Abstract
Purpose
To evaluate suggested metastasis-related microRNAs (miRNAs) for their association with disease-free survival (DFS) and overall survival (OS) of triple negative breast cancer (TNBC).
Methods
In a cohort of 456 TNBC cases, we systematically evaluated 57 previously-reported metastasis-related microRNAs in tumor tissue using the NanoString nCounter assay. Cox regression was applied to evaluate miRNA expression in association with DFS and OS. In vitro assays using the TNBC cell line MDA-MB-231 were also conducted to validate epidemiological study findings.
Results
During a median follow-up of 5.3 years, 112 deaths and 97 recurrences were documented. High levels of miR-374b-5p, miR-218-5p, or miR-126-3p, or low levels of miR-27b-3p were independently associated with a favorable TNBC outcome (P<0.01 for all). A composite score based on the levels of these 4 microRNAs was associated with DFS, with hazard ratios (95% confidence interval) of 0.70 (0.43–1.15), 0.51 (0.29–0.90), and 0.18 (0.09–0.37) for the second, third and fourth compared to the lowest quartile. Incorporating the miRNA score with known TNBC predictors, i.e., age at diagnosis, tumor stage and basal-like subtype, increased the C-index for predicting DFS from 0.68 to 0.74. Additionally, miR-126-3p was correlated with basal-like breast cancer, and miR-374b-5p modified the therapeutic effects of 5-Fluorouracil and Cyclophosphamide treatments in basal-like breast cancer patients. Restoring miR-126-3p, miR-218-5p, or miR-374b-5p, or inhibiting miR-27b-3p in MDA-MB-231 cells reduced cell proliferation. miR-374b-5p suppressed cell invasion and miR-218-5p inhibited colonization.
Conclusion
This study provides strong evidence that the levels of miR-374b-5p, miR-27b-3p, miR-126-3p, and miR-218-5p in tumor tissues predict TNBC outcomes.
Keywords: miRNA, TNBC, breast cancer, disease-free survival, overall survival
Introduction
Triple negative breast cancer (TNBC) is an aggressive subtype of breast cancer that does not express the estrogen receptor (ER), progesterone receptor (PR), or human epidermal growth factor receptor 2 (HER2) genes, and has a high rate of metastasis and poorer prognosis [1]. Due to the lack of a known molecular therapeutic target, currently there are no specific targeted therapies for TNBC [2]. Therefore, understanding the biology of TNBC and identifying its prognostic and predictive biomarkers are pivotal in managing TNBC.
MicroRNA (miRNA), a class of noncoding small RNA that post-transcriptionally regulates gene expression [3], plays an important role in cell proliferation, differentiation, and apoptosis by targeting multiple downstream genes [4]. Impaired miRNA expression contributes to the development and progression of breast and other cancers [5, 6]. Several miRNA signatures have previously been linked to response to treatments, progression and recurrence of the disease [7–9], Thus, miRNA signatures could potentially predict survival.
Comparing miRNA expression levels from breast cancer tumor tissue with adjacent normal tissue, and comparing lymph nodes from the metastatic lesion or highly-metastatic cell line derivatives with their parental cell line, recent studies have reported several breast cancer metastasis-associated miRNAs [10–17]. However, only a few of these miRNAs have been evaluated in humans for their prognostic and predictive values in breast cancer outcomes and results are controversial [10, 11, 13, 14, 17]. To our knowledge, only two studies with relatively small numbers of patients have specifically evaluated tumor miRNA markers in association with TNBC prognosis [11, 18].
In this study, we systematically evaluated 57 putative metastasis-related miRNAs for their association with recurrence and mortality in a cohort study of 456 TNBC patients.
Methods
Study population, sample and data collection
Subjects of the current study were among the participants of the Shanghai Breast Cancer Survival Study (SBCSS), a population-based cohort study of 5,042 breast cancer survivors described in detail elsewhere [19]. Participants of the SBCSS were recruited approximately 6 months after diagnosis and were followed up by in-person surveys at 18, 36, 60 and 120 months after cancer diagnosis, in combination with periodic record linkage with the Shanghai Vital Statistics Registry. The study protocol was approved by the institutional review boards of Vanderbilt University and the Shanghai Municipal Center for Disease Control and Prevention, and all participants provided written informed consent.
Tumor characteristics, including stage, grade, and ER/PR status were determined from medical charts. HER2 status was assessed in the Vanderbilt Molecular Epidemiology Lab [20]. Tumor sections were collected from diagnostic hospitals for 4,036 SBCSS participants, of which 525 had TNBC. Due to inadequate quantity of tumor tissue, 28 cases were excluded, leaving 497 participants for the current study. Participants’ hematoxylin and eosin (H&E) slides were reviewed by a study pathologist. Tumor tissue was dissected to ensure that samples contained more than 80% tumor cells for RNA extraction. Total RNA was isolated and purified using an miRNeasy FFPE Kit (Qiagen, Valencia, CA) and quality and quantity were checked with Nanodrop and an Agilent BioAnalyzer.
The Human v2 miRNA Expression Assay (NanoString), which includes CodeSets of probes specific to 800 common human miRNAs was used for the assays. The assays were conducted in NanoString’s in-house service lab (Seattle, WA) following a standard protocol [21]. The sample quality assurance and data normalization were performed using the R package NanoStringNorm (version 1.1.16) [22]. The background count level was estimated as mean + 2 standard deviations (SD) of the 6 negative controls and was subtracted from each sample to correct the level of non-specific binding. We used the ratio of the geometric mean of the top-100-expressed miRNAs across all samples over that of the individual sample, i.e., , where Yi = geometric mean of the top-100-expressed miRNAs of a given sample i and N is the sample size, to normalize RNA content in the study. This ratio was multiplied by the original counter of each miRNA sample to derive the normalized miRNA expression.
Thirty-two samples were excluded from further analysis due to: (1) average level of the sample’s 6 negative controls was >3 SD of the mean of all samples’ negative controls; (2) >95% miRNA was not detected in the sample; or (3) it had a RNA content normalization factor that was >3 SD of the mean of the normalization factors. Additionally, we excluded 9 samples in TNM stage 0 (in situ).
Of the 456 samples remaining for the study, 403 were collected before chemotherapy or from non-chemotherapy patients and 33 after chemotherapy. Timing of treatment cannot be determined for 20 samples. The calling algorithm developed by Parker et al. was applied to classify tumors into Luminal A, Luminal B, HER2-enriched, Basal-like, or Normal-like breast cancer based on PAM50 genes [23].
Statistical analysis
Outcomes of the study were recurrence/breast cancer-specific mortality (disease-free survival [DFS]) and all-cause mortality (overall survival [OS]). Event-free participants were censored at the date of last in-person contact or November 30, 2013 (date of latest record linkage). Distribution of miRNA expression is skewed for most miRNAs, and thus the miRNA expression was categorized into deciles before analysis. For miRNAs that were not detectable in >10% of samples, those with zero count were classified into one category and the remainder categorized based on their decile distribution. For the initial analysis, the categorized ordinal variables (e.g., with value of 0 to 9) were treated as continuous ones in the model. For significant miRNAs, we subsequently reduced the categories to quartiles. We applied the Cox Regression model to evaluate the associations of miRNA expression with DFS and OS. Adjustments were made for age at diagnosis, TNM stage (five levels: stage I, IIA, IIB, III-IV, and missing), and basal-like subtype.
To evaluate the aggregated effort of multiple miRNAs, we created miRNA scores based on the 4 miRNAs that were significantly associated with DFS in our study by summing the products of each miRNA expression level with its β-coefficient derived from the Cox regression analysis for DFS and OS. The association of the miRNA scores with DFS and OS were evaluated categorically based on quartile distribution and continuously for trend analysis. We further carried out the receiver operating curve analysis and estimated the C-index for predicting breast cancer recurrence/mortality by adding the miRNA score in the base predictive mode that only included known predictors, i.e., age at diagnosis, TNM stage and basal-like subtype. We applied a likelihood ratio test to compare the differences between the base model and the model with addition of the miRNA score.
All statistical analyses were performed using SAS software (version 9.3; SAS Institute, Inc., Cary, NC).
In vitro studies
We carried out the following in vitro assays to evaluate the effects of the 4 significant miRNAs on TNBC cell proliferation, invasion and colonization.
Cell line and tissue culture
TNBC cell line MDA-MB-231, purchased from American Type Culture Collection (ATCC), was used in the studies and was cultured in Dulbecco’s Modified Eagle Medium (DMEM) supplemented by 10% FBS and 1% P/S. miRNAs, anti-miRNAs, control miRNA, and lipofectamin RNAiMAX were purchased from Life Technologies (Grand Island, NY).
Transfection and cell proliferation assay
1.5×105 MDA-MB-231 cells were seeded in 6-well plates one day before transfection. Sixty pmol of miRNA were transfected with lipofectamin RNAiMAX in OPI medium. Twenty-four hours after transfection, the complete medium was replaced. Cells were counted at 2 and 4 days after transfection, using a hemacytometer.
Invasion assay
Transwell inserts were obtained from Corning (Corning, NY) and the bottom inserts were coated with matrigel. 5×104 miRNA transfected MDA-MB-231 cells were suspended in a serum-free medium and loaded into inserts. The bottom chambers were filled with DMEM (10% FBS). The cells were incubated at 37°C for 4-5 hours. Cells were fixed using formalin and stained with crystal violet solution. Non-migrated cells were removed from the top of the inserts. Numbers of migrated cells transfected with miR-374b-5p were compared with those of control miRNA transfected cells.
Clonogenic assay
Five hundred miRNA transfected MDA-MB-231 cells were seeded in 6-well plates. Cells were cultured for 10 days, and then stained with crystal violet solution. Number of colonies formed with diameters greater than 3 mm were compared to those of controls.
For all above functional in vitro assays, three independent experiments were performed and the differences were examined by a Student T-test (two groups) or one-way ANOVA (>two groups), P ≤ 0.05 was considered as statistically significant.
Ingenuity Pathway Analysis
To gain further knowledge on the potential functional mechanisms for the significant miRNAs, we conducted bioinformatics analysis based on the Ingenuity Pathway Analysis (IPA) tool (version17199142, http://www.ingenuity.com). We applied the miRNA Target Filter function in IPA to retrieve those predicted, experimentally validated, and literature reported targets of each miRNA.
Results
Over a median follow-up of 5.3 years (range: 0.7–8.9 years), 112 deaths and 97 recurrences or breast cancer deaths were observed. As expected, 5-year DFS and OS rates were inversely associated with advanced TNM stage and disease grade. Patients with basal-like subtype had lower DFS and OS rates compared to those with the non-basal-like subtype (Table 1).
Table 1.
Characteristics of participants in the TNBC cohort of the Shanghai Breast Cancer Survival Study
| Characteristics | Number | 5-yr DFS | 5-yr OS |
|---|---|---|---|
| No. of cases | 456 | 80.02% | 81.31% |
| Age at diagnosis, median (range) | 51.6 (26.1–74.3) | ||
| TNM stage | |||
| I | 137 | 88.92% | 89.05% |
| IIA | 164 | 83.26% | 84.05% |
| IIB | 95 | 74.50% | 73.68% |
| III – IV | 47 | 54.67% | 63.69% |
| Unknown | 13 | 76.92% | 84.62% |
| Grade | |||
| 1 | 51 | 88.00% | 94.12% |
| 2 | 140 | 79.82% | 82.01% |
| 3 | 263 | 78.80% | 78.70% |
| Unknown | 2 | 50% | 50% |
| Chemotherapy | |||
| Yes | 430 | 80.72% | 82.28% |
| No | 26 | 68.11% | 65.38% |
| Radiotherapy | |||
| Yes | 126 | 66.14% | 68.76% |
| No | 330 | 85.29% | 86.06% |
| Sample collection before chemo | |||
| Yes | 403 | 83.42% | 84.36% |
| No | 33 | 56.30% | 59.38% |
| Unknown | 20 | 50.00% | 55.00% |
| Basal-like | |||
| Yes | 189 | 71.61% | 73.94% |
| No | 242 | 87.46% | 88.01% |
| Unknown | 25 | 71.15% | 72.00% |
| No. of deaths | 112 | ||
| No. of recurrence/breast cancer deaths | 97 | ||
For the 57 miRNAs examined, expression levels of miR-27b-3p, miR-126-3p, miR-142-5p, miR-218-5p, and miR-374b-5p were significantly associated with DFS of TNBC, independent of age at diagnosis and TNM stage (Table S1); hazard ratios associated with per decile increments of miRNA were 1.12 (1.04 – 1.20), 0.91 (0.84 – 0.97), 0.87 (0.76 – 0.99), 0.90 (0.84 – 0.97) and 0.89 (0.83 – 0.96), respectively. Due to the high proportion of zero value (56%) and overall low expression (count range: 0–541 with 91% lower than 20) for miR-142-5p, it was excluded from further analyses. Further analysis based on quartile cuts showed a significant dose-response association for DFS for all 4 miRNAs, with HRs being 2.10 (1.17 – 3.76), 0.48 (0.25 – 0.91), 0.47 (0.25 – 0.87) and 0.51 (0.28 – 0.92) for the highest compared to the lowest quartiles (Table 2).
Table 2.
Association of top four miRNAs with disease-free survival in the Shanghai Breast Cancer Survival Study
| miRNA | Events/Total | 5-yr DFS (%) | HR1 (95% CI) | HR2 (95% CI) |
|---|---|---|---|---|
| miR-126b Q1 | 26/114 | 76.67 | 1 | 1 |
| Q2 | 29/114 | 75.07 | 1.10 (0.64 – 1.89) | 1.17 (0.68 – 2.01) |
| Q3 | 26/114 | 80.54 | 0.87 (0.50 – 1.52) | 0.95 (0.54 – 1.66) |
| Q4 | 16/114 | 87.69 | 0.48 (0.25 – 0.91) | 0.59 (0.31 – 1.13) |
| P for trend | 0.017 | 0.09298 | ||
| miR-218-5p Q1 | 29/114 | 75.84 | 1 | 1 |
| Q2 | 30/118 | 76.84 | 0.93 (0.55 – 1.56) | 0.90 (0.54 – 1.52) |
| Q3 | 22/110 | 81.54 | 0.65 (0.37 – 1.14) | 0.65 (0.37 – 1.14) |
| Q4 | 16/114 | 85.95 | 0.47 (0.25 – 0.87) | 0.48 (0.26 – 0.89) |
| P for trend | 0.0072 | 0.00967 | ||
| miR-27b-3p Q1 | 18/114 | 85.94 | 1 | 1 |
| Q2 | 19/114 | 86.71 | 1.00 (0.52 – 1.91) | 0.96 (0.50 – 1.84) |
| Q3 | 26/115 | 76.96 | 1.42 (0.77 – 2.60) | 1.39 (0.75 – 2.55) |
| Q4 | 34/113 | 70.24 | 2.10 (1.17 – 3.76) | 2.02 (1.13 – 3.63) |
| P for trend | 0.0046 | 0.00684 | ||
| miR-374b-5p Q1 | 31/119 | 76.95 | 1 | 1 |
| Q2 | 27/113 | 77.84 | 0.84 (0.50 – 1.40) | 0.87 (0.52 – 1.46) |
| Q3 | 21/111 | 81.82 | 0.57 (0.32 – 0.99) | 0.58 (0.33 – 1.01) |
| Q4 | 18/113 | 83.77 | 0.51 (0.28 – 0.92) | 0.51 (0.28 – 0.91) |
| P for trend | 0.0098 | 0.00897 |
Participants were categorized based on the quartiles of the miRNA values.
Adjusted for age at diagnosis and TNM stage
Additionally adjusted for basal-like breast cancer subtype
When further adjusted for basal-like breast cancer subtype, the associations of miR-27b-3p, miR-218-5p and miR-374b-5p with DFS remained statistically significant. However, the association of miR-126-3p with DFS lost its significance (Table 2). The expression level of miR-126-3p was lower in basal-like breast cancer [median (Q1–Q3): 2250 (1796–3124)] than in non-basal-like breast cancer [median (Q1–Q3): 2726 (1944–3649); p=0.0007]. The expression of the other 3 miRNAs did not differ between basal-like and non-basal-like breast cancer subtypes (data not shown). Similar association patterns were observed for OS (Table S2).
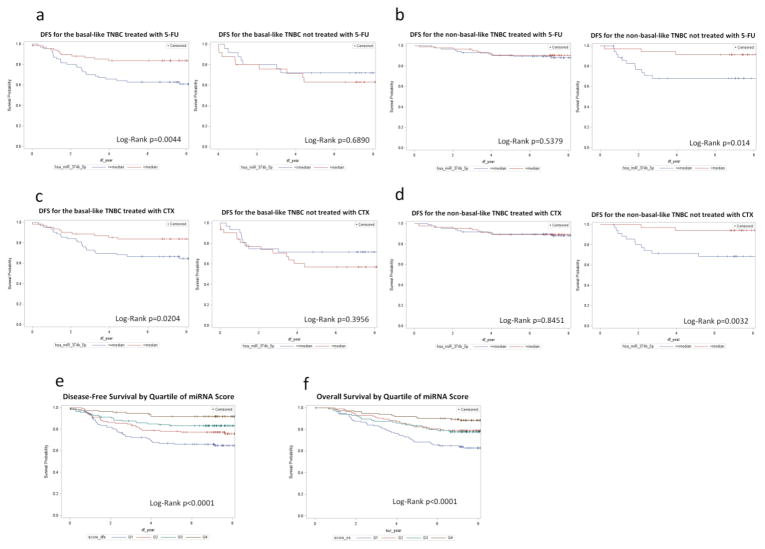
The association of miR-374b-5p with breast cancer was modified by chemotherapy and basal-like breast cancer subtype (Pinteraction=0.03). For patients with basal-like breast cancer and treated with 5-Fluorouracil (5-FU), a higher level of miR-374b-5p expression was significantly associated with DFS, while this association was not observed among patients who did not receive 5-FU treatment (Fig. 1A). For non-basal-like breast cancer, miR-374b-5p was only associated with DFS among subjects who had not received 5-FU (Fig. 1B). Another drug, Cyclophosphamide (CTX), exhibited a similar association pattern (Pinteraction=0.02) (Fig. 1C and 1D). Since co-administration of 5-FU and CTX was common in our study population (60%), we mutually adjusted for each of the drugs during the assessment of interaction between 5-FU or CTX and miR374b-5p. We found that among the basal-like breast cancer subtype, P values were 0.09 and <0.01, respectively, for interaction between 5-FU and miR374b-5p and between CTX and miR374b-5p.
Fig. 1.
Fig. 1 a-d Kaplan-Meier curves for disease free survival (DFS) by miR-374b-5p level for basal-like breast cancer patients treated by 5-FU or CTX. Figures 1 A and B showed an interaction between miR-374b-5p and 5-FU treatment, and Figures C and D showed an interaction between miR-374b-5p and CTX treatment on DFS for basal-like and non-basal-like TNBC
Fig. 1 e and f Kaplan-Meier curves for disease-free survival (E) and overall survival (F) by miRNA scores
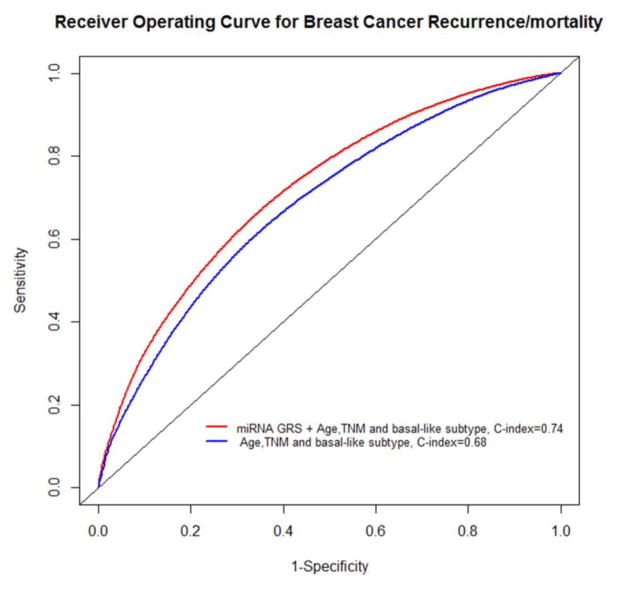
To examine the joint effects of these 4 miRNAs on TNBC outcomes, we derived 2 miRNA scores for DFS and OS based on the categorized expression of miR-126-3p, miR-27b-3p, miR218-5p, and miR374b-5p and evaluated their association with DFS and OS. HRs crossing the first to fourth quartile of the risk score were 1.0, 0.70 (95% CI=0.43 – 1.15), 0.51 (95% CI=0.29 – 0.90), and 0.18 (95% CI=0.09 – 0.37) (Ptrend<0.0001) for DFS and 1.0, 0.57 (95% CI=0.35 – 0.94), 0.64 (95% CI=0.39 – 1.05), and 0.29 (95% CI=0.16 – 0.52) (Ptrend<0.0001) for OS. Graphic presentation of the association between the miRNA score and DFS and OS are shown in Fig. 1E and 1F. Addition of the miRNA score to a Cox-regression model that included age, TNM and basal-like subtype significantly improved the model performance (likehood ratio test, P<0.01) and increased the C-index for predicting the DFS from 0.68 to 0.74 (Figure 2).
Fig. 2.
Receiver operating curves (ROC) for breast cancer recurrence and breast cancer specific mortality for model with only known predictors. The model additionally included the miRNA score
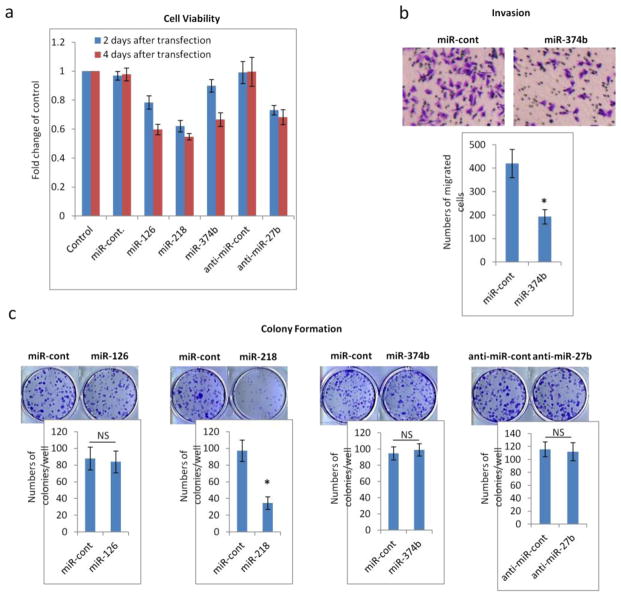
To further substantiate the evidence obtained from the epidemiological study, we over-expressed miR-126-3p, miR-218-5p, miR-374b-5p, and anti-miR-27b-3p individually in a TNBC cell line MDA-MB-231 and carried out proliferation, invasion, and colony formation assays. We found that increased expression of miR-126-3p, miR-218-5p, and miR-374b-5p or inhibition of miR-27b-3p reduced the rate of tumor cell growth (Fig. 3A). Overexpression of miR-374b-5p suppressed metastatic invasion (Fig. 3B), and overexpression of miR-218-5p reduced cell colony formation (Fig. 3C).
Fig. 3.
In vitro studies on influences of miRNAs on MDA-MB-231 cell proliferation, invasion and colony formation. miR-126-3p, miR-218-5p, miR-374b-5p, or anti-miR-27b-3p was transfected into MDA-MB-231 breast cancer cells. miR-cont and anti-miR-cont, which did not target any human mRNA, were used as negative controls for all assays. Mean and standard deviation were shown (n=3). * indicates P≤0.05. NS indicates P>0.05
Fig. 3a Proliferation: Two days and four days after transfection, cell numbers decreased for cells transfected with miR-126, miR-218, miR374b and anti-miR-27b
Fig. 3b Invasion: MDA-MB-231 cells transfected with miR-374b migrated less compared to controls
Fig. 3c Colony formation: 10 days after culture, miR-128 transfected MDA-MB-231 cells formed significantly fewer colonies compared to control cells
The Ingenuity Pathway Analysis (IPA) suggests that 126-3p targets were enriched in the SAPK/JNK signaling pathway; miR-27b-3p was correlated with PPARs and PTEN signaling; miR-218-5p targets were enriched in the Wnt pathway; and miR-374b-5p targets were enriched in fibroblast growth factor (FGF) and transforming growth factor (TGF) pathways.
Discussion
Previous animal and small-scale human studies have investigated the role of several miRNAs on breast cancer metastasis [10–15, 18]. However, few studies have investigated associations of miRNAs with breast cancer DFS and OS, especially for TNBC patients. In this study of 456 TNBC patients, we found that miR-126-3p, miR-218-5p, and miR374b-5p were positively associated with, and miR-27b-3p was inversely related with DFS and OS. We also found that the aggregate miRNA scores provided a better prediction of TNBC outcomes than individual miRNA. Addition of the miRNA score to the known TNBC prognostic factors, i.e., age, TNM and basal-like subtype, increased the C-index for predicting DFS from 0.68 to 0.74. Additionally, miR-126-3p was related to basal-like breast cancer, and the association of miR-374b-5p with DFS and OS was modified by 5-FU or CTX treatment and by basal-like breast cancer subtype.
Of these 4 significant miRNAs, miR-126-3p has been well-studied for its role in breast cancer prognosis [24, 25]. It has also been shown in vivo that restoration of miR-126-3p or the miR-126/miR-126* pair reduced recruitment of endothelial cells to metastatic breast cancer tissue in animals [12], and inflammatory monocytes and mesenchymal stem cells into tumor stroma [15]. Consistent with the previous report [13], our in vitro study showed that miR-126 reduced breast cancer cell proliferation. However, our study did not find it affected tumor cells’ invasive behavior directly.
miR-126-3p targets were enriched in the SAPK/JNK signaling pathways. miR-126 targeted VEGFA and PIK3R2 genes and regulated the VEGF/PI3K/AKT signaling pathway which is highly relevant to TNBC [26]. miR-126-3p is also correlated with MELK gene expression, which is related to breast cancer stem cells [27]. In our study, miR-126-3p expression was significantly lower in basal-like than in non-basal-like breast cancer tissue and adjustment for the basal-like subtype attenuated the outcome association, suggesting that miR-126-3p may exert its effects on recurrence and mortality through influencing genes that determine the basal-like phenotype.
miR-27b-3p is an onco-miRNA and its inhibitor (anti-miR-27b) reduces tumor growth and metastasis in vivo and cell migration and invasion in ER and PR positive breast cancer cell line ZR-75 [17]. We observed that anti-miR-27b only reduced cell growth but had no effect on cancer cell invasion and colonization in vitro in the TNBC cell line MDA-MB-231. IPA indicates that miR-27b-3p is correlated with PPARs and PTEN signaling. Further studies are needed to understand the molecular mechanism underlying the effect of dysregulation of miR-27b-3p on TNBC.
The biological functions of miR-218-5p and miR-374b-5p are unknown. Our in vitro studies showed that over-expression of miR-218-5p suppressed tumor cell colony formation, and over-expression of miR-374b-5p reduced tumor cell invasion, supporting our epidemiological findings that both miRNAs were associated with better outcomes for TNBC. The host genes of miR-218-5p, SLIT2 and SLIT3, were reported to be inactive in breast and lung cancers due to hypermethylation in the promoter region [28], suggesting hypermethylation in the host genes of this miRNA might be a potential mechanism. IPA suggests that miR-218-5p targets are enriched in the Wnt pathway and miR-374b-5p targets are enriched in the FGF and TGF pathways.
Only 4 of the 57 previously reported metastasis-related miRNAs were associated with prognosis for TNBC in our study. Another miRNA, miR-142-5p, was significantly associated with TNBC outcomes but was not pursued further because it was expressed in very few samples and at very low levels (56% undetectable and 91% < 20 miRNA counts). This low confirmation rate may be due to the major methodological differences between our and previous studies. Our study is a population-based study that specifically focused on TNBC while previous studies were primarily conducted in metastatic human breast cancer cell lines/mouse models, or comparing miRNA levels of human breast cancer/adjacent tissues [10–13, 15]. Metastasis and recurrence are time-dependent events and different miRNAs may be involved in early or late metastasis. Not taking time-dependent effects into consideration may have contributed to the inconsistent results. Another possible explanation is that although some miRNAs can promote or suppress metastasis in vitro or in vivo, the levels of miRNA in these experiments may not reflect what is commonly observed in human tissue and thus may not be informative predictors for TNBC outcomes in humans. Furthermore, miRNAs with a small inter-individual variation are unlikely to predict prognosis well even though they are biologically involved in cancer metastasis. The latter is exemplified by miR-335, a metastasis suppressor, often silenced in human breast cancer [29], which had a very low level of expression in all of our study samples (data not shown) and thus cannot be used to predict outcomes.
To our knowledge, our study is the largest study on miRNA and TNBC outcomes. The population-based cohort study design and ability to adjust for a wide range of potential confounders increased the validity of our findings. The additional in vitro studies and bioinformatics studies further strengthen the biological evidence and shed light on potential molecular mechanisms. Our study is limited by its low statistical power to evaluate predictors for TNBC by molecular subtypes and interactions between miRNA and cancer treatments. Because our study was designed to validate previously-reported metastasis-related miRNAs and some of the miRNAs are correlated, we did not apply multiple comparison adjustment in our study. Although chance findings cannot be completely ruled out, evidence from our functional studies suggests against such a possibility.
In summary, we found that 4 miRNAs were associated with DFS and OS of TNBC. Aggregately, these 4 miRNAs provide a better prediction for TNBC outcomes than any single miRNA, and show great potential for improving the prediction for TNBC recurrence and mortality.
Supplementary Material
Acknowledgments
Funding information: This work was supported by the Department of Defense Breast Cancer Research Program (DAMD 17-02-1-0607); the National Institutes of Health (R01CA118229, P50CA098131); and the Vanderbilt Molecular and Genetic Epidemiology of Cancer training program (R25CA160056 to Yan Liu). RNA sample preparation was conducted at the Survey and Biospecimen Shared Resources, supported in part by the Vanderbilt-Ingram Cancer Center (P30CA068485).
Footnotes
Conflict of Interest Statement: The authors declare that they have no conflict of interest.
References
- 1.Ossovskaya V, Wang Y, Budoff A, et al. Exploring Molecular Pathways of Triple-Negative Breast Cancer. Genes Cancer. 2011;2:870–879. doi: 10.1177/1947601911432496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dent R, Trudeau M, Pritchard KI, et al. Triple-negative breast cancer: clinical features and patterns of recurrence. Clin Cancer Res Off J Am Assoc Cancer Res. 2007;13:4429–4434. doi: 10.1158/1078-0432.CCR-06-3045. [DOI] [PubMed] [Google Scholar]
- 3.Lim LP, Lau NC, Garrett-Engele P, et al. Microarray analysis shows that some microRNAs downregulate large numbers of target mRNAs. Nature. 2005;433:769–773. doi: 10.1038/nature03315. [DOI] [PubMed] [Google Scholar]
- 4.Bartel DP. MicroRNAs: Genomics, Biogenesis, Mechanism, and Function. Cell. 2004;116:281–297. doi: 10.1016/S0092-8674(04)00045-5. [DOI] [PubMed] [Google Scholar]
- 5.Kumar MS, Lu J, Mercer KL, et al. Impaired microRNA processing enhances cellular transformation and tumorigenesis. Nat Genet. 2007;39:673–677. doi: 10.1038/ng2003. [DOI] [PubMed] [Google Scholar]
- 6.Iorio MV, Ferracin M, Liu C-G, et al. MicroRNA Gene Expression Deregulation in Human Breast Cancer. Cancer Res. 2005;65:7065–7070. doi: 10.1158/0008-5472.CAN-05-1783. [DOI] [PubMed] [Google Scholar]
- 7.Calin GA, Ferracin M, Cimmino A, et al. A MicroRNA signature associated with prognosis and progression in chronic lymphocytic leukemia. N Engl J Med. 2005;353:1793–1801. doi: 10.1056/NEJMoa050995. [DOI] [PubMed] [Google Scholar]
- 8.Calin GA, Croce CM. MicroRNA signatures in human cancers. Nat Rev Cancer. 2006;6:857–866. doi: 10.1038/nrc1997. [DOI] [PubMed] [Google Scholar]
- 9.Yu S-L, Chen H-Y, Chang G-C, et al. MicroRNA Signature Predicts Survival and Relapse in Lung Cancer. Cancer Cell. 2008;13:48–57. doi: 10.1016/j.ccr.2007.12.008. [DOI] [PubMed] [Google Scholar]
- 10.Cai J, Guan H, Fang L, et al. MicroRNA-374a activates Wnt/β-catenin signaling to promote breast cancer metastasis. J Clin Invest. 2013;123:566–579. doi: 10.1172/JCI65871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cascione L, Gasparini P, Lovat F, et al. Integrated MicroRNA and mRNA Signatures Associated with Survival in Triple Negative Breast Cancer. PLoS ONE. 2013;8:e55910. doi: 10.1371/journal.pone.0055910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Png KJ, Halberg N, Yoshida M, Tavazoie SF. A microRNA regulon that mediates endothelial recruitment and metastasis by cancer cells. Nature. 2012;481:190–194. doi: 10.1038/nature10661. [DOI] [PubMed] [Google Scholar]
- 13.Tavazoie SF, Alarcón C, Oskarsson T, et al. Endogenous human microRNAs that suppress breast cancer metastasis. Nature. 2008;451:147–152. doi: 10.1038/nature06487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Volinia S, Galasso M, Sana ME, et al. Breast cancer signatures for invasiveness and prognosis defined by deep sequencing of microRNA. Proc Natl Acad Sci. 2012;109:3024–3029. doi: 10.1073/pnas.1200010109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhang Y, Yang P, Sun T, et al. miR-126 and miR-126* repress recruitment of mesenchymal stem cells and inflammatory monocytes to inhibit breast cancer metastasis. Nat Cell Biol. 2013;15:284–294. doi: 10.1038/ncb2690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ma L, Teruya-Feldstein J, Weinberg RA. Tumour invasion and metastasis initiated by microRNA-10b in breast cancer. Nature. 2007;449:682–688. doi: 10.1038/nature06174. [DOI] [PubMed] [Google Scholar]
- 17.Jin L, Wessely O, Marcusson EG, et al. Prooncogenic Factors miR-23b and miR-27b Are Regulated by Her2/Neu, EGF, and TNF-α in Breast Cancer. Cancer Res. 2013;73:2884–2896. doi: 10.1158/0008-5472.CAN-12-2162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gasparini P, Cascione L, Fassan M, et al. microRNA expression profiling identifies a four microRNA signature as a novel diagnostic and prognostic biomarker in triple negative breast cancers. Oncotarget. 2014;5:1174–1184. doi: 10.18632/oncotarget.1682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shu XO, Zheng Y, Cai H, et al. Soy food intake and breast cancer survival. JAMA. 2009;302:2437–2443. doi: 10.1001/jama.2009.1783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Su Y, Zheng Y, Zheng W, et al. Distinct distribution and prognostic significance of molecular subtypes of breast cancer in Chinese women: a population-based cohort study. BMC Cancer. 2011;11:292. doi: 10.1186/1471-2407-11-292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Geiss GK, Bumgarner RE, Birditt B, et al. Direct multiplexed measurement of gene expression with color-coded probe pairs. Nat Biotechnol. 2008;26:317–325. doi: 10.1038/nbt1385. [DOI] [PubMed] [Google Scholar]
- 22.Waggott D, Chu K, Yin S, et al. NanoStringNorm: an extensible R package for the pre-processing of NanoString mRNA and miRNA data. Bioinforma Oxf Engl. 2012;28:1546–1548. doi: 10.1093/bioinformatics/bts188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Parker JS, Mullins M, Cheang MCU, et al. Supervised risk predictor of breast cancer based on intrinsic subtypes. J Clin Oncol Off J Am Soc Clin Oncol. 2009;27:1160–1167. doi: 10.1200/JCO.2008.18.1370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Guo C, Sah FJ, Beard L, et al. The Non-coding RNA, miR-126, Suppresses the Growth of Neoplastic Cells by Targeting Phosphatidylinositol 3-Kinase Signaling and is Frequently Lost in Colon Cancers. Genes Chromosomes Cancer. 2008;47:939–946. doi: 10.1002/gcc.20596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Feng R, Chen X, Yu Y, et al. miR-126 functions as a tumour suppressor in human gastric cancer. Cancer Lett. 2010;298:50–63. doi: 10.1016/j.canlet.2010.06.004. [DOI] [PubMed] [Google Scholar]
- 26.Zhu N, Zhang D, Xie H, et al. Endothelial-specific intron-derived miR-126 is down-regulated in human breast cancer and targets both VEGFA and PIK3R2. Mol Cell Biochem. 2011;351:157–164. doi: 10.1007/s11010-011-0723-7. [DOI] [PubMed] [Google Scholar]
- 27.Hebbard LW, Maurer J, Miller A, et al. Maternal Embryonic Leucine Zipper Kinase Is Upregulated and Required in Mammary Tumor-Initiating Cells In vivo. Cancer Res. 2010;70:8863–8873. doi: 10.1158/0008-5472.CAN-10-1295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dallol A, Silva NFD, Viacava P, et al. SLIT2, a Human Homologue of the Drosophila Slit2 Gene, Has Tumor Suppressor Activity and Is Frequently Inactivated in Lung and Breast Cancers. Cancer Res. 2002;62:5874–5880. [PubMed] [Google Scholar]
- 29.Png KJ, Yoshida M, Zhang XH-F, et al. MicroRNA-335 inhibits tumor reinitiation and is silenced through genetic and epigenetic mechanisms in human breast cancer. Genes Dev. 2011;25:226–231. doi: 10.1101/gad.1974211. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.