Abstract
Arthropods form a broad phylum within the animal kingdom, comprising widely varying members such as insects, arachnids, crabs and centipedes. In addition to common allergies to house dust mites or hymenoptera venom, there are also rarer allergies that can be attributed to three major sources of allergens: cockroaches, ticks and storage mites. Other less known allergen sources include spiders, mosquitos, horseflies, red chironomid larvae, silverfish and ladybugs, as well as a variety of storage pests. At present, only extract-based test systems are available for the majority of allergens in IgE-based diagnostics. Molecular characterisation of numerous individual allergens has already been carried out. However, these individual allergens are only available for a small number of allergen sources (e. g. cockroaches and storage mites) in routine diagnostics. Particularly in the case of allergen sources with known high cross-reactivity, the use of marker allergens is believed to improve diagnostics. The currently known individual allergens of the above-mentioned allergy triggers from the arthropod realm are summarized and their potential use in allergy diagnostics discussed.
Introduction
Arthropods form an extensive phylum of the animal kingdom, comprising widely varying members such as insects, arachnids, crabs and centipedes (Fig. 1). The molecular diagnostics of the frequent house dust mite allergy and the hymenoptera venom allergy has already been discussed in separate articles [1, 2]. The present article deals with rarer allergies to specific members of the arthropod realm, describes the extracts available for diagnostic purposes as well as the currently known individual allergens and discusses their potential application in allergy diagnostics.
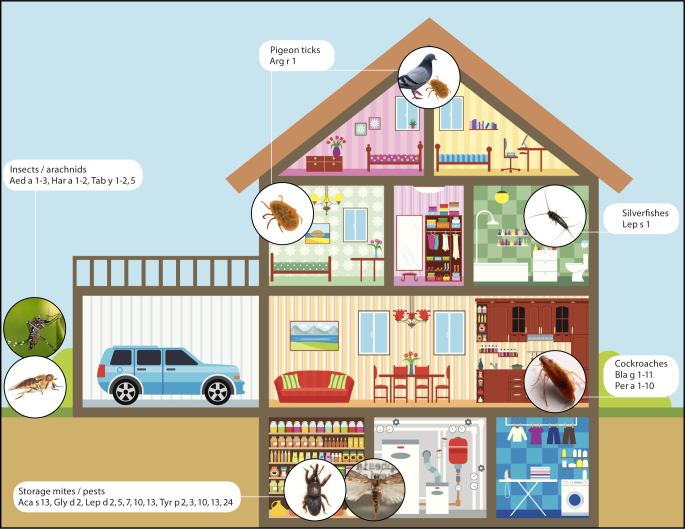
Fig. 1.
Favored habitations of various allergenic arthropods around the house and the garden. Characterized allergens are indicated.
© [M] House: mylisa / fotolia.com; all other pictures: see tables
Cockroach allergy
Exposure and distribution
The cockroach order (Blattodea) is made up of more than 4,600 species and its members are found worldwide. Cockroaches are nocturnal and are found primarily in the Tropics and Subtropics. The domestic cockroaches best investigated as allergen sources include the German cockroach (Blattella germanica), which dominates in the US in terms of numbers, as well as the American cockroach (Periplaneta americana) and the oriental cockroach (Blatta orientalis). The Periplaneta fuliginosa cockroach, which was originally indigenous only to Japan, Southeast Asia and the Southern United States, has spread worldwide by infesting containers transported either by ship or air. The frequency of cockroach allergies depends to a great extent on the level of exposure to cockroach allergens [3]. Allergen exposure in urban areas is on the whole significantly higher than in suburban areas, where, nevertheless, allergen concentrations are measured in as much as 30 % of US households [4, 5, 6].
Allergen identification
The official allergen database (www.allergen.org) includes important allergens from the German and US cockroaches: Bla g 1–11 and Per a 1–10. These cockroach allergens can be subdivided into more than 10 protein groups on the basis of highly varying physiological functions [3] (Tab. 1). They have been identified in cockroach faeces, eggs or exoskeletons. Homologous, possibly cross-reactive allergens have been described in other cockroach species (www.allergome.org).
Table 1.
Individual allergens of the German and US cockroach identified according to the IUIS Allergen Nomenclature Sub-Committee
| Allergen | Name | Molecular weight (kDa) |
|---|---|---|
| Blattella germanica a, c, d, e, f (German cockroach) |

|
|
| Bla g 1b | 46 | |
| Bla g 2b | Aspartate protease | 36 |
| Bla g 3 | Hemocyanin | 78,9 (Mass spectrometry) |
| Bla g 4 | Calycine | 21 |
| Bla g 5b | Glutathione S-transferase | 23 |
| Bla g 6 | Troponin C | 21 |
| Bla g 7b | Tropomyosin | 31 |
| Bla g 8 | Myosin, light chain | |
| Bla g 11 | α-Amylase | 57 |
| Periplaneta americana a, c, d (American cockroach) |

|
|
| Per a 1 | 45 | |
| Per a 3 | Arylphorin/hemocyanin | 72 |
| Per a 6 | Troponin C | 17 |
| Per a 7 | Tropomyosin | 33 |
| Per a 9 | Arginine kinase | 43 |
| Per a 10 | Serine protease | 28 |
a ImmunoCAP®, Phadia/Thermo Scientific, Freiburg; b ImmunoCAP®ISAC, Phadia/Thermo Scientific, Freiburg; c 3gAllergy™/Immunlite, Siemens Healthcare, Eschborn; d ALLERG-O-LIQ®, Dr. Fooke Laboratorien GmbH, Neuss; e Allergozyme®, Omega Diagnostics, Reinbek; f Allercoat™, EuroImmune, Lübeck
Relevance
Whilst cockroach allergen sensitization is one of the greatest risk factors for increased asthma-related morbidity among the low-income population in the US (referred to as the inner-city asthma problem in the US), sensitization rates in Germany and Europe are generally far lower [6]. A study by Hirsch et al. [7] found that only 4.2 % of approximately 3,000 children in Dresden, Germany, had specific immunoglobulin E (IgE) (> 0.7 kU/l) to the German cockroach (Blattella germanica), whereby the prevalence of sensitization among asthmatic children was 6.1 %. Most cockroach-sensitized children in this study were also sensitized to other allergens. Also in a patient-based study carried out by several European centres, where skin tests were performed using different indoor and outdoor allergens in over 3,000 patients, an overall prevalence of sensitization to cockroach (in this case Blattella germanica) of 8.9 % was found, and of 12 % in German patients [8]. The prevalence of specific IgE-antibodies to individual cockroach allergens varies considerably; this appears to depend on regional exposure to cockroach allergens [9, 10]. The major allergens are found in the protein groups 1–5 (Bla g 1–5). Since group-1 and -2 cockroach allergens (Bla g 1 and Bla g 2) are released into the environment, they serve well as detection molecules in the assessment of allergen exposure [3].
Cross-reactive allergens
Homologous allergens from different cockroach species, e. g. Bla g 1 and Per a 1, exhibit high — albeit variable — cross-reactivity. The tropomyosins Bla g 7 and Per a 7, as well as arginine kinase (Per a 9), are highly similar to the homologous allergens of other arthropods (> 80 % identity). The clinical significance of IgE cross-reactivity between tropomyosins and arginine kinases of cockroaches, shellfish and house dust mites has not been fully elucidated as yet [11, 12].
Tick allergy
Exposure and distribution
Cases of anaphylactic reactions to pigeon ticks have been consistently reported in recent years, most notably in France, Poland and Italy, but also in Germany [13, 14]. The pigeon tick (Argas reflexus), which belongs to the soft-tick family, is a temporary ectoparasite of wild pigeons in southern and central Europe. It feeds primarily on blood from its host at night and seeks refuge in wall crevices and wood cracks during the day. If the pigeon is unable to return to its nest due to, e. g. building work, the tick will seek new hosts by invading homes, where it will infest humans. Adult ticks can survive for several years without food and are extremely challenging to combat. In addition to the severe anaphylactic reactions described in the literature, there are also many instances of mild local reactions. A study carried out in Leipzig, Germany found an 8 % rate of severe systemic reactions and a 99 % rate of local reactions [14].
In addition to pigeon ticks, occasional cases of classic immediate-type reactions are described following bites from the common wood tick (Ixodes ricinus), the Australian paralysis tick (Ixodes holocyclus) and the brown dog tick (Rhipicephalus sanguineus), which is found primarily in southern Europe. Such cases involve an IgE-mediated reaction to protein in tick saliva.
One particular form of allergy, the delayed red-meat allergy, is also associated with tick bites. This allergy involves IgE sensitization to a sugar epitope, galactose-α-1.3-galactose, which is believed to be triggered by tick bites [15]. Whilst in the US the American lone star tick (Amblyomma americanum) and in Australia the Australian paralysis tick (Ixodes holocyclus) are discussed as allergy triggers, the common wood tick (Ixodes ricinus) and the marsh tick (Dermacentor) are associated with sensitization to galactose-α-1.3-galactose in Europe.
Allergen identification
The major allergen of the pigeon tick, Arg r 1, is a lipocalin. A number of major mammalian allergens also belong to the lipocalin family, such as Can f 1, Can f 2, Equ c 1 and Mus m 1, among others. However, since their amino acid sequences are highly distinct, any cross-reactivity appears to be ruled out.
No allergens from other tick species have been isolated or characterized to date.
Galactose-α-1.3-galactose, the allergen relevant in delayed red-meat allergy, is found on bovine thyroglobulin. Galactose-α-1.3-galactose bovine thyroglobulin is available for the diagnosis of galactose-α-1.3-galactose sensitization in the ImmunoCAP® system (Phadia/Thermo Scientific).
Storage mite allergy
Exposure and distribution
Storage mites, microscopic arachnids that feed on plant and animal matter, are typical storage pests. Depending on the species, they are found in grain and animal feed, as well as in hay and straw. The mites most commonly found in Europe include the storage mite (Lepidoglyphus destructor), the flour mite (Acarus siro), the house mite (Glycyphagus domesticus) and the mould mite (Tyrophagus putrescentiae). The last of these has a predilection for foods containing protein and fat, such as ham or cheese. All species thrive optimally at temperatures of 20–30°C and at a relative air humidity of > 65 % [16, 17, 18].
Allergen identification
For both the storage mite and the mould mite, five different molecules are stored in the allergen database (Tab. 2), including the panallergen tropomyosin (Lep d 10, Try p 10). However, with around 13 % IgE recognition, tropomyosin is a minor allergen. Allergens have been identified in carcasses as well as in faeces. The major allergen belongs to group 2 (Lep d 2, Tyr p 2, Gly d 2) and has been found in mite intestine; its function, however, is unknown.
Table 2.
Individual storage mite allergens identified according to the IUIS Allergen Nomenclature Sub-Committee
| Allergen | Name | Molecular weight (kDa) |
|---|---|---|
| Acarus siro a, c, d, e, f (Flour mite) | ||
| Aca s 13 | Fatty-acid binding protein | 15 |
| Glycyphagus domesticus a, c, d, e, f (House mite) |
|
|
| Gly d 2 | 15 | |
| Lepidoglyphus destructor a, c, d, e, f (Storage mite) | ||
| Lep d 2b | NPC2 family | 16 |
| Lep d 5 | ||
| Lep d 7 | ||
| Lep d 10 | Tropomyosin | |
| Lep d 13 | Fatty-acid binding protein | |
| Tyrophagus putrescentiae a, c, d, e, f (Mould mite) | ||
| Tyr p 2 | NPC2 family | 16 |
| Tyr p 3 | Trypsin | 26 |
| Tyr p 10 | Tropomyosin | |
| Tyr p 13 | Fatty-acid binding protein | 15 |
| Tyr p 24 | Troponin C | 18 |
a ImmunoCAP®, Phadia/Thermo Scientific, Freiburg; b ImmunoCAP®ISAC, Phadia/Thermo Scientific, Freiburg; c 3gAllergy™/Immunlite, Siemens Healthcare, Eschborn; d ALLERG-O-LIQ®, Dr. Fooke Laboratorien GmbH, Neuss; e Allergozyme®, Omega Diagnostics, Reinbek; f Allercoat™, EuroImmune, Lübeck
Relevance
Storage mite allergies frequently affect farmers and individuals working in the animal-feed industry. Symptoms often include bronchial asthma and allergic rhinitis. Occasional cases of oral dust mite allergy have been described. Severe allergic symptoms occurred following the ingestion of flour-based foods baked using contaminated ingredients [19]. These reports related to contamination with storage mites as well as with house dust mites.
Cross-reactive allergens
Although there is strong cross-reactivity between extracts from storage, house dust and flour mites, there is little IgE cross-reactivity between house dust and storage mites. However, co-sensitizations appear to be common. Group-2 allergens in particular (Lep d 2 and Gly d 2) exhibit high sequence identity. The tropomyosin from storage mites (Lep d 10) has a high degree of identity with Der f 10 and Der p 10 in the house dust mite, such that cross-reactivity is highly likely.
Allergies to other arthropods
A variety of other arachnids and insects can also cause allergies in rare cases (Tab. 3). Individuals who work in barns or stables, where spiders are found in abundance, can experience allergic reactions to the spiders themselves as well as to their cobwebs. Salivary proteins from mosquitos and horseflies can cause strong local allergic reactions and, more rarely, systemic reactions [20, 21]. To date, three mosquito allergens from Aedes aegypti have been included in the International Union of Immunological Societies (IUIS) database: Aed a 1, an apyrase (68 kDa), Aed a 2 (37 kDa) and Aed a 3 (30 kD), of as yet unknown function [20]. Other allergens, such as tropomyosin Aed a 7, have been described in the Allergome database. Three major allergens have been identified so far for the horsefly (Tabanus spp.): Tab y 1, an apyrase, Tab y 2, a hyaluronidase and Tab y 5, an antigen-5 protein [21]. The last two show cross-reactivity with hyaluronidase and antigen 5 of the Vespidae family [22] and offer an explanation for presumed cross-reactions between wasp venom and horsefly saliva.
Table 3.
Individual allergens of other arthropods identified according to the IUIS Allergen Nomenclature Sub-Committee
| Allergen | Name | Molecular weight (kDa) |
|---|---|---|
| Aedes aegypti (Yellow fever mosquito), Aedes spp., Culex pipiens a, c, d, e, f (Common house mosquito) |

|
|
| Aed a 1 | Apyrase | 68 |
| Aed a 2 | 37 | |
| Aed a 3 | 30 | |
| Argas reflexus e (Pigeon tick) |

|
|
| Arg r 1 | Lipokalin | 17 |
| Chironomus thummi thummi a, c, d, e, f (Red chironomid larvae) |

|
|
| Chi t 1 | Hämoglobinkomponente III/IV | 16 |
| Chi t 1 | Hämoglobinkomponente I/IA | 16 |
| Chi t 3 | Hämoglobinkomponenten II-β, VI, VIII, IX | 16 |
| Chi t 4 | Hämoglobinkomponente IIIA | 16 |
| Chi t 9 | Hämoglobinkomponente X | 16 |
| Harmonia axyridis (Asian ladybug) |

|
|
| Har a 1 | 10 | |
| Har a 2 | Aldehyddehydrogenase | 55 |
| Lepisma saccharina (Silverfish) |

|
|
| Lep s 1 | Tropomyosin | 36 |
| Tabanus yao, Tabanus spp. a, c, d, e, f (Horsefly) |

|
|
| Tab y 1 | Apyrase | 70 |
| Tab y 2 | Hyaluronidase | 35 |
| Tab y 5 | Antigen-5-verwandtes Protein | 26 |
a ImmunoCAP®, Phadia/Thermo Scientific, Freiburg; b ImmunoCAP®ISAC, Phadia/Thermo Scientific, Freiburg; c 3gAllergy™/Immunlite, Siemens Healthcare, Eschborn; d ALLERG-O-LIQ®, Dr. Fooke Laboratorien GmbH, Neuss; e Allergozyme®, Omega Diagnostics, Reinbek; f Allercoat™, EuroImmune, Lübeck
The red chironomid larvae (Chironomus thummi thummi) are popular as fishing bait and are known to trigger allergic respiratory symptoms in individuals working in fish-food manufacture and in hobby aquarists [23]. Their various haemoglobin components are recorded in the IUIS database as allergens Chi t 1–4 and Chi t 9.
The silverfish (Lepisma saccharina) is found primarily in the kitchen, bathroom and cellar. In the case of high levels of infestation, allergens may be present in house dust. The tropomyosin Lep s 1 is the only known allergen to date. It exhibits cross-reactivity with tropomyosin of other arthropods, such as the house dust mite, the cockroach and the shrimp [24].
The multicoloured Asian ladybug (Harmonia axyridis) was introduced in the US between 1960 and 1990 as a means of pest control. Since then, it has assumed plague proportions, as the animals swarm out and invade houses and other buildings in their hundreds in order to hibernate. They have become a new and significant source of seasonal indoor allergens in the US [25]. Extract-based diagnostics of ladybug allergy exhibits high cross-reactivity with cockroach extract (Blatella germanica). Two major allergens have been identified to date: Har a 1 (10 kDa), a protein believed to be specific for ladybug sensitisation and Har a 2 (55 kDa), a protein related to the aldehyde dehydrogenase of the red flour beetle [25].
Storage pests, such as the wheat weevil (Sitophilus granarius), the mealworm beetle (Tenebrio molitor), the confused flour beetle (Tribolium confusum), the Berlin beetle (Trogoderma angustum) or the Mediterranean flour moth (Ephestia kuehniella) have also been described as allergen sources (Tab. 4). Since these storage pests are found primarily in stored grain, occupational groups such as farmers, bakers, millers and grain storage workers are particularly affected and, depending on the duration of exposure, can develop symptoms like allergic rhinitis and bronchial asthma [6]. It has not been possible as yet to include any IgE-binding proteins — characterized in the context of case descriptions using immunoblot or inhibition experiments — in the IUIS allergen database.
Table 4.
Available storage pest extracts
| Latin name | Name | |
|---|---|---|
| Ephestia kuehniella a, c, e |

|
Mediterranean flour moth |
| Sitophilus granarius a, e |

|
Wheat weevil |
| Tenebrio molitor a |

|
Mealworm beetle |
| Tribolium confosum a, d, e, f |

|
Confused flour beetle |
| Trogoderma angustuma, e, f | Berlin beetle | |
a ImmunoCAP®, Phadia/Thermo Scientific, Freiburg; b ImmunoCAP®ISAC, Phadia/Thermo Scientific, Freiburg; c 3gAllergy™/Immunlite, Siemens Healthcare, Eschborn; d ALLERG-O-LIQ®, Dr. Fooke Laboratorien GmbH, Neuss; e Allergozyme®, Omega Diagnostics, Reinbek; f Allercoat™, EuroImmune, Lübeck
Diagnostics and treatment
Routine diagnosis of the rarer allergies to arthropods is carried out by means of skin testing or specific IgE antibody detection using extracts. At present, extracts from three cockroach species (Periplaneta americana, Blatella germanica and Blatta orientalis), four storage mite species (Lepidoglyphus destructor, Acarus siro, Glycyphagus domesticus and Tyrophagus putrescentiae) and a number of storage pests (e. g. Sitophilus granarius, Tribolium confusum, Trogoderma angustum and Ephestia kuehniella) are available from a variety of manufacturers for the purposes of in vitro diagnostics; Argas reflexus extract is available from only one manufacturer (Omega Diagnostics, Reinbeck). However, clinical history can provide a strong indication of pigeon tick allergy: night-time tick bite, typically during the warm season and direct proximity to wild pigeons. Allergen components (Lep d 2, Bla g 1, Bla g 2, Bla g 5 and Bla g 7) are only available as yet in the ISAC test system (Thermo Scientific), not however in the ImmunoCAP® system (Thermo Scientific).
Preparations for specific immunotherapy are offered at present in Germany only for storage mite allergy. Studies on subcutaneous and sublingual immunotherapy of cockroach allergy are currently underway in the US [26] and the results are promising.
Perspectives
Given that a variety of allergens (e. g. cockroach, tick and storage mite) are already well characterized and for the most part available as recombinant molecules, the way has been paved for the development of IgE-based diagnostic tests using individual allergen components.
Conclusion
Although not well standardized, the available extracts permit IgE-based diagnosis of allergies to cockroaches, storage mites and storage pests. A broadening of IgE-based diagnostics in the future with various individual allergens would be beneficial.
However, the aim of further developments in molecular testing systems should be to use marker allergens for the unequivocal detection of sensitization and differentiation from cross-reactions. Marker allergens for tick sensitization, such as Arg r 1 from the pigeon tick, could be used to exclude pigeon tick allergy in cases of unexplained anaphylaxis.
Footnotes
Conflict of interest
The corresponding author states that there are no conflicts of interest.
Cite this as
Hilger C, Kuehn A, Raulf M, Jakob T. Cockroach, tick, storage mite and other arthropod allergies: Where do we stand with molecular allergy diagnostics? Part 15 of the Series Molecular Allergology. Allergo J Int 2014;23:172–8 DOI: 10.1007/s40629-014-0024-2
References
- 1.Vrtala S., Kleine-Tebbe J. Hausstaubmilbenallergene und ihre Bedeutung. Allergo J. 2013;22:546–9. doi: 10.1007/s15007-013-0441-4. [DOI] [Google Scholar]
- 2.Spillner E., Blank S., Jakob T. Potenzial, Fallstricke und aktueller Status der molekularen Diagnostik am Beispiel der Insektengiftallergie. Allergo J. 2012;21:249–56. doi: 10.1007/s15007-012-0128-2. [DOI] [Google Scholar]
- 3.Pomés A., Arruda L.K. Investigating cockroach allergens: aiming to improve diagnosis and treatment of cockroach allergic patients. Methods. 2013;66:75–85. doi: 10.1016/j.ymeth.2013.07.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Matsui E.C., Wood R.A., Rand C., Kanchanaraksa S., Swartz L., Curtin-Brosnan J., Eggleston P.A. Cockroach allergen exposure and sensitization in suburban middle-class children with asthma. J Allergy Clin Immunol. 2003;112:87–92. doi: 10.1067/mai.2003.1588. [DOI] [PubMed] [Google Scholar]
- 5.Cohn R.D., Arbes S.J., Jr, Jaramillo R., Reid L.H., Zeldin D.C. National prevalence and exposure risk for cockroach allergen in U.S. households. Environ Health Perspect. 2006;114:522–6. doi: 10.1289/ehp.8561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Raulf M., Sander I., Gonnissen D., Zahradnik E., Brüning T. Schaben und Co. Die Rolle von Gesundheitsschädlingen als Allergenquelle. Bundesgesundheitsbl. 2014;57:585–92. doi: 10.1007/s00103-013-1926-8. [DOI] [PubMed] [Google Scholar]
- 7.Hirsch T., Stappenbeck C., Neumeister V., Weiland S.K., von Mutius E., Keil U., Leupold W. Exposure and allergic sensitization to cockroach allergen in East Germany. Clin Exp Allergy. 2000;30:529–37. doi: 10.1046/j.1365-2222.2000.00785.x. [DOI] [PubMed] [Google Scholar]
- 8.Heinzerling L.M., Burbach G.J., Edenharter G., Bachert C., Bindslev-Jensen C., Bonini S., et al. GA(2)LEN skin test study I: GA(2)LEN harmonization of skin prick testing: novel sensitization patterns for inhalant allergens in Europe. Allergy. 2009;64:1498–506. doi: 10.1111/j.1398-9995.2009.02093.x. [DOI] [PubMed] [Google Scholar]
- 9.Sohn M.H., Kim K.E. The cockroach and allergic diseases. Allergy Asthma Immunol Res. 2012;4:264–9. doi: 10.4168/aair.2012.4.5.264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Barbosa M.C., Santos A.B., Ferriani V.P., Pomés A., Chapman M.D., Arruda L.K. Efficacy of recombinant allergens for diagnosis of cockroach allergy in patients with asthma and/or rhinitis. Int Arch Allergy Immunol. 2013;161:213–9. doi: 10.1159/000346318. [DOI] [PubMed] [Google Scholar]
- 11.Binder M., Mahler V., Hayek B., Sperr W.R., Schöller M., Prozell S., et al. Molecular and immunological characterization of arginine kinase from the Indian meal moth, Plodia interpunctella, a novel cross-reactive invertebrate pan-allergen. J Immunol. 2001;167:5470–7. doi: 10.4049/jimmunol.167.9.5470. [DOI] [PubMed] [Google Scholar]
- 12.Wang J., Calatroni A., Visness C.M., Sampson H.A. Correlation of specific IgE to shrimp with cockroach and dust mite ex-posure and sensitization in an inner-city population. J Allergy Clin Immunol. 2011;128:834–7. doi: 10.1016/j.jaci.2011.07.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hilger C., Bessot J.C., Hutt N., Grigioni F., De Blay F., Pauli G., Hentges F. IgE-mediated anaphylaxis caused by bites of the pigeon tick Argas reflexus: cloning and expression of the major allergen Arg r 1. J Allergy Clin Immunol. 2005;115:617–22. doi: 10.1016/j.jaci.2004.11.052. [DOI] [PubMed] [Google Scholar]
- 14.Kleine-Tebbe J., Heinatz A., Gräser I., Dautel H., Hansen G.N., Kespohl S., et al. Bites of the European pigeon tick (Argas reflexus): Risk of IgE-mediated sensitizations and anaphylactic reactions. J Allergy Clin Immunol. 2006;117:190–5. doi: 10.1016/j.jaci.2005.08.056. [DOI] [PubMed] [Google Scholar]
- 15.Commins S.P., James H.R., Kelly L.A., Pochan S.L., Workman L.J., Perzanowski M.S., et al. The relevance of tick bites to the production of IgE antibodies to the mammalian oligosaccharide galactose-α-1,3-galactose. J Allergy Clin Immunol. 2011;127:1286–93. doi: 10.1016/j.jaci.2011.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.van Hage-Hamsten M., Johansson E. Clinical and immunologic aspects of storage mite allergy. Allergy. 1998;53:49–53. doi: 10.1111/j.1398-9995.1998.tb04997.x. [DOI] [PubMed] [Google Scholar]
- 17.FernÄndez-Caldas E., Iraola V., Carnés J. Molecular and biochemical properties of storage mites (except Blomia species) Protein Pept Lett. 2007;14:954–9. doi: 10.2174/092986607782541033. [DOI] [PubMed] [Google Scholar]
- 18.Franz J.T., Masuch G., Müsken H., Bergmann K.C. Mite fauna of German farms. Allergy. 1997;52:1233–7. doi: 10.1111/j.1398-9995.1997.tb02529.x. [DOI] [PubMed] [Google Scholar]
- 19.SÄnchez-Borges M., SuÄrez Chacón R., Capriles-Hulett A., Caballero-Fonseca F., FernÄndez-Caldas E. Anaphylaxis from ingestion of mites: pancake anaphylaxis. J Allergy Clin Immunol. 2013;131:31–5. doi: 10.1016/j.jaci.2012.09.026. [DOI] [PubMed] [Google Scholar]
- 20.Simons F.E., Peng Z. Mosquito allergy: recombinant mosquito salivary antigens for new diagnostic tests. Int Arch Allergy Immunol. 2001;124:403–5. doi: 10.1159/000053771. [DOI] [PubMed] [Google Scholar]
- 21.Ma D., Li Y., Dong J., An S., Wang Y., Liu C., et al. Purification and characterization of two new allergens from the salivary glands of the horsefly, Tabanus yao. Allergy. 2011;66:101–9. doi: 10.1111/j.1398-9995.2010.02435.x. [DOI] [PubMed] [Google Scholar]
- 22.An S., Chen L., Wei J.F., Yang X., Ma D., Xu X., et al. Purification and characterization of two new allergens from the venom of Vespa magnifica. PLoS One. 2012;7:e31920. doi: 10.1371/journal.pone.0031920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Baur X., Liebers V. Insect hemoglobins (Chi tI) of the diptera family Chironomidae are relevant environmental, occupational, and hobby-related allergens. Int Arch Occup Environ Health. 1992;64(3):185–8. doi: 10.1007/BF00380907. [DOI] [PubMed] [Google Scholar]
- 24.Barletta B., Butteroni C., Puggioni E.M., Iacovacci P., Afferni C., Tinghino R., et al. Immunological characterization of arecombinant tropomyosin from a new indoor source, Lepisma saccharina. Clin Exp Allergy. 2005;35:483–9. doi: 10.1111/j.1365-2222.2005.02214.x. [DOI] [PubMed] [Google Scholar]
- 25.Nakazawa T., Satinover S.M., Naccara L., Goddard L., Dragulev B.P., Peters E., Platts-Mills T.A. Asian ladybugs (Harmonia axyridis): a new seasonal indoor allergen. J Allergy Clin Immunol. 2007;119:421–7. doi: 10.1016/j.jaci.2006.11.633. [DOI] [PubMed] [Google Scholar]
- 26.Wood R.A., Togias A., Wildfire J., Visness C.M., Matsui E.C., Gruchalla R., et al. Development of cockroach immunotherapy by the Inner-City Asthma Consortium. J Allergy Clin Immunol. 2014;133:846–52. doi: 10.1016/j.jaci.2013.08.047. [DOI] [PMC free article] [PubMed] [Google Scholar]