SUMMARY
The hypothalamus integrates information required for the production of a variety of innate behaviors such as feeding, mating, aggression and predator avoidance. Despite an extensive knowledge of hypothalamic function, how embryonic genetic programs specify circuits that regulate these behaviors remains unknown. Here, we find that in the hypothalamus the developmentally regulated homeodomain-containing transcription factor Dbx1 is required for the generation of specific subclasses of neurons within the lateral hypothalamic area/zona incerta (LH) and the arcuate (Arc) nucleus. Consistent with this specific developmental role, Dbx1 hypothalamic-specific conditional-knockout mice display attenuated responses to predator odor and feeding stressors but do not display deficits in other innate behaviors such as mating or conspecific aggression. Thus, activity of a single developmentally regulated gene, Dbx1, is a shared requirement for the specification of hypothalamic nuclei governing a subset of innate behaviors.
INTRODUCTION
Seminal studies over the past twenty years, first in the spinal cord and hindbrain, then later in the forebrain, have revealed that neuronal subclass identity is established via the combinatorial actions of transcription factors expressed in ventricular and subventricular zone neural progenitors (Briscoe et al., 2000; Dasen and Jessell, 2009; Flames and Hobart, 2009; Hébert and Fishell, 2008; Shirasaki and Pfaff, 2002; Wonders and Anderson, 2006). Programs of migration and connectivity are then carried out as development proceeds, ultimately resulting in a fully formed and functional nervous system. While the developmental logic for specification of a variety of neuronal populations throughout the neuraxis has now been largely identified, how embryonic transcriptional programs are linked to the emergence of complex behaviors remains unknown.
Innate behaviors, defined as those that manifest without prior training, are controlled via the coordinated actions of hypothalamic nuclei. Depending on the context, different hypothalamic nuclei are activated and function to integrate biologically relevant information to achieve appropriate behavioral outputs (Gross and Canteras, 2012; Sokolowski and Corbin, 2012). For example, the arcuate (Arc) nucleus and the lateral hypothalamic area/zona incerta (LH) are dedicated to controlling feeding through the coordinated actions of orexigenic (feeding-promoting) and anorexigenic (feeding-inhibiting) neurons (Sohn et al., 2013; Sternson et al., 2013; Yeo and Heisler, 2012; Zeltser et al., 2012). In addition, neurons in the Arc and LH have been implicated in the regulation of stress responses, such as in response to food deprivation and predator odor (Maniam and Morris, 2012; Canteras et al., 1997; Beijamini and Guimarães, 2006). These nuclei send direct projections to the paraventricular nucleus (PVN), which acts as the hypothalamic gate of the hypothalamic-pituitary-adrenal (HPA) axis (Maniam and Morris, 2012; Atasoy et al., 2012; Betley et al., 2013; Sohn et al., 2013; Sternson et al., 2013). Other hypothalamic nuclei, such as the ventral medial hypothalamus (VMH) and premammillary nucleus (PMN) perform overlapping and non-overlapping functions with the Arc and LH in not only regulating different types of responses to innate stressors such as predator odor, but also conspecific aggressive, mating and maternal care behaviors (Canteras et al., 1997; Lin et al., 2011; Silva et al., 2013; Takahashi, 2014; Yang et al., 2013). Despite an understanding of these aspects of hypothalamic control of behavior, the link between developmental mechanisms specifying neuronal identity and manifestation of innate behaviors remains unexplored.
To explore the relationship between genetic mechanisms governing neuronal specification and regulation of hypothalamic-driven innate behaviors, we focused on the developmentally regulated transcription factor Dbx1. Dbx1 is widely expressed in hypothalamic progenitors (Alvarez-Bolado et al., 2012; Causeret et al. 2011; Flames et al., 2007; Hirata et al., 2009; Lu et al., 1992; Shoji et al., 1996) and is required for specification of neuronal subgroups in the spinal cord, midbrain and hindbrain (Bouvier et al., 2010; Gray et al., 2010; Inamata and Shirasaki, 2014; Pierani et al., 2001). Employing a combination of approaches, we find that Dbx1 functions in a highly selective manner in hypothalamic development acting at three interrelated levels. First, at the developmental level, via regulation of a select set of embryonic patterning genes, Dbx1 function is restricted to specification of orexigenic neurons in the Arc and LH. Second, at the circuit level, Dbx1 is required for the ability of the Arc and LH, and subsequently the HPA axis, to mount appropriate physiological responses to fasting and predator odor. Third, this specificity of Dbx1 function in hypothalamic development and circuit function translates to select behavioral deficits in responses to innate feeding and predator-odor stressors. Thus, our data reveal that Dbx1-dependent transcriptional control is a common developmental mechanism for specification of functionally related neurons in two distinct hypothalamic nuclei and links embryonic patterning to the manifestation of innate behaviors.
RESULTS
Hypothalamic-specific conditional deletion of Dbx1
In the embryonic ventral diencephalon, Dbx1 is expressed from approximately embryonic day (E) 9.5 to E13.5 from the rostral preoptic domain to the caudal mammillary domain (Alvarez-Bolado et al., 2012; Causeret et al., 2011; Flames et al., 2007; Hirata et al., 2009; Figure 1B.i–G.i). Dbx1−/− knockout mice do not survive beyond birth (Bouvier et al., 2010; Pierani et al., 2001; Gray et al., 2010), precluding assessment of the long-term consequences of Dbx1 loss-of-function. To overcome this limitation, we generated a Dbx1 conditional-knockout allele (Dbx1flox/flox) (Figure 1A), which were crossed to previously generated Nkx2.1Cre mice (Xu et al., 2008) to generate hypothalamic-specific conditional-knockout mice. The resulting conditional-knockout progeny (Nkx2.1cre;Dbx1c/− mice, herein referred to as Dbx1cKO mice) were viable, and for all experimental analyses Nkx2.1Cre;Dbx1c/+ mice produced from the same cross served as controls (herein referred to as Ctrl).
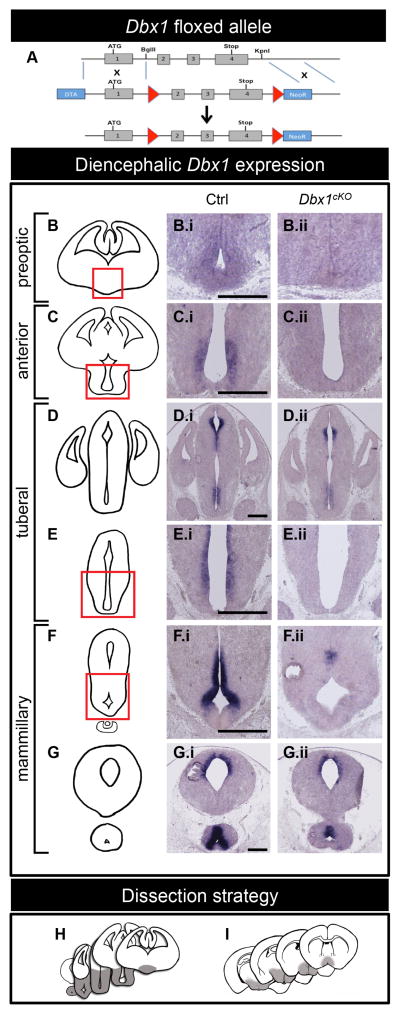
Figure 1. Dbx1cKO targeting construct and embryonic Dbx1 expression.
(A) Schematic of Dbx1 conditional targeting approach. LoxP sites flank exons 2, 3 and 4 of the Dbx1 gene. The DNA-binding homeodomain is encoded by exons 3 and 4.
(B–G) Schematic of rostral (top) to caudal (bottom) coronal views of the embryonic forebrain. Dbx1 expression at E13.5 is shown in Ctrl (B.i–G.i) and Dbx1cKO (B.ii–G.ii) embryos in serial coronal sections. Complete loss of Dbx1 expression is observed in the preoptic (B.ii), anterior (C.ii), posterior tuberal (E.ii) and anterior mammillary (F.ii) regions, with partial loss of expression in the anterior tuberal and sparing of the dorsal diencephalon (D.ii) and posterior mammillary regions (G.ii).
(H–I) Regions of the E13.5 embryonic (H) and P90 (I) diencephalon (shaded grey) dissected for RNA extraction and microarray analyses. The scale bar represents 500 μm.
In the E13.5 Dbx1cKO forebrain, Dbx1 expression was maintained in progenitor zones that contribute to the dorsal diencephalon (Figure 1D.i-ii), amygdala and septum (Figure S1A.i–C.ii). In the ventral diencephalon, loss of Dbx1 expression was observed across multiple domains including the preoptic, anterior, posterior tuberal and mammillary regions (Figure 1B.i–G.ii), areas previously established to generate the postnatal POA, AH, PVN, Arc, LH and part of the VMH and PMN nuclei (Alvarez-Bolado et al., 2012; Caqueret et al., 2006; Kurrasch et al., 2007; Shimogori et al., 2010; Yee et al., 2009). Partial recombination was observed in the anterior tuberal and posterior mammillary regions. Thus, Nkx2.1Cre-driven recombination resulted in loss of Dbx1 function across the majority of the hypothalamic primordium and not in the telencephalon.
To assess the consequences of conditional loss of Dbx1 function on embryonic patterning and postnatal gene expression in an unbiased manner, we performed four separate microarray screens of mRNA isolated from microdissected Dbx1cKO and Ctrl, male and female embryonic hypothalamic primordium and postnatal hypothalamus (Figure 1H–I). From this analysis, we identified cohorts of genes potentially misregulated in Dbx1cKO mice at both embryonic and postnatal stages (Table S1). We refined the candidate genes for subsequent validation based on three criteria: 1) known or hypothesized role in neural development or hypothalamic function, 2) known expression in the developing or postnatal hypothalamus based on either the published literature or gene expression atlases (e.g., Allen Brain Atlas) and 3) significant fold-changes ≥1.5 in the microarray data.
Altered patterning in the embryonic Dbx1cKO and Dbx1−/− hypothalamus
We next performed in situ hybridization (ISH) (or immunohistochemistry when antibodies were available) on ≥3 Dbx1cKO and Ctrl males and females at four different ages: E13.5, E17.5, postnatal day (P) 21 and P90 (Table S2). Four of the validated genes expressed in the Arc or LH – the orexigenic neuromodulators agouti related protein (Agrp), neuropeptide Y (Npy), hypocretin/orexin (Hcrt) and pro-melanin concentrating hormone (Pmch) – are highly implicated in stress responses, feeding and arousal (Nahon 2006; Berridge et al., 2010; Pankevich et al., 2010; Maniam and Morris, 2012; Williams and Elmquist, 2012; Domingos et al., 2013). The other validated genes included Nkx2.4, which encodes a developmentally regulated transcription factor expressed in the ventral diencephalon with no known function (Small et al., 2000); Ctnnb1, encoding a mediator of the canonical Wnt signaling pathway (Valenta et al., 2012); and Dbx1, the targeted gene. In addition to these markers, we assayed known markers that define hypothalamic patterning (Table S2). For embryonic analyses, we conducted our assays using both Dbx1cKO and Dbx1−/− embryos, which importantly provided two genotypes for validating gene expression changes assigned to Dbx1 function.
At E13.5, we observed a significant decrease in Npy expression in Dbx1 mutant embryos in the primordial Arc, which was combined with significant decrease in expression of the homeodomain-containing transcriptional regulator Bsx (Figure 2B–C.iv), a gene previously shown to be required for generation of the Npy+/Agrp+ population (Sakkou et al., 2007). In contrast, we observed no changes in the expression of the anorexigenic marker Pomc in Dbx1cKO and Dbx1−/− embryos (Figure 2D–D.iv). Analysis of Dbx1−/− embryos importantly confirmed that the spared Pomc expression at embryonic Dbx1cKO stages was not due to remnant Dbx1 expression in Dbx1cKO mice.
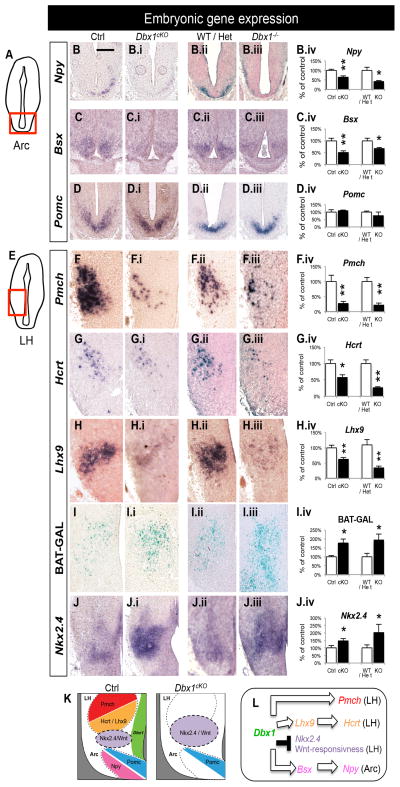
Figure 2. Embryonic gene expression changes in Dbx1cKO and Dbx1−/− mice.
Schematic of a coronal view of the E13.5 brain at the level of the Arc (A) and LH (E) show the regions analyzed in (B–D.iv) and (F–K.ii), respectively.
(A–D) In the Arc, significant decreases in Npy (B–B.iv) and Bsx (C–C.iv) expression are observed in both Dbx1cKO and Dbx1−/− embryos at E13.5 and E17.5, respectively. No changes in expression of Pomc are observed in either Dbx1cKO or Dbx1−/− embryos at E13.5 (D–D.iv).
(E–K) In the LH, a significant decrease in Pmch (F–F.iv), Hcrt (G–G.iv) and Lhx9 (H–H.iv) expression is observed in both Dbx1cKO and Dbx1−/− embryos at E13.5 or E17.5, respectively. Significant increases in numbers of Wnt-responding cells as revealed by LacZ staining in E17.5 Dbx1cKO;BAT-GAL+/− and Dbx1−/−;BAT-GAL+/− embryos (I–I.iv), expansion of Nkx2.4 expression domain (D–D.iv) in Dbx1cKO and Dbx1−/− embryos at E13.5, and increases in Calbindin+ cells in the E15.5 Dbx1−/−;BAT-GAL+/− embryos (K–K.ii) were observed.
(L) Summary diagram of the changes in embryonic gene expression in Dbx1 mutants.
Mean ± SEM; n = 3 – 22 per experimental group; *, p < 0.05; **, p < 0.01
The scale bar represents 500 μm.
In the LH, we observed a dramatic decrease in Pmch expression in the LH (Figure 2F–F.iv). In addition, Hcrt expression, which along with Pmch marks the two major LH output populations, was also significantly decreased during embryogenesis (Figure 2G–G.iv). This corresponded with a significant decrease in expression of Lhx9 (Figure 2H–H.iv), a LIM-homeodomain containing transcription factor required for the specification of Hcrt+ neurons (Dalal et al., 2013). In the primordial LH, using previously generated BAT-GAL mice as a readout of Wnt-signaling (Maretto et al., 2003), we also observed increased numbers of Wnt-responsive cells in Dbx1 mutant embryos (Figure 2I–I.iv). This increase was combined with an expansion of Nkx2.4 expression (Figure 2J–J.iv). Together, these data reveal that Dbx1 is required for repression of Nkx2.4 expression and over-production of Wnt-responsive cells in the LH. In the E15.5 LH, some of these Wnt-responsive cells express Calbindin, a marker of inhibitory interneurons (Figure S2B–F). The number of co-labeled cells, as well as the number of Calbindin+ neurons, was significantly increased in the embryonic Dbx1−/− LH (Figure 2K–K.ii; Figure S2). This increase in Calbindin+ neurons persisted in the postnatal Dbx1cKO LH (Figure 3I–I.ii). Thus, Dbx1 both positively and negatively regulates the expression of patterning genes and markers of select Arc and LH neuronal subpopulations in the hypothalamic primordium (Figure 2L).
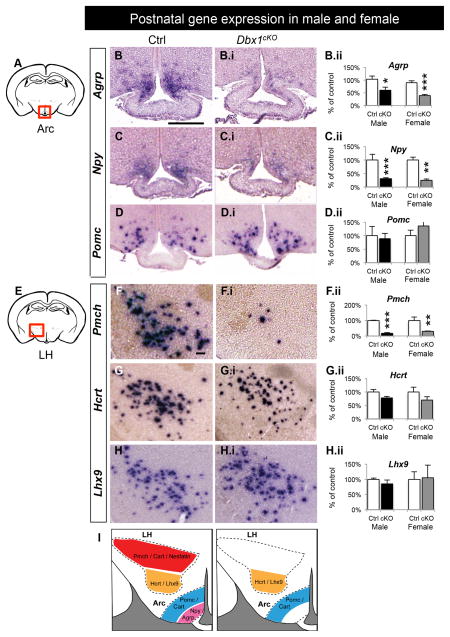
Figure 3. Postnatal decreases in orexigenic gene expression in Dbx1cKO males and females.
Schematic of a coronal view of the postnatal brain at the level of the Arc (A) and LH (E) show regions analyzed at P90 in (B–D.ii) and (F–I.ii), respectively.
(A–D) In the Arc of both male and female Dbx1cKO brains, a significant decrease in expression of Agrp (B–B.ii) and Npy (C–C.ii) is observed with no change in expression of Pomc (D–D.ii).
(E–I) In the LH of both male and female Dbx1cKO brains, a significant decrease in expression of Pmch (B–B.ii), with no changes in expression of Hcrt and Lhx9 and increases in Calbindin+ cells (I–I.ii) is observed.
(J) Summary diagram of the changes in postnatal gene expression in Dbx1cKO mice.
Mean ± SEM; n = 4 – 17 per sex, per experimental group; * p < 0.05; ** p < 0.01, *** p < 0.0001
The scale bars represent 250 μm.
Despite the widespread expression of Dbx1 in a broad portion of the embryonic ventral diencephalon, patterning of other hypothalamic nuclei including the PVN, VMH and PMN were surprisingly unaffected in both Dbx1cKO and Dbx1−/− embryos (Figure S3). Thus, Dbx1 function appears to be restricted to specification of orexigenic neurons and generation of proper numbers of Calbindin+ neurons.
Changes in expression of orexigenic neuromodulators in postnatal Dbx1cKO mice
To determine if the select embryonic changes mentioned above resulted in alterations in neuronal populations in the postnatal Arc and LH, we assessed expression of a series of genes that mark these populations. As the hypothalamus contains many sexually dimorphic neuronal populations (Manoli et al., 2013; Simerly 2002), we quantified neuronal changes in both sexes. In the Arc, orexigenic neurons are characterized by their co-expression of Agrp and Npy, whereas anorexigenic neurons are characterized by their expression of Pomc and Cart (Broberger, 1999; Horvath et al., 1997; Ovejso et al., 2001); both populations are Dbx1-derived (data not shown). In both male and female postnatal Dbx1cKO mice, we observed a significant decrease in expression of Agrp and Npy (Figure 3B–C.ii). This corresponded with a decrease in the level of Agrp detected in Agrp+ projection fields (Figure S4A.i–G). In contrast, we observed no changes in expression of Pomc mRNA (Figure 3D–D.ii), or Pomc or Cart protein (Figure S4H–I.ii) in the Dbx1cKO Arc. Moreover, expression of tyrosine hydroxylase (TH), which marks dopaminergic neurons in the Arc (Chan-Palay et al., 1984), was also unchanged in Dbx1cKO mice (Figure S4J–J.ii).
Differential expression of the neuropeptides Pmch and Hcrt define the two major neuronal output populations in the LH (Burdakov et al., 2005b). At P21 and P90, we observed a significant decrease in the expression of Pmch mRNA (Figure 3F–Fii) and Pmch protein (Figure S4K–K.ii) in both male and female Dbx1cKO mice. Pmch neurons also co-express Nesfatin and Cart (Croizier et al., 2010; Elias et al., 2001; Fort et al., 2008), which were also decreased in Dbx1cKO LH (Figure S4L–M.ii); populations which are also Dbx1-derived (data not shown). Surprisingly, however, the decrease in Hcrt and Lhx9 observed in the embryonic LH (Figure 2G–H.iv) was not observed in the postnatal LH of Dbx1cKO mice (Figure 3G–H.ii), indicating that postnatal expression of these genes are Dbx1-independent. Collectively, these data show that in the LH and Arc, Dbx1 is selectively required for the postnatal expression of most orexigenic, but not anorexigenic or dopaminergic, neuromodulators.
To explore whether loss of Dbx1 function affected postnatal gene expression in the PVN, VMH and PMN, we assessed expression of Avp, Sim1, Oxt, Fezf1, Nr5a1 and Lef1, markers that define these nuclei (Caqueret et al., 2006; Goshu et al., 2004; Shimogori 2010). Comporting with the lack of changes in embryonic patterning of these nuclei (Figure S3), the expression of each of these markers was unchanged in postnatal Dbx1cKO mice (Figure S5). This finding further illustrates the restricted function of Dbx1 in specification of neuronal subsets in the Arc and LH.
Altered hypothalamic circuit function in Dbx1cKO mice
Given the observed alterations in embryonic expression of Lhx9, Hcrt, Pmch and Calbindin (Figures 2 and S2) and in postnatal expression of Pmch, Nesfatin, Cart and Calbindin (Figures 3 and S4) in Dbx1 mutants, we hypothesized that function of LH would be disrupted. Unique to Pmch+ and Hcrt+ neurons in the LH are their evoked action potentials in response to glucose. Pmch+ neurons are electrophysiologically silent at low physiological levels of extracellular glucose but generate action potentials at higher levels. In contrast, Hcrt+ neurons respond to low glucose but are silent at increased concentrations (Burdakov and Alexopoulos, 2005; Burdakov et al, 2005a; Burdakov et al, 2005b). To investigate the function of these two neuronal populations, we performed 60-grid multiple electrode array (MEA) extracellular field recordings of the LH in acute brain slices from postnatal Dbx1cKO and Ctrl mice bathed in low- and high- glucose medium (Figure 4A–B). Compared with Ctrl brain slices, slices from Dbx1cKO brains displayed ~85% lower average spike density in low-glucose aCSF (0.2mM). Similarly, when the superfusion medium was switched to high-glucose aCSF (5.0 mM), the average spike frequency in Dbx1cKO mice was significantly lower (by ~50%) than in Ctrl mice (Figure 4C–H). These data demonstrate that glucose-sensitive LH neuron function is impaired in Dbx1cKO mice. Despite this dysfunction, glucose tolerance, a process primarily mediated by the pancreas (Efendić et al., 1976), was unaffected in Dbx1cKO mice (Figure 4I). This functional defect in the ability of the Dbx1cKO LH to respond to high glucose levels is likely due to reduced Pmch+ neuron number. In addition, despite recovery of expression of Lhx9 and Hcrt in postnatal Dbx1cKO mice, the mutant LH also fails to properly respond to low glucose.
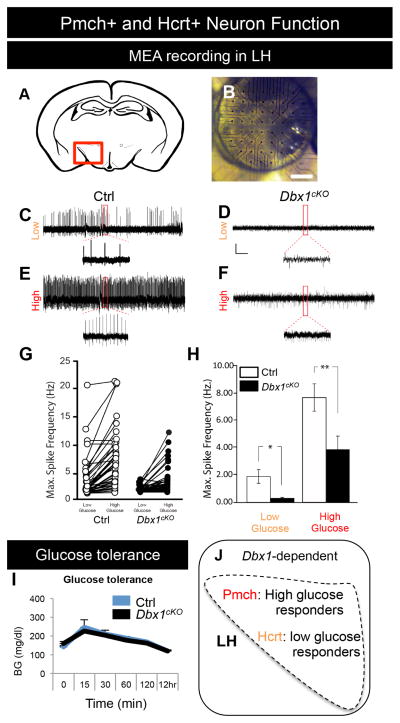
Figure 4. Altered function of LH neurons in postnatal Dbx1cKO males.
(A) Schematic indicating location of the placement of the 60-channel multiple electrode array (MEA) at the region of the LH (red square). (B) Image of the MEA on the hypothalamus of a coronal brain.
(C–F) Prototypical traces from extracellular recordings of glucose-responsive units (neurons) from the LH of Ctrls (C, E) and Dbx1cKO (D, F) male mice at P17 – P20. Traces show the response of the units in low (0.2 mM; C–D) and high (5.0 mM; E–F) glucose aCSF.
(G) Maximum spike frequency of glucose responsive units obtained during a 15 min recording in 0.2 mM glucose and subsequent 15 min recording in 5.0 mM glucose.
(H) Averages of the maximum spike frequency of glucose-responsive units shown in panel G.
(I) Glucose tolerance was unaffected in adult Dbx1cKO males after glucose injection (2g/kg bw) or a 12 hr fast.
(J) Diagram showing the known function of high glucose sensing (Pmch+) and low glucose sensing (Hcrt+) neurons in the LH.
Mean ± SEM; n = 3 – 5 per experimental group; *, p < 0.05; **, p < 0.01
Scale bar represents 200 μm.
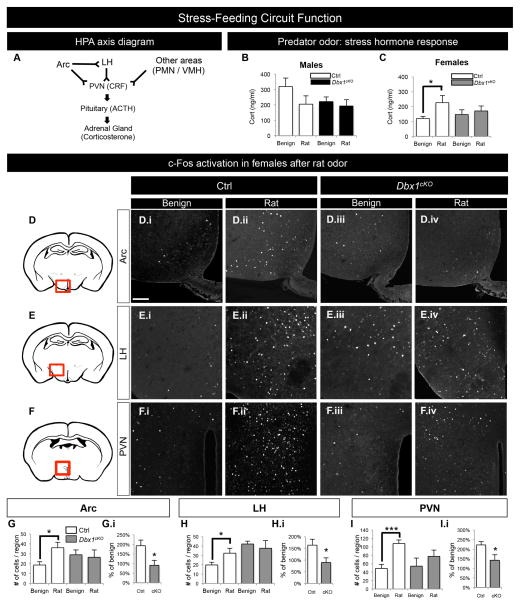
The Arc and the LH form a circuit with each other (Larsen et al., 1994; Peyron et al., 1998; Atasoy et al., 2012; Betley et al., 2013 and Figure S6A–E.ii) and with the PVN, which initiates corticosterone (Cort) release in response to stress via the HPA axis (Takahashi, 2014 and Figure 5A). The Arc-LH-PVN circuit regulates homeostatic responses to a variety of stressors as well as regulation of food intake (Maniam and Morris 2012; Canteras et al., 1997; Berridge et al., 2010). In addition, Agrp neurons are activated during fasting (Wagner et al., 2004; Wu et al., 2014), and the LH is activated in response to predator-induced stress (Canteras et al., 1997; Beijamini and Guimarães, 2006). Because our above findings revealed deficits in specification and function of Arc and LH neuronal subpopulations (Figures 2–4, S2, S4), we next wanted to examine if these alterations resulted in deficits in Arc, LH and PVN responsiveness to various stressors: innate predator stress (exposure to rat odor) and a food deprivation stressor (fasting). After a single exposure to the rat odor, Ctrl females, but not Ctrl males, displayed the expected increase in plasma levels of Cort (Figure 5B–C, white bars). Interestingly, Dbx1cKO females had an attenuated Cort response to this innate stressor that is unchanged from the response to benign bedding. (Figure 5C, gray bars). Thus, Dbx1cKO females did not display the typical physiological response to this stimulus. To determine whether neurons in the Arc-LH-PVN circuit are activated normally in the presence of both predator odor and fasting stressors, we next assessed c-Fos expression. After exposure to predator odor, female Ctrl animals displayed the expected significant increase in the number of c-Fos+ cells in the Arc, LH and PVN (Canteras et al., 1997; Beijamini and Guimarães, 2006). Strikingly, the number of c-Fos+ cells in these regions in Dbx1cKO females did not increase (Figure 5D–I.i). To further probe the activation of the Arc-LH-PVN circuit in another stress paradigm, we assessed stress-feeding responsiveness in which animals were fasted for 12 hr and then analyzed for c-Fos expression. Similar to the lack of neuronal activation in response to predator odor, Dbx1cKO mice also displayed a lack of increase in numbers of c-Fos+ cells in all three regions after fasting (Figure S6F–N.i). Together, these data reveal deficits in the level of neuronal activation within the Arc, LH and PVN in Dbx1cKO mice in response to two different stressors and links misspecification of subpopulations of Arc and LH neurons to circuit dysfunction.
Figure 5. Altered function of LH and Arc circuit in postnatal Dbx1cKO males and females.
(A) Diagram of the hypothalamic-pituitary-adrenal (HPA) axis, with neurons in the Arc and LH projecting to the PVN forming the stress-feeding circuit. During a stressful event, the PVN stimulates the release of CRF, triggering the release of ACTH from the pituitary, which causes the release of corticosterone from the adrenal glands (Herman et al., 1996).
(B) Male plasma corticosterone (Cort) levels in Ctrl mice exposed to benign odors (clean bedding; white bars) are not significantly changed compared to Ctrl mice exposed to rat odor (bedding from a rat cage; white bars). Male Dbx1cKO mice also display no changes in Cort response (black bars).
(C) Female Cort levels in Ctrl mice (white bars) are significantly increased in the presence of rat odor as compared to benign odor. In contrast, in female Dbx1cKO mice (gray bars) Cort levels do not increase in the presence of rat odor compared to benign odor.
(D–F) Schematic of a coronal view of the postnatal brain at the level of the Arc (D), LH (E) and PVN (F) with a red box indicating corresponding areas of IHC images.
(D.i–F.iv) Representative images of c-Fos expression in the Arc, LH and PVN 1 hr after exposure to benign (D.i–F.i and D.iii–F.iii) or rat odors (D.ii–F.ii and D.iv–F.iv) in Ctrl (D.i–F.ii) and Dbx1cKO (D.iii–F.iv) mice.
(G–I.i) Significant increases in numbers of c-Fos+ cells in Ctrl females exposed to rat odor compared to benign odor are observed in the Arc (G), LH (H) and PVN (I) (white bars), with no change in numbers of c-Fos+ cells in Dbx1cKO females (gray bars). Compared to Ctrl, the fold change in c-Fos+ cells after exposure to rat odor is significantly lower in Dbx1cKO female Arc (G.i), LH (H.i) and PVN (I.i).
Mean ± SEM; n= 3–11, *p value < 0.05, **p<0.01, ***p<0.001
Dbx1 is required for innate stress behavioral responses
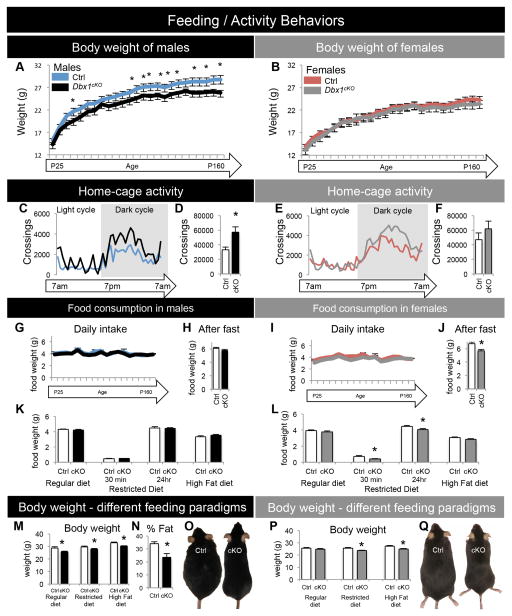
As our above data revealed, Arc and LH neuronal populations and circuits that are essential for feeding and stress responses were altered in Dbx1cKO mice. To determine the behavioral effect of these alterations, we next conducted a comprehensive battery of assays to examine the status of energy homeostasis and predator avoidance responses in Dbx1cKO mice. First, we observed a subtle decrease in body weight in male Dbx1cKO mice after the weaning period (P>30), a phenotype not observed in females (Figure 6A–B). This sexually dimorphic weight decrease correlated with increased activity (Figure 6C–D) rather than changes in food intake or metabolism as measured by our assays (Figure 6G and S7E–G). No changes in these measures were detected in female Dbx1cKO mice (Figure 6E–F, I and S7H–J). Therefore, despite causing a similar decrease in orexigenic neurons in both sexes, loss of Dbx1 function only affects male activity, representing a potential contributor to lower body weight. Interestingly, however, female Dbx1cKO mice ate less than Ctrl after fasting or restricted-feeding paradigms (Figure 6J, L), and a decrease in body weight was detected in both males and females after restricted-feeding or high-fat diet paradigms (Figure 6M–Q). Together these findings indicate that although Dbx1 functions to specify the same neuronal subgroups in both sexes, there is a sexually dimorphic difference in weight/activity and stress-related feeding behaviors.
Figure 6. Body weight, activity and food consumption in Dbx1cKO males and females.
(A–B) Body weight (in grams) of mice on a regular chow diet was assessed from P25 until P160. A significant decrease in body weight of Dbx1cKO males (A), but not females (B), is observed at later postnatal ages.
(C–F) Home-cage activity was assessed in P30 mice over a 24 hr period in a metabolic chamber in P30 mice given a regular chow diet. A significant increase in the number of horizontal line crossings is observed in Dbx1cKO males (D), but not females (F).
(G, I) Daily food consumption of mice on a regular chow diet was assessed from P25 until P160, with no significant changes observed in either sex.
(H, J) After a 12 hr fast, food consumption was recorded for 24 hr. A significant decrease in the amount of food consumed during the 24 hr re-feeding period is observed in Dbx1cKO females (J), but not males (H).
(K, L) Food consumption was recorded during different feeding paradigms (regular, restricted, or high-fat diet) after a 24 hr (and 30 min with restricted diet) re-feeding period. Dbx1cKO females ate significantly less than Ctrl while on a restricted diet (L) with no changes in food consumption while on a regular or high-fat diet. In contrast, Dbx1cKO males showed no changes in feeding under any condition (K).
(M, P) Body weight was recorded daily during different feeding paradigms (regular, restricted, or high-fat diet). (M) Body weight in Dbx1cKO males is significantly lower compared to Ctrl males under all feeding paradigms tested. (P) Significant decreases in body weights of Dbx1cKO females were only observed after restricted or high fat feeding paradigms.
(N) Significant decrease in body fat composition in Dbx1cKO males after 7 weeks on a high fat diet. Body weight and fat composition was matched in each subject to give a % fat for each subject (n = 5 mice per group). (O, Q) Images of Ctrl and Dbx1cKO mice after 7 weeks on a high-fat diet.
Mean ± SEM; unless stated otherwise n = 9 – 15 per experimental group; *, p < 0.05
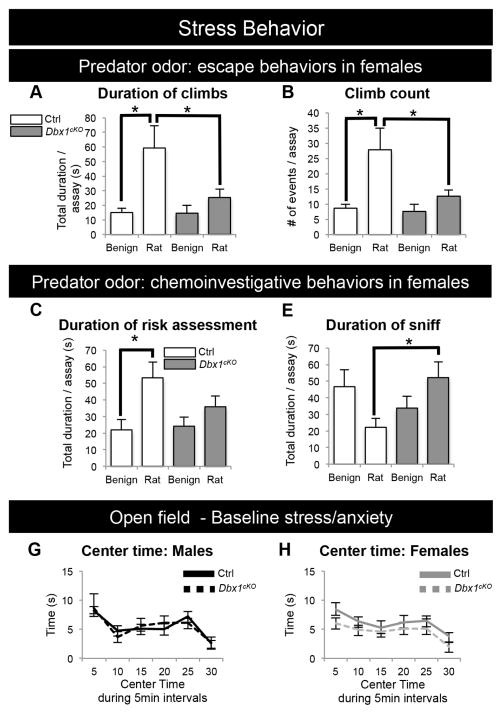
To examine behavioral responses to predator-induced stress responses, we next exposed Dbx1cKO and Ctrl mice to rat odor. Responses to this strong innate stressor are typically characterized by stereotyped fear behaviors that begin with an initial risk assessment (cautious approach), followed by escape responses including climbing (Apfelbach et al., 2005; Blanchard et al., 2005; Martinez et al., 2008; Silva et al., 2013). Because of the female-specific stress response (i.e., Cort release) in this paradigm (Figure 5B–C), we focused our behavioral assessments on female mice. Ctrl female mice presented with rat odor displayed a significant increase in escape behaviors compared to presentation with a benign odor (Figure 7A–B). In striking contrast, Dbx1cKO female mice responded equivalently to rat odor and benign stimulus; these females displayed significantly fewer and shorter escape behaviors compared to Ctrl (Figure 7A–B; Supplemental Movie). Similarly, when exposed to rat odor, Dbx1cKO females did not display the normal increased duration of risk assessments (Figure 7C). Instead, Dbx1cKO females displayed more casual chemoinvestigative behavior with a significantly longer duration of sniffing the rat odor compared to Ctrl females (Figure 7D). No differences were observed in the open field assay, suggesting that baseline stress was unaffected in Dbx1cKO mice (Figure 7E–F). In summary, these data show that loss of Dbx1 strikingly correlates with a lack of appropriate behavioral responses to a strong stressor stimulus.
Figure 7. Altered innate stress behaviors of Dbx1cKO females.
(A, B) Female Ctrl mice spend significantly more time climbing (A) and have increased number of climbs (B) during a 15 min exposure to rat odor compared to benign odor (white bars). In contrast, female Dbx1cKO mice display no significant difference in the amount of time climbing (A) or number of climbs (B) when exposed to rat odor compared to benign odors (gray bars).
(C–D) Female Ctrl mice (white bars) in the presence of rat odor compared to benign odor spend more time engaged in cautious chemoinvestigative (risk assessment) behaviors (C) and less time engaged in casual chemoinvestigation (sniffing) (D). In contrast, female Dbx1cKO mice (gray bars) in the presence of rat odor have no change in the time spent risk assessing (C) and spend significantly more time sniffing the rat odor compared to Ctrl mice (D).
(E–F) Baseline stress levels as assessed by time spent in the center of an open field are unchanged in both male and female Dbx1cKO mice compared to Ctrls.
Mean ± SEM; n = 11 – 15 per experimental group, *, p value < 0.05. See also Supplemental Movie.
The hypothalamus also regulates a number of other innate behaviors such as mating, male conspecific aggression, maternal aggression, pup retrieval and territorial urine marking (Yang and Shah, 2014; Stowers et al., 2013; Sokolowski and Corbin, 2012; Dulac and Wagner, 2006). Although Dbx1 was deleted from the VMH and PMN progenitor zones (Figures S1), nuclei that are involved in these behaviors (Gross and Canteras, 2012; Sokolowski and Corbin, 2012), we found that they were not altered (Figure S8). These data also importantly indicate that Dbx1cKO mice do not lack the ability to sense and process specific modalities of chemosensory information. This is further supported by the ability of Dbx1cKO mice to find hidden food in a basic olfaction test (Figure S8G, O). Furthermore, the narrow range of observed behavioral phenotypes – namely, those involved in stress responses – is consistent with the restricted function of Dbx1 in specification of subsets of Arc and LH neurons.
DISCUSSION
A central goal in neuroscience is to understand the mechanisms by which information encoded during embryogenesis is translated into building circuitry that mediates the vast array of behaviors displayed by complex organisms. Here, we used a multidisciplinary approach combining conditional gene deletion, gene expression analyses, electrophysiology and animal behavior to demonstrate that a single developmentally regulated transcription factor, Dbx1, specifies a restricted subset of neurons forming hypothalamic circuits that selectively control innate physiological and behavioral responses to stress. Collectively, our results suggest a model in which Dbx1-dependent processes link developmental genetic control of hypothalamic development with circuit function and innate behaviors (Figure 8).
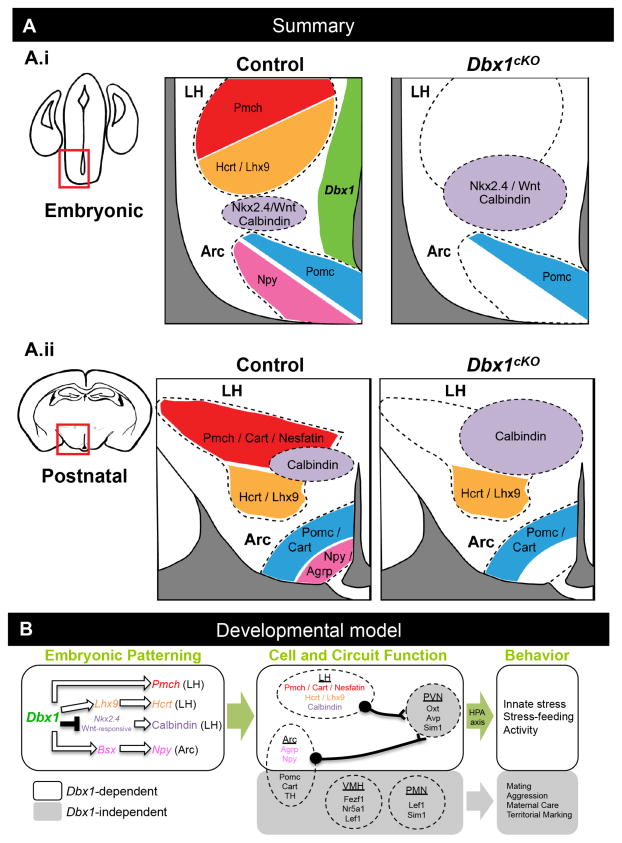
Figure 8. Summary and Model.
(A) Summary of findings in Dbx1 mutant embryonic and postnatal brains. Changes in gene expression are schematically shown at embryonic and (A.i) and postnatal stages (A.ii). Dbx1 expression is shown in the embryonic VZ (A.i. green). (B) Proposed model of Dbx1-dependent and Dbx1-independent specification of hypothalamic neurons, nuclei and behaviors (B.i) Dbx1 appears to function via repression of Nkx2.4 and positive genetic interaction with Lhx9 and Bsx to specify orexigenic neuropeptide (Pmch, Hcrt, Npy) expressing neurons. Dbx1 also negatively regulates the number of Wnt-responsive cells, either directly or via an intermediary such as Nkx2.4, resulting in regulation of the appropriate number of Calbindin+ neurons. White arrows indicate putative genetic interactions, but do not detail direct or indirect mechanisms. (B.ii) Defects in the specification of LH and Arc in Dbx1cKO are correlated with alterations in postnatal orexigenic peptide expression, neuronal function, and stress-feeding circuit function after fasting and predator odor exposure (white areas). Dbx1-independent pathways (grey areas) presumably act in parallel to specify anorexigenic peptide (Pomc/Cart) expressing neurons in the Arc, as well as other genes and innate behaviors associated with PVN, PMN and VMH hypothalamic nuclei. (B.iii) Behaviors altered in the adult Dbx1cKO mice are shown in white area while behaviors unaltered in adult Dbx1cKO mice are termed Dbx1-independent (grey area). Large green arrows indicate presumed link between Dbx1-dependent embryonic patterning, neuron and circuit function and behavior.
Dbx1 specifies orexigenic neurons
While the hypothalamus is less well studied than other regions of the neuraxis such as the spinal cord and cerebral cortex, a series of relatively recent genetic loss- and gain-of-function studies have identified some of the genes critical for development of diverse sets of hypothalamic neuronal subpopulations. Collectively, these studies have revealed that in a manner similar to those seen in other regions of the nervous system, both developmentally regulated extrinsic (e.g. Shh, BMPs, Wnts) and intrinsic (e.g. bHLH and homeodomain-containing transcription factors such as Lhx2, Rax, Nkx2.1, Mash1 and Otp) factors are essential for either regional patterning of the hypothalamus or specification of neuronal subpopulations (Caqueret et al., 2006; McNay et al., 2006; Lu et al., 2013; Peng et al., 2012; Shimogori et al, 2010; Szabó et al., 2009; Wang et al., 2012; Wolf and Ryu, 2013; reviewed in Hoch et al., 2009; Kaji and Nonogaki, 2013; Lee and Blackshaw, 2014; Salvatierra et al., 2014). Here, we find that Dbx1 is required for the specification of orexigenic Npy+/Agrp+ neurons in the Arc and Pmch+ and Hcrt+ output neurons in the LH. This selective function for Dbx1 in the hypothalamus is reminiscent of its role in the specification of select subsets of neurons in the spinal cord, hindbrain and midbrain. In each of these areas, Dbx1 is required for the expression of cohorts of specific effector genes, which work together in a region-specific manner to direct the formation of either V0 spinal cord interneurons, hindbrain Pre-Bötzinger complex neurons, or midbrain commissural neurons (Bouvier et al., 2010; Gray et al., 2010; Inamata and Shirasaki, 2014; Pierani et al., 2001). Our results further suggest a molecular mechanism by which Dbx1 may act to specify orexigenic hypothalamic neuronal subgroups in the Arc and LH via regulation of select embryonic effector genes (i.e., Bsx, Nkx2.4 and Lhx9).
The Arc complex contains molecularly and functionally distinct neuronal populations operating within the feeding and stress neural circuitry (Maniam and Morris, 2012; Atasoy et al., 2012; Betley et al., 2013; Sohn et al., 2013; Sternson et al., 2013). Previous studies have revealed that development of the Npy+/Agrp+ neurons requires the action of the homeodomain-containing gene Bsx (Sakkou et al., 2007). We find a reduction of Bsx expression in Dbx1 mutant embryos that correlates with the reduction in Npy/Agrp expression. This reveals a genetic interaction between Dbx1 and Bsx as part of a combinatorial transcriptional cascade specifying Arc orexigenic neurons. However, whether this is direct transcriptional control remains unknown, and will prove interesting to explore. In contrast, we find that development of the anorexigenic Pomc+ and TH+ neuronal populations is Dbx1-independent.
In the LH, the two major projection neuron populations are the Pmch+ and Hcrt+ neurons. With regard to the Pmch+ population, we find a dramatic reduction of Pmch, Cart and Nesfatin expression in the postnatal Dbx1cKO LH. Since Cart and Nesfatin are co-expressed with Pmch (Croizier et al., 2010; Elias et al., 2001; Fort et al., 2008), the most parsimonious explanation is that this population is almost completely absent in the postnatal LH. Moreover, this loss correlates with an expansion of the Calbindin+ population in the Dbx1 mutant LH. These changes are also linked to an expansion of embryonic expression of the homeodomain-containing gene Nkx2.4 and an increase in the number of Wnt-responsive cells that co-label with Calbindin. This suggests that Dbx1 is required for the specification of the progenitor pool that generates the Pmch+ population possibly via repression of Nkx2.4. This positive and negative regulation by Dbx1 is similar to its function in the spinal cord where it acts to positively regulate V0 genetic programs and to repress genetic programs required for the development of V1 neurons derived from the adjacent progenitor domain (Pierani et al., 2001).
Although we have yet to understand the full cohort of genes that are directly and indirectly regulated by Dbx1, our data show that Dbx1 is required high in the hierarchy of genes that control development of orexigenic neuronal subpopulations (Figure 8).
Dbx1 is required for stress-feeding circuit function
Altered specification of the Agrp+/Npy+, Pmch+ and Hcrt+ neurons in the Arc and LH would be expected to lead to a dysfunctional circuit. Here we demonstrate by a number of criteria that Dbx1 is required for proper circuit function in the postnatal hypothalamus. Unlike most other neurons, a known function of Pmch+ and Hcrt+ neurons is to respond to high and low glucose levels, respectively (Burdakov et al, 2005a; Burdakov et al, 2005b). A notable decrease in the electrophysiological response to high glucose was observed in the Dbx1cKO LH, demonstrating an impaired function of the Pmch+ population, most likely due to the reduced Pmch+ population. Likewise, the postnatal Dbx1cKO LH also fails to respond properly to low glucose; therefore, the Hcrt+ population remains electrophysiologically dysfunctional despite the postnatal recovery of Hcrt and Lhx9 expression.
The LH, Arc and paraventricular nucleus (PVN) form a circuit that regulates normal responses to a variety of stressors. In response to stressors, including predator stress and fasting, the PVN stimulates peripheral Cort release as part of the hypothalamic-pituitary-adrenal (HPA) axis (Herman et al., 1996; Takahashi, 2014). LH neuronal activation is also part of the hypothalamic response to predator-odor stressors (Canteras et al., 1997; Beijamini and Guimarães, 2006), and the LH sends projections directly to the PVN (Larsen et al., 1994; Figure S6A–E.ii). Moreover, Pmch signaling in the LH acts as an anxiogen to facilitate the stress response, including Cort release (Borowsky et al., 2002; Smith et al., 2006). Thus in Dbx1 mutants, misspecification and dysfunction of the Pmch+ and Hcrt+ populations, both of which comprise projection neurons, can manifest as an impairment in HPA axis activation. The PVN is also a major projection target of Npy+/Agrp+ neurons (Atasoy et al., 2012; Betley et al., 2013; and Figure S4C.i), which we show to be under Dbx1 regulatory control. In Dbx1cKO mice, the reduced Npy+/Agrp+ population produces fewer Agrp+ projections to all known targets, including LH and PVN. Moreover, previous studies have revealed links between stressors, Agrp expression and HPA axis activation (Maniam and Morris, 2012; Vieau et al., 2007). In addition to revealing a function for Dbx1 in specification of Arc and LH populations and in producing normal responses to glucose, we demonstrate that c-Fos activation in the Arc, LH and PVN of Dbx1cKO mice is impaired in response to predator odor and fasting, two well characterized stressors that engage the HPA axis. Correlating with this finding is the lack of normal Cort release in Dbx1cKO mice after exposure to the predator odor stressor. Together, these data suggest that Dbx1 is required for normal development and function of the HPA axis and provides an important link between the molecular and circuit deficits.
In addition to the PVN, two other major nuclei regulating predator-stress responses are the VMH and PMN (Gross and Canteras, 2012; Sokolowski and Corbin, 2013). Although we cannot rule out subtle defects in neuronal specification within the PVN, VMH, or PMN, gross patterning of these nuclei as assessed by multiple markers appears unaffected in Dbx1 mutants. Most significantly, we find normal expression of Nr5a1, a marker of neurons that comprise the VMH innate fear circuit (Silva et al., 2013). This is consistent with the notion that the deficit in the stress-feeding circuit arises from defects in the LH and Arc. Thus, the observed decreases in Agrp+ projections to the PVN, combined with defects in LH function, provide the most direct explanation for the altered physiological responses to food deprivation and predator odor stressors (Figure 8). Despite being transiently expressed only during the progenitor stage within the hypothalamic primordium, Dbx1 appears to set in motion a genetic program whose disruption carries significant consequences for later circuit function. However, whether this is through direct consequence of mis-specification of orexigenic neurons or by other secondary compensatory mechanisms is still unknown.
Dbx1 is required for stress-feeding behaviors
Considering the known roles of the Npy+/Agrp+, Pmch+ and Hcrt+ populations in regulating feeding behavior, we anticipated a decrease in feeding in Dbx1cKO mutants. However, under normal feeding conditions, we observed a subtle decrease in weight only in male mutants. This decrease was not correlated to changes in food intake or metabolism, but instead was associated with hyperactivity. However, the precise cause of the decrease in weight remains unclear and may be due to a combination of activity and subtle metabolic changes undetected in our assays. Nevertheless, the relatively mild decrease in body weight observed in male Dbx1cKO mice is generally consistent with studies uncovering differences in the severity of phenotypes between embryonic versus postnatal manipulations of feeding systems. For example, developmental ablation of Agrp+/Npy+ or Pmch+ neurons or embryonic deletion of the genes encoding Agrp, Npy, or Pmch has mild to no effect on adult feeding (Luquet et al., 2005; Shimada et al., 1998; Erickson et al., 1996a; Erickson et al., 1996b; Qian et al., 2002; Palmiter et al., 1998). In contrast, adult neuronal ablation leads to dramatic effects on feeding (Luquet et al., 2005; Whiddon and Palmiter, 2013; Gropp et al., 2005).
Interestingly, in response to restricted access to food or fasting, we observed a deficit in food consumption in female Dbx1cKO mice. This suggests that the Dbx1-dependent developmental defect in neuronal specification manifests as a feeding deficit only under stress-related feeding conditions, such as limited food sources, and in a sexually dimorphic manner. The apparent critical role for Dbx1 in specific stress pathways is most strongly supported by the striking finding of a profound deficit in predator odor avoidance in Dbx1cKO mice. This dramatically correlates with a lack of elevated plasma levels of Cort, suggesting that the Dbx1cKO hypothalamus is deficient in its ability to process aversive information imparted by predator odor. These select behavioral deficits are consistent with the above mentioned molecular and circuit deficits and suggests a model which links Dbx1-dependent deficits at molecular, circuit and behavioral levels (Figure 8).
In summary, although the precise mechanistic function of Dbx1 in executing hypothalamic developmental programs remains to be elucidated, our findings reveal a common requirement for Dbx1 in establishing circuits that regulate innate responses to select stressors. This appears to occur via requirement for this transcription factor in specification of hypothalamic neuronal subpopulations critical for normal HPA axis function.
EXPERIMENTAL PROCEDURES
Animals
Conditional-knockout mice (Dbx1cKO: Nkx2.1Cre+/−;Dbx1c/−) and controls (Ctrl: Nkx2.1Cre+/−; Dbx1c/+) were obtained by crossing Nkx2.1Cre+/−; Dbx1+/− males with Dbx1flox/flox females. Dbx1 knockout mice (KO: Dbx1−/−) and controls (WT/Het: Dbx1+/+ and Dbx1+/−) were obtained by crossing male and female Dbx1+/− mice. See Supplemental Experimental Procedures for more details.
Gene Expression Profiling
RNA was isolated from E13.5 hypothalamic primordium or 3–4 month old hypothalamic tissue and analyzed using Illumina® (San Diego, CA) Gene Expression BeadChip Array technology. See Supplemental Experimental Procedures for more details.
Histology
We performed ISH and IHC on serial coronal sections spanning the rostro-caudal extent of the hypothalamus. All comparable sections (e.g. Ctrl vs. Dbx1cKO) were on the same slide and treated/imaged under the same conditions. See Supplemental Experimental Procedures for more details.
Multiple Electrode Array Electrophysiology
300 μM P17–20 brain slices (bregma −2.06 to −2.30 mm) were transfered to a 60 channel MEA. The chamber was maintained at 30°C under continuous perfusion (2 ml/min) of oxygenated aCSF for 1 hr prior to data acquisition. A 15-min recording of spontaneous spiking activity of each slice was acquired. The superfusion solution was then switched to a high-glucose (5.0 mM) aCSF solution, and a 1 hr recording was obtained. See Supplemental Experimental Procedures for more details.
Glucose Challenge
Tail vein blood was used to determine blood glucose levels (BG) with a glucometer (Accu-Chek Compact Plus, Roche Diagnostics, IN). BG was determined at time 0 before an ip injection of glucose (2 mg/kg) and then 15, 30, 45 and 120 min afterwards. Mice were then fasted 12 hr and BG was taken again.
Behavior Assays
All non-feeding behavior assays were video-recorded and scored independently by two investigators blind to genotype using the Scorevideo program for MatLab (Wu et al., 2009). Unless otherwise stated, all animals were group-housed by sex after weaning and then singly housed and habituated to the behavioral assay 3 days prior to experiment, which took place >1 hr after the beginning of the dark cycle. See Supplemental Experimental Procedures for more details.
ELISA
Serum from blood was collected 1 hr after first exposure to bedding (benign or rat). Samples were run in duplicate using corticosterone ELISA kits (Abcam ab108821) per manufacturer’s recommendations. See Supplemental Experimental Procedures for more details.
Statistical evaluation
Quantitation of data was performed blind to relevant variables, including genotype and exposure group. Using GraphPad Prism 6 statistical software, a Repeated Measure or One-way ANOVA followed by Tukey–Kramer multiple comparison test was used for analysis of experiments involving three groups or more (Figures 5B, C, G, H, I; 6A, B; 7; S6I, J, K, L, M, N), and an unpaired t-test with Welch’s correction was used for analysis of experiments involving two groups (Figures 2–4; 5G.i, H.i, I.i; 6D, F, H, J, K–P; S2–S5; S6I.i, J.i, K.i, L.i, M.i, N.i; S7–S8).
Supplementary Material
Highlights.
Dbx1 is widely expressed in the hypothalamic primordium
Dbx1 specifies select neuronal subpopulations in the Arc and LH hypothalamic nuclei
Pmch-, Hcrt-, Npy- and Agrp-expressing orexigenic neurons require Dbx1 function
Dbx1 regulates innate stress responses but not other innate behaviors
Acknowledgments
We thank N. Betley, V. Gallo, S. Lima, B. Martin, L. Oboti, J. Triplett, I. Zohn and M. Texada for constructive input and/or critical reading of the manuscript. We thank members of the Corbin and Triplett labs for input during the course of this study and N. Swarup for technical assistance. We thank S. Blackshaw for his insightful input on hypothalamic development. We thank S. Anderson for Nkx2.1cre mice and S. Blackshaw, K. Campbell, C.M. Fan, P. Gray, K. Hashimoto-Torii, M. Hayhurst, O. Marin and Y. Nakagawa for ISH probes. We thank the CNMC Transgenic Core for generation of conditional-knockout mice and the UPenn Mouse Phenotyping, Physiology and Metabolism Core (supported by P30DK19525). This work was partially supported by NIH grants RO1DC12050, RO1NIDA020140, RO1NIDA020140S1, RO1NIDA020140S2 and ARRA R01DA020140-S1 (J.G.C.). N.M.S. is supported by R01NS049488, R01NS083872 and the Ellison Medical Foundation. K.S. is supported by F32DA035754. S.E. was the Uehara Memorial Foundation, Takeda science foundation and JSPS Institutional Program for Young Researcher Overseas Visits, S-C.B. and J.M. were supported by the NIDA summer research program to promote diversity (RO1020140S2). The CNMC IDDRC Imaging Core microscopy is funded by NIH IDDRC P30HD040677.
Footnotes
AUTHOR CONTRIBUTIONS
K.S. conceived, executed, analyzed, interpreted the majority of behavioral and gene expression experiments, scored all behavioral videos, oversaw the genomic screen, imaged and analyzed the viral tracing experiment with L.O., supervised T.T, A.L., M.Z and J.M., and co-wrote the manuscript with J.G.C. S.E. conceived and executed a subset of behavioral and gene expression experiments, generated ISH probes, oversaw the genomic screen and supervised Y.K. during the course of this study. T.H. generated the Dbx1cKO mice. Y.K. carried out a subset of behavioral and gene expression experiments. T.T. carried out a subset of gene expression studies and scored a subset of behavioral videos. A.L., J. M. and M.Z. performed a subset of ISH experiments. L.O. conducted the viral tracing study with K.S. S.-C.B. performed the MEA electrophysiology experiments under the supervision of K.J. S.G. and S.K performed the microarray screen and bioinformatics. A.P. provided Dbx1cre mice and technical input. K.J. designed, executed, oversaw and analyzed the electrophysiology experiments. N.T. co-mentored S.E. during the course of this study. N.M.S provided input and training on execution, analysis and interpretation of behavioral experiments and provided intellectual input. J.G.C. originated, conceived coordinated and oversaw all aspects of the design, implementation and interpretation of the project and co-wrote the manuscript with K.S.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Alvarez-Bolado G, Paul FA, Blaess S. Sonic hedgehog lineage in the mouse hypothalamus: from progenitor domains to hypothalamic regions. Neural Dev. 2012;7:4. doi: 10.1186/1749-8104-7-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Apfelbach R, Blanchard CD, Blanchard RJ, Hayes RA, McGregor IS. The effects of predator odors in mammalian prey species: a review of field and laboratory studies. Neurosci Biobehav Rev. 2005;29:1123–44. doi: 10.1016/j.neubiorev.2005.05.005. [DOI] [PubMed] [Google Scholar]
- Atasoy D, Betley JN, Su HH, Sternson SM. Deconstruction of a neural circuit for hunger. Nature. 2012;488:172–7. doi: 10.1038/nature11270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailey KR, Crawley JN. Chapter 5: Anxiety-Related Behaviors in Mice. In: Buccafusco JJ, editor. Methods of Behavior Analysis in Neuroscience. 2. Boca Raton, FL: 2009. [Google Scholar]
- Beijamini V, Guimarães FS. c-Fos expression increase in NADPH-diaphorase positive neurons after exposure to a live cat. Behav Brain Res. 2006;170:52–61. doi: 10.1016/j.bbr.2006.01.025. [DOI] [PubMed] [Google Scholar]
- Betley JN, Cao ZF, Ritola KD, Sternson SM. Parallel, redundant circuit organization for homeostatic control of feeding behavior. Cell. 2013;155:1337–50. doi: 10.1016/j.cell.2013.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanchard DC, Blanchard RJ, Griebel G. Defensive responses to predator threat in the rat and mouse. Curr Protoc Neurosci. 2005;Chapter 8:Unit 8.19. doi: 10.1002/0471142301.ns0819s30. [DOI] [PubMed] [Google Scholar]
- Borowsky B, Durkin MM, Ogozalek K, Marzabadi MR, DeLeon J, Lagu B, Heurich R, Lichtblau H, Shaposhnik Z, Daniewska I, Blackburn TP, Branchek TA, Gerald C, Vaysse PJ, Forray C. Antidepressant, anxiolytic and anorectic effects of a melanin-concentrating hormone-1 receptor antagonist. Nat Med. 2002;8:825–30. doi: 10.1038/nm741. [DOI] [PubMed] [Google Scholar]
- Bouvier J, Thoby-Brisson M, Renier N, Dubreuil V, Ericson J, Champagnat J, Pierani A, Chédotal A, Fortin G. Hindbrain interneurons and axon guidance signaling critical for breathing. Nat Neurosci. 2010;13:1066–74. doi: 10.1038/nn.2622. [DOI] [PubMed] [Google Scholar]
- Briscoe J, Pierani A, Jessell TM, Ericson J. A homeodomain protein code specifies progenitor cell identity and neuronal fate in the ventral neural tube. Cell. 2000;101:435–45. doi: 10.1016/s0092-8674(00)80853-3. [DOI] [PubMed] [Google Scholar]
- Broberger C. Hypothalamic cocaine- and amphetamine-regulated transcript (CART) neurons: histochemical relationship to thyrotropin-releasing hormone, melanin-concentrating hormone, orexin/hypocretin and neuropeptide Y. Brain Res. 1999;848:101–13. doi: 10.1016/s0006-8993(99)01977-0. [DOI] [PubMed] [Google Scholar]
- Burdakov D, Alexopoulos H. Metabolic state signalling through central hypocretin/orexin neurons. J Cell Mol Med. 2005;9:795–803. doi: 10.1111/j.1582-4934.2005.tb00380.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burdakov D, Gerasimenko O, Verkhratsky A. Physiological changes in glucose differentially modulate the excitability of hypothalamic melanin-concentrating hormone and orexin neurons in situ. J Neurosci. 2005a;25:2429–33. doi: 10.1523/JNEUROSCI.4925-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burdakov D, Luckman SM, Verkhratsky A. Glucose-sensing neurons of the hypothalamus. Philos Trans R Soc Lond B Biol Sci. 2005b;360:2227–35. doi: 10.1098/rstb.2005.1763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canteras NS, Chiavegatto S, Ribeiro do Valle LE, Swanson LW. Severe reduction of rat defensive behavior to a predator by discrete hypothalamic chemical lesions. Brain Res Bull. 1997;44:297–305. doi: 10.1016/s0361-9230(97)00141-x. [DOI] [PubMed] [Google Scholar]
- Caqueret A, Boucher F, Michaud JL. Laminar organization of the early developing anterior hypothalamus. Dev Biol. 2006;298:95–106. doi: 10.1016/j.ydbio.2006.06.019. [DOI] [PubMed] [Google Scholar]
- Causeret F, Ensini M, Teissier A, Kessaris N, Richardson WD, Lucas de Couville T, Pierani A. Dbx1-expressing cells are necessary for the survival of the mammalian anterior neural and craniofacial structures. PLoS One. 2011;6:e19367. doi: 10.1371/journal.pone.0019367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan-Palay V, Záborszky L, Köhler C, Goldstein M, Palay SL. Distribution of tyrosine-hydroxylase-immunoreactive neurons in the hypothalamus of rats. J Comp Neurol. 1984;227:467–96. doi: 10.1002/cne.902270403. [DOI] [PubMed] [Google Scholar]
- Croizier S, Franchi-Bernard G, Colard C, Poncet F, La Roche A, Risold PY. A comparative analysis shows morphofunctional differences between the rat and mouse melanin-concentrating hormone systems. PLoS One. 2010;5:e15471. doi: 10.1371/journal.pone.0015471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalal J, Roh JH, Maloney SE, Akuffo A, Shah S, Yuan H, Wamsley B, Jones WB, de Strong CG, Gray PA, Holtzman DM, Heintz N, Dougherty JD. Translational profiling of hypocretin neurons identifies candidate molecules for sleep regulation. Genes Dev. 2013;27:565–78. doi: 10.1101/gad.207654.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dasen JS, Jessell TM. Hox networks and the origins of motor neuron diversity. Curr Top Dev Biol. 2009;88:169–200. doi: 10.1016/S0070-2153(09)88006-X. [DOI] [PubMed] [Google Scholar]
- Domingos AI, Sordillo A, Dietrich MO, Liu ZW, Tellez LA, Vaynshteyn J, Ferreira JG, Ekstrand MI, Horvath TL, de Araujo IE, Friedman JM. Hypothalamic melanin concentrating hormone neurons communicate the nutrient value of sugar. Elife. 2013;2:e01462. doi: 10.7554/eLife.01462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dulac C, Wagner S. Genetic analysis of brain circuits underlying pheromone signaling. Annu Rev Genet. 2006;40:449–67. doi: 10.1146/annurev.genet.39.073003.093937. [DOI] [PubMed] [Google Scholar]
- Elias CF, Lee CE, Kelly JF, Ahima RS, Kuhar M, Saper CB, Elmquist JK. Characterization of CART neurons in the rat and human hypothalamus. J Comp Neurol. 2001;432:1–19. doi: 10.1002/cne.1085. [DOI] [PubMed] [Google Scholar]
- Efendić S, Cerasi E, Luft R, Gladnikoff G. Potentiation of glucose-induced insulin release by glucose in the isolated pancreas of fed and fasted rats. Diabetes. 1976;25:949–54. doi: 10.2337/diab.25.10.949. [DOI] [PubMed] [Google Scholar]
- Erickson JC, Clegg KE, Palmiter RD. Sensitivity to leptin and susceptibility to seizures of mice lacking neuropeptide Y. Nature. 1996a;381:415–421. doi: 10.1038/381415a0. [DOI] [PubMed] [Google Scholar]
- Erickson JC, Hollopeter G, Palmiter RD. Attenuation of the obesity syndrome of ob/ob mice by the loss of neuropeptide Y. Science. 1996b;274:1704–1707. doi: 10.1126/science.274.5293.1704. [DOI] [PubMed] [Google Scholar]
- Flames N, Pla R, Gelman DM, Rubenstein JL, Puelles L, Marín O. Delineation of multiple subpallial progenitor domains by the combinatorial expression of transcriptional codes. J Neurosci. 2007;27:9682–95. doi: 10.1523/JNEUROSCI.2750-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flames N, Hobert O. Gene regulatory logic of dopamine neuron differentiation. Nature. 2009;458:885–9. doi: 10.1038/nature07929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fort P, Salvert D, Hanriot L, Jego S, Shimizu H, Hashimoto K, Mori M, Luppi PH. The satiety molecule nesfatin-1 is co-expressed with melanin concentrating hormone in tuberal hypothalamic neurons of the rat. Neuroscience. 2008;155:174–81. doi: 10.1016/j.neuroscience.2008.05.035. [DOI] [PubMed] [Google Scholar]
- Gradinaru V, Zhang F, Ramakrishnan C, Mattis J, Prakash R, Diester I, Goshen I, Thompson KR, Deisseroth K. Molecular and cellular approaches for diversifying and extending optogenetics. Cell. 2010;141:154–65. doi: 10.1016/j.cell.2010.02.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gropp E, Shanabrough M, Borok E, Xu AW, Janoschek R, Buch T, Plum L, Balthasar N, Hampel B, Waisman A, Barsh GS, Horvath TL, Brüning JC. Agouti-related peptide-expressing neurons are mandatory for feeding. Nat Neurosci. 2005;8:1289–1291. doi: 10.1038/nn1548. [DOI] [PubMed] [Google Scholar]
- Goshu E, Jin H, Lovejoy J, Marion JF, Michaud JL, Fan CM. Sim2 contributes to neuroendocrine hormone gene expression in the anterior hypothalamus. Mol Endocrinol. 2004;18:1251–62. doi: 10.1210/me.2003-0372. [DOI] [PubMed] [Google Scholar]
- Gray PA, Hayes JA, Ling GY, Llona I, Tupal S, Picardo MC, Ross SE, Hirata T, Corbin JG, Eugenín J, Del Negro CA. Developmental origin of preBötzinger complex respiratory neurons. J Neurosci. 2010;30:14883–95. doi: 10.1523/JNEUROSCI.4031-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gross CT, Canteras NS. The many paths to fear. Nat Rev Neurosci. 2012;13:651–8. doi: 10.1038/nrn3301. [DOI] [PubMed] [Google Scholar]
- Hébert JM, Fishell G. The genetics of early telencephalon patterning: some assembly required. Nat Rev Neurosci. 2008;9:678–85. doi: 10.1038/nrn2463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herman JP, Prewitt CM, Cullinan WE. Neuronal circuit regulation of the hypothalamo-pituitary-adrenocortical stress axis. Crit Rev Neurobiol. 1996;10:371–94. doi: 10.1615/critrevneurobiol.v10.i3-4.50. [DOI] [PubMed] [Google Scholar]
- Hirata T, Li P, Lanuza GM, Cocas LA, Huntsman MM, Corbin JG. Identification of distinct telencephalic progenitor pools for neuronal diversity in the amygdala. Nat Neurosci. 2009;12:141–9. doi: 10.1038/nn.2241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoch RV, Rubenstein JL, Pleasure S. Genes and signaling events that establish regional patterning of the mammalian forebrain. Semin Cell Dev Biol. 2009;20:378–86. doi: 10.1016/j.semcdb.2009.02.005. [DOI] [PubMed] [Google Scholar]
- Horvath TL, Bechmann I, Naftolin F, Kalra SP, Leranth C. Heterogeneity in the neuropeptide Y-containing neurons of the rat arcuate nucleus: GABAergic and non-GABAergic subpopulations. Brain Res. 1997;756:283–6. doi: 10.1016/s0006-8993(97)00184-4. [DOI] [PubMed] [Google Scholar]
- Inamata Y, Shirasaki R. Dbx1 triggers crucial molecular programs required for midline crossing by midbrain commissural axons. Development. 2014;141:1260–71. doi: 10.1242/dev.102327. [DOI] [PubMed] [Google Scholar]
- Kaji T, Nonogaki K. Role of homeobox genes in the hypothalamic development and energy balance. Front Biosci (Landmark Ed) 2013;18:740–7. doi: 10.2741/4136. [DOI] [PubMed] [Google Scholar]
- Kurrasch DM, Cheung CC, Lee FY, Tran PV, Hata K, Ingraham HA. The neonatal ventromedial hypothalamus transcriptome reveals novel markers with spatially distinct patterning. J Neurosci. 2007;27:13624–34. doi: 10.1523/JNEUROSCI.2858-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larsen PJ, Hay-Schmidt A, Mikkelsen JD. Efferent connections from the lateral hypothalamic region and the lateral preoptic area to the hypothalamic paraventricular nucleus of the rat. J Comp Neurol. 1994;342:299–319. doi: 10.1002/cne.903420211. [DOI] [PubMed] [Google Scholar]
- Luquet S, Perez FA, Hnasko TS, Palmiter RD. NPY/AgRP neurons are essential for feeding in adult mice but can be ablated in neonates. Science. 2005;310:683–5. doi: 10.1126/science.1115524. [DOI] [PubMed] [Google Scholar]
- Lee DA, Blackshaw S. Feed your head: neurodevelopmental control of feeding and metabolism. Annu Rev Physiol. 2014;76:197–223. doi: 10.1146/annurev-physiol-021113-170347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin D, Boyle MP, Dollar P, Lee H, Lein ES, Perona P, Anderson DJ. Functional identification of an aggression locus in the mouse hypothalamus. Nature. 2011;470:221–6. doi: 10.1038/nature09736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu S, Bogarad LD, Murtha MT, Ruddle FH. Expression pattern of a murine homeobox gene, Dbx, displays extreme spatial restriction in embryonic forebrain and spinal cord. Proc Natl Acad Sci U S A. 1992;89:8053–7. doi: 10.1073/pnas.89.17.8053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu F, Kar D, Gruenig N, Zhang ZW, Cousins N, Rodgers HM, Swindell EC, Jamrich M, Schuurmans C, Mathers PH, Kurrasch DM. Rax is a selector gene for mediobasal hypothalamic cell types. J Neurosci. 2013;33:259–72. doi: 10.1523/JNEUROSCI.0913-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maniam J, Morris MJ. The link between stress and feeding behaviour. Neuropharmacology. 2012;63:97–110. doi: 10.1016/j.neuropharm.2012.04.017. [DOI] [PubMed] [Google Scholar]
- Manoli DS, Fan P, Fraser EJ, Shah NM. Neural control of sexually dimorphic behaviors. Curr Opin Neurobiol. 2013;23:330–8. doi: 10.1016/j.conb.2013.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maretto S, Cordenonsi M, Dupont S, Braghetta P, Broccoli V, Hassan AB, Volpin D, Bressan GM, Piccolo S. Mapping Wnt/beta-catenin signaling during mouse development and in colorectal tumors. Proc Natl Acad Sci U S A. 2003;100:3299–304. doi: 10.1073/pnas.0434590100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez RC, Carvalho-Netto EF, Amaral VC, Nunes-de-Souza RL, Canteras NS. Investigation of the hypothalamic defensive system in the mouse. Behav Brain Res. 2008;192:185–90. doi: 10.1016/j.bbr.2008.03.042. [DOI] [PubMed] [Google Scholar]
- McNay DE, Pelling M, Claxton S, Guillemot F, Ang SL. Mash1 is required for generic and subtype differentiation of hypothalamic neuroendocrine cells. Mol Endocrinol. 2006;20:1623–32. doi: 10.1210/me.2005-0518. [DOI] [PubMed] [Google Scholar]
- Nahon JL. The melanocortins and melanin-concentrating hormone in the central regulation of feeding behavior and energy homeostasis. C R Biol. 2006;329:623–38. doi: 10.1016/j.crvi.2006.03.021. [DOI] [PubMed] [Google Scholar]
- Palmiter RD, Erickson JC, Hollopeter G, Baraban SC, Schwartz MW. Life without neuropeptide Y. Recent Prog Horm Res. 1998;53:163–199. [PubMed] [Google Scholar]
- Peng CY, Mukhopadhyay A, Jarrett JC, Yoshikawa K, Kessler JA. BMP receptor 1A regulates development of hypothalamic circuits critical for feeding behavior. J Neurosci. 2012;32:17211–24. doi: 10.1523/JNEUROSCI.2484-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peyron C, Tighe DK, van den Pol AN, de Lecea L, Heller HC, Sutcliffe JG, Kilduff TS. Neurons containing hypocretin (orexin) project to multiple neuronal systems. J Neurosci. 1998;18:9996–10015. doi: 10.1523/JNEUROSCI.18-23-09996.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierani A, Moran-Rivard L, Sunshine MJ, Littman DR, Goulding M, Jessell TM. Control of interneuron fate in the developing spinal cord by the progenitor homeodomain protein Dbx1. Neuron. 2001;29:367–84. doi: 10.1016/s0896-6273(01)00212-4. [DOI] [PubMed] [Google Scholar]
- Qian S, Chen H, Weingarth D, Trumbauer ME, Novi DE, Guan X, Yu H, Shen Z, Feng Y, Frazier E, Chen A, Camacho RE, Shearman LP, Gopal-Truter S, MacNeil DJ, Van der Ploeg LHT, Marsh DJ. Neither Agouti-Related Protein nor Neuropeptide Y is critically required for the regulation of energy homeostasis in mice. Mol Cell Biol. 2002;22:5027–5035. doi: 10.1128/MCB.22.14.5027-5035.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakkou M, Wiedmer P, Anlag K, Hamm A, Seuntjens E, Ettwiller L, Tschöp MH, Treier M. A role for brain-specific homeobox factor Bsx in the control of hyperphagia and locomotory behavior. Cell Metab. 2007;5:450–63. doi: 10.1016/j.cmet.2007.05.007. [DOI] [PubMed] [Google Scholar]
- Salvatierra J, Lee DA, Zibetti C, Duran-Moreno M, Yoo S, Newman EA, Wang H, Bedont JL, de Melo J, Miranda-Angulo AL, Gil-Perotin S, Garcia-Verdugo JM, Blackshaw S. The LIM homeodomain factor Lhx2 is required for hypothalamic Tanycyte specification and differentiation. J Neurosci. 2014;34:16809–16820. doi: 10.1523/JNEUROSCI.1711-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimada M, Tritos NA, Lowell BB, Flier JS, Maratos-Flier E. Mice lacking melanin-concentrating hormone are hypophagic and lean. Nature. 1998;396:670–4. doi: 10.1038/25341. [DOI] [PubMed] [Google Scholar]
- Silva BA, Mattucci C, Krzywkowski P, Murana E, Illarionova A, Grinevich V, Canteras NS, Ragozzino D, Gross CT. Independent hypothalamic circuits for social and predator fear. Nat Neurosci. 2013;16:1731–3. doi: 10.1038/nn.3573. Epub 2013 Nov 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simerly RB. Wired on hormones: endocrine regulation of hypothalamic development. Curr Opin Neurobiol. 2005;15:81–5. doi: 10.1016/j.conb.2005.01.013. [DOI] [PubMed] [Google Scholar]
- Shimogori T, Lee DA, Miranda-Angulo A, Yang Y, Wang H, Jiang L, Yoshida AC, Kataoka A, Mashiko H, Avetisyan M, Qi L, Qian J, Blackshaw S. A genomic atlas of mouse hypothalamic development. Nat Neurosci. 2010;13:767–75. doi: 10.1038/nn.2545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shirasaki R, Pfaff SL. Transcriptional codes and the control of neuronal identity. Annu Rev Neurosci. 2002;25:251–81. doi: 10.1146/annurev.neuro.25.112701.142916. [DOI] [PubMed] [Google Scholar]
- Shoji H, Ito T, Wakamatsu Y, Hayasaka N, Ohsaki K, Oyanagi M, Kominami R, Kondoh H, Takahashi N. Regionalized expression of the Dbx family homeobox genes in the embryonic CNS of the mouse. Mech Dev. 1996;56:25–39. doi: 10.1016/0925-4773(96)00509-6. [DOI] [PubMed] [Google Scholar]
- Small EM, Vokes SA, Garriock RJ, Li D, Krieg PA. Developmental expression of the Xenopus Nkx2-1 and Nkx2-4 genes. Mech Dev. 2000 Sep;96(2):259–62. doi: 10.1016/s0925-4773(00)00400-7. [DOI] [PubMed] [Google Scholar]
- Smith DG, Davis RJ, Rorick-Kehn L, Morin M, Witkin JM, McKinzie DL, Nomikos GG, Gehlert DR. Melanin-concentrating hormone-1 receptor modulates neuroendocrine, behavioral, and corticolimbic neurochemical stress responses in mice. Neuropsychopharmacology. 2006;31:1135–45. doi: 10.1038/sj.npp.1300913. [DOI] [PubMed] [Google Scholar]
- Sohn JW, Elmquist JK, Williams KW. Neuronal circuits that regulate feeding behavior and metabolism. Trends Neurosci. 2013;36:504–12. doi: 10.1016/j.tins.2013.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sokolowski K, Corbin JG. Wired for behaviors: from development to function of innate limbic system circuitry. Front Mol Neurosci. 2012;5:55. doi: 10.3389/fnmol.2012.00055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sternson SM, Nicholas Betley J, Cao ZF. Neural circuits and motivational processes for hunger. Curr Opin Neurobiol. 2013;23:353–60. doi: 10.1016/j.conb.2013.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stowers L, Cameron P, Keller JA. Ominous odors: olfactory control of instinctive fear and aggression in mice. Curr Opin Neurobiol. 2013;23:339–45. doi: 10.1016/j.conb.2013.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szabó NE, Zhao T, Cankaya M, Theil T, Zhou X, Alvarez-Bolado G. Role of neuroepithelial Sonic hedgehog in hypothalamic patterning. J Neurosci. 2009;29:6989–7002. doi: 10.1523/JNEUROSCI.1089-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi LK. Olfactory systems and neural circuits that modulate predator odor fear. Front Behav Neurosci. 2014;8:72. doi: 10.3389/fnbeh.2014.00072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valenta T, Hausmann G, Basler K. The many faces and functions of β-catenin. EMBO J. 2012;31:2714–36. doi: 10.1038/emboj.2012.150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vieau D, Sebaai N, Léonhardt M, Dutriez-Casteloot I, Molendi-Coste O, Laborie C, Breton C, Deloof S, Lesage J. HPA axis programming by maternal undernutrition in the male rat offspring. Psychoneuroendocrinology. 2007;32(Suppl 1):S16–20. doi: 10.1016/j.psyneuen.2007.03.014. [DOI] [PubMed] [Google Scholar]
- Wagner CG, McMahon CD, Marks DL, Daniel JA, Steele B, Sartin JL. A role for agouti-related protein in appetite regulation in a species with continuous nutrient delivery. Neuroendocrinology. 2004;80:210–8. doi: 10.1159/000082735. [DOI] [PubMed] [Google Scholar]
- Wang X, Kopinke D, Lin J, McPherson AD, Duncan RN, Otsuna H, Moro E, Hoshijima K, Grunwald DJ, Argenton F, Chien CB, Murtaugh LC, Dorsky RI. Wnt signaling regulates postembryonic hypothalamic progenitor differentiation. Dev Cell. 2012;23:624–36. doi: 10.1016/j.devcel.2012.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whiddon BB, Palmiter RD. Ablation of neurons expressing melanin-concentrating hormone (MCH) in adult mice improves glucose tolerance independent of MCH signaling. J Neurosci. 2013;33:2009–16. doi: 10.1523/JNEUROSCI.3921-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams KW, Elmquist JK. From neuroanatomy to behavior: central integration of peripheral signals regulating feeding behavior. Nat Neurosci. 2012;15:1350–5. doi: 10.1038/nn.3217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf A, Ryu S. Specification of posterior hypothalamic neurons requires coordinated activities of Fezf2, Otp, Sim1a and Foxb1.2. Development. 2013;140:1762–73. doi: 10.1242/dev.085357. [DOI] [PubMed] [Google Scholar]
- Wonders CP, Anderson SA. The origin and specification of cortical interneurons. Nat Rev Neurosci. 2006;7:687–96. doi: 10.1038/nrn1954. [DOI] [PubMed] [Google Scholar]
- Wu Q, Lemus MB, Stark R, Bayliss JA, Reichenbach A, Lockie SH, Andrews ZB. The temporal pattern of c-Fos activation in hypothalamic, cortical, and brainstem nuclei in response to fasting and refeeding in male mice. Endocrinology. 2014;155:840–53. doi: 10.1210/en.2013-1831. [DOI] [PubMed] [Google Scholar]
- Xu X, Coats JK, Yang CF, Wang A, Ahmed OM, Alvarado M, Izumi T, Shah NM. Modular genetic control of sexually dimorphic behaviors. Cell. 2012;148:596–607. doi: 10.1016/j.cell.2011.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Q, Tam M, Anderson SA. Fate mapping Nkx2.1-lineage cells in the mouse telencephalon. J Comp Neurol. 2008;506:16–29. doi: 10.1002/cne.21529. [DOI] [PubMed] [Google Scholar]
- Yang CF, Chiang MC, Gray DC, Prabhakaran M, Alvarado M, Juntti SA, Unger EK, Wells JA, Shah NM. Sexually dimorphic neurons in the ventromedial hypothalamus govern mating in both sexes and aggression in males. Cell. 2013;153:896–909. doi: 10.1016/j.cell.2013.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang CF, Shah NM. Representing Sex in the Brain, One Module at a Time. Neuron. 2014;82:261–278. doi: 10.1016/j.neuron.2014.03.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yee CL, Wang Y, Anderson S, Ekker M, Rubenstein JL. Arcuate nucleus expression of NKX2.1 and DLX and lineages expressing these transcription factors in neuropeptide Y(+), proopiomelanocortin(+), and tyrosine hydroxylase(+) neurons in neonatal and adult mice. J Comp Neurol. 2009;517:37–50. doi: 10.1002/cne.22132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeo GS, Heisler LK. Unraveling the brain regulation of appetite: lessons from genetics. Nat Neurosci. 2012;15:1343–9. doi: 10.1038/nn.3211. [DOI] [PubMed] [Google Scholar]
- Zeltser LM, Seeley RJ, Tschöp MH. Synaptic plasticity in neuronal circuits regulating energy balance. Nat Neurosci. 2012;15:1336–42. doi: 10.1038/nn.3219. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.