Currents were low-pass filtered at 5 kHz, digitized at 2–10 kHz (Digidata 1322A, Molecular Devices) and recorded on a PC running Clampex 8.0 software (Molecular Devices). Series resistance was not compensated.
GABA and etomidate were prepared as stock solutions and diluted to desired concentrations with external solution on the day of the experiment. Drugs were delivered via a multi-channel superfusion pipette coupled to piezo-electric elements that switched solution among channels with the solution exchange time at an open electrode consistently less than 2 ms (Desai et al., 2009). The external solution and drug solutions were driven by gravity. Washout after each GABA or drug application was at least 60 s to minimize accumulation of desensitization (Scheller and Forman, 2002, Feng et al., 2004, Laha et al., 2013). GABA concentration-responses were examined in the absence or presence of 3.2 μM etomidate, without etomidate pre-application. In kinetic studies, currents evoked by GABA as well as by co-application of GABA and etomidate were recorded from the same cells, and etomidate was pre-applied (2 s) prior to co-application of GABA plus etomidate (4 s or 30 s).
1.3. Chemicals and solutions
Chemicals were purchased from either Sigma-Aldrich (St. Louis, MO) or Fisher Scientific (Fair Lawn, NJ), unless otherwise mentioned. R(+)-etomidate [2 mg/ml in 35% propylene glycol/water (v/v) formulation] was obtained from Hospira Inc. (Lake Forest, IL).
1.4. Data analysis
Whole-cell currents were analyzed offline using Clampfit 8.1 (Molecular Devices) and GraphPad Prism 5.0d (GraphPad Software Inc., La Jolla, CA). Peak currents were measured directly from the baseline to the peak, and residual currents at “steady state” were measured from the baseline to the end of 30-s application. Etomidate enhancement of GABA-evoked currents was calculated by dividing the peak current during etomidate+GABA co-application by the peak current evoked by the same concentration of GABA alone. For GABA concentration-responses, peak currents were normalized to control currents evoked with 1 mM GABA. Normalized concentration-response data were fitted using a logistic equation with a variable slope: I = Imax/(1 + 10(LogEC50−Log[GABA])*Hill slope), where I represents normalized peak current with or without etomidate co-application, Imax denotes the maximal normalized GABA response. Current desensitization was calculated as a percent reduction from peak current to that at the end of the GABA/drug application. Rates of deactivation and desensitization (30 s-application) were analyzed by fitting the relevant current phases to single or multiple exponential decay functions, using Levenberg-Marquardt non-linear least squares method (Clampfit 8.1, Molecular Devices). For multi-exponential processes, weighted τ (τw) was calculated using the formula Σ(ai×τi)/Σai (i = 1–4), where ai represent the relative amplitudes, and τi the corresponding exponential time constants. In 3 cells, we were unable to fit exponentials to the desensitization portion of the currents. Therefore, we excluded these cells from rate analysis but included them for extent of desensitization.
Data are reported as mean ± S.E.M. Results from studies in receptors expressed under different transfection conditions were compared using one-way ANOVA with posthoc Tukey’s multiple comparison test. Paired Student’s t tests were used to compare results before and after etomidate treatment. Mann-Whitney U test was used to compare the rates of desensitization prior to and after etomidate treatment. Differences were considered statistically significant when p was less than 0.05.
2. RESULTS
2.1. Etomidate evoked similar changes in β3-α1-δ/β3-α1 receptors expressed in HEK293T cells under different transfection conditions
2.1.1 Etomidate modulation of maximal GABA responses
There is evidence that assembly of αβδ receptors from free subunits may vary depending on the ratio and total amounts of cDNA or mRNA used in different expression systems (Botzolakis et al., 2007, Wagoner and Czajkowski, 2010, Feng et al., 2014). We therefore examined whether GABA and etomidate effects in HEK293T cells were affected by the total amount of transfected cDNAs encoding concatenated β3-α1-δ trimer and β3-α1 dimer GABAA receptor subunit assemblies. After transfecting HEK293T cells with three different amounts of cDNA mix (0.6, 2.0 and 6.0 μg/3.5-cm dish; trimer:dimer = 1:1 molar ratio), we examined the effect of etomidate (3.2 μM) on maximal GABA responses, desensitization and deactivation. Saturating GABA (1 mM) evoked similar small currents from cells transfected with the different amounts of β3-α1-δ/β3-α1 cDNAs (Figure 1A). The current amplitude was unaffected (p > 0.05) by the amount of cDNAs used: 0.6 (65.4 ± 24.2 pA, n = 8), 2.0 (93.3 ± 26.9 pA, n = 8) and 6.0 μg (72.6 ± 15.2 pA, n = 8). Etomidate at 3.2 μM substantially enhanced the peak currents evoked by 1 mM GABA from β3-α1-δ/β3-α1 receptors. The fold of current enhancement by etomidate was independent of the transfected cDNA amount: 0.6 (16.0 ± 2.9), 2.0 (11.3 ± 1.1) and 6.0 μg cDNA (9.7 ± 0.7) (Figure 1B).
Figure 1. Etomidate produced similar modulation on β3-α1-δ/β3-α1 receptors expressed with different amount of cDNA transfections.
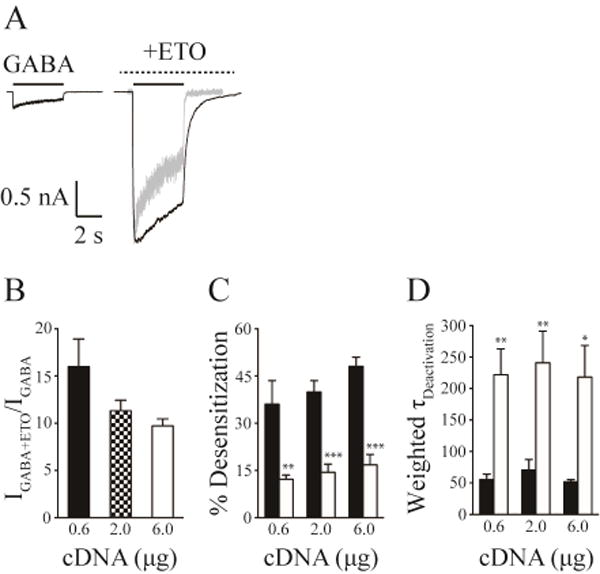
A, Representative whole cell current traces evoked by saturating GABA (1 mM) and co-application of saturating GABA (1 mM) and etomidate (3.2 μM) with etomidate pre-applied for β3-α1-δ/β3-α1 receptors transfected with 2.0 µg cDNAs. The solid lines indicated the application of GABA, and the dashed lines denoted the application of etomidate. The grey trace was the GABA current, whose peak current amplitude was normalized to that of the current evoked by GABA and etomidate to show the alterations of desensitization and deactivation induced by etomidate. B, The mean fold of enhancement of GABA (1 mM) currents by etomidate for β3-α1-δ/β3-α1 receptors transfected with 0.6, 2.0 and 6.0 µg cDNAs, respectively. C, The reduction of the extent of desensitization by etomidate for β3-α1-δ/β3-α1 receptors transfected with 0.6, 2.0 and 6.0 µg cDNAs, respectively. D, The increase in the weighted deactivation time constant (τw) by etomidate for β3-α1-δ/β3-α1 receptors transfected with 0.6, 2.0 and 6.0 μg cDNAs, respectively. In panel C and D, the filled bars represented the properties of currents evoked by GABA, and the open bars represented those by GABA plus etomidate. Error bars denoted S.E.M. *, Significantly different from GABA control at p < 0.05; ** p < 0.01; *** p < 0.001.
2.1.2 Desensitization of maximal GABA responses
Consistent with previous studies (Feng, 2010), the amount of desensitization evoked by 4-s application of saturating GABA for β3-α1-δ/β3-α1 receptors was relatively small. Different transfection conditions resulted in similar percentage desensitization after 4-s GABA application: 0.6 (36.1 ± 7.4%), 2.0 (39.9 ± 3.6%) and 6.0 μg cDNA (48.1 ± 2.9%). Independent of the cDNA amount, addition of etomidate significantly reduced the extent of desensitization relative to maximal GABA alone during a 4-s application: 12.2 ± 1.3% for 0.6 (p < 0.01), 14.4 ± 2.6% for 2.0 (p < 0.001) and 16.8 ± 3.3% for 6.0 μg cDNA (p < 0.001) (Figure 1C).
To further assess the amount of desensitization and determine the rate of desensitization, we also performed prolonged application (30 s) of 1 mM GABA in the absence and presence of 3.2 µM etomidate for β3-α1-δ/β3-α1 receptors (transfected with 2.0 µg cDNA per dish) (Figure 3A). The extent of desensitization after 30 s application of 1 mM GABA averaged 73.7 ± 4.6% (n = 9). Co-application of GABA and etomidate significantly reduced the desensitization to 24.2 ± 2.8% (p < 0.001). The rates of desensitization were not significantly different between the currents evoked by 1 mM GABA and those evoked by co-application of GABA and etomidate (weighted median time constant, τw: 4654 ms vs. 4961 ms; n = 6).
Figure 3. Etomidate evoked a greater enhancement of steady-state currents than that of peak currents.
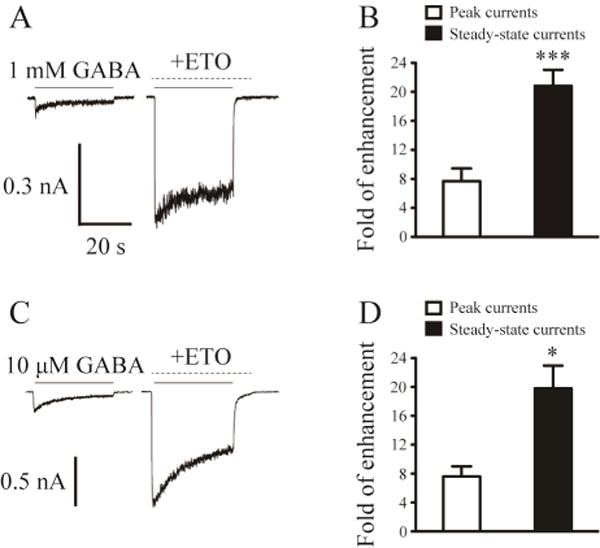
A, Representative current traces evoked by prolonged application (30 s) of 1 mM GABA as well as 1 mM GABA and 3.2 μM etomidate (with etomidate pre-applied) to show the steady-state current change evoked by etomidate for β3-α1-δ/β3-α1 receptors. The solid lines indicated the application of GABA, and the dashed lines denoted the application of etomidate. B, The mean fold of peak current and steady-state current enhancement by etomidate in the presence of 1 mM GABA for β3-α1-δ/β3-α1 receptors. C, Representative current traces evoked by prolonged application (30 s) of 10 µM GABA as well as 10 µM GABA and 3.2 µM etomidate (with etomidate pre-applied) to show the steady-state current change evoked by etomidate for β3-α1-δ/β3-α1 receptors. D, The mean fold of peak current and steady-state current enhancement by etomidate in the presence of 10 µM GABA for β3-α1-δ/β3-α1 receptors. *, Significantly different from peak current enhancement at p < 0.05; *** p < 0.001.
2.1.3 Deactivation of maximal GABA responses
Current deactivation (τw) following 4-s application of 1 mM GABA was also unaffected by cDNA amount: 0.6 (55.3 ± 8.8 ms), 2.0 (70.7 ± 16.4 ms) and 6.0 μg cDNA (51.8 ± 3.7 ms). Compared with deactivation after 4-s application of maximal GABA, addition of 3.2 µM etomidate significantly increased τw to 221.9 ± 41.1 ms for 0.6 (p < 0.01), 240.9 ± 50.2 ms for 2.0 (p < 0.01) and 218.2 ± 50.2 ms for 6.0 μg cDNA (p < 0.05) (Figure 1D).
Because the amount of cDNA used in transfections did not significantly affect β3-α1-δ/β3-α1 receptor functions, we utilized only 2.0 µg cDNA per dish in additional experiments.
2.2. Etomidate modulation of GABA concentration-responses in β3-α1-δ/β3-α1 receptors expressed in HEK293T cells
The EC50 for GABA-dependent peak responses in β3-α1-δ/β3-α1 receptors was 69.4 µM (n = 5; Figure 2A, B). When GABA was simultaneously applied with etomidate (3.2 μM), an upward and leftward shift of GABA concentration-response was observed. Etomidate reduced GABA EC50 to 35.8 μM (n = 4) and produced a ~4-fold (4.2 ± 0.9) increase in maximal GABA response (maximal GABA currents: 269.6 ± 64.0 pA vs. 974.4 ± 112.5 pA in the absence and presence of etomidate) (Figure 2A, B).
Figure 2. GABA concentration-response in the absence and presence of etomidate.
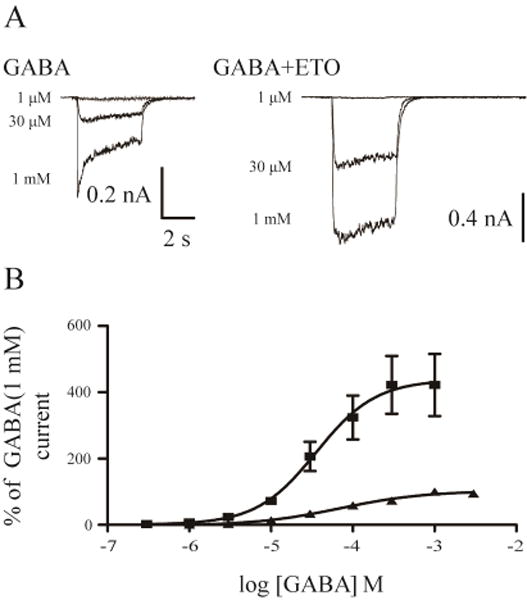
A, Representative current traces evoked by increasing concentrations of GABA as well as by increasing concentrations of GABA and 3.2 µM etomidate for β3-α1-δ/β3-α1 receptors. B, GABA concentration-response curves of β3-α1-δ/β3-α1 receptors in the absence and presence of 3.2 µM etomidate.
2.3. Etomidate produced a greater effect on “steady-state” than peak current in β3-α1-δ/β3-α1 receptors
Given that native α1βδ receptors mediate GABAergic tonic currents in response to constant GABA exposure (Glykys et al., 2007), we examined the effects of etomidate on β3-α1-δ/β3-α1 receptor “steady state” currents (Feng et al., 2009). The effects of etomidate on peak vs. steady-state currents of β3-α1-δ/β3-α1 receptors were compared in experiments using prolonged GABA applications (30 s) (Figure 3A, C). In currents stimulated with 1 mM GABA, etomidate enhanced peak currents by 7.7 ± 1.8 fold and augmented the steady-state currents by 20.8 ± 2.2 fold (n = 9). The enhancement of the steady-state currents by etomidate was significantly greater than that of the peak currents (p < 0.001) (Figure 3B). The αβδ receptors are physiologically exposed to low ambient GABA in the brain (< 1 µM) (Farrant and Nusser, 2005). Therefore, we also examined the effect of etomidate on the steady-state currents of β3-α1-δ/β3-α1 receptors during prolonged exposure to low concentrations of GABA. Compared with α4β3δ and free α1β3δ receptors, β3-α1-δ/β3-α1 receptors have high GABA EC50 values (Kaur et al., 2009, Baur et al., 2010), and sub-micromolar GABA did not elicit detectable whole-cell currents. Following the approach used by others (Stell et al., 2003), we therefore used 10 μM GABA, which reliably evoked small but quantifiable currents (Figure 3C). Etomidate enhanced the peak currents of β3-α1-δ/β3-α1 receptors exposed to 10 µM GABA by 7.6 ± 1.4 fold but increased the steady-state currents by 19.8 ± 3.1 fold (n = 5). The enhancement of steady-state currents was again significantly greater than that of peak currents (p < 0.05) (Figure 3D).
3. DISCUSSION
In the current study of concatenated α1β3δ receptors expressed in HEK293T cells, we observed that etomidate substantially enhanced maximal GABA-evoked currents, while producing a small leftward shift in GABA concentration-responses. Etomidate also prolonged deactivation and decreased desensitization, resulting in a greater enhancement of steady-state current than that of peak current in this receptor isoform.
3.1. Concatenated α1β3δ receptors exhibit similar functional properties in HEK293T cells and oocytes
The functional expression of free αβδ receptors is apparently affected by the ratio of the δ subunit used (Botzolakis et al., 2007, You and Dunn, 2007, Wagoner and Czajkowski, 2010, Feng et al., 2014). We previously reported that the functions of both concatenated β3-α1-δ/β3-α1 receptors and α1β3δ receptors assembled from free subunits in Xenopus oocytes are similar, consistent with other proposed αβδ subunit assemblies (Barrera et al., 2008, Botzolakis et al., 2008, Shu et al., 2012, Feng et al., 2014, Patel et al., 2014). We also found that in oocytes, concatenated assemblies are less affected than free subunits by injected mRNA amount (Feng et al., 2014). In the current study, we therefore studied β3-α1-δ/β3-α1 receptors expressed in HEK293T cells. Our data demonstrate that β3-α1-δ/β3-α1 receptors expressed in HEK293T cells function similarly to those expressed in oocytes. In both systems, GABA is a partial agonist for these receptors, and etomidate enhances apparent GABA potency while dramatically increasing maximal GABA-induced currents up to 20-fold under some conditions. Moreover, the function and allosteric modulation of β3-α1-δ/β3-α1 receptors are relatively independent of the amount of cDNA/mRNA used. These findings are in agreement with a recent report that concatenated α4β2δ receptors display consistent functional properties in both oocyte and HEK293 cell expression systems (Eaton et al., 2014). It should be noted that, in the same study, α4β2δ receptors assembled from free α4, β2 and δ subunits exhibit different responses to neurosteroid modulation in HEK293 cells, oocytes and native neurons (Eaton et al., 2014), indicating that α4β2δ receptors in vivo may be structurally different from recombinant counterparts formed in expression systems. Although several studies suggest that β3-α1-δ/β3-α1 receptors may be the predominant stoichiometry in free recombinant α1β3δ receptors (see above), it is possible that this subunit arrangement is different from that in native α1β3δ receptors.
3.2. Etomidate uniquely modulates the desensitization of concatenated α1β3δ receptors
An important new finding in our kinetic studies is that etomidate decreased the extent of GABA-induced desensitization of β3-α1-δ/β3-α1 receptors. Another previous study showed that etomidate reduced the desensitization of GABAA receptors expressed on dissociated spinal cord neurons (Zhang et al., 2002). It is not known what receptor isoforms are predominantly expressed on these dissociated neurons, but the δ subunit expression is detectable in rodent spinal cord (Ma et al., 1993). Our prior studies of etomidate and derivatives indicate little or no effect on the desensitization of α1β2γ2L receptors (Zhong et al., 2008). It was also reported that the barbiturate pentobarbital and propofol modestly slowed desensitization of synaptic GABAA receptors (Bai et al., 1999, Feng et al., 2004).
In contrast to etomidate, pentobarbital and the neurosteroid tetrahydrodeoxycorticosterone (THDOC) increased the desensitization of free α1β3δ receptors (Wohlfarth et al., 2002, Feng et al., 2004). Structural studies indicate that pentobarbital requires the δ subunit sequence from the N terminus to the N-terminal portion of the first transmembrane domain to enhance the desensitization of free α1β3δ receptors (Feng and Macdonald, 2010). Given that etomidate decreases the desensitization of β3-α1-δ/β3-α1 receptors, it is likely that different receptor structural elements mediate the desensitization effects of etomidate vs. pentobarbital. Indeed, in αβγ GABAA receptors, etomidate binds exclusively to transmembrane β+/α− interfacial pockets while barbiturates bind preferentially to homologous α+/β− and γ+/β− sites (Chiara et al., 2013). Modulation of α1β3δ receptor desensitization by general anesthetics may also be influenced by the presence of receptors assembled with different numbers of the δ subunit in HEK293 cells (Wagoner and Czajkowski, 2010). However, concatenated α1β3δ receptors designed to incorporate two copies of δ subunit produced very small maximal GABA currents, which were not sensitive to THDOC modulation (Kaur et al., 2009). The inconsistent properties of αβδ receptors expressed in HEK293 cells using free subunits at variable ratios significantly limit the strength of inferences in comparing different drug effects. The use of concatenated subunit assemblies appears to be a strategy that provides more consistent results. Future studies of barbiturate and neurosteroid modulation in concatenated β3-α1-δ/β3-α1 receptors are therefore warranted.
Etomidate prolonged the deactivation of β3-α1-δ/β3-α1 receptors in the current study. This observation is consistent with previous studies in native neurons (Yang and Uchida, 1996, Bai et al., 1999, Zhang et al., 2002) and in free recombinant αβδ and αβγ receptors that general anesthetics slowed the deactivation of GABAA receptors (Li and Pearce, 2000, Wohlfarth et al., 2002, Feng et al., 2004, Zhong et al., 2008). The mechanistic basis of prolonged deactivation is likely that etomidate stabilizes open conductive receptor states relative to the closed state. Thus, prolonged deactivation is mechanistically linked to other changes in experimental parameters, such as enhanced GABA potency and increased GABA efficacy, which also reflect etomidate effects on channel gating.
3.3. Etomidate favorably modulates the steady-state current of concatenated α1β3δ receptors
In the current study, we demonstrated that etomidate at a clinically relevant concentration substantially enhanced the peak currents evoked by saturating or low concentrations of GABA for β3-α1-δ/β3-α1 receptors. These observations are consistent with previous studies on modulation of αβδ receptors by etomidate in both whole cells and single channels (Brown et al., 2002, Seymour et al., 2012, Feng et al., 2014). Importantly, we also observed that the steady-state currents of HEK293T-expressed β3-α1-δ/β3-α1 receptors were potentiated by etomidate to a much greater extent than peak currents. Increased gating efficacy contributes to etomidate enhancement of both peak and steady-state currents, while reduced desensitization results in greater overall enhancement of steady-state current. A recent study observed that etomidate enhanced the steady-state currents of α1β2γ2L receptors evoked by low concentration of GABA (Li and Akk, 2015), suggesting that modulation of tonically activated synaptic-type receptors also contributes to the clinical action of etomidate. Interestingly, comparing etomidate effects in β3-α1-δ/β3-α1 receptors expressed in oocytes (Feng et al., 2014) vs. HEK293T cells (present study) indicates that peak current enhancement is greater in oocytes, and comparable in magnitude to steady-state enhancement of HEK293T currents. This may be because peak currents in oocytes evolve more slowly than the fast desensitization phases that are readily observed using smaller HEK293T cells and rapid solution exchange.
In summary, etomidate at clinically relevant concentrations produced substantial enhancement of maximal GABA-induced currents for β3-α1-δ/β3-α1 receptors expressed in HEK293T cells, in line with our previous findings in oocytes. Etomidate also reduced the extent of desensitization and prolonged the deactivation of these receptors. The effects of etomidate on channel desensitization resulted in greater enhancement of the steady-state current relative to the peak phasic current of β3-α1-δ/β3-α1 receptors. Thus, its desensitization effects may contribute significantly to etomidate modulation of GABAergic tonic inhibition in the CNS, as observed in both thalamus and cortex (Belelli et al., 2005, Drasbek et al., 2007).
Highlights.
The function of concatenated αβδ receptors is similar in HEK cells and oocytes.
Etomidate reduces desensitization and prolongs deactivation of αβδ receptors.
Etomidate produces a greater enhancement of steady-state vs. peak currents.
Acknowledgments
This work was supported by National Institutes of Health National Institute of General Medical Sciences (R01 GM089745 and P01 GM058448) to S.A.F. We thank the support from the Department of Anesthesia, Critical Care and Pain Medicine, Massachusetts General Hospital.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Bai D, Pennefather PS, MacDonald JF, Orser BA. The general anesthetic propofol slows deactivation and desensitization of GABA(A) receptors. J Neurosci. 1999;19:10635–10646. doi: 10.1523/JNEUROSCI.19-24-10635.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrera NP, Betts J, You H, Henderson RM, Martin IL, Dunn SM, Edwardson JM. Atomic force microscopy reveals the stoichiometry and subunit arrangement of the alpha4beta3delta GABA(A) receptor. Mol Pharmacol. 2008;73:960–967. doi: 10.1124/mol.107.042481. [DOI] [PubMed] [Google Scholar]
- Baur R, Kaur KH, Sigel E. Diversity of structure and function of alpha1alpha6beta3delta GABAA receptors: comparison with alpha1beta3delta and alpha6beta3delta receptors. J Biol Chem. 2010;285:17398–17405. doi: 10.1074/jbc.M110.108670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belelli D, Peden DR, Rosahl TW, Wafford KA, Lambert JJ. Extrasynaptic GABAA receptors of thalamocortical neurons: a molecular target for hypnotics. J Neurosci. 2005;25:11513–11520. doi: 10.1523/JNEUROSCI.2679-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bianchi MT, Haas KF, Macdonald RL. Structural determinants of fast desensitization and desensitization-deactivation coupling in GABAa receptors. J Neurosci. 2001;21:1127–1136. doi: 10.1523/JNEUROSCI.21-04-01127.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Botzolakis EJ, Stanic AK, Feng HJ, Gurba KN, Tian M, Macdonald RL. Assembly and stoichiometry of alphabetadelta GABAA receptors. Soc Neurosci Abstr. 2007;33:441–443. [Google Scholar]
- Botzolakis EJ, Stanic AK, Gurba KN, Lagrange AH, Feng HJ, Hu NN, Macdonald RL. Flow cytometric analysis of GABAA receptor surface expression: evidence that alphabetagamma and alphabetadelta isoforms have similar subunit stoichiometries and arrangements. Soc Neurosci Abstr. 2008;34:427–414. [Google Scholar]
- Brown N, Kerby J, Bonnert TP, Whiting PJ, Wafford KA. Pharmacological characterization of a novel cell line expressing human alpha(4)beta(3)delta GABA(A) receptors. Br J Pharmacol. 2002;136:965–974. doi: 10.1038/sj.bjp.0704795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiara DC, Dostalova Z, Jayakar SS, Zhou X, Miller KW, Cohen JB. Mapping general anesthetic binding site(s) in human alpha1beta3 gamma-aminobutyric acid type A receptors with [(3)H]TDBzl-etomidate, a photoreactive etomidate analogue. Biochemistry. 2012;51:836–847. doi: 10.1021/bi201772m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiara DC, Jayakar SS, Zhou X, Zhang X, Savechenkov PY, Bruzik KS, Miller KW, Cohen JB. Specificity of intersubunit general anesthetic-binding sites in the transmembrane domain of the human alpha1beta3gamma2 gamma-aminobutyric acid type A (GABAA) receptor. J Biol Chem. 2013;288:19343–19357. doi: 10.1074/jbc.M113.479725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desai R, Ruesch D, Forman SA. Gamma-amino butyric acid type A receptor mutations at beta2N265 alter etomidate efficacy while preserving basal and agonist-dependent activity. Anesthesiology. 2009;111:774–784. doi: 10.1097/ALN.0b013e3181b55fae. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drasbek KR, Hoestgaard-Jensen K, Jensen K. Modulation of extrasynaptic THIP conductances by GABAA-receptor modulators in mouse neocortex. J Neurophysiol. 2007;97:2293–2300. doi: 10.1152/jn.00651.2006. [DOI] [PubMed] [Google Scholar]
- Eaton MM, Bracamontes J, Shu HJ, Li P, Mennerick S, Steinbach JH, Akk G. gamma-aminobutyric acid type A alpha4, beta2, and delta subunits assemble to produce more than one functionally distinct receptor type. Mol Pharmacol. 2014;86:647–656. doi: 10.1124/mol.114.094813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farrant M, Nusser Z. Variations on an inhibitory theme: phasic and tonic activation of GABA(A) receptors. Nat Rev Neurosci. 2005;6:215–229. doi: 10.1038/nrn1625. [DOI] [PubMed] [Google Scholar]
- Feng HJ. Allosteric Modulation of alphabetadelta GABAA Receptors. Pharmaceuticals. 2010;3:3461–3477. [Google Scholar]
- Feng HJ, Bianchi MT, Macdonald RL. Pentobarbital differentially modulates alpha1beta3delta and alpha1beta3gamma2L GABAA receptor currents. Mol Pharmacol. 2004;66:988–1003. doi: 10.1124/mol.104.002543. [DOI] [PubMed] [Google Scholar]
- Feng HJ, Botzolakis EJ, Macdonald RL. Context-dependent modulation of alphabetagamma and alphabetadelta GABA A receptors by penicillin: implications for phasic and tonic inhibition. Neuropharmacology. 2009;56:161–173. doi: 10.1016/j.neuropharm.2008.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng HJ, Jounaidi Y, Haburcak M, Yang X, Forman SA. Etomidate Produces Similar Allosteric Modulation in alpha1beta3delta and alpha1beta3gamma2L GABAA Receptors. Br J Pharmacol. 2014;171:789–798. doi: 10.1111/bph.12507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng HJ, Macdonald RL. Barbiturates require the N terminus and first transmembrane domain of the delta subunit for enhancement of alpha1beta3delta GABAA receptor currents. J Biol Chem. 2010;285:23614–23621. doi: 10.1074/jbc.M110.122564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glykys J, Peng Z, Chandra D, Homanics GE, Houser CR, Mody I. A new naturally occurring GABA(A) receptor subunit partnership with high sensitivity to ethanol. Nat Neurosci. 2007;10:40–48. doi: 10.1038/nn1813. [DOI] [PubMed] [Google Scholar]
- Haas KF, Macdonald RL. GABAA receptor subunit gamma2 and delta subtypes confer unique kinetic properties on recombinant GABAA receptor currents in mouse fibroblasts. J Physiol. 1999;514(Pt 1):27–45. doi: 10.1111/j.1469-7793.1999.027af.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones MV, Westbrook GL. Desensitized states prolong GABAA channel responses to brief agonist pulses. Neuron. 1995;15:181–191. doi: 10.1016/0896-6273(95)90075-6. [DOI] [PubMed] [Google Scholar]
- Kaur KH, Baur R, Sigel E. Unanticipated structural and functional properties of delta-subunit-containing GABAA receptors. J Biol Chem. 2009;284:7889–7896. doi: 10.1074/jbc.M806484200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laha KT, Ghosh B, Czajkowski C. Macroscopic kinetics of pentameric ligand gated ion channels: comparisons between two prokaryotic channels and one eukaryotic channel. PloS one. 2013;8:e80322. doi: 10.1371/journal.pone.0080322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li GD, Chiara DC, Sawyer GW, Husain SS, Olsen RW, Cohen JB. Identification of a GABAA receptor anesthetic binding site at subunit interfaces by photolabeling with an etomidate analog. J Neurosci. 2006;26:11599–11605. doi: 10.1523/JNEUROSCI.3467-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li P, Akk G. Synaptic-type alpha1beta2gamma2L GABAA receptors produce large persistent currents in the presence of ambient GABA and anesthetic drugs. Mol Pharmacol. 2015;87:776–781. doi: 10.1124/mol.114.096453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X, Pearce RA. Effects of halothane on GABA(A) receptor kinetics: evidence for slowed agonist unbinding. J Neurosci. 2000;20:899–907. doi: 10.1523/JNEUROSCI.20-03-00899.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma W, Saunders PA, Somogyi R, Poulter MO, Barker JL. Ontogeny of GABAA receptor subunit mRNAs in rat spinal cord and dorsal root ganglia. The Journal of comparative neurology. 1993;338:337–359. doi: 10.1002/cne.903380303. [DOI] [PubMed] [Google Scholar]
- Meera P, Olsen RW, Otis TS, Wallner M. Etomidate, propofol and the neurosteroid THDOC increase the GABA efficacy of recombinant alpha4beta3delta and alpha4beta3 GABA A receptors expressed in HEK cells. Neuropharmacology. 2009;56:155–160. doi: 10.1016/j.neuropharm.2008.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mody I, Pearce RA. Diversity of inhibitory neurotransmission through GABA(A) receptors. Trends Neurosci. 2004;27:569–575. doi: 10.1016/j.tins.2004.07.002. [DOI] [PubMed] [Google Scholar]
- Olsen RW, Sieghart W. International Union of Pharmacology. LXX. Subtypes of gamma-aminobutyric acid(A) receptors: classification on the basis of subunit composition, pharmacology, and function. Update. Pharmacol Rev. 2008;60:243–260. doi: 10.1124/pr.108.00505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel B, Mortensen M, Smart TG. Stoichiometry of delta subunit containing GABA(A) receptors. Br J Pharmacol. 2014;171:985–994. doi: 10.1111/bph.12514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rusch D, Zhong H, Forman SA. Gating allosterism at a single class of etomidate sites on alpha1beta2gamma2L GABA A receptors accounts for both direct activation and agonist modulation. J Biol Chem. 2004;279:20982–20992. doi: 10.1074/jbc.M400472200. [DOI] [PubMed] [Google Scholar]
- Scheller M, Forman SA. Coupled and uncoupled gating and desensitization effects by pore domain mutations in GABA(A) receptors. J Neurosci. 2002;22:8411–8421. doi: 10.1523/JNEUROSCI.22-19-08411.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seymour VA, Curmi JP, Howitt SM, Casarotto MG, Laver DR, Tierney ML. Selective modulation of different GABAA receptor isoforms by diazepam and etomidate in hippocampal neurons. Int J Biochem Cell Biol. 2012;44:1491–1500. doi: 10.1016/j.biocel.2012.06.001. [DOI] [PubMed] [Google Scholar]
- Shu HJ, Bracamontes J, Taylor A, Wu K, Eaton MM, Akk G, Manion B, Evers AS, Krishnan K, Covey DF, Zorumski CF, Steinbach JH, Mennerick S. Characteristics of concatemeric GABA(A) receptors containing alpha4/delta subunits expressed in Xenopus oocytes. Br J Pharmacol. 2012;165:2228–2243. doi: 10.1111/j.1476-5381.2011.01690.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stell BM, Brickley SG, Tang CY, Farrant M, Mody I. Neuroactive steroids reduce neuronal excitability by selectively enhancing tonic inhibition mediated by delta subunit-containing GABAA receptors. Proc Natl Acad Sci U S A. 2003;100:14439–14444. doi: 10.1073/pnas.2435457100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagoner KR, Czajkowski C. Stoichiometry of expressed alpha(4)beta(2)delta gamma-aminobutyric acid type A receptors depends on the ratio of subunit cDNA transfected. J Biol Chem. 2010;285:14187–14194. doi: 10.1074/jbc.M110.104257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wohlfarth KM, Bianchi MT, Macdonald RL. Enhanced neurosteroid potentiation of ternary GABA(A) receptors containing the delta subunit. J Neurosci. 2002;22:1541–1549. doi: 10.1523/JNEUROSCI.22-05-01541.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang J, Uchida I. Mechanisms of etomidate potentiation of GABAA receptor-gated currents in cultured postnatal hippocampal neurons. Neuroscience. 1996;73:69–78. doi: 10.1016/0306-4522(96)00018-8. [DOI] [PubMed] [Google Scholar]
- You H, Dunn SM. Identification of a domain in the delta subunit (S238-V264) of the alpha4beta3delta GABAA receptor that confers high agonist sensitivity. J Neurochem. 2007;103:1092–1101. doi: 10.1111/j.1471-4159.2007.04817.x. [DOI] [PubMed] [Google Scholar]
- Zhang ZX, Lu H, Dong XP, Liu J, Xu TL. Kinetics of etomidate actions on GABA(A) receptors in the rat spinal dorsal horn neurons. Brain Res. 2002;953:93–100. doi: 10.1016/s0006-8993(02)03274-2. [DOI] [PubMed] [Google Scholar]
- Zhong H, Rusch D, Forman SA. Photo-activated azi-etomidate, a general anesthetic photolabel, irreversibly enhances gating and desensitization of gamma-aminobutyric acid type A receptors. Anesthesiology. 2008;108:103–112. doi: 10.1097/01.anes.0000296074.33999.52. [DOI] [PMC free article] [PubMed] [Google Scholar]


