Abstract
REM sleep decreases dramatically during development. We tested the hypothesis that some of this decrease may be due to GABAergic inhibition of reticular activating system neurons. Recordings of pedunculopontine neurons in vitro showed that the GABAa receptor agonist muscimol depolarized non-cholinergic cells early in the developmental decrease in REM sleep, and hyperpolarized them later. Most cholinergic cells were hyperpolarized throughout the period tested. The GABAb receptor agonist baclofen hyperpolarized both cholinergic and non-cholinergic cells, although the degree of polarization decreased with age. Part of the gradual decrement in REM sleep during development may be due in part to the increasing inhibition mediated by GABAa receptors on pedunculopontine neurons. However, this influence appears to be mainly on non-cholinergic cells.
Keywords: arousal, rapid eye movement (REM) sleep, reticular activating system
Introduction
Rapid eye movement (REM) sleep may help direct the course of brain maturation [1]. REM sleep decreases from ~8 hrs (50% of sleep time) to ~1 hour (15%) in the adult, mostly from birth until the end of puberty [2]. In the rat, the decrease in REM sleep occurs between 10–30 days postnatally, declining from ~70% to ~15% of sleep time [3]. This may be due to a REM sleep inhibitory process during the first two weeks of life [4]. We hypothesize that, if the developmental decrease in REM sleep does not occur, a lifelong increase in REM sleep drive may ensue [5]. Such a mechanism may be responsible for the postpubertal onset of disorders marked by increased REM sleep drive, such as schizophrenia, anxiety disorder, and depression [5].
The pedunculopontine nucleus (PPN), the cholinergic arm of the reticular activating system (RAS), modulates waking and REM sleep [6]. Stimulation of this region increased REM sleep [7], while lesions of the PPN decreased REM sleep [8]. PPN neurons show tonic activity in waking, tonic and bursting activity in REM sleep, and reduced activity during slow wave sleep [6, 9]. In the rat, PPN neurons are active during waking and REM sleep, but with little bursting activity [10]. We have been studying developmental changes in the PPN to determine those factors most correlated with the developmental decrease in REM sleep [5]. This study tested the hypothesis that PPN neurons are differentially modulated during development by the γ-amino-butyric acid (GABA) agonist muscimol (MUS) (specific for GABAa receptors) and the GABA agonist baclofen (BAC) (specific for GABAb receptors). Preliminary findings were reported [11].
Methods
Sprague-Dawley rats (n=80, ages 12–21 days) were anaesthetized (ketamine, 60–70 mg/kg, im, until reflexes were absent) and decapitated. Sagittal brainstem slices were cut at 400 μm and maintained in oxygenated artificial cerebrospinal fluid [12]. Intracellular recordings were carried out with 80–100 MΩ microelectrodes filled with 3M K acetate and 1% neurobiotin. Selected cells were injected intracellularly (500 msec, 0.5 nA cathodal pulses at 1 Hz for 10 min), the slice fixed in 4% paraformaldehyde and processed for immunocytochemical labeling of neurobiotin-filled neurons using Texas Red-avidin and histochemically for localization of NADPH diaphorase-labeled PPN neurons [12]. PPN neurons were identified as type I (low threshold spike- LTS), type II (A current) or type III (A+LTS) [12]. Cellular responses to MUS (50μM) or BAC (30μM) were tested by micropressure application. Tetrodotoxin (TTX, 0.3μM) was used to block sodium channels and determine if responses were directly postsynaptic. Chi square tests compared distributions of responses over age, and analysis of variance assessed differences in response amplitudes. Procedures were approved by the UAMS Animal Care Committee and comply with NIH Guidelines for the ethical and humane care of laboratory animals.
Results
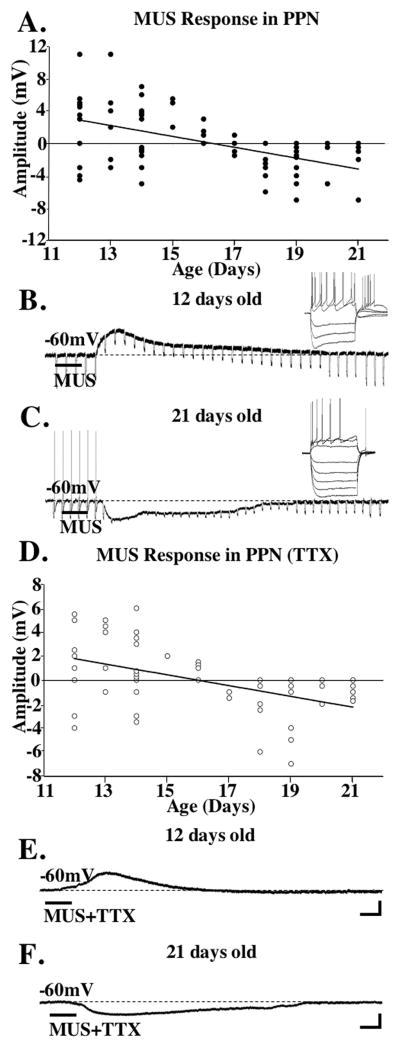
We recorded from 84 PPN neurons, 67/84 were identified as type II (A current) and 17/84 were type III (A+LTS currents), with no type I cells sampled [12]. Of these, 48/84 were identified as to phenotype, 13/37 type II cells were cholinergic (ACh+) and 24/37 type II neurons were non-cholinergic (ACh−), while 2/11 type III cells were ACh+ and 9/11 type III cells were ACh−. MUS depolarized 56% of type II PPN cells 12–16 days old (Mean±SE; +3.5±0.8mV, n=14), while 20% were hyperpolarized (−2.6±0.4mV, n=5), and 6 did not respond (n=6). Of the 17–21 day cells, 45% were hyperpolarized (−1.6±0.3mV, n=20), 50% had no response (n=10) and one, a 17-day cell, was depolarized. In TTX, 40% of 12–16 day cells showed direct depolarizing responses (2.9±0.5mV, n=10), 52% had no response to MUS (n=13), and 8% were depolarized (2.0±0.3mV, n=2). However, 31% of 17–21 day cells were directly hyperpolarized (−1.4±0.3mV, n=5), 69% had no response (n=11), and none were depolarized.
Figure 1A shows a plot of responses over age using MUS, and 1D shows a plot of cells that responded to MUS in TTX. Figures 1B and 1E show a representative ACh− type III 12 day cell that was depolarized by MUS. Figures 1C and 1F show the hyperpolarization induced by MUS on a ACh+ type II 21 day cell. Although MUS depolarized some PPN cells early during the period tested, only one or no action potential was induced (as in this case), followed by an apparent shunting of the membrane probably due to a decrease in membrane resistance [13]. The input resistance for cells depolarized by MUS decreased by 15±7% (n=4, 12–15 day cells depolarized by MUS).
Figure 1. Responses of PPN cells to MUS.
A. Plot of PPN cells recorded after application of MUS across age. Depolarizing responses are above “0”, hyperpolarizing responses are below “0” on the x-axis. Unresponsive cells shown as filled circles on the x-axis with overlapping circles close to “0” denoting no response. The y-axis indicates the maximum membrane change during the response. The regression line is y = −0.6716x + 10.928, R squared = 0.2622. B. Recording from a 12 day type III PPN cell after MUS application. The inset shows depolarizing and hyperpolarizing steps in this LTS cell. C. Recording from a 21 day type II PPN neuron showing a hyperpolarization in response to MUS. The inset shows the presence of Ia conductance in this type II cell. D. Plot of PPN cells recorded after MUS+TTX. The distribution of responses across the two age ranges (12–15 and 17–21 days) was significantly different after MUS (Chi square= 13.02, df=2, p<0.004), and after MUS in TTX (Chi square=7.57, df=2, p<0.02). The amplitude of the depolarization at 12–16 days under MUS before and after TTX was not statistically different (ANOVA df=23, F=0.33, p=0.6). The amplitude of the hyperpolarization at 12–16 days did not differ from that at 17–21 days (ANOVA df=4, F=2.21, p=0.2). The amplitude of the hyperpolarization at 17–21 days under MUS before and after TTX was not statistically different (ANOVA df=13, F=2.21, p=0.16). The regression line is y = −0.4503x + 7.1882, R squared=0.2299. E. Recording from the same cell as in 1B except in TTX. Note the depolarization persisted after sodium channel blockade. F. Recording from the same cell as in 1C showing a hyperpolarization after MUS in TTX. Calibration bars 250 msec and 25 mV for the inset in B, and 2 sec and 3 mV for B and E; 250 msec and 25 mV for the inset in C, and 2 sec and 2 mV for C and F.
Cholinergic (ACh+) PPN neurons showed mainly hyperpolarizing effects following MUS. Of the type II ACh+ cells, 8 were hyperpolarized and were aged 12–21 days, while only 3 were depolarized and were aged 12–16 days, and 2 aged 19 and 21 days showed no significant effect. Of the type III ACh+ cells, 1 aged 12 days was depolarized, and 1 aged 18 days showed no response. ACh+ cells showed mainly hyperpolarization, with some depolarization early in development. Non-cholinergic (ACh−) PPN neurons showed most of the early depolarizing responses. Of the type II ACh− cells, 9 were depolarized and were aged 12–16 days, 9 were hyperpolarized and were aged 13–21 days, and 6 were aged 13–21 days and showed no response. Of the type III ACh− cells, 4 were depolarized and were aged 14–18 days, 3 were hyperpolarized and were 14–21 days, and 2 showed no response. These results suggest that ACh− PPN cells contributed more to the early depolarizing responses to MUS than ACh+ cells.
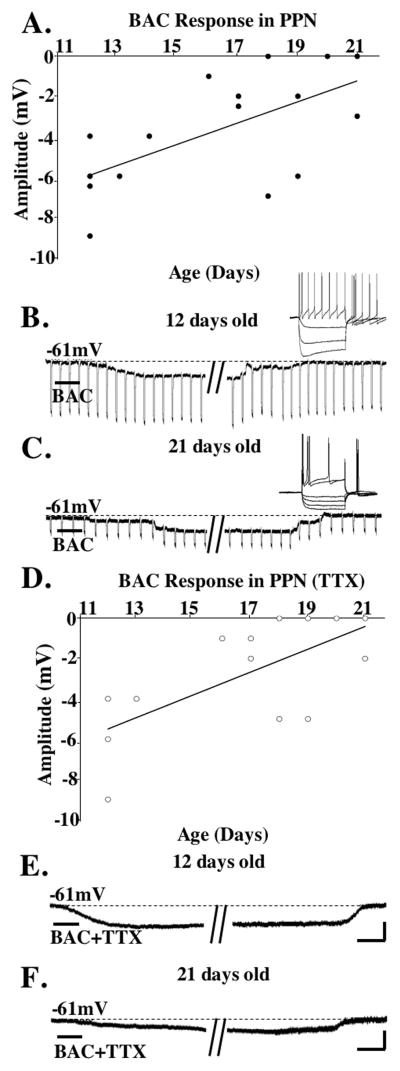
BAC hyperpolarized 92% of 12–16 day cells (−5.3±1.3mV, n=11) and 1 cell failed to show a response (8%), while 69% of 17–21 day cells were hyperpolarized (−3.2±0.7mV, n=9), and 31% had no response (n=4). In TTX, 89% of the 12–16 day cells were hyperpolarized (−4.2±0.9mV, n=8) and 11% had no response to BAC (n=1), while 31% of 17–21 day cells were hyperpolarized (−2.8±0.7mV, n=4) and 68% showed no response (n=9). This suggests that many of the GABAb responses observed later in development were not directly on the neurons recorded but may have been presynaptic.
Figure 2A shows a plot of the responses to BAC, and 2D shows a plot of responses to BAC in TTX. Figures 2B and 2E show the hyperpolarization induced on a ACh− type II cell after BAC. Figures 2C and 2F show a hyperpolarization induced on a ACh+ type II cell after BAC.
Figure 2. Responses of PPN cells to BAC.
A. Plot of PPN cells after application of BAC across age. Hyperpolarizing responses shown below “0” on the x-axis. Unresponsive cells shown as filled circles on the x-axis with overlapping circles denoting no response. The y-axis indicates the maximum membrane change induced during the response. The regression line was y= 0.5249x + 12.25, R squared= 0.3925. B. Recording from a 12 day type II PPN cell after BAC. C. Recording from a 21 day type II PPN neuron showing a hyperpolarization after BAC. The inset shows depolarizing and hyperpolarizing steps showing the presence of Ia conductance. D. Plot of PPN cells after BAC+TTX. The distribution of responses in the two age ranges following BAC were not statistically different before (Chi square=1.97, df=2, p=0.16) or after TTX (Chi square=2.48, df=2, p=0.11). The amplitude of the hyperpolarization to BAC was not statistically different across age (ANOVA df=19, F=2.93, p=0.1) or before and after TTX (ANOVA df=17, F=0.45, p=0.5). The regression line is y=0.5665x + 12.295, R squared= 0.4552. E. Recording from the same cell as in 2B except in TTX. Note the hyperpolarization persisted after sodium channel blockade. F. Recording from the same cell as in C showing a hyperpolarization after BAC in TTX. Calibration bars 250 msec and 25 mV for the inset in B, and 15 sec and 6 mV for B and E; 250 msec and 10 mV for the inset in C, and 15 sec and 3 mV for C and F.
Both ACh+ and ACh− PPN neurons showed mainly hyperpolarization after BAC. Of the type II ACh+ cells, 5/5 were hyperpolarized and were aged 12–21 days, while none were depolarized across 12–21 days. The single type III ACh+ cell tested was aged 18 days and showed no response. ACh+ cells showed mainly hyperpolarization. Similarly, 7/7 ACh− type II PPN neurons were hyperpolarized and were aged 12–21 days, and 2 ACh− type II cells were aged 20–21 days and showed no response. These results suggest that ACh− PPN cells also showed mainly hyperpolarization after BAC.
A group of ACh+ (n=6, 5 type II and 1 type III) and ACh− (n=9, all type II) cells were tested for responses to both MUS and BAC. Of the ACh+ cells, 3 were hyperpolarized by both MUS and BAC, one 12-day cell was depolarized by MUS and hyperpolarized by BAC, one 19-day cell was not affected by MUS but hyperpolarized by BAC, and one 18-day cell, the type III cell sampled, was not responsive. Of the ACh− cells, 3 were depolarized by MUS and hyperpolarized by BAC (aged 12–13 days), 3 were hyperpolarized by both MUS and BAC, 1 was not affected by MUS but hyperpolarized by BAC, 1 was not affected by BAC but hyperpolarized by MUS, and 1 not affected. Input resistance following BAC was decreased by both MUS (27±15%) and BAC (11±4%) (n=4 cells hyperpolarized by both MUS and BAC).
Discussion
Our findings suggest that GABAa receptor activation leads to depolarization of PPN neurons early in development (12–16 days), but to hyperpolarization later (17–21 days) during the developmental decrease in REM sleep. These effects persisted in TTX and were assumed to be postsynaptic. The majority of depolarized cells early in development were ACh− PPN cells, while most ACh+ PPN cells were hyperpolarized in both early and later periods. GABAb receptor activation hyperpolarized both ACh+ and ACh− PPN neurons during both early and later periods. GABA, the primary inhibitory influence in the adult brain, was found to be excitatory during development due to the delayed expression of chloride co-transporters [13]. Ben-Ari showed that GABAa receptor regulated channels represent the earliest excitatory influence in the neonatal brain [13]. Our results suggest that PPN neurons, especially ACh− cells, show depolarization early in development shifting to hyperpolarization later during the developmental decrease in REM sleep. We do not know if the differential responses in ACh+ neurons (i.e. mostly hyperpolarization with few cells depolarizing) are due to earlier maturation of chloride transporters in ACh+ compared to ACh− cells, or to other factors. This developmental shift occurred only in response to the GABAa agonist MUS and not to the GABAb agonist BAC. It is unknown if a delay in maturation of chloride transporters in PPN cells is limited to channels activated by GABAa and not to GABAb receptors.
GABAergic neurons in the PPN are intermingled with ACh+ neurons [14] and express c-fos during carbachol-induced REM sleep in the cat [15]. In the cat, there is co-localization of GABA and ACh in PPN cells, suggesting possibile co-release by PPN neurons involved in the regulation of behavioral states [16]. The PPN also receives GABAergic projections from the substantia nigra [17, 18], which terminate mostly on ACh− neurons [19], but also terminate on ACh+ neurons [20]. While we cannot speculate as to the origin of the GABAergic afferents to the cells recorded, our results suggest that both ACh+ and ACh− PPN cells are modulated by GABAergic input, and that these responses persist in the presence of TTX, i.e. these responses are postsynaptic and/or there is a TTX-resistant presynaptic mechanism present at least in some cases.
Injection of picrotoxin, a GABAa receptor antagonist, into the PPN reduced REM sleep [21], while injection of MUS increased the frequency and duration of REM sleep [22]. Our results with MUS showed that maturation of GABAa responses in the PPN led to hyperpolarization of both ACh+ and ACh− cells. It is not known how these cells may modulate the frequency and duration of REM sleep episodes. On the other hand, a recent study in awake rats showed that the GABAb agonist BAC suppressed REM sleep [23]. These authors did not observe modulation of REM sleep using a GABAa agonist, in keeping with other results [24]. Our findings showed that both ACh+ and ACh− PPN cells were hyperpolarized by BAC, both of which would be affected by infusions of BAC into the region, but cannot speculate if both receptor types may be involved in modulating REM sleep, which remains a limitation of work on slices. Future studies need to determine the roles of identified ACh+ and ACh− cells during changes in sleep-wake state, which has only been possible using extracellular recordings of unidentified cells. Our findings agree with the suggestion that the sedative effects of anesthesia may be mediated by GABAergic receptors in the sleep-wake control pathway [25]. GABAa responses in the adult are clearly inhibitory, and our studies suggest that both ACh+ and ACh− PPN neurons could both be affected by anesthetics. However, the hyperpolarization induced by MUS decreased with age, suggesting that this inhibition is modest.
Additional studies are needed to determine if this degree of hyperpolarization persists in adulthood. Our results indicate that, if anesthetics act via GABA receptors in this pathway, the effects would be less effective (may actually produce excitation) if anesthesia is induced before the developmental maturation of chloride co-transporters. Interestingly, GABA becomes excitatory in adult patients with epilepsy [13], suggesting that a reversal of the developmental shift from depolarization to hyperpolarization could be a factor in some pathological states. Future studies need to address the potential effects of dysregulation in this process that could prevent or reduce the developmental decrease in REM sleep, and lead to a number of devastating disorders [5].
Conclusion
These results show a shift from excitation to inhibition by GABAa receptor activation, while GABAb receptor activation is inhibitory but becomes less pronounced, across the developmental decrease in REM sleep. The gradual decrement in the percent of REM sleep during this period may be due in part to the increasing inhibition mediated by GABAa receptors postsynaptically on PPN neurons. However, this influence appears to be mainly on ACh− PPN cells.
Acknowledgments
Supported by USPHS grants NS20246 and RR020146.
References
- 1.Marks GA, Shaffery JP, Oksenberg A, Speciale SG, Roffwarg HP. A functional role for REM sleep in brain maturation. Behav Brain Res. 1995;69:1–11. doi: 10.1016/0166-4328(95)00018-o. [DOI] [PubMed] [Google Scholar]
- 2.Roffwarg HP, Muzio JN, Dement WC. Ontogenetic development of the human sleep-dream cycle. Science. 1966;152:604–619. doi: 10.1126/science.152.3722.604. [DOI] [PubMed] [Google Scholar]
- 3.Jouvet-Mounier D, Astic L, Lacote D. Ontogenesis of the states of sleep in rat, cat, and guinea pig during the first postnatal month. Dev Psychobiol. 1970;2:216–239. doi: 10.1002/dev.420020407. [DOI] [PubMed] [Google Scholar]
- 4.Vogel GW, Feng P, Kinney GG. Ontogeny of REM sleep in rats: possible implications for endogenous depression. Physiol Behav. 2000;68:453–461. doi: 10.1016/s0031-9384(99)00207-3. [DOI] [PubMed] [Google Scholar]
- 5.Garcia-Rill E, Kobayashi T, Good C. The developmental decrease in REM sleep. Thal & Rel Syst. 2003;2:115–131. [Google Scholar]
- 6.Steriade M, McCarley RW. Brainstem Control of Wakefulness and Sleep. Plenum Press; New York, NY: 1990. p. 499. [Google Scholar]
- 7.Thakkar M, Portas C, McCarley RW. Chronic low-amplitude electrical stimulation of the laterodorsal tegmental nucleus of freely moving cats increases REM sleep. Brain Research. 1989;723:223–227. doi: 10.1016/0006-8993(96)00256-9. [DOI] [PubMed] [Google Scholar]
- 8.Webster HH, Jones BE. Neurotoxic lesions of the dorsolateral pontomesencephalic tegmentum-cholinergic cell area in cat. II. Effects upon sleep-waking states. Brain Res. 1988;458:285–302. doi: 10.1016/0006-8993(88)90471-4. [DOI] [PubMed] [Google Scholar]
- 9.El Mansari M, Sakai K, Jouvet M. Unitary characteristics of presumptive cholinergic tegmental neurons during the sleep-waking cycle in freely moving cats. Exptl Brain Res. 1989;76:519–529. doi: 10.1007/BF00248908. [DOI] [PubMed] [Google Scholar]
- 10.Datta S, Siwek DF. Single cell activity patterns of pedunculopontine tegmentum neurons across the sleep-wake cycle in the freely moving rat. J Neurosci Res. 2002;70:611–621. doi: 10.1002/jnr.10405. [DOI] [PubMed] [Google Scholar]
- 11.Bay K, Skinner RD, Garcia-Rill E. The roles of GABAa and GABAb in the pedunculopontine nucleus (PPN) during REM sleep development. Neusoci Abst. 2005;31:63.20. [Google Scholar]
- 12.Kobayashi T, Good C, Biedermann J, Barnes C, Skinner RD, Garcia-Rill E. Developmental changes in pedunculopontine (PPN) neurons. J Neurophysiol. 2004;91:1470–1481. doi: 10.1152/jn.01024.2003. [DOI] [PubMed] [Google Scholar]
- 13.Cossart R, Bernard C, Ben-Ari Y. Multiple facets of GABAergic neurons and synapses: multiple fates of GABA signalling in epilepsies. Trends Neurosci. 2005;28:108–115. doi: 10.1016/j.tins.2004.11.011. [DOI] [PubMed] [Google Scholar]
- 14.Ford B, Holmes CJ, Mainville L, Jones BE. GABAergic neurons in the rat mesencephalic tegmentum: codistribution with cholinergic and other tegmental neurons projecting to the lateral hypothalamus. J Comp Neurol. 1995;363:177–196. doi: 10.1002/cne.903630203. [DOI] [PubMed] [Google Scholar]
- 15.Torterolo P, Yamuy J, Sampogna S, Morales FR, Chase MH. GABAergic neurons of the laterodorsal and pedunculopontine tegmental nuclei of the cat express c-fos during carbachol-induced active sleep. Brain Res. 2001;892:309–319. doi: 10.1016/s0006-8993(00)03264-9. [DOI] [PubMed] [Google Scholar]
- 16.Jia HG, Yamuy J, Sampogna S, Morales FR, Chase MH. Colocalization of γ-amino-butyric acid and acetylcholine in neurons in the laterodorsal and pedunculopontine tegmental nuclei in the cat: a light and electron microscopy study. Brain Res. 2003;992:205–219. doi: 10.1016/j.brainres.2003.08.062. [DOI] [PubMed] [Google Scholar]
- 17.Beckstead RM, Domesick VB, Nauta WJH. Efferent connections of the substantia nigra and ventral tegmental area in the rat. Brain Res. 1979;175:191–217. doi: 10.1016/0006-8993(79)91001-1. [DOI] [PubMed] [Google Scholar]
- 18.Rye DB. Contributions of the pedunculopontine region to normal and altered REM sleep. Sleep. 1997;20:757–788. doi: 10.1093/sleep/20.9.757. [DOI] [PubMed] [Google Scholar]
- 19.Steininger TL, Rye DB, Wainer BH. Afferent projections to the cholinergic pedunculopontine nucleus and adjacent midbrain extrapyramidal area in the albino rat. J Comp Neurol. 1992;321:515–543. doi: 10.1002/cne.903210403. [DOI] [PubMed] [Google Scholar]
- 20.Grofova I, Zhou M. Nigral innervation of cholinergic and glutamatergic cells in the rat mesopontine tegmentum: light and electron microscopic anterograde tracing and immunohistochemical studies. J Comp Neurol. 1998;395:359–379. [PubMed] [Google Scholar]
- 21.Pal D, Mallick BN. GABA in pedunculo pontine tegmentum regulates spontaneous rapid eye movement sleep by acting on GABAA receptors in freely moving rats. Neurosci Lett. 2004;365:200–204. doi: 10.1016/j.neulet.2004.04.080. [DOI] [PubMed] [Google Scholar]
- 22.Torterolo P, Morales FR, Chase MH. GABAergic mechanisms in the pedunculopontine tegmental nucleus of the cat promote active (REM) sleep. Brain Res. 2002;944:1–9. doi: 10.1016/s0006-8993(02)02475-7. [DOI] [PubMed] [Google Scholar]
- 23.Ulloor J, Mavanji V, Saha S, Siwek DF, Datta S. Spontaneous REM sleep is modulated by the activation of the pedunculopontine tegmental GABAb receptors in the freely moving rat. J Neurophysiol. 2004;91:1822–1831. doi: 10.1152/jn.01104.2003. [DOI] [PubMed] [Google Scholar]
- 24.Sanford LD, Tang X, Xiao J, Ross RJ, Morrison AR. Microinjection into the pedunculopontine tegmentum: effects of the GABA-A antagonist, bicuculline, on sleep, PGO waves and behavior. Arch Ital Biol. 1998;136:205–214. [PubMed] [Google Scholar]
- 25.Lydic R, Baghdoyan H. Sleep, anesthesiology, and the neurobiology of arousal state control. Anesthesiology. 2005;103:1268–1295. doi: 10.1097/00000542-200512000-00024. [DOI] [PubMed] [Google Scholar]