Abstract
Fungal infections remain a threat due to the lack of broad spectrum fungal vaccines and protective antigens. Recent studies showed that attenuated Blastomyces dermatitidis confers protection via T cell recognition of an unknown, but conserved antigen. Using transgenic CD4+ T cells recognizing this antigen, we identify an amino acid determinant within the chaperone calnexin that is conserved across diverse fungal ascomycetes. Calnexin, typically an ER protein, also localizes to the surface of yeast, hyphae and spores. T cell epitope mapping unveiled a 13-residue sequence conserved across Ascomycota. Infection with divergent ascomycetes including dimorphic fungi, opportunistic molds, and the agent causing white nose syndrome in bats induces expansion of calnexin-specific CD4+ T cells. Vaccine delivery of calnexin in glucan particles induces fungal antigen-specific CD4+ T cell expansion and resistance to lethal challenge with multiple fungal pathogens. Thus, the immunogeneticity and conservation of calnexin make this fungal protein a promising vaccine target.
Keywords: Fungi, immunity, T cells, tetramers, vaccines
INTRODUCTION
Major killers such as poliomyelitis have been eradicated, but new pathogens are emerging. Fungi are one such group, which is linked partly to modern medical practices. Fungi, from yeasts colonizing the skin or mucosa, to molds from soil or water, are often harmless in the context of normal host responses. However, the success of cancer chemotherapy, as well as the AIDS pandemic, has led to immune deficiencies in a growing segment of the human population. Likewise, the routine use of intravenous catheters in hospitals provides a route of access for microbes that otherwise might not be able to infect human hosts. Candida is now among the leading agents of nosocomial blood stream infections (Pfaller et al., 2011). Infection with the mold Aspergillus is among the most feared complications in patients with hematological malignancies (Walsh et al., 2008). Over one million new cases per year of cryptococcosis are estimated worldwide in patients with AIDS, and over half those affected die of the infection (Park et al., 2009). Fungal infections have thus become an important cause of morbidity and mortality, and represent an increasing burden on the medical system. Effective ways to treat and prevent these infections are badly needed.
Vaccines have been hailed as one of the greatest achievements in public health during the past century. The global eradication of Smallpox virus in humans and Rinderpest virus in animals, and the near eradication or successful prevention of other viral or bacterial infections, for example meningitis in children due to Hemophilus influenze Type B, offer compelling examples. Yet, the development of safe and efficacious vaccines against fungi has been a major hurdle. This difficulty stems from the relative genetic complexity and intractability of fungi in the laboratory, limited knowledge of the mechanisms that underpin anti-fungal protective immunity, and a lack of defined antigen (Ag) candidates for vaccine protetion against fungal pathogens. To date, only two vaccines against fungi have moved into clinical trials (Cassone and Casadevall, 2012). An investigational candidate vaccine containing rAls3p-N (NDV-3), directed against Candida (and also S. aureus), has been tested for safety and immunogenicity in volunteers in a Phase I trial. Another candidate vaccine containing rSap2p was found to be tolerated and effective in inducing specific antibodies and B cell memory in women with recurrent vulvovaginitis in a European clinical trial (Edwards, 2012). Highly conserved Ags that are shared across fungal pathogens in a family or taxon would be preferable, but the only such component that has shown promise is β-glucan. Cassone et. al. (Torosantucci et al., 2005) reported that this shared cell wall component served as the basis for a glyco-conjugate vaccine against Candida and Aspergillus. This preparation has not yet moved into clinical trials, but β-glucan particles (GPs) could serve as an experimental platform for the delivery of candidate vaccines against fungi.
We described an effective live, attenuated vaccine against infection with Blastomyces dermatitidis (Wüthrich et al., 2000). This dimorphic fungus causes the systemic mycosis blastomycosis and exhibits genetic and morphological similarities to six related dimorphic fungi that cause human disease: Histoplasma capsulatum, Coccidioides posadasii and immitis, Penicillium marneffei, Sporothrix schenkii and Paracoccidiodes brasiliensis. The dimorphic fungi are in the fungal taxon Ascomycota, which includes diverse members such as A. fumigatus and also the white nose fungus, Pseudogymnoascus destructans, the cause of epidemic fatal disease spreading among bats across the U.S. Analysis of the attenuated vaccine against blastomycosis revealed that resistance is mediated by CD4+ T cells; cloning of the protective T cells disclosed the identity of the T cell receptor (TCR) and enabled the generation of a TCR (Tg) transgenic mouse, termed 1807. TCR Tg 1807 cells recognize and respond to all the dimorphic fungi of North America (Blastomyces, Histoplasma, Coccidiodes) and confer resistance against lethal experimental infection with each of them (Wüthrich et al., 2011a; Wüthrich et al., 2011b). These findings imply that the T cells recognize a conserved Ag in dimorphic fungi and perhaps fungal Ascomycetes.
Here, we sought to identify a conserved Ag in pathogenic fungi. We used broadly reactive, protective 1807 cells to probe for such an Ag. We report that calnexin, which is generally thought of as an intracellular resident of endoplasmic reticulum, is displayed on the fungal surface and represents the shared Ag of 1807 cells. We also describe that the calnexin epitope is highly conserved in the taxon Ascomycota. Finally, by using calnexin-peptide MHCII tetramers, we show that fungal display of this sequence across numerous ascomycetes induces the expansion of calnexin-specific CD4+ T cells that can be harnessed for vaccine immunity against multiple fungal pathogens.
RESULTS
Steps used to identify calnexin as the shared Ag
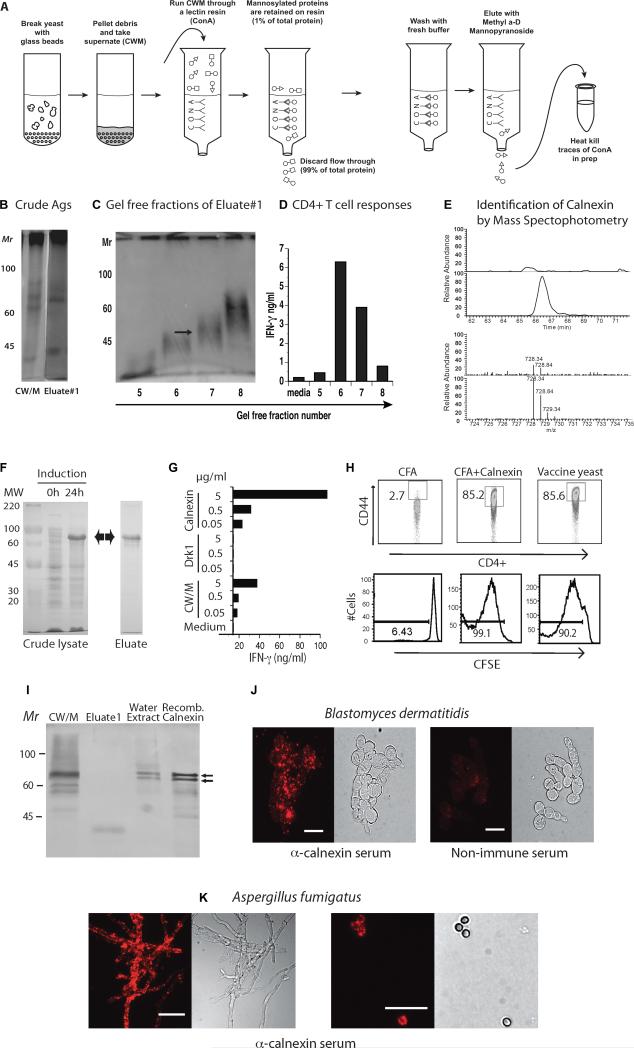
1807 TCR Tg cells recognize a protective Ag that is shared among systemic dimorphic fungi (Wüthrich et al., 2012; Wüthrich et al., 2011b). To identify the shared Ag, we prepared a cell wall membrane (CW/M) extract from B. dermatitidis vaccine yeast (Wüthrich et al., 2000). After running CW/M through a Con A column that retains mannosylated proteins, we collected Eluate 1, which contained 1 % of the protein present in the starting material (Fig. 1A). Trace Con A released from the column into Eluate #1 was heated to destroy its mitogenic activity (not shown). Eluate #1 (Fig. 1B) was fractioned in a gel free system to separate constituents by size (Fig. 1C). Fractions 6 and 7 stimulated 1807 T cells to produce IFN-γ whereas medium control and fractions 5 and 8 did not (Fig. 1D). To identify the T cell reactive Ag, we subjected fraction 7 to mass spec analysis. Proteins were identified by cross-referencing the mass of detected peptides against a database of the B. dermatitidis proteome. Proteins in non-stimulatory fractions and proteins diverging from the mass parameters of the gel-free fraction were discounted. This technique yielded a roster of five protein candidates potentially representing the shared Ag. Calnexin was one of these five proteins (Fig. 1E).
Fig. 1. Identity of shared fungal Ag.
A. Generation of eluate #1. B. Silver stain of PAGE of B. dermatitidis Ags. C. Gel free separation of Eluate #1 into fractions. D. Stimulation of 1807 TCR Tg cells in vitro by fractions from panel C, as measured by IFN-γ response. Arrow in fraction 7 denotes material analyzed by MS/MS. E. Identification of calnexin by MS/MS. The panel shows data collected for one calnexin-derived peptide, as an example. The top set of paired traces is a comparison of the HPLC separation of the non-stimulatory control fraction (upper) and the stimulatory fraction #7 (lower). The peak in fraction #7 is not present in the control. MS of this peak (bottom traces) identified the peptide: LQNSLNCGGAYMK [728.34Da; +2H] and this mass is better represented in stimulatory fraction #7 (lower) vs. non-stimulatory control (upper). Adjacent peaks are representative of isotopic variants. E. Induction of E. coli produced r-calnexin (63kD). F. r-calnexin stimulates 1807 T cells to produce IFN-γ in vitro. G. r-calnexin activates (CD44) and induces proliferation (CFSE) of transferred 1807 cells in vivo. H. Western of crude Ags. I&J. Surface stain of B. dermatitidis yeast (strain#55) and A. fumigatus hyphae (left) and spores (right) with anti-calnexin oligospecific antibody. Bar = 10 microns.
Proof that calnexin is the Ag
To investigate whether calnexin is the shared Ag that stimulates 1807 T cells, we cloned and expressed fungal calnexin in E. coli. 24 h after induction, the crude lysate from E. coli harbored an additional prominent band that migrated between 60 -70 kD, which corresponds with the predicted Mr of 63 kD for recombinant calnexin (r-calnexin) (Fig. 1F). We purified r-calnexin over a Ni-NTA column (Fig. 1F) and used the protein to stimulate 1807 cells in an in vitro co-culture system with bone marrow dendritic cells (BMDC). 1807 T cells produced IFN-γ in response to r-calnexin in a concentration-dependent manner. The response to r-calnexin exceeded that to CW/M extract, which also harbors calnexin at a lower concentration (Fig. 1G). In contrast, r-Drk1, a hybrid histidine kinase of B. dermatitidis (Nemecek et al., 2006) expressed and purified from E. coli as a control, did not induce IFN-γ production by 1807 T cells. Thus, r-calnexin (not LPS from E. coli) induced cytokine production by 1807 T cells specifically and in a concentration-dependent manner.
To assess whether r-calnexin induces activation and proliferation of 1807 cells in vivo, we transferred 1807 TCR Tg T cells into naïve wild-type recipient mice prior to vaccination. Similar to live B. dermatitidis vaccine yeast, r-calnexin emulsified in complete Freund's adjuvant (CFA) activated and stimulated proliferation of > 85% of the transferred 1807 cells (Fig. 1H), but adjuvant alone did not. These results identify calnexin as the shared Ag recognized by 1807 TCR Tg T cells, which confer immunity to multiple systemic dimorphic fungi (Wüthrich et al., 2012; Wüthrich et al., 2011b).
Evidence that calnexin is displayed on the surface of fungi
Among fungal pathogens, virulence factors and antigenic proteins are largely secreted or associated with the cell wall or surface. Despite the fact that calnexin is a molecular chaperone and folding sensor that regulates the transport of proteins from the ER to the Golgi (Ellgaard and Helenius, 2003), vaccination with B. dermatitidis yeast efficiently stimulates 1807 T cell responses in vivo. To address this unexpected finding, we investigated whether calnexin is displayed on the yeast surface. During our early search for the shared Ag, we found that a water-soluble extract of surface proteins from the vaccine strain yeast activated 1807 T cells (data not shown). Western analysis of the water-soluble extract detected a doublet that migrated on SDS-PAGE at the same position as r-calnexin produced by E. coli (Fig. 1I). To investigate whether B. dermatitidis vaccine yeast harbor calnexin on their surface, we stained yeast with polyclonal anti-calnexin antibody. Vaccine yeast stained positively with the anti-calnexin serum (Fig. 1J). The virulent parental strain 26199 used for the pulmonary challenge of mice also harbored calnexin on the yeast surface (data not shown). Since calnexin is shared among ascomycetes, we tested whether it is also expressed on the surface of Aspergillus fumigatus. Exposure of hyphae and spores to anti-calnexin antibody showed punctate surface staining and fluorescence (Fig. 1K). Thus, calnexin is detectable on the surface of B. dermatitidis yeast and A. fumigatus hyphae and spores.
Identification of T cell epitopes in calnexin
We aligned the amino acid (aa) sequence of calnexin from the fungal species that we have reported stimulate 1807 T cells in vivo (Wüthrich et al., 2011b), including B. dermatitidis, H. capsulatum, C. posadasii and P. brasiliensis. We investigated regions of sequence conservation that might represent the shared epitope for the 1807 T-cell receptor. We found that calnexin is highly conserved across the entire protein sequence among these dimorphic fungi (Fig. S1). Thus, the identification of highly conserved areas of the protein was not a sufficient measure to hone in on the 1807 epitope-containing sequence. To narrow the focus of possible peptides to test for 1807 reactivity, we subjected Blastomyces calnexin to two class II I-Ab restricted-epitope prediction algorithms (Methods and Fig. S1). The IEBD algorithm predicted six regions of overlapping peptide with binding affinity values (IC50) less than 500nM. In a second analysis, an algorithm developed by Marc Jenkins (unpublished data) refined the above analysis, predicting 10 strong H2-IAb epitopes in B.dermatitidis calnexin (Fig. S1). We synthesized peptides of 13 aa length, representing these 10 predicted epitopes (named peptide #1 to #10) and tested them to define the cognate epitope for the 1807 T-cell receptor.
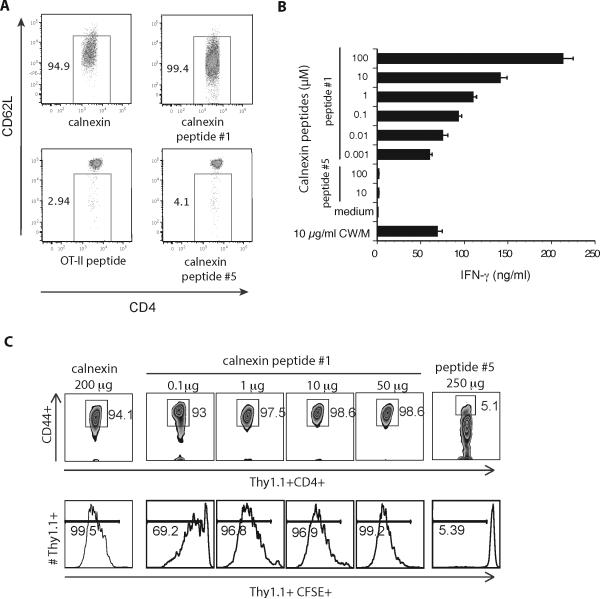
To test whether the synthetic peptides activate naïve 1807 T cells in vitro, we loaded BMDC with peptides and cultured them with 1807 cells. Peptide #1 comprised of the sequence LVVKNPAAHHAIS activated naïve 1807 T cells as measured by their reduced expression of CD62L (Fig. 2A) and increased expression of CD44 (data not shown). An irrelevant control, ovalbumin (OT-II) peptide, and all other synthetic calnexin peptides did not activate 1807 cells. Peptide #1 also stimulated the production of IFN-γ by 1807 cells in a concentration-dependent manner (Fig. 2B). As little as 1 to 10 nM of peptide #1 stimulated as much IFN-γ as 10 μg/ml of CW/M Ag, which has been shown to induce substantial amounts of the cytokine (data not shown). None of the other calnexin peptides induced IFN-γ production by 1807 cells. In vivo, 0.1 to 1 μg of peptide #1 was enough to activate and induce the proliferation of naïve 1807 T cells (Fig. 2C). Thus, peptide #1 is the T-cell epitope recognized by 1807 cells.
Fig. 2. Identification of calnexin's T cell epitope recognized by 1807 cells.
A. In vitro activation of 1807 T cells by calnexin peptide #1. 105 BMDC were loaded with calnexin (50μg/ml) or peptide (10μM) and co-cultured with 3 × 105 CD4+ purified 1807 T cells. 3d later T-cells were analyzed for activation by flow cytometry. B. Naïve 1807 T cells were co-cultured as in Panel A, and culture supernatants analyzed for IFN-γ. C. Mice received 106 naïve, CFSE-labeled 1807 cells prior to s.c. vaccination with 200 μg rcalnexin, a dilution series of peptide #1 and 250 μg peptide #5 (as a negative control). 7d later the skin draining lymph nodes were harvested and CFSE profiles and CD44-expression of 1807 cells analyzed.
Calnexin peptide #1 in fungi and activation of T cells in vivo
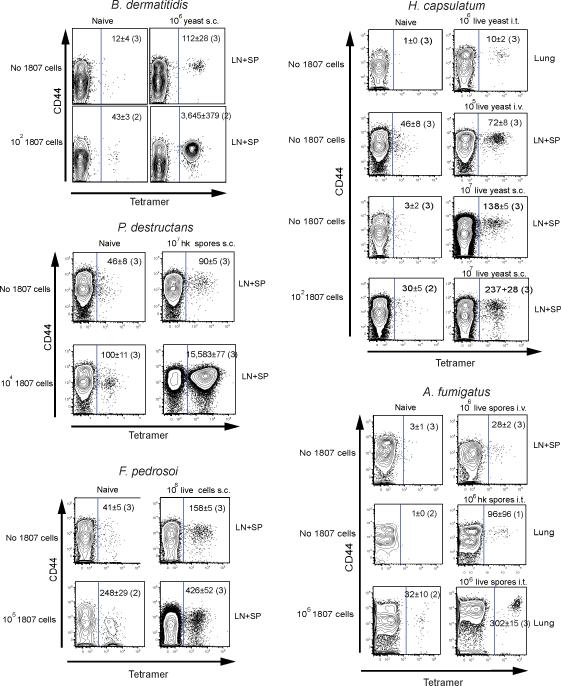
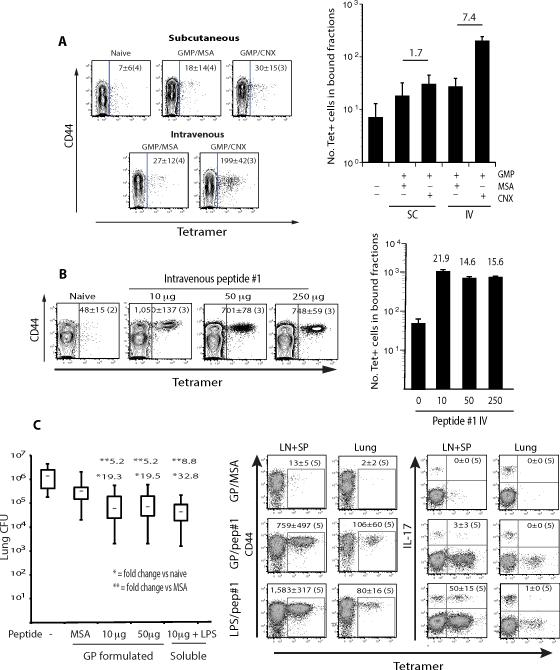
We analyzed conservation of the sequence of peptide #1 broadly throughout fungi. The 13 aa sequence is found in four phyla including Ascomycota, Basidiomycota, Chytridiomycota and Glomeromycota (Tables 1 and S1). The highest conservation of the peptide was found in ascomycetes. To investigate biological relevance, and test whether medically important fungi with conserved peptide #1 sequences trigger the expansion and activation of TCR Tg 1807 and endogenous, polyclonal, peptide #1-specific CD4+ T-cells in vivo, we transferred naïve 1807 T cells into mice before infection or vaccination with these fungi. One week later, we analyzed activation of 1807 and also endogenous Ag-specific CD4+ T-cells using a calnexin peptide-MHC class II tetramer. B. dermatitidis, A. fumigatus, H. capsulatum, C. posadasii, Fonsecaea pedrosoi causing chromoblastomycosis (da Gloria Sousa et al., 2011), and Pseudogymnoascus (Geomyces) destructans causing white nose syndrome and death in bats in the U.S. (Lorch et al., 2011) expanded and activated 1807 and tetramer positive CD4+ T cells in vivo (Figs. 3 and S2, and data not shown). Fungi that did not trigger expansion of tetramer positive CD4+ T cells included Candida albicans, Cryptococcus neoformans, and Pneumocystis jiroveci, none of which are ascomycetes. Naïve mice harbored 29 ± 10 tetramer positive CD4+ T cells per animal; hardly any tetramer positive CD8+ T-cells were detected in vaccinated mice (Fig. S2A). Thus, the tetramer recognizes and binds the T-cell receptor of calnexin peptide #1-specific CD4+ T-cells in a specific manner and can be used as a tool to monitor Ag-specific T cells in vivo in response to a number of pathogenic fungal ascomycetes.
Table 1.
The 9 amino acid core sequence of calnexin peptide#1 is conserved among fungal ascomycetes
| Peptide #1 residue position |
||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Genus/species/strain | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 1807 reactiveg |
||||
| Blastomyces dermatitidis a | L | V | V | K | N | P | A | A | H | H | A | I | S | + |
| Histoplasma capsulatum b | - | - | - | - | - | - | - | - | - | - | - | - | - | + |
| Paracoccidioides brasilliensis_Pb18 | - | - | I | - | - | A | - | - | - | - | - | - | - | |
| Paracoccidioides lutzii_ Pb01 | - | - | I | - | - | A | - | - | - | - | - | - | - | + |
| Coccidioides immitis._RS | - | - | - | - | - | A | - | - | - | - | - | - | - | |
| Coccidioides posadasii c | - | - | - | - | - | A | - | - | - | - | - | - | - | + |
| Penicillium marneffei | - | L | - | - | - | - | - | - | - | - | - | - | ||
| Penicillium chrysogenum | - | - | - | - | A | - | - | - | - | - | - | - | ||
| Aspergillus sp.1d | - | - | - | - | - | - | - | - | - | - | - | - | - | |
| Aspergillus sp.2e | - | - | - | - | - | V | - | - | - | - | - | - | - | + |
| Pseudogymnoascus destructans | - | - | - | - | - | A | - | - | - | - | - | - | - | + |
| Magnaportha orysae_70-15 | - | - | - | - | - | - | - | - | - | - | - | - | - | |
| Fonsecaea pedrosoi f | + | |||||||||||||
| Cladophiliaphora carrionii f | - | - | - | - | - | A | - | - | - | - | - | - | - | |
| Exophiala dermatitidis f | - | - | - | - | - | A | - | - | - | - | - | - | - | |
| Pneumocystis carinii_(rat form 1) | - | - | L | - | - | E | - | - | - | - | - | - | - | - |
| Neuroaspora crassa_OR74A | - | - | - | - | - | A | - | - | - | - | - | - | - | |
| Cryptococcus neoformans | - | - | L | - | T | K | - | - | - | - | - | - | - | - |
| Schizophullum commune_H4-8 | - | - | A | - | T | K | - | - | - | - | - | - | - | |
| Candida albicans_5314 | - | - | M | - | S | R | - | S | - | Y | - | - | - | - |
| Homo sapiens (Calnexin) | - | - | L | M | S | R | - | K | - | - | - | - | - | |
| Homo sapiens (Calmegin) | - | - | L | - | S | R | - | K | - | - | - | - | - | |
 identical to B. dermatitidis at all 13 peptide#1 amino acids
identical to B. dermatitidis at all 13 peptide#1 amino acids
B. dermatitidis strains: 26199, 18808, Er-3, 14081
H. capsulatum strains: G186AR, Nam1, H88, H143
C. posadasii strains: C35 Δ SOWgp, Silveira
Aspergillus species group.1: A. flavus, A., oryzae, A. terreus
Aspergillus species group 2: A. nidulans, A. kawachii, A. niger, A. fumigatus 293, A. clavatus
F. pedrosoi calnexin sequence is not available; Peptide#1 sequences of two related species from the family Herpotrichiellaceae are shown below F. pedrosoi.
The data for P. lutzii and C. posadasii were originally reported in Wüthrich et al., 2011b.
Fig. 3. Tetramer enrichment of endogenous, fungal-specific T cells ex vivo.
Mice received naïve 1807 T cells or not and were infected by doses and routes shown for B. dermatitidis yeast, F. pedrosoi spores, A. fumigatus spores, H. capsulatum yeast and P. destructans spores. 7d post-infection, the skin draining lymph nodes (LN), spleen (SP) or lungs were collected. The number of calnexin peptide #1-specific CD4+ T cells were analyzed and quantified after tetramer enrichment as detailed in the Methods. Tetramer positive cells are shown to the right of the gate in each dot plot. The number represents the geometric mean ± SEM of tetramer-positive cells, with number of mice studied in parenthesis.
The basis for variable expansion of peptide-specific T cells by fungi
We sought to explain the effect of calnexin peptide #1 variation in fungi. It is likely that the nonamer core for peptide #1 is VKNPAAHHA (Table 1). For the class II MHC, I-Ab, P1, 3, 4, 5, 7, 9 make contacts with I-Ab, and P2, 5, 7, and 8 are usually the most important TCR contacts, especially P5 (Nelson et al., 2014). Calnexin from C. immitis and Aspergillus can be detected by VKNPAAHHA:I-Ab-specific T cells because A or V at P4 are permissive for I-Ab binding and these peptides have the same TCR contact amino acids at P2, 5, 7, and 8 as calnexin from B. dermatitidis. Conversely, P. carinii may not be recognized because E at P4 is not permissive for I-Ab binding, and the peptide likely does not bind I-Ab. Calnexin from C. albicans is not recognized because R at P4 is not permissive for I-Ab binding, and thus, this peptide likely does not bind I-Ab. Candida also has a Y for H substitution at P8, which should make the peptide unrecognizable to VKNPAAHHA:I-Ab-specific T cells even if it does bind to I-Ab.
Response to calnexin in humans
In a pilot study, we assayed the CD4+ T cell response to calnexin in human subjects with either a history of confirmed infection due to dimorphic fungi or residence in an endemic area and laboratory evidence of prior infection (immune) vs. healthy subjects that lacked the above features (non-immune) (Fig. S3A-D). Five of six immune subjects responded to calnexin vs. one of four non-immune subjects. The response to calnexin in immune subjects was dose-dependent, similar to that for the immunodominant fungal Ag heat shock protein 60 (Hsp60) and not due to contaminating LPS.
Functions of calnexin specific T cell responses
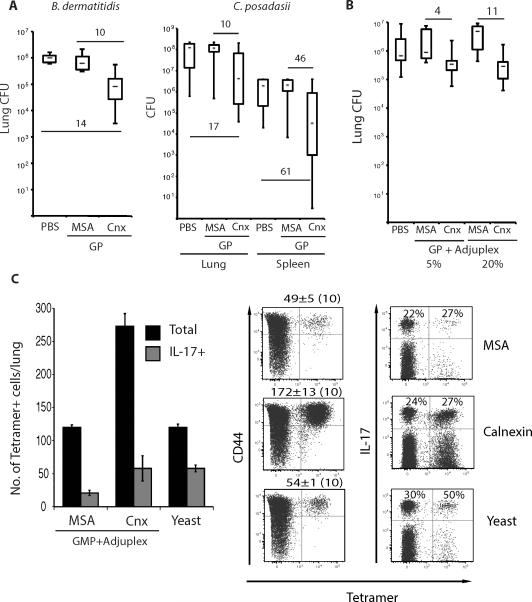
To test whether vaccination with calnexin induces protective immunity against lethal, pulmonary fungal infection, we immunized mice with r-calnexin. We investigated selected adjuvants empirically such as glucan particles (GP) to promote type 17 immunity and adjuplex, type 1 immunity. Vaccination with calnexin formulated in GP or adjuplex reduced lung and spleen CFU ≥ 10-fold vs. control mice after infection with B. dermatitidis or C. posadasii (Fig. 4A+B); reduced lung CFU correlated with prolonged survival (Fig. S4A). Vaccination with calnexin lead to increased numbers of tetramer-positive cells recruited to the lung at day 4 post-infection (Fig. 4C). Of the CD44hi CD4+ T cells recruited to lung after fungal challenge of Blastomyces yeast-vaccinated mice, about 1% are tetramer positive and that proportion more than doubles after vaccination with calnexin (Fig. S5A). After vaccination with calnexin, 15-20% of the tetramer-positive cells in the draining lymph nodes display the chemokine receptors CCR6 or CXCR3 (Fig. S5B), which are respectively linked with Th17 and Th1 cell recruitment (Hirota et al., 2007; Nanjappa et al., 2012a; Nanjappa et al., 2012b). Nearly 30% of tetramer-positive cells recruited to the lung were IL-17 producers in calnexin-vaccinated mice (Fig 4C). Thus, vaccination with calnexin induces the development of Ag-specific CD4+ T cells that are recruited to the lung after challenge and this response is linked to reduced CFU and prolonged survival in association with features of Th17 and Th1 immunity.
Fig. 4. Vaccine-induced resistance mediated by calnexin.
A. Mice were vaccinated s.c. thrice, 2 wks apart with 108 glucan particles (GP) loaded with 10μg r-calnexin (Cnx) or mouse serum albumin (MSA) as a control. 2wk after the last boost, mice were challenged with 2×103 B. dermatitidis 26199 yeast or 86 spores of C. posadasii strain C735. Lung and spleen (latter for C. posadasii infection) CFU were assessed 2wk post-infection. Numbers indicate the fold difference in lung CFUs vs. controls. B. Mice were vaccinated s.c. with 25 μg r-calnexin or MSA mixed with 5 or 20% Adjuplex. 2 wk after the last boost, mice were challenged with 2×103 B. dermatitidis and lung CFU measured as in A. Numbers are the fold difference in lung CFUs vs. controls. C. IL-17 reporter mice were vaccinated thrice with 25μg of calnexin encapsulated in GMP and mixed with 5% Adjuplex. The histogram shows the mean number of tetramer-positive cells from the bound and unbound fractions combined. Dot plots show the mean ± SEM number of tetramer-positive and percent of IL-17+ (eYFP+) CD4+ T cells among tetramer-positive and -negative cells from the bound fraction, enumerated by FACS. Dot plots represent an overlay of 10 samples/group.
The role of T cell precursor frequency and expansion in calnexin induced protection
The frequency of naïve CD4+ T cell populations affects the size of the T-cell response after immunization with the relevant peptide (Moon et al., 2007). We tested whether better expansion and recruitment of calnexin peptide #1 specific CD4+ T cells would improve vaccine protection. With calnexin vaccination above, we observed ≈100-200 tetramer positive cells recruited to the lung after infection, but only about 50 of these cells produced IL-17, implying that type 17 responses could be further enhanced.
We first compared different routes of vaccine delivery. The intravenous (i.v.) route with particles bearing calnexin triggered better expansion than the subcutaneous (s.c.) route (Fig. 5A). Delivery of soluble peptide #1 with LPS i.v. prompted a further increase in the number of tetramer-positive cells at the peak of expansion (Fig. 5B), especially at the lowest dose of 10 μg peptide. Improved expansion of calnexin-specific T cells did not translate into better protection against infection compared to the preceding approaches (Fig. 5C), perhaps because only a small fraction of tetramer-positive cells were recalled to the lungs and fate-mapping mice demonstrated that essentially none maintained production of IL-17. Thus, i.v. delivery promoted better expansion, but differentiation or persistence of IL-17 effectors wavered despite vaccine protection.
Fig. 5. Intravenous delivery of calnexin peptide, expansion of endogenous, tetramer-specific T cells, and resistance to infection.
A. Wild type C57BL6 mice were vaccinated s.c. or i.v. with 108 glucan mannan particles (GMP) loaded with 10 μg of r-calnexin (Cnx) or MSA as a negative control. B. Mice were vaccinated i.v. with 10-250 μg soluble calnexin peptide #1 and 5 μg LPS. 7d after vaccination in panels A and B, the skin draining lymph nodes and spleen were harvested and the number and activation (CD44) of tetramer-positive T cells assessed. The dot plots represent concatenated samples for 3 - 4 mice (noted in parenthesis) per group. The numbers of tetramer+ CD4+ T cells per concatenated sample is indicated inside the dot plots. The mean ± SEM of tetramer+ CD4+ T cells per mouse is indicated in the histogram (right). The number over a bar denotes the fold change of tetramer+ T cells vs. indicated control mice. C. To assess resistance after i.v. delivery of calnexin peptide, mice were vaccinated thrice with 10 μg soluble peptide #1 plus 5 μg LPS or GP loaded with 10 or 50 μg peptide #1 or MSA as a control. 2wk after the last boost, mice were challenged with 2×103 B. dermatitidis 26199 yeast. Lung CFU was assayed 2wk post-infection. * and **, denote fold change vs. the GMP/MSA or naïve control groups, respectively. Dot plots show the mean ± SEM number of tetramer+, activated (CD44+) and IL-17 differentiated cells (as determined by eYFP fluorescence with IL-17A fate-reporter mice) in the draining lymph nodes and spleen at the time of challenge, and recalled to the lung 4d post-infection, concatenated for 5 mice/group.
Enhanced vaccine-induced expansion of calnexin specific T cells
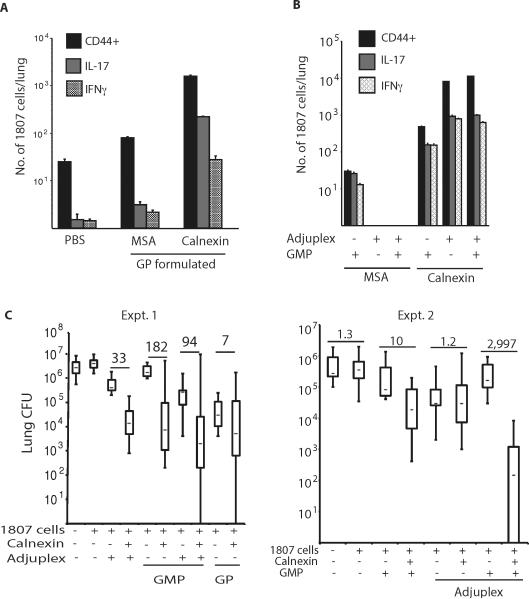
We sought an alternate approach to promote expansion, differentiation and maintenance of calnexin-specific T cells to explore their role in vaccine protection. We transferred naïve 1807 T cells prior to s.c. vaccination to increase the pool of Ag-experienced CD4+ T cells that persist. In mice given GP-encapsulated calnexin, we enumerated the number of activated (CD44+) and cytokine-producing 1807 T cells upon recall in the lung at day 4 post-infection. The number of CD44+ Ag-specific lung CD4+ T cells increased 41-fold in mice that received 1807 T cells (11,240 ± 298 1807 cells; Fig. 6A) vs. those that did not (273 ± 19 tetramer positive cells; Fig. 4C). Encouraged by this finding, we empirically tested different calnexin vaccine formulations to boost the number of Ag-experienced 1807 cells in the lung upon recall and sway their polarization. Mannan was added to GP to sway type 17 responses and adjuplex to drive type 1 responses. Glucan mannan particles (GMP), adjuplex and the combination of the two together yielded maximal numbers of IL-17- and IFN-γ producing 1807 T cells in the lung (Fig. 6B), with ≥ 104 recalled 1807 T cells showing an activated phenotype and ≥103 T cells each producing IL-17 or IFN-γ. To test whether increased numbers of calnexin-primed CD4+ T cells translate into improved vaccine resistance, we determined the lung burden after infection in mice that received transferred, naïve 1807 T cells before vaccination. Calnexin formulated with GMP and adjuplex together yielded ≈3,000-fold less lung CFU than adjuvant-control mice (Fig. 6C). Thus, calnexin is a conserved Ag capable of inducing vaccine resistance against infection with multiple fungal ascomycetes if the conditions are optimized for precursor frequency, expansion and maintenance of T cells that produce IL-17, IFN-γ or both.
Fig. 6. Naïve T cell precursor frequency and adjuvant formulation impact the pool size of calnexin primed T cells and resistance to infection.
A. Mice received 106 naïve 1807 cells prior to vaccination s.c. with 108 glucan particles (GP) loaded with 10μg r-calnexin or MSA as a negative control. 2wk after the last boost, mice were challenged with 2×103 B. dermatitidis 26199 yeast and the number of activated (CD44+) and cytokine-producing 1807 cells determined by FACS. B. Mice received 106 naïve 1807 cells before vaccination s.c. with 50 μg calnexin or MSA formulated in GMP or Adjuplex or in GMP and Adjuplex together. At d4 post-challenge, the number of CD44+, IL-17 and IFN-γ producing 1807 cells were determined by FACS. C. Mice received 106 naïve 1807 cells and were vaccinated as in B. 2wk after the last boost, mice were challenged with B. dermatitidis and lung CFU assayed 2wk post-infection when unvaccinated controls were moribund. Numbers in bold are the fold-change vs. MSA vaccinated controls.
DISCUSSION
We report the discovery of an immunodominant Ag – calnexin – that is conserved among numerous members of the fungal taxon Ascomycota. The peptide sequence that induces CD4+ T cell responses is conserved among the endemic, systemic dimorphic fungi, as well as clinically important Aspergillus species, Fonsecea pedrosoi, and even P. destructans, also referred to as the white nose fungus, which is sweeping across North America and devastating bat populations. This sequence is functionally important for inducing the expansion of Ag-specific T cells following exposure to each of these fungi, and the responses stemmed progression of ascomycete fungal infections that we studied, including Blastomyces and Coccidioides. The calnexin sequence diverges in fungi of other taxa, such as the basidiomycetes, and importantly also in mammals. The calnexin CD4+ T cell epitope is conserved for the inbred mouse strain studied here. Likewise, humans that have recovered from certain fungal infections demonstrate recall responses to calnexin in their CD4+ T cells.
Most of the major fungal antigens reported to date are either secreted or cell wall associated molecules (Rappleye and Goldman, 2008). In Blastomyces, the chief Ag BAD-1 is both released and yeast cell wall associated. In Histoplasma, the skin test Ag histoplasmin is a cell culture filtrate that contains H and M Ags, which are encoded by a β-glucosidase and catalase, respectively (Deepe and Durose, 1995; Zancope-Oliveira et al., 1999). In Cryptococcus sp., mannoproteins in or on the cell wall, or accumulated in the supernatant, trigger immunity to this fungus (Levitz and Specht, 2006). In Candida, the principal Ag targets of vaccines currently under study are Als3, which is a surface adhesin, and Sap2, which is a secreted aspartyl proteinase (Cassone and Casadevall, 2012). Thus, we were surprised that a protein such as calnexin, which monitors protein folding and glycosylation in the ER of cells, would serve as a major trigger of host cellular immune responses. We found that although calnexin normally resides in interior cell compartments, anti-calnexin antisera detected this protein on the surface of Blastomyces yeast and Aspergillus spores and hyphae. While unexpected, this result is not unprecedented. In Histoplasma, HIS62, a heat-shock protein (HSP), triggers CD4+ T cells that confer immunity in response to the fungus (Gomez et al., 1991). HSPs have been detected on the surface of Histoplasma yeast and mediate adherence to host integrin receptors (Long et al., 2003). Likewise, histone-like proteins have been detected on the surface of this fungus and antibodies directed against these proteins confer immunity (Nosanchuk et al., 2003). The localization of calnexin on the fungal surface could be due to protein shedding from dead or dying fungi, followed by non-specific adherence to the surface of viable cells. Alternatively, surface localization could be due to the trafficking of intracellular molecules through the cell wall in vesicles, as described in other fungi (Casadevall et al., 2009). The route notwithstanding, intracellular proteins including calnexin may unexpectedly appear at the fungal surface and induce immune recognition by the host.
In mapping the T cell epitope of calnexin, we synthesized peptide-MHCII tetramers and exploited this tool to study endogenous CD4+ T cells specific for this sequence on multiple pathogenic fungi. The pool of naïve calnexin specific cells in a C57BL/6 mouse is about 30 CD4+ T cells. This pool of T cells expands in response to exposure to a wide range of fungal ascomycetes, including the white nose fungus P. destructans. Our results supporting the conserved nature of the Ag were confirmed with TCR transgenic T cells that were adoptively transferred in parallel into infected mice. While the availability of transgenic T cells enables the monitoring of Ag specific immune responses, transfer of large numbers of T cells has pitfalls and limitations that may introduce artifacts that distort or misrepresent the true nature of the immune response to microbes (Moon et al., 2009). Peptide-MHCII tetramers offer a powerful tool to circumvent such limitations. We validated this tool for detecting and tracking endogenous fungal Ag specific CD4+ T cell responses to multiple fungi, in a manner that has not been previously available for the study of immunity to fungi. This tool will offer investigators studying various fungal pathogens a level of resolution that has not previously been possible. We show that this tool can be applied to study fungal diseases that vary from the endemic, systemic mycoses such as blastomycosis and histoplasmosis, to the opportunistic fungal disease Aspergillosis, to the tropical mycosis chromoblastomycosis, and unexpectedly, even to the fatal bat disease caused by the white nose fungus.
We used calnexin peptide-MHCII tetramers to track the behavior of IL-17-producing, Ag-specific CD4+ T cells with the benefit of fate mapping mice. We previously demonstrated that IL-17 production by CD4+ T cells is indispensable in vaccine immunity against dimorphic fungi that cause North American systemic mycoses ((Nanjappa et al., 2012a; Wüthrich et al., 2011a). We have found that IL-17 producing T cells are maintained and persist after vaccination with attenuated yeast in CD4-sufficient and -deficient mice (Nanjappa et al., 2012a; Wüthrich et al., 2011a). In contrast, others have reported that IL-17 producing T cells are short lived and dwindle due to death or conversion to type 1 cytokine producing T cells (Hirota et al., 2011; Pepper et al., 2010). Here, we exploited tetramers to track fungal Ag-specific, IL-17 producing T cells after vaccination. Calnexin vaccination induced T cells to differentiate into IL-17 producers, and tetramer positive cells recalled to the lung after challenge included IL-17 producers. These cells dwindled after i.v. peptide vaccination. In contrast, mice that received transferred 1807 T cells and s.c. vaccination with GMP and adjuplex evinced a large population of IL-17 producers during recall. Thus, fungal Ag-specific CD4+ T cells that produce IL-17 in response to vaccination were maintained in the latter setting. In a murine model of cutaneous Candida infection, IL-17 producing CD4+ T cells did not persist (Hirota et al., 2011). Our findings are in line with data in humans where Candida responsive, IL-17 producing T cells persist (Acosta-Rodriguez et al., 2007). Tetramers developed here should allow us to elucidate strategies to promote the persistence of memory T cells that confer anti-fungal immunity after vaccine administration.
In view of the conserved nature of calnexin, and its potential clinical utility for vaccination against pathogenic fungi, we immunized mice with calnexin or its epitopes and tested efficacy against pulmonary challenge with Blastomyces or Coccidioides. We encapsulated calnexin in GPs due to the potential advantages of polarizing the immune response toward IL-17 producing CD4+ T cells (Soto and Ostroff, 2008). Calnexin vaccine protected mice against lethal blastomycosis or coccidioidomycosis, reducing lung CFU by at least 1 log vs. control mice. In addition to calnexin delivery in GPs, we explored adjuvants such as mannan, LPS and adjuplex that may polarize T cells differently; each gave similar levels of calnexin-induced resistance and our results suggest a role for both type 17 and type 1 immunity. Thus, calnexin could prove to be a valuable component for a “pan-fungal” vaccine.
The size of the pool of naïve precursors specific for calnexin peptide #1 is an average size (Nelson et al., 2014) of 30 cells. Because the size of this precursor pool dictates the ultimate number of Ag-specific T cells in the expanded pool after vaccination (Moon et al., 2007), we sought to expand this pool to boost calnexin vaccine efficacy. Delivery of peptide via the i.v. route lead to an expanded pool of calnexin-specific T cells. In the latter circumstance, the pool of calnexin-specific T cells increased to >1000 cells in the draining lymph nodes and spleen of calnexin-vaccinated mice, or more than 20-fold higher than the number of cells in control mice. However, tetramers showed that Ag-specific effectors were poorly maintained based on recall and vaccine efficacy was unchanged.
We investigated cell transfer as an alternate maneuver to increase the size of the precursor pool and boost vaccine efficacy. Transfer of 1807 T cells lead to a 10-fold enhancement of calnexin peptide-specific T cells recruited to the lungs on challenge; ≈10,000 of these cells exhibited an activated (CD44+) phenotype and ≈10% produced IL-17 or IFN-γ (1,000 each). These mice also had vaccine given s.c. in GMPs in association with adjuplex adjuvant so that the independent role of each of these conditions -precursor number vs. adjuvant - could not be discerned. These combined conditions yielded improved vaccine efficacy, with levels that far exceeded other conditions, resulting in a 3-4 log reduction in lung CFU in a model of lethal experimental fungal infection. We cannot exclude that TCR affinity played a role in better protection after transfer of transgenic T cells and vaccination. Nevertheless, T cell transfer has been used to treat immune suppressed patients with CMV infections in the setting of bone marrow or stem cell transplantation (Blyth et al., 2013; Peggs et al., 2011). Such patients receive donor T cells after expansion of Ag specific T cells in vitro, followed by magnetic bead enrichment of activated cytokine producing T cells. Another major risk in these patients is pulmonary aspergillosis (Singh and Paterson, 2005). Because calnexin is conserved in Aspergillus and displayed on the fungal surface, and because the fungus induces expansion of calnexin specific CD4+ T cells during infection, transfer of calnexin-specific T cells that are activated, expanded, and enriched in vitro may offer immunotherapeutic benefit to patients with invasive fungal infection (Beck et al., 2006).
MATERIALS AND METHODS
Fungi
Strains used were wild-type B. dermatitidis ATCC 26199 and strain #55, the isogenic, attenuated mutant lacking BAD1 (Brandhorst et al., 1999); H. capsulatum strain G217B; C. posadasii strain C735; C. albicans strain #5314 (Wüthrich et al., 2011b); P. destructans ATCC 20631-21; A. fumigatus Af293; and F. pedrosoi strain ATCC 46428. Growth conditions are in supplemental methods.
Mouse strains
Inbred C57BL/6, IL-17atm1.1(icre)Stck/J (stock # 16879) and Gt(ROSA)26Sortm1(EYFP)Cos reporter mice (stock # 6148) were obtained from Jackson laboratory, Bar Harbor, ME. Breeding IL-17atm1.1(icre)Stck/J to Gt(ROSA)26Sortm1(EYFP)Cos reporter mice enabled us to fluorescently label and track IL-17A expressing cells as described for fate mapping (Hirota et al., 2011). Blastomyces-specific TCR Tg 1807 mice were bred to B6.PL (Thy1.1+) mice to obtain Thy1.1+ 1807 cells (Wüthrich et al., 2012).
Generation of Eluate #1
Cell wall membrane (CW/M) Ag was extracted from BAD1-null vaccine yeast as described (Wüthrich et al., 2000). Briefly, yeast were broken open with glass beads, debris pelleted, and the aqueous supernatant harvested. CW/M Ag was diluted to a protein concentration of 1.5mg/ml in binding buffer containing 20mM Tris, pH7.6, 0.3M NaCl, 1mM MnCl2, 1mM MgCl2, 1mM CaCl2 and centrifuged to remove insoluble complexes. To enrich the mannosylated proteins in the CW/M Ag preparation, we used a Con A column (Fig. 1A). See supplement for details.
Enrichment of the shared Ag by Gel-free separation and identification by mass spectroscopy analysis
Eluate #1 was applied to a Gel-free 8100 fractionation system (Protein Discovery, Knoxville, TN), and separated on a 10% Tris-Acetate cartridge. Fractions were collected that corresponded to separately eluted MW markers. These fractions were surveyed for protein content by PAGE analysis and silver stain. The fractions that activated 1807 T cells (quantified by production of INF-γ) were concentrated by FASP for mass spectroscopy analysis (below).
Filter aided sample preparation [FASP] method
FASP sample preparation (Universal sample preparation method for proteome analysis) and mass spectrometric analysis was done at the Mass Spectrometry Facility, University of Wisconsin-Madison. Peptides were analyzed by nanoLC-MS/MS using the Agilent 1100 nanoflow system (Agilent Technologies) connected to a hybrid linear ion trap-orbitrap mass spectrometer (LTQ-Orbitrap XL, Thermo Fisher Scientific) equipped with a nanoelectrospray ion source. See supplemental methods for detail.
Generation and purification of r-calnexin
Calnexin was cloned and expressed in E. coli using standard recombinant techniques described in supplemental methods.
GP-calnexin-MSA/yR, GMP-calnexin-MSA/yR, GP-MSA/yR, and GP-MSA/yR Vaccine Formulations
Glucan Particles (GP) and Glucan Mannan Particles (GMP) were purified from Baker's yeast using chemical and organic extractions (Soto and Ostroff, 2008; Young et al., 2007). GPs and GMPs containing encapsulated r-calnexin-mouse serum albumin (MSA; Equitech-Bio, Kerrville, TX) and yeast RNA (yR; Sigma, St. Louis, MO) (G(M)P-calnexin-MSA/yR) or control MSA/yR (G(M)P-MSA/tR) were synthesized (Huang et al., 2010; Soto and Ostroff, 2008). Vaccine formulations were adjusted to 109 particles/ml in saline for injection (Baxter, Deerfield, IL) and flash frozen in single use aliquots to deliver 10 μg calnexin complexed with 50 μg MSA/108 particles per 0.1 ml dose. Vaccine Ag identity and encapsulation efficiency (>95%) were established by SDS-PAGE. GMP calnexin peptide 1-MSA/yR vaccine formulations were synthesized as described for calnexin protein.
Generation of MHC class II tetramer
To create tetramer, we covalently linked the peptide Ag by a fusion to the N-terminus of the MHCII chain via a flexible glycine-serine linker as described http://www.jenkinslab.umn.edu/Jenkins_Lab/Protocols_files/New%20tetramer%20production%20052212.pdf and (Moon et al., 2007). Detail on cloning and expression is provided in supplemental methods.
Enrichment, staining and analysis of rare epitope-specific T cells
To enrich epitope-specific T cells in mice we used a magnetic bead-based procedure that results in about a 100-fold increase in the frequency of the target population (Moon et al., 2009; Moon et al., 2007). Enriched cells were stained with a cocktail of fluorochrome-labeled antibodies specific for B220, CD11b, CD11c, F4/80, CD3, CD8, CD4 and CD44. The entire stained sample was collected on an LSRII flow cytometer and live cells analyzed by FlowJo software (Treestar) following the gating strategy described (Moon et al., 2009). The total number of tetramer positive cells from a mouse was calculated from the percent of tetramer-positive events multiplied by the total number of cells in the enriched fraction as described (Moon et al., 2009) and in the enriched plus unbound fraction when larger numbers of tetramer positive cells are present.
Stimulation of 1807 T cells in vitro
To test the antigenicity of the calnexin protein and peptides, we loaded bone marrow derived dendritic cells (BMDC) with Ag and cultured them with naïve 1807 T cells to assess T-cell activation and cytokine production. After three days of co-culture, cell supernatants were harvested and analyzed for cytokines by ELISA and 1807 T cells stained for the activation markers CD44 and CD62L (Wüthrich et al., 2012). Blastomyces CW/M-reactive T-cell clone #5, whose TCR was cloned to generate 1807 transgenic mice (Wüthrich et al., 2007), was also used as a reporter T-cell to identify the presence of the Ag. Cell-culture supernatants were generated in 96-well plates in 0.2 ml containing 105 BMDC, 0.05 to 10 μg/ml of CW/M Ag (Wüthrich et al., 2000), 0.05 to 50 μg/ml calnexin and Drk1 (as a negative control) (Nemecek et al., 2006) and 0.001 to 100 μM calnexin peptides #1-10 (Fig. S1). Supernatants were collected after 72 hrs of co-culture. IFN-γ and IL-17A were measured by ELISA (R&D System, Minneapolis, MN; detection limits were 0.05ng/ml).
Generation of a water-soluble extract from vaccine yeast
Yeast surface proteins were extracted three times with three yeast-pellet volumes of water by agitating the yeast for 1hr at 4°C. Yeast were separated from the supernatant by centrifugation and filtration through a 0.2 μm filter. The water soluble-extract was concentrated by a Centricon column with a 30 kD cutoff.
Vaccination and infection
Mice were vaccinated as described (Wüthrich et al., 2000) twice, 2 wk apart, s.c. with 10 to 200 μg r-calnexin protein or calnexin peptide. Adjuvants included CFA, 5-20% adjuplex (Advanced BioAdjuvants), GP without or with mannan (GMP). Some mice were vaccinated i.v. with 50 μg calnexin or 10 μg peptide #1 mixed with 5 μg LPS or loaded onto 5 × 107 GMP. Mice were infected intratracheally (i.t.) with 2 × 103 or 2 × 104 wild-type yeast of B. dermatitidis strain 26199, 2 × 105 H. capsulatum G217B, 2 × 105 FKS or 60 spores of the virulent C. posadasii isolate C735 (Wüthrich et al., 2000; Wüthrich et al., 2011a). To assess infiltration of primed CD4 T cells into the lungs, challenged mice were analyzed at d 4 post-infection. To quantify lung infection, homogenized lungs were plated and yeast colony forming units (CFU) enumerated on BHI agar (Difco, Detroit, MI), sheep-blood containing Mycosel plates, or GYE plates containing 50 μg/ml of chloramphenicol.
Adoptive transfer of 1807 cells and experimental challenge
To assess the T helper cytokine phenotype of calnexin-specific CD4+ T cells after vaccination with r-calnexin and various adjuvants, we transferred 106 naïve 1807 Tg cells into C57BL/6 wild-type mice before vaccination. On the same day, recipients were vaccinated, boosted two weeks later and challenged 2 wks after the boost.
In vitro stimulation and identification of activated human T cells
Peripheral blood mononuclear cells (PBMC) were isolated from heparinized whole blood collected over histopaque 1119 and 1077. Studies were approved by UW-Madison IRB (protocol 2014-1167). Patients provided informed consent. PBMC were stimulated with 10 μg/ml r-calnexin, 107/ml heat killed C. albicans or crude or purified fungal Ag (10 μg/ml Blastomyces CW/M, 5 μg/ml Histoplasma CW/M, 100 μg/ml Blastomyces alkali-soluble, water-soluble (ASWS) Ag, 10 μg/ml Coccidioidin, and 5 μg/ml Histoplasma Hsp60) plus 5U/ml IL-2 and 1 μg/ml α-human CD40 mAb for 14hr at 37°C/5% CO2. After stimulation, cells were bead-enriched by CD154+ selection (Miltenyi). Enriched cells were stained with live/dead blue fluorescent dye (Life Technologies), and α-CD8 PerCP, -CD4 PeCy-7, -CD3 BV785, -B220 Pacblue, -CD154 PE and -CD137 APC. B220-, CD8-, CD3+, CD4+ T cells were analyzed for CD137 and CD154 expression using FlowJo.
Statistical Analysis
The number and percent of activated, proliferating or cytokine producing T-cells and differences in number of CFU were analyzed using the Wilcoxon rank test for nonparametric data (Fisher and van Belle, 1993) or the T-test when data were normally distributed. A P value of < 0.05 is considered statistically significant.
Supplementary Material
ACKNOWLEDGEMENTS
Supported by NIH grants AI040996 and AI105816 to B.K.; AI093553 to M.W.; AI103760 to M.K.J.; AI-071118 to G.C. T.L.; and a Royal Golden Jubilee Ph.D. Scholarship Grant PHD/0092/2553 from Mahidol University, Bangkok, Thailand to T.L. We thank Robert Gordon for help with illustrations; Paul Ahlquist for loaning the gel free system; the Nett and Huttenlocher labs for helping collect blood samples; and Drs. Nancy Keller, David Blehert and Gordon Brown for providing fungal strains.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Acosta-Rodriguez EV, Rivino L, Geginat J, Jarrossay D, Gattorno M, Lanzavecchia A, Sallusto F, Napolitani G. Surface phenotype and antigenic specificity of human interleukin 17-producing T helper memory cells. Nature immunology. 2007;8:639–646. doi: 10.1038/ni1467. [DOI] [PubMed] [Google Scholar]
- Beck O, Topp MS, Koehl U, Roilides E, Simitsopoulou M, Hanisch M, Sarfati J, Latge JP, Klingebiel T, Einsele H, et al. Generation of highly purified and functionally active human TH1 cells against Aspergillus fumigatus. Blood. 2006;107:2562–2569. doi: 10.1182/blood-2005-04-1660. [DOI] [PubMed] [Google Scholar]
- Blyth E, Clancy L, Simms R, Ma CK, Burgess J, Deo S, Byth K, Dubosq MC, Shaw PJ, Micklethwaite KP, et al. Donor-derived CMV-specific T cells reduce the requirement for CMV-directed pharmacotherapy after allogeneic stem cell transplantation. Blood. 2013;121:3745–3758. doi: 10.1182/blood-2012-08-448977. [DOI] [PubMed] [Google Scholar]
- Brandhorst TT, Wüthrich M, Warner T, Klein B. Targeted gene disruption reveals an adhesin indispensable for pathogenicity of Blastomyces dermatitidis. The Journal of experimental medicine. 1999;189:1207–1216. doi: 10.1084/jem.189.8.1207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casadevall A, Nosanchuk JD, Williamson P, Rodrigues ML. Vesicular transport across the fungal cell wall. Trends in microbiology. 2009;17:158–162. doi: 10.1016/j.tim.2008.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cassone A, Casadevall A. Recent progress in vaccines against fungal diseases. Current opinion in microbiology. 2012;15:427–433. doi: 10.1016/j.mib.2012.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- da Gloria Sousa M, Reid DM, Schweighoffer E, Tybulewicz V, Ruland J, Langhorne J, Yamasaki S, Taylor PR, Almeida SR, Brown GD. Restoration of pattern recognition receptor costimulation to treat chromoblastomycosis, a chronic fungal infection of the skin. Cell Host Microbe. 2011;9:436–443. doi: 10.1016/j.chom.2011.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deepe GS, Jr., Durose GG. Immunobiological activity of recombinant H antigen from Histoplasma capsulatum. Infection and immunity. 1995;63:3151–3157. doi: 10.1128/iai.63.8.3151-3157.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards JE., Jr. Fungal cell wall vaccines: an update. Journal of medical microbiology. 2012;61:895–903. doi: 10.1099/jmm.0.041665-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellgaard L, Helenius A. Quality control in the endoplasmic reticulum. Nat Rev Mol Cell Biol. 2003;4:181–191. doi: 10.1038/nrm1052. [DOI] [PubMed] [Google Scholar]
- Fisher LD, van Belle G. Biostatistics: A Methodology for the Health Sciences. John Wiley & Sons; New York: 1993. pp. 611–613. [Google Scholar]
- Gomez FJ, Gomez AM, Deepe GS., Jr. Protective efficacy of a 62-kilodalton antigen, HIS-62, from the cell wall and cell membrane of Histoplasma capsulatum yeast cells. Infection and immunity. 1991;59:4459–4464. doi: 10.1128/iai.59.12.4459-4464.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirota K, Duarte JH, Veldhoen M, Hornsby E, Li Y, Cua DJ, Ahlfors H, Wilhelm C, Tolaini M, Menzel U, et al. Fate mapping of IL-17-producing T cells in inflammatory responses. Nature immunology. 2011;12:255–263. doi: 10.1038/ni.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirota K, Yoshitomi H, Hashimoto M, Maeda S, Teradaira S, Sugimoto N, Yamaguchi T, Nomura T, Ito H, Nakamura T, et al. Preferential recruitment of CCR6-expressing Th17 cells to inflamed joints via CCL20 in rheumatoid arthritis and its animal model. The Journal of experimental medicine. 2007;204:2803–2812. doi: 10.1084/jem.20071397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang H, Ostroff GR, Lee CK, Specht CA, Levitz SM. Robust stimulation of humoral and cellular immune responses following vaccination with antigen-loaded beta-glucan particles. MBio. 2010;1 doi: 10.1128/mBio.00164-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levitz SM, Specht CA. The molecular basis for the immunogenicity of Cryptococcus neoformans mannoproteins. FEMS yeast research. 2006;6:513–524. doi: 10.1111/j.1567-1364.2006.00071.x. [DOI] [PubMed] [Google Scholar]
- Long KH, Gomez FJ, Morris RE, Newman SL. Identification of heat shock protein 60 as the ligand on Histoplasma capsulatum that mediates binding to CD18 receptors on human macrophages. J Immunol. 2003;170:487–494. doi: 10.4049/jimmunol.170.1.487. [DOI] [PubMed] [Google Scholar]
- Lorch JM, Meteyer CU, Behr MJ, Boyles JG, Cryan PM, Hicks AC, Ballmann AE, Coleman JT, Redell DN, Reeder DM, et al. Experimental infection of bats with Geomyces destructans causes white-nose syndrome. Nature. 2011;480:376–378. doi: 10.1038/nature10590. [DOI] [PubMed] [Google Scholar]
- Moon JJ, Chu HH, Hataye J, Pagan AJ, Pepper M, McLachlan JB, Zell T, Jenkins MK. Tracking epitope-specific T cells. Nat Protoc. 2009;4:565–581. doi: 10.1038/nprot.2009.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moon JJ, Chu HH, Pepper M, McSorley SJ, Jameson SC, Kedl RM, Jenkins MK. Naive CD4(+) T cell frequency varies for different epitopes and predicts repertoire diversity and response magnitude. Immunity. 2007;27:203–213. doi: 10.1016/j.immuni.2007.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nanjappa SG, Heninger E, Wuthrich M, Gasper DJ, Klein BS. Tc17 cells mediate vaccine immunity against lethal fungal pneumonia in immune deficient hosts lacking CD4+ T cells. PLoS pathogens. 2012a;8:e1002771. doi: 10.1371/journal.ppat.1002771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nanjappa SG, Heninger E, Wuthrich M, Sullivan T, Klein B. Protective antifungal memory CD8(+) T cells are maintained in the absence of CD4(+) T cell help and cognate antigen in mice. J Clin Invest. 2012b;122:987–999. doi: 10.1172/JCI58762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson RW, Beisang D, Tubo NJ, Dileepan T, Wiesner DL, Nielsen KN, Spanier JA, Fife BT, Moon JJ, Wuethrich M, et al. TCR cross-reactivity between similar foreign and self peptides influences naïve cell population size and autoimmunity. Immunity. 2014 doi: 10.1016/j.immuni.2014.12.022. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nemecek JC, Wuthrich M, Klein BS. Global control of dimorphism and virulence in fungi. Science. 2006;312:583–588. doi: 10.1126/science.1124105. [DOI] [PubMed] [Google Scholar]
- Nosanchuk JD, Steenbergen JN, Shi L, Deepe GS, Jr., Casadevall A. Antibodies to a cell surface histone-like protein protect against Histoplasma capsulatum. J Clin Invest. 2003;112:1164–1175. doi: 10.1172/JCI19361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park BJ, Wannemuehler KA, Marston BJ, Govender N, Pappas PG, Chiller TM. Estimation of the current global burden of cryptococcal meningitis among persons living with HIV/AIDS. AIDS. 2009;23:525–530. doi: 10.1097/QAD.0b013e328322ffac. [DOI] [PubMed] [Google Scholar]
- Peggs KS, Thomson K, Samuel E, Dyer G, Armoogum J, Chakraverty R, Pang K, Mackinnon S, Lowdell MW. Directly selected cytomegalovirus-reactive donor T cells confer rapid and safe systemic reconstitution of virus-specific immunity following stem cell transplantation. Clin Infect Dis. 2011;52:49–57. doi: 10.1093/cid/ciq042. [DOI] [PubMed] [Google Scholar]
- Pepper M, Linehan JL, Pagan AJ, Zell T, Dileepan T, Cleary PP, Jenkins MK. Different routes of bacterial infection induce long-lived TH1 memory cells and short-lived TH17 cells. Nature immunology. 2010;11:83–89. doi: 10.1038/ni.1826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfaller MA, Moet GJ, Messer SA, Jones RN, Castanheira M. Candida Bloodstream Infections: Comparison of Species Distributions and Antifungal Resistance Patterns in Community-Onset and Nosocomial Isolates in the SENTRY Antimicrobial Surveillance Program, 2008-2009. Antimicrobial Agents and Chemotherapy. 2011;55:561–566. doi: 10.1128/AAC.01079-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rappleye CA, Goldman WE. Fungal stealth technology. Trends in immunology. 2008;29:18–24. doi: 10.1016/j.it.2007.10.001. [DOI] [PubMed] [Google Scholar]
- Singh N, Paterson DL. Aspergillus infections in transplant recipients. Clinical microbiology reviews. 2005;18:44–69. doi: 10.1128/CMR.18.1.44-69.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soto ER, Ostroff GR. Characterization of multilayered nanoparticles encapsulated in yeast cell wall particles for DNA delivery. Bioconjug Chem. 2008;19:840–848. doi: 10.1021/bc700329p. [DOI] [PubMed] [Google Scholar]
- Torosantucci A, Bromuro C, Chiani P, De Bernardis F, Berti F, Galli C, Norelli F, Bellucci C, Polonelli L, Costantino P, et al. A novel glyco-conjugate vaccine against fungal pathogens. The Journal of experimental medicine. 2005;202:597–606. doi: 10.1084/jem.20050749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walsh TJ, Anaissie EJ, Denning DW, Herbrecht R, Kontoyiannis DP, Marr KA, Morrison VA, Segal BH, Steinbach WJ, Stevens DA, et al. Treatment of aspergillosis: clinical practice guidelines of the Infectious Diseases Society of America. Clin Infect Dis. 2008;46:327–360. doi: 10.1086/525258. [DOI] [PubMed] [Google Scholar]
- Wüthrich M, Ersland K, Sullivan T, Galles K, Klein BS. Fungi subvert vaccine T cell priming at the respiratory mucosa by preventing chemokine-induced influx of inflammatory monocytes. Immunity. 2012;36:680–692. doi: 10.1016/j.immuni.2012.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wüthrich M, Filutowicz HI, Allen HL, Deepe GS, Klein BS. V{beta}1+ J{beta}1.1+/V{alpha}2+ J{alpha}49+ CD4+ T Cells Mediate Resistance against Infection with Blastomyces dermatitidis. Infection and immunity. 2007;75:193–200. doi: 10.1128/IAI.01148-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wüthrich M, Filutowicz HI, Klein BS. Mutation of the WI-1 gene yields an attenuated Blastomyces dermatitidis strain that induces host resistance. J Clin Invest. 2000;106:1381–1389. doi: 10.1172/JCI11037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wüthrich M, Gern B, Hung CY, Ersland K, Rocco N, Pick-Jacobs J, Galles K, Filutowicz H, Warner T, Evans M, et al. Vaccine-induced protection against 3 systemic mycoses endemic to North America requires Th17 cells in mice. J Clin Invest. 2011a;121:554–568. doi: 10.1172/JCI43984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wüthrich M, Hung CY, Gern BH, Pick-Jacobs JC, Galles KJ, Filutowicz HI, Cole GT, Klein BS. A TCR Transgenic Mouse Reactive with Multiple Systemic Dimorphic Fungi. J Immunol. 2011b;187:1421–1431. doi: 10.4049/jimmunol.1100921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young SH, Ostroff GR, Zeidler-Erdely PC, Roberts JR, Antonini JM, Castranova V. A comparison of the pulmonary inflammatory potential of different components of yeast cell wall. J Toxicol Environ Health A. 2007;70:1116–1124. doi: 10.1080/15287390701212224. [DOI] [PubMed] [Google Scholar]
- Zancope-Oliveira RM, Reiss E, Lott TJ, Mayer LW, Deepe GS., Jr. Molecular cloning, characterization, and expression of the M antigen of Histoplasma capsulatum. Infection and immunity. 1999;67:1947–1953. doi: 10.1128/iai.67.4.1947-1953.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.