Abstract
The γ-aminobutyric acid-A (GABAA) and N-methyl-D-aspartate (NMDA) receptors mediate aspects of the behavioural effects of alcohol. Prior studies reported drugs that block NMDA receptors or facilitate GABAA receptor function produce ethanol-like effects in humans. The purpose of this study was to compare the ethanol-related effects of two pharmacological agents with known NMDA and GABAA receptor activity. As part of an ongoing, larger study, 28 subjects (age, 21–30) with no personal or family histories of alcoholism were administered subanesthetic doses of the GABAA receptor agonist thiopental, the NMDA receptor antagonist, ketamine and placebo on three separate test days. Various ethanol-related measures were administered. At doses of thiopental and ketamine that produced similar levels of sedation and cognitive effects, both agents produced significant ethanol-like effects and subjective intoxication. However, the intensity of the ethanol-like effects of ketamine was greater than that of thiopental. In addition, ketamine produced alterations in perception that were not produced by thiopental. These data provide further support for a model where GABAA receptor facilitation may contribute significantly to ethanol effects associated with social drinking, whereas NMDA receptor antagonism may contribute to relatively greater extent to features of ethanol ‘intoxication’.
Keywords: ethanol, GABA, ketamine, NMDA, thiopental
Introduction
A growing body of evidence has identified the γ-aminobutyric acid-A (GABAA) and N-methyl-D-aspartate (NMDA) receptors as important sites for mediating ethanol’s effects (Mehta and Ticku, 1990; Aguayo, et al., 2002; Krystal, et al., 2003a; Krystal, et al., 2003b; Krystal, et al., 2003c; Boehm, et al., 2006; Krystal, et al., 2006). Ethanol has direct and indirect effects on GABAA receptors. For example, ethanol enhances the activation of synaptic GABAA receptors by stimulating GABA release. It also may enhance the stimulation of extrasynaptic GABAA receptors by directly stimulating these receptors and by raising the levels of neurosteroids (Morrow, et al., 2001; Criswell and Breese, 2005; Siggins, et al., 2005; Weiner and Valenzuela, 2006). Animals with genetic deletions of several GABAA receptor subunits show reduced ethanol response (Boehm, et al., 2004). Also, GABAA receptor inverse agonist drugs appear to have the ability to block aspects of the physiologic and behavioural effects of ethanol (Krystal, et al., 2006).
NMDA glutamate receptors are among the highest affinity alcohol targets in the brain (Grant and Lovinger, 1995). NMDA receptor antagonists substitute for alcohol, particularly at higher doses of alcohol (Grant, 1999). Also, mice strains differing in their NMDA receptor density in several brain regions (Valverius, et al., 1990) also differ in their capacity to discriminate alcohol from other substances (Balster, et al., 1993). In humans, NMDA receptor antagonists, including ketamine (Krystal and Raskin, 1970; Krystal, et al., 1998; Krupitsky, et al., 2001; Petrakis, et al., 2004a) and dextromethorphan (Schutz and Soyka, 2000), have ethanol-like effects in healthy human subjects and recovering alcohol-dependent patients.
Psychopharmacologic techniques have been useful for characterising ethanol-like effects mediated by GABAA and NMDA receptors in humans, including studies of the GABAA agonists, benzodiazepines and barbiturates (McCaul, et al., 1990; McCaul, et al., 1991; Schuckit, et al., 1991; Cowley, et al., 1992; Cowley, et al., 1994; Volkow, et al., 1995; Cowley, et al., 1996; Krystal, et al., 1998), and of the NMDA antagonist, ketamine (Krystal, et al., 2003b; Petrakis, et al., 2004a). These studies suggest that the interplay of ethanol actions at GABA and NMDA receptors influence the expression of ethanol intoxication at varying levels of ethanol consumption (Grant and Colombo, 1993; Krystal, et al., 1998; Krystal, et al., 2003c). At lower ethanol doses, the facilitation of GABAA receptor function may be important in mediating the stimulatory and anxiolytic effects. Higher ethanol doses that often produce dysphoric mood, impaired coordination and cognitive impairments may be medicated predominately by blockade of NMDA receptors (Krystal, et al., 2003a).
The purpose of this study was to compare the subjective behavioural effects of GABAA receptor stimulation, using the short-acting barbiturate, thiopental, and NMDA receptor blockade, using the NMDA receptor antagonist ketamine, in healthy volunteers with no family or personal history of alcohol dependence. We used doses which produced similar levels of sedation and hypothesised that thiopental would better approximate effects associated with lower levels of ethanol intoxication, and ketamine would better approximate higher levels of ethanol intoxication.
Methods
Subjects
As part of an ongoing study of the neurobiology of the heritable risk for alcoholism, healthy individuals (n = 28) were recruited by advertisement and compensated for their participation. Inclusion criteria included 1) no lifetime axis I psychiatric or substance use disorder, 2) medically and neurologically healthy on the basis of history, physical examination, electrocardiogram and screening laboratories and 3) no family history of alcoholism in any first-or second-degree relatives. Exclusion criteria included 1) individuals with a history of counseling or psychotherapy, except family therapy centered around another family member, 2) extended unwillingness to remain alcohol-free for three days prior to testing, 3) positive urine toxicology for drugs including marijuana, cocaine, benzodiazepines, amphetamines or opioids on test days, 4) for women, positive pregnancy test at screening or intention to engage in unprotected sex during the study, 5) being alcohol naïve, 6) previous unpleasant experience with thiopental or ketamine and 7) adoptees with no contact with family members. This study was approved by the Institutional Review Boards of the VA Connecticut Healthcare System and of Yale University, School of Medicine.
After signing informed consent, subjects underwent baseline screening, including structured interview, physical examination and laboratory assessment including urine toxicology. Prior to administration of any study medication, subjects were warned that both thiopental and ketamine had addictive potential, and that their effects resembled the effects of alcohol. Individuals were encouraged not to participate if they were concerned about an increased risk for the subsequent development of a substance use disorder. Subjects were then scheduled to receive thiopental, ketamine and placebo on three separate test days at least three days apart in a randomized order under double-blind conditions. Prior to each test session, participants fasted overnight and remained in a fasting state during the test session. They presented to the Biological Studies Unit at VA Connecticut Healthcare System, West Haven campus, at approximately 8:30 a.m. Prior to testing, subjects underwent urine drug screening for toxicology and breathalyzer screening, and after all tests were negative, an intravenous line was placed. On the test day, patients received a 60-minute infusion of thiopental at a 1.5 mg/kg loading dose and infusion rate of 40 mcg/kg/min; ketamine at a 0.23 mg/kg loading dose and infusion rate of 58 mcg/kg/min; or a saline solution (infusion commenced at time point 0). The dose of each medication was chosen to achieve a desired sedation (relaxed with eyes open), whereby patients were able to complete challenge paperwork. The medications were administered by an anaesthesiologist (AP) in accordance with hospital conscious sedation policies.
Subjective intoxication ratings were assessed at 15, 45, 80, 100, 170 and 230 minutes after the start of infusion using the Number of Drinks Scale (NDS), the Biphasic Alcohol Affects Scale (BAES) and for euphoric effects, the Visual Analog Scale (VAS) for ‘buzzed’. Subjects also reported on similarity to drugs of abuse (alcohol and marijuana) as measured by the VASs of Similarity to Drugs of Abuse (VASSDA). All subjects had used alcohol and were evaluated on the alcohol-like effects; only those subjects familiar with marijuana use were asked to rate the similarity to marijuana. The VASSDA consisted of VASs (0 = not at all, 7 = extremely) measuring the perceived similarity of the administered agent to ethanol and marijuana and has been used in a previous challenge study conducted by this group (Krystal, et al., 1998). Subjects were asked to report on the number of drinks they felt they had consumed using the NDS, which has also been used in several previous challenge studies conducted by this group (Krystal, et al., 1998). The BAES measures both the stimulating effects and sedating effects associated with ethanol intoxication (Martin, et al., 1993). The stimulating effects are associated with the ascending limb of ethanol intoxication and include feeling energised, excited, stimulated, talkative, ‘up’, and vigorous. The sedating effects are associated with the descending limb of ethanol intoxication and include difficulty in concentrating, ‘down’, heavy headed, inactive, sedated, having slow thoughts and feeling sluggish. In addition, the perceived intensity of feeling ‘buzzed’ was assessed using a VAS (0 = not at all, 7 = extremely). This mood rating scale, which has also been used for other mood states, has shown convergent validity with other measures of mood states during similar challenge studies (Krystal, et al., 1996; Krystal, et al., 1998; Petrakis, et al., 2001).
Mood ratings were assessed using VASs. These scales are VASs marked proportionately to the perceived intensity of the subjective experience (0 = not at all, 7 = extremely) for the following mood states: depression, anxiety and drowsiness. These mood rating scales have shown convergent validity with other measures of mood states in other previous studies (Krystal, et al., 1996; Krystal, et al., 1998; Petrakis, et al., 2004a). Drowsiness was assessed as an initial measure in this study to substantiate the basis of the comparison of these two agents for the remaining measures.
Dissociative states were assessed using the Clinician Administered Dissociative States Scale (CADSS). The CADSS measures perceptual alterations (Bremner, et al., 1998). This scale consists of 19 self-report items and 8 clinician-rated items (0 = not at all, 4 = extremely), which was shown to be sensitive to the effects of ketamine (Krystal, et al., 1994). The scale yields both an objective and a subjective score, the latter has been divided into five factors including body perception, time perception, external perception, sense of impaired memory and sense of unreality.
Data analysis
Data were checked for normality prior to analysis using Kolmogorov-Smirnov test statistics and normal probability plots. All outcome measures were heavily skewed. Therefore, non-normal outcomes were analysed using the nonparametric approach for repeated measures data by Brunner (Brunner, et al., 2002), where the data were first ranked and then fitted using a mixed effects model with an unstructured variance-covariance matrix and P values adjusted for ANOVA-type statistics (ATS). The models for CADSS, BAES, NDS and the similarity to alcohol scale included both drug (placebo, ketamine, thiopental) and time (study time points) as within-subjects explanatory factors. These models allowed for testing of all main and interactive effects of drug and time. When appropriate, post-hoc comparisons were performed. In the above models, subject was used as the clustering factor. All reported P values are Bonferroni adjusted applied within but not across domains. Data were analysed using SAS, version 9.1 (SAS Institute Inc., Cary, North Carolina, USA).
Results
Subject characteristics
As shown in Table 1, 28 individuals participated in this study. Twenty-one (75%) completed all 3 test days, 4 completed 2 test days (of those, 2 completed ketamine and placebo test days, 2 completed thiopental and placebo test day) and 3 completed only 1 test day (2 completed only the thiopental test day and 1 completed only the ketamine test day). Three subjects reported the reason for dropout was a scheduling conflict. One subject experienced nausea and vomiting on the second test day (ketamine) and opted not to complete that test day; another completed 2 full test days, but on the 3rd test day (ketamine), experienced nausea, and data was unable to be collected; one did not want to continue after feeling ‘uncomfortable’ with the medication on test day 1 (thiopental); another subject completed 2 test days but was dropped administratively because the subject revealed more alcohol use than that had been disclosed on intake. All subjects were included in the data analysis (n = 28).
Table 1.
Basic characteristics
| Variable | Sample (N = 28)
|
|
|---|---|---|
| Mean | (SD) | |
| Age | 23.8 | 2.5 |
| Education (years) | 15.2 | 1.8 |
| Age began drinking | 17.1 | 2.2 |
| Number of drinking days in the past 30 days | 5.4 | 4.8 |
| Number of standard drinks in the past 30 days | 16.5 | 19.8 |
| Variable | n | % |
| Gender | ||
| Male | 15 | 53.6 |
| Female | 13 | 46.4 |
| Ethnicity | ||
| White | 19 | 67.9 |
| African American | 4 | 14.3 |
| Hispanic | 2 | 7.1 |
| Asian | 2 | 7.1 |
| Other | 1 | 3.6 |
| Marital status | ||
| Never married | 25 | 89.3 |
| Married | 2 | 7.1 |
| Living with partner | 1 | 3.6 |
The average age was 23.8 ± 2.5. In all 15 (53.6%) were male and 13 (46.4%) were female. Nineteen (67.9%) were Caucasian, 4 (14.3%) African American, 2 (7.1%) Hispanic, 2 (7.1%) Asian, 1 (3.6%) were unknown with regards to race. The majority (25 or 89.3%) were never married. All subjects had at least a high school education (12 years) with an average of 15.2 ± 1.8 years of education. The average age at which subjects began drinking was 17.1 ± 2.2 years. The average number of drinking days within the past 30 days before infusion was 5.4 ± 4.8 (range, 0–17), and the average number of standard drinks within the past 30 days before infusion was 16.5 ± 19.8 (range, 0–75).
Sedation
Both ketamine and thiopental doses were chosen to cause similar levels of sedation. To validate this level, subjects were asked to rate their level of sedation based on a VAS for ‘drowsiness’. On the basis of VAS for drowsiness, there was a significant treatment by time effect [ATS = 2.4, df = 4.4, P = 0.012]. Both ketamine and thiopental produced increased sedative effects compared with placebo at 15, 45, 80 and 110 minutes post drug infusion (all P < 0.05). There were no significant differences observed between thiopental and ketamine on this measure.
Subjective intoxication
Perceived similarity to ethanol and marijuana
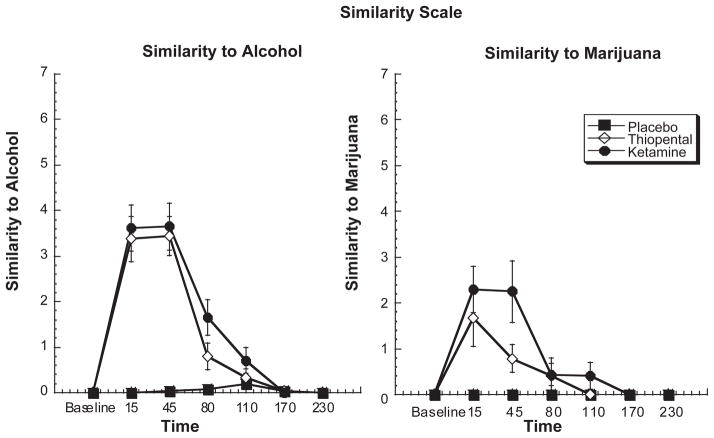
Both ketamine and thiopental showed similarities to ethanol in a dose-related manner [treatment by time effect, ATS = 21.7, df = 7.2, P < 0.0006 and ATS = 8.21, df = 4, P < 0.0006, respectively] (see Figure 1). Post-hoc analyses showed that both ketamine and thiopental significantly resembled ethanol compared with placebo at 15, 45 and 80 minutes post infusion (all P < 0.5). The perceived similarity to ethanol during ketamine and thiopental administration was more robust than the observed similarity to marijuana (see Figure 1); nonetheless, both ketamine and thiopental were rated as more marijuana-like than placebo in a dose-related manner [treatment by time effect, ATS = 8.21, df = 4, P < 0.0006]. Ketamine produced more sustained effects with continuing similarity to alcohol at 110 minutes after drug infusion and to marijuana at 80 minutes after drug infusion.
Figure 1.
Similarity to alcohol and marijuana based on VAS (0–7) in response to a 60-minute infusion of ketamine, thiopental or placebo. Infusion begins at T = 0 and ends at T = +60.
Number of Drinks Scale
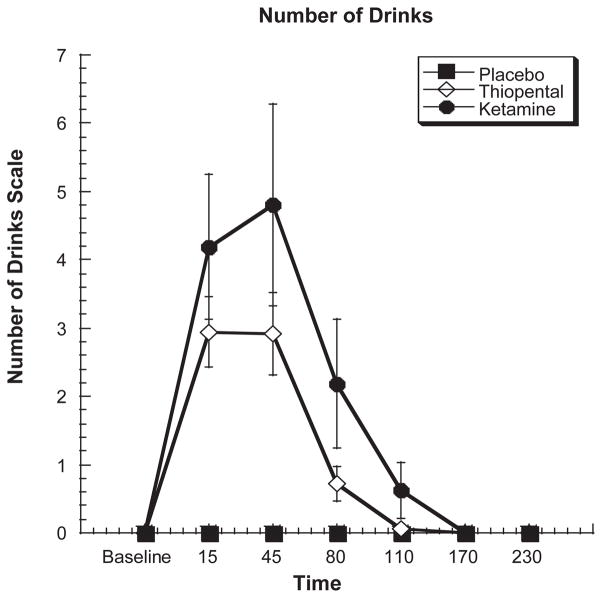
As shown in Figure 2, both ketamine and thiopental produced similar increases in the perceived number of standard drinks scale on the basis of the NDS [treatment by time interaction, ATS = 13.3, df = 5.6, P < 0.0006]. At 15 minutes, ketamine infusion was rated as the equivalent of 4.19 standard ethanol drinks (SD = 4.22), whereas thiopental was rated as the equivalent of 2.94 standard drinks (SD = 2.18). Although the duration of the effect was larger for ketamine, differences in the perceived number of drinks between ketamine and thiopental were not statistically distinguishable.
Figure 2.
Self report of the perceived number of standard drinks in response to a 60-minute infusion of ketamine, thiopental or placebo. Infusion begins at T = 0 and ends at T = +60.
Biphasic Alcohol Effects Scale
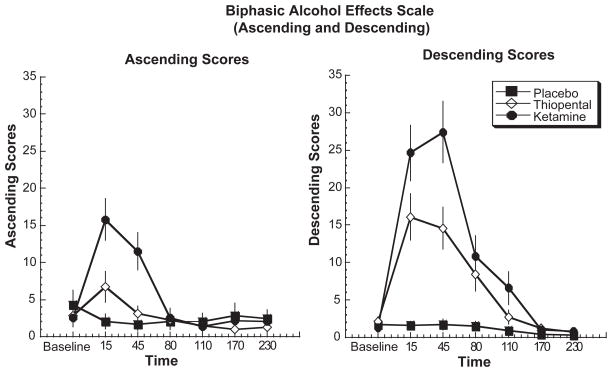
As shown in Figure 3, both ketamine and thiopental produced significant effects associated with the ascending limb of alcohol intoxication [treatment by time interaction, ATS = 6.3, df = 7.21, P < 0.0006] and significant effects associated with the descending limb of alcohol intoxication [treatment by time interaction: ATS = 15.1, df = 7.1, P < 0.0006] effects over time. Ketamine and thiopental produced significantly greater stimulatory effects compared with placebo at 15 minutes after drug infusion (P < 0.05); ketamine however produced significantly greater stimulatory effects compared with both thiopental and placebo at 15 and 45 minutes after drug infusion (P < .05). With regards to the descending limb of alcohol intoxication, both ketamine and thiopental produced significant effects compared with placebo at time points 15, 45, 80 and 110 minutes post infusion but no differences between active drug conditions. These results are consistent with the results found with the VAS for sedation (see above). However, ketamine had sustained effects and had significantly greater effects compared with thiopental at both 15 and 45 minutes post infusion.
Figure 3.
Biphasic Alcohol Effects Scale (Ascending and Descending) in response to a 60-minute infusion of ketamine, thiopental or placebo. Infusion begins at T = 0 and ends at T = +60.
Self-reported high
On the ‘buzzed’ VAS, both ketamine and thiopental showed significant dose-related effects [treatment by time effect, ATS = 20.9, df = 5.5, P < 0.0006]. These increases were most pronounced during the ketamine condition where ‘buzzed’ levels were significantly greater compared with both placebo and thiopental at 15, 45, 80 and 110 minutes post infusion (all P < 0.5). In addition, thiopental produced more significant increases compared with placebo at 15 and 45 minutes post infusion (all P < 0.05).
Intensity of mood states
On the basis of VAS for anxiousness, there was a significant treatment by time effect [ATS = 11.5, df = 4.9, P < 0.0004]. Ketamine produced significant effects compared with placebo and thiopental at 15 and 45 minutes (all P < 0.05). However, no significant differences were observed between thiopental and placebo.
No significant effects were observed between ketamine and thiopental relative to placebo or between ketamine and thiopental for VAS measures of depression and irritability.
CADSS
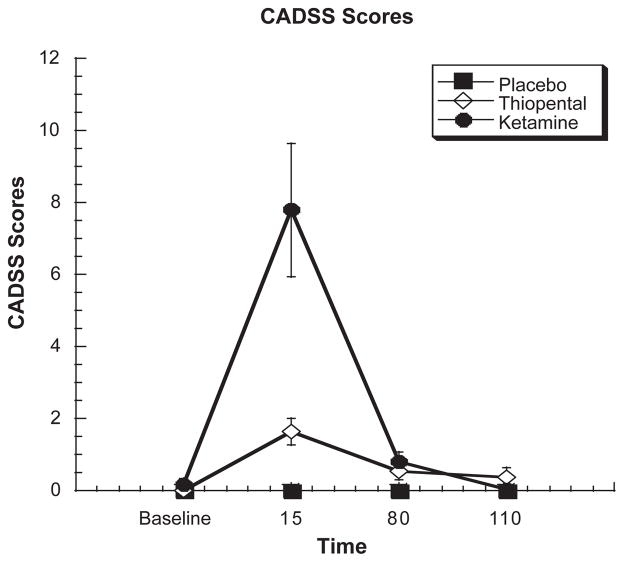
For both the patient- and clinician-rated components of the CADSS scale, there was a significant treatment by time effect (ATS = 24.9, df = 3.41, P < .0004 and ATS = 10.0, df = 2.64, P < .0004) (see Figure 4). For the patient-rated component, post-hoc analyses showed ketamine as having greater increased effects compared with placebo at 15 and 80 minutes post infusion (all P < 0.05) and thiopental at 15 minutes post infusion (P < 0.05). Thiopental also produced greater effects relative to placebo at 15 and 80 minutes post infusion (all P < 0.05). For the clinician-rated component, both ketamine and thiopental produced significant increases compared with placebo at 15 minutes post infusion (P < 0.05). Furthermore, ketamine showed greater effects compared with thiopental 15 minutes post infusion (P < 0.05).
Figure 4.
Clinical Administered Dissociative States Scale subject’s response to a 60-minute infusion of ketamine, thiopental or placebo. Infusion begins at T = 0 and ends at T = +60.
Discussion
The principle findings from this study are that in healthy individuals, subanesthetic doses of the GABAA agonist thiopental and the NMDA antagonist ketamine both demonstrate ethanol-like effects. Both thiopental and ketamine produced subjective effects that were judged to be more similar to ethanol than marijuana. However, at doses where ketamine and thiopental produced similar levels of sedation, ketamine effects were perceived as being more similar to ethanol and producing more intense ethanol-like effects than thiopental. These findings are consistent with prior preclinical research studies suggesting that ethanol actions at GABAA receptors are relatively more important than NMDA receptors for low doses of ethanol, and that the reverse is true for higher doses of ethanol (Grant, 1999). There were also qualitative differences between ketamine and thiopental effects. As expected, ketamine, but not thiopental, was associated with perceptual changes (Krystal, et al., 1994; Krupitsky, et al., 2001).
Previous studies conducted by our group and others have been able to show the ability of ketamine to cause euphoria (Krystal, et al., 1998; Krupitsky, et al., 2001; Krystal, et al., 2003a; Petrakis, et al., 2004a) and ethanol-like effects (Krystal, et al., 1998; Krupitsky, et al., 2001) and have identified NMDA receptor dysfunction as a primary cause for ethanol withdrawal syndrome (Hendricson, et al., 2007). The results from this study further substantiate the importance of the NMDA site as an important site of ethanol action in the brain. However, the therapeutic significance of this action remains to be determined. Some of ketamine’s effects may be mediated by enhancing glutamate release onto non-NMDA receptors (Anand, et al., 2000; Deakin, et al., 2008). NMDA antagonists block excitation of GABA interneurons and may have an excitotoxic effect on posterior cingulated pyramidal cells (Olney and Farber, 1995; Deakin, et al., 2008). Thus, neurobiological mechanisms other than NMDA antagonism may also contribute to ketamine’s effects.
The dysphoric effects of ketamine appear to be reduced in alcohol-dependent patients (Krystal, et al., 2003b) and in healthy individuals with a family history of alcoholism (Petrakis, et al., 2004b), suggesting that NMDA receptor is implicated in the pathophysiology of alcohol dependence and/ or in the vulnerability to develop alcohol dependence. It has further been hypothesised that medications with action on the NMDA receptor blockade may play a therapeutic role in alcohol dependence (Krystal, et al., 2003a; Krystal, et al., 2003b; Krystal, et al., 2003c). On the one hand, the NMDA receptor antagonist, memantine, appears to reduce craving under baseline conditions and following the exposure to ethanol-related cues (Bisaga and Evans, 2004; Krupitsky, et al., 2007). However, memantine does not appear to reduce alcohol craving following a priming dose of ethanol (Bisaga and Evans, 2004) or be effective in decreasing alcohol use as a treatment for alcoholism (Evans, et al., 2007). Thus, although NMDA receptor blockade appears to contribute to the subjective effects associated with ethanol intoxication, it is not yet clear that NMDA receptors are an important target for the treatment of alcoholism.
The GABAA agonist, thiopental, also produced ethanol-like effects including euphoria and sedation. This is consistent with a large literature describing the ethanol-like discriminative effects of benzodiazepines and barbiturates in animals (Porcu and Grant, 2004; Besheer and Hodge, 2005; McMahon and France, 2005) and a smaller human research literature describing the euphoric effects of these drugs (McCaul, et al., 1990; McCaul, et al., 1991; Cowley, et al., 1992; Cowley, et al., 1996). There is some evidence that ethanol has very low affinity for synaptic GABAA receptors and does not act upon the same class of GABAA receptors as alcohol (Krystal, et al., 2006). The ethanol-like effects of thiopental may reflect the ability of ethanol to stimulate GABA release and to indirectly enhance the stimulation of synaptic GABAA receptors. Medications with GABAergic effect receptors, such as the benzodiazepines, play a role in the treatment of alcohol withdrawal. There is theoretical interest in the development of partial inverse agonists for the benzodiazepine receptor as treatments for alcoholism, but these medications are limited by their anxiogenic and pro-convulsant effect. The development of other agents that influence GABA, including steroid anaesthetic agents such as ganaxolone, may hold promise for drugs that target extrasynaptic receptors as therapeutic tools for alcoholism (Krystal, et al., 2006).
This study illustrated the utility of thiopental as a probe of GABA function in laboratory-based testing. In this study, thiopental did not cause any adverse events and was well tolerated by subjects. This agent may be more useful in terms of its rapid onset and short duration of action compared with previous studies that used oral GABAergic agents as probes including secobarbital (McCaul, et al., 1990; McCaul, et al., 1991) and diazepam (McCaul, et al., 1990; McCaul, et al., 1991). Future studies using thiopental at different doses may assist in providing further information regarding the potential utility of this agent as a reliable probe in studying the role of GABAA receptor activity in alcoholism.
There were several methodologic limitations of this study. For example, although ketamine is an uncompetitive antagonist of NMDA receptors, it does not bind to the same site of the NMDA receptor as ethanol (Krystal, et al., 2003a; Krystal, et al., 2003b; Krystal, et al., 2003c). Similarly, thiopental does not act upon the same subclass of GABAA receptors as ethanol (Krystal, et al., 2006). For the sake of feasibility, a single dose of ketamine and thiopental were studied. However, a comparison of the effects of multiple doses of each drug would have provided a more accurate assessment of their respective ethanol-like effects. In addition, this study was restricted to healthy subjects without a family history of alcoholism. It is possible that the most important population, those individuals at increased familial risk for alcoholism, would show a different pattern of response to each agent.
In summary, this study characterises the behavioural effects of medications that act on NMDA and GABAergic receptors. Both agents produced ethanol-like effects in addition to perceived similarities to ethanol. However, ketamine produced more robust findings that suggest a more predominant role of the NMDA receptor in mediating ethanol’s effects and a greater role in mediating ethanol’s effects at higher levels of ethanol intoxication than GABAA receptor facilitation, which may contribute significantly to ethanol effects associated with social drinking. The profiles of these medications differed somewhat, as ketamine was associated with more perceptual alterations than with thiopental administration. These findings highlight the potential importance of NMDA and GABAA receptors in human alcohol intoxication. Future studies are needed to understand better how this insight informs our understanding of the heritable risk for alcoholism and the treatment of alcohol use disorders.
Acknowledgments
The authors acknowledge the important contributions of Angelina Genovese, R.N.C., M.B.A., Elizabeth O’Donnell, R.N., and Brenda Breault, R.N., B.S.N., of the Neurobiological Studies Unit of the VA Connecticut Healthcare System, West Haven Campus, West Haven, Connecticut. In addition, the authors acknowledge support for this work from the Department of Veterans Affairs (VA Merit Review Grant, Alcohol Research Center) and National Institute on Alcohol Abuse and Alcoholism (KO5 AA 14906-04, 2P50-AA012870-07, T-32 AA 015496-02). Dr Krystal has served as a paid scientific consultant for years 2005, 2006, 2007 to Alkermes, Astra Zeneca, Aventis Pharmaceuticals, Bristol-Myers Squibb, Cypress Bioscience Inc, Eli Lilly and Co., Fidelity Bioscience, Forest Laboratories Inc., Glaxo-SmithKline, Janssen Research Foundation, Merz Pharmaceuticals, Organon Pharmaceuticals Inc., Pfizer Pharmaceuticals, Sumitomo Pharmaceuticals America Ltd., Takeda Industries, UCB Pharma and US Micron. He is also co-sponsor of two pending patents related to the use of glutamatergic agents to treat psychiatric disorders and anti-depressant effects of oral ketamine. Dr Petrakis holds a grant funded through Forest Laboratories Inc.
Contributor Information
D Dickerson, UCLA Integrated Substance Abuse Programs, Los Angeles, CA, USA.
B Pittman, NIAAA Center for the Translational Neuroscience of Alcoholism and Department of Psychiatry, Yale University School of Medicine, New Haven, CT, USA.
E Ralevski, NIAAA Center for the Translational Neuroscience of Alcoholism and Department of Psychiatry, Yale University School of Medicine, New Haven, Connecticut, USA; Department of Veterans Affairs Alcohol Research Center, VA Connecticut Healthcare System (116-A), West Haven, CT, USA.
A Perrino, NIAAA Center for the Translational Neuroscience of Alcoholism and Department of Anesthesiology, Yale University School of Medicine, New Haven, CT, USA; Department of Veterans Affairs Alcohol Research Center, VA Connecticut Healthcare System (116-A), West Haven, CT, USA.
D Limoncelli, NIAAA Center for the Translational Neuroscience of Alcoholism and Department of Psychiatry, Yale University School of Medicine, New Haven, CT, USA; Department of Veterans Affairs Alcohol Research Center, VA Connecticut Healthcare System (116-A), West Haven, CT, USA.
J Edgecombe, NIAAA Center for the Translational Neuroscience of Alcoholism and Department of Psychiatry, Yale University School of Medicine, New Haven, CT, USA; Department of Veterans Affairs Alcohol Research Center, VA Connecticut Healthcare System (116-A), West Haven, CT, USA.
G Acampora, NIAAA Center for the Translational Neuroscience of Alcoholism and Department of Psychiatry, Yale University School of Medicine, New Haven, CT, USA; Department of Veterans Affairs Alcohol Research Center, VA Connecticut Healthcare System (116-A), West Haven, CT, USA.
JH Krystal, NIAAA Center for the Translational Neuroscience of Alcoholism and Department of Psychiatry, Yale University School of Medicine, New Haven, CT, USA; Department of Veterans Affairs Alcohol Research Center, VA Connecticut Healthcare System (116-A), West Haven, CT, USA; Clinical Neuroscience Research Unit, Abraham Ribicoff Research Facilities, New Haven, CT, USA.
I Petrakis, NIAAA Center for the Translational Neuroscience of Alcoholism and Department of Psychiatry, Yale University School of Medicine, New Haven, CT, USA; Department of Veterans Affairs Alcohol Research Center, VA Connecticut Healthcare System (116-A), West Haven, CT, USA.
References
- Aguayo LG, Peoples RW, Yeh HH, Yevenes GE. GABA(A) receptors as molecular sites of ethanol action. Direct or indirect actions. Curr Top Med Chem. 2002;2:869–885. doi: 10.2174/1568026023393426. [DOI] [PubMed] [Google Scholar]
- Anand A, Charney DS, Oren DA, Berman RM, Hu XS, Cappiello A. Attenuation of the neuropsychiatric effects of ketamine with lamotrigine: support for hyperglutamatergic effects of N-methyl-D-aspartate receptor antagonists. Arch Gen Psychiatry. 2000;57:270–276. doi: 10.1001/archpsyc.57.3.270. [DOI] [PubMed] [Google Scholar]
- Balster R, Wiley J, Tokarz M, Tabakoff B. Effects of ethanol and NMDA antagonists on operant behavior in ethanol withdrawal seizure-prone and -resistant mice. Behav Pharmacol. 1993;4:107–113. [PubMed] [Google Scholar]
- Besheer J, Hodge CW. Pharmacological and anatomical evidence for an interaction between mGluR5- and GABA(A) alpha1-containing receptors in the discriminative stimulus effects of ethanol. Neuropsychopharmacology. 2005;30:747–757. doi: 10.1038/sj.npp.1300616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bisaga A, Evans SM. Acute effects of memantine in combination with alcohol in moderate drinkers. Psychopharmacology. 2004;172:16–24. doi: 10.1007/s00213-003-1617-5. [DOI] [PubMed] [Google Scholar]
- Boehm SL, 2nd, Ponomarev I, Blednov YA, Harris RA. From gene to behavior and back again: new perspectives on GABAA receptor subunit selectivity of alcohol actions. [reprint of Biochem Pharmacol. 2004 Oct 15;68(8):1581–602; PMID: 15451402] Adv Pharmacol. 2006;54:171–203. doi: 10.1016/s1054-3589(06)54008-6. [DOI] [PubMed] [Google Scholar]
- Boehm SL, 2nd, Ponomarev I, Jennings AW, Whiting PJ, Rosahl TW, Garrett EM, et al. Gamma-Aminobutyric acid A receptor subunit mutant mice: new perspectives on alcohol actions. Biochem Pharmacol. 2004;68:1581–1602. doi: 10.1016/j.bcp.2004.07.023. [DOI] [PubMed] [Google Scholar]
- Bremner JD, Krystal JH, Putnam FW, Southwick SM, Marmar C, Charney DS, et al. Measurement of dissociative states with the Clinician-Administered Dissociative States Scale (CADSS) J Trauma Stress. 1998;11:125–136. doi: 10.1023/A:1024465317902. [DOI] [PubMed] [Google Scholar]
- Brunner E, Domhof S, Langer F. Nonparametric Analysis of Longitudinal Data in Factorial Experiments. New York, NY: John Wiley & Sons; 2002. [Google Scholar]
- Cowley DS, Roy-Byrne PP, Godon C, Greenblatt DJ, Ries R, Walker RD, et al. Response to diazepam in sons of alcoholics. Alcohol Clin Exp Res. 1992;16:1057–1063. doi: 10.1111/j.1530-0277.1992.tb00699.x. [DOI] [PubMed] [Google Scholar]
- Cowley DS, Roy-Byrne PP, Greenblatt DJ, Kramer GL, Petty F. Effect of diazepam on plasma gamma-aminobutyric acid in sons of alcoholic fathers. Alcohol Clin Exp Res. 1996;20:343–347. doi: 10.1111/j.1530-0277.1996.tb01650.x. [DOI] [PubMed] [Google Scholar]
- Cowley DS, Roy-Byrne PP, Radant A, Hommer DW, Greenblatt DJ, Vitaliano PP, et al. Eye movement effects of diazepam in sons of alcoholic fathers and male control subjects. Alcohol Clin Exp Res. 1994;18:324–332. doi: 10.1111/j.1530-0277.1994.tb00021.x. [DOI] [PubMed] [Google Scholar]
- Criswell HE, Breese GR. A conceptualization of integrated actions of ethanol contributing to its GABAmimetic profile: a commentary. Neuropsychopharmacology. 2005;30:1407–1425. doi: 10.1038/sj.npp.1300750. [DOI] [PubMed] [Google Scholar]
- Deakin JF, Lees J, McKie S, Hallak JE, Williams SR, Dursun SM. Glutamate and the neural basis of the subjective effects of ketamine: a pharmaco-magnetic resonance imaging study. Arch Gen Psychiatry. 2008;65:154–164. doi: 10.1001/archgenpsychiatry.2007.37. [DOI] [PubMed] [Google Scholar]
- Evans SM, Levin FR, Brooks DJ, Garawi F. A pilot double-blind treatment trial of memantine for alcohol dependence. Alcohol Clin Exp Res. 2007;31:775–782. doi: 10.1111/j.1530-0277.2007.00360.x. [DOI] [PubMed] [Google Scholar]
- Grant K. Strategies for understanding the pharmacological effects of ethanol with drug discrimination procedures. Pharmacol Biochem Behav. 1999;64:261–267. doi: 10.1016/s0091-3057(99)00075-1. [DOI] [PubMed] [Google Scholar]
- Grant KA, Colombo G. Discriminative stimulus effects of ethanol: effect of training dose on the substitution of N-methyl-D-aspartate antagonists. J Pharmacol Exp Ther. 1993;264:1241–1247. [PubMed] [Google Scholar]
- Grant KA, Lovinger DM. Cellular and behavioral neurobiology of alcohol: receptor-mediated neuronal processes. Clin Neurosci. 1995;3:155–164. [PubMed] [Google Scholar]
- Hendricson AW, Maldve RE, Salinas AG, Theile JW, Zhang TA, Diaz LM, et al. Aberrant synaptic activation of N-methyl-D-aspartate receptors underlies ethanol withdrawal hyperexcitability. J Pharmacol Exp Ther. 2007;321:60–72. doi: 10.1124/jpet.106.111419. [DOI] [PubMed] [Google Scholar]
- Krupitsky E, Neznova O, Masalov D, Burakov A, Didenko T, Romanova T, et al. Effects of memantine on cue-induced alcohol craving in recovering alcohol dependent patients. Am J Psychiatry. 2007;164:1–5. doi: 10.1176/ajp.2007.164.3.519. [DOI] [PubMed] [Google Scholar]
- Krupitsky EM, Burakov AM, Romanova TN, Grinenko NI, Grinenko AY, Fletcher J, et al. Attenuation of ketamine effects by nimodipine pretreatment in recovering ethanol dependent men: psychopharmacologic implications of the interaction of NMDA and L-type calcium channel antagonists. Neuropsycho-pharmacology. 2001;25:936–947. doi: 10.1016/S0893-133X(01)00346-3. [DOI] [PubMed] [Google Scholar]
- Krystal J, Staley J, Mason G, Petrakis I, Kaufman J, Harris R, et al. GABAA receptors and alcoholism: Intoxication, dependence, vulnerability, and treatment. Arch Gen Psychiatry. 2006;63:957–968. doi: 10.1001/archpsyc.63.9.957. [DOI] [PubMed] [Google Scholar]
- Krystal JH, Karper LP, Seibyl JP, Freeman GK, Delaney R, Bremner JD, et al. Subanesthetic effects of the noncompetitive NMDA antagonist, ketamine, in humans. Psychotomimetic, perceptual, cognitive, and neuroendocrine responses. Arch Gen Psychiatry. 1994;51:199–214. doi: 10.1001/archpsyc.1994.03950030035004. [DOI] [PubMed] [Google Scholar]
- Krystal JH, Petrakis IL, Krupitsky E, Schutz C, Trevisan L, DSouza DC. NMDA receptor antagonism and the ethanol intoxication signal: from alcoholism risk to pharmacotherapy. Ann N Y Acad Sci. 2003a;1003:176–184. doi: 10.1196/annals.1300.010. [DOI] [PubMed] [Google Scholar]
- Krystal JH, Petrakis IL, Limoncelli D, Webb E, Gueorguieva R, DSouza DC, et al. Altered NMDA glutamate receptor antagonist response in recovering ethanol-dependent patients. Neuropsychopharmacology. 2003b;28:2020–2028. doi: 10.1038/sj.npp.1300252. [DOI] [PubMed] [Google Scholar]
- Krystal JH, Petrakis IL, Mason G, Trevisan L, DSouza DC. N-methyl-D-aspartate glutamate receptors and alcoholism: reward, dependence, treatment, and vulnerability. Pharmacol Ther. 2003c;99:79–94. doi: 10.1016/s0163-7258(03)00054-8. [DOI] [PubMed] [Google Scholar]
- Krystal JH, Petrakis IL, Webb E, Cooney NL, Karper LP, Namanworth S, et al. Dose-related ethanol-like effects of the NMDA antagonist, ketamine, in recently detoxified alcoholics. Arch Gen Psychiatry. 1998;55:354–360. doi: 10.1001/archpsyc.55.4.354. [DOI] [PubMed] [Google Scholar]
- Krystal JH, Raskin HA. Drug Dependence: Aspects of Ego Functions. Detroit, MI: Wayne State University Press; 1970. [Google Scholar]
- Krystal JH, Webb E, Cooney NL, Kranzler HR, Southwick SW, Heninger GR, et al. Serotonergic and noradrenergic dysregulation in alcoholism: m-chlorophenylpiperazine and yohimbine effects in recently detoxified alcoholics and healthy comparison subjects. Am J Psychiatry. 1996;153:83–92. doi: 10.1176/ajp.153.1.83. [DOI] [PubMed] [Google Scholar]
- Martin CS, Earleywine M, Musty RE, Perrine MW, Swift RM. Development and validation of the Biphasic Alcohol Effects Scale. Alcohol Clin Exp Res. 1993;17:140–146. doi: 10.1111/j.1530-0277.1993.tb00739.x. [DOI] [PubMed] [Google Scholar]
- McCaul ME, Turkkan JS, Svikis DS, Bigelow GE. Alcohol and secobarbital effects as a function of familial alcoholism: acute psychophysiological effects. Alcohol Clin Exp Res. 1990;14:704–712. doi: 10.1111/j.1530-0277.1990.tb01230.x. [DOI] [PubMed] [Google Scholar]
- McCaul ME, Turkkan JS, Svikis DS, Bigelow GE. Alcohol and secobarbital effects as a function of familial alcoholism: extended intoxication and increased withdrawal effects. Alcohol Clin Exp Res. 1991;15:94–101. doi: 10.1111/j.1530-0277.1991.tb00524.x. [DOI] [PubMed] [Google Scholar]
- McMahon LR, France CP. Combined discriminative stimulus effects of midazolam with other positive GABAA modulators and GABAA receptor agonists in rhesus monkeys. Psychopharmacology. 2005;178:400–409. doi: 10.1007/s00213-004-2022-4. [DOI] [PubMed] [Google Scholar]
- Mehta AK, Ticku MK. Are GABAB receptors involved in the pharmacological effects of ethanol. Eur J Pharmacol. 1990;182:473–480. doi: 10.1016/0014-2999(90)90044-7. [DOI] [PubMed] [Google Scholar]
- Morrow AL, VanDoren MJ, Penland SN, Matthews DB. The role of GABAergic neuroactive steroids in ethanol action, tolerance and dependence. Brain Res Brain Res Rev. 2001;37:98–109. doi: 10.1016/s0165-0173(01)00127-8. [DOI] [PubMed] [Google Scholar]
- Olney JW, Farber NB. NMDA antagonists as neurotherapeutic drugs, psychotogens, neurotoxins, and research tools for studying schizophrenia. Neuropsychopharmacology. 1995;13:335–345. doi: 10.1016/0893-133X(95)00079-S. [DOI] [PubMed] [Google Scholar]
- Petrakis IL, Limoncelli D, Gueorguieva R, Jatlow P, Boutros NN, Trevisan L, et al. Altered NMDA glutamate receptor antagonist response in individuals with a family vulnerability to alcoholism [see comment] Am J Psychiatry. 2004a;161:1776–1782. doi: 10.1176/ajp.161.10.1776. [DOI] [PubMed] [Google Scholar]
- Petrakis IL, OMalley S, Rounsaville B, Poling J, McHugh-Strong C, Krystal JH. Naltrexone augmentation of neuroleptic treatment in alcohol abusing patients with schizophrenia. Psychopharmacology. 2004b;172:291–297. doi: 10.1007/s00213-003-1658-9. [DOI] [PubMed] [Google Scholar]
- Petrakis IL, Trevisan L, Boutros NN, Limoncelli D, Cooney NL, Krystal JH. Effect of tryptophan depletion on alcohol cue-induced craving in abstinent alcoholic patients. Alcohol Clin Exp Res. 2001;25:1151–1155. [PubMed] [Google Scholar]
- Porcu P, Grant KA. Discriminative stimulus effects of ethanol in rats using a three-choice ethanolmidazolam-water discrimination. Behav Pharmacol. 2004;15:555–567. doi: 10.1097/00008877-200412000-00004. [DOI] [PubMed] [Google Scholar]
- Schuckit MA, Greenblatt D, Gold E, Irwin M. Reactions to ethanol and diazepam in healthy young men. J Stud Alcohol. 1991;52:180–187. doi: 10.15288/jsa.1991.52.180. [DOI] [PubMed] [Google Scholar]
- Schutz CG, Soyka M. Dextromethorphan challenge in alcohol-dependent patients and controls. Arch Gen Psychiatry. 2000;57:291–292. doi: 10.1001/archpsyc.57.3.291. [DOI] [PubMed] [Google Scholar]
- Siggins GR, Roberto M, Nie Z. The tipsy terminal: presynap-tic effects of ethanol. Pharmacol Ther. 2005;107:80–98. doi: 10.1016/j.pharmthera.2005.01.006. [DOI] [PubMed] [Google Scholar]
- Valverius P, Crabbe J, Hoffman P, Tabakoff B. NMDA receptors in mice bred to be prone or resistant to ethanol withdrawal seizures. Eur J Pharmacol. 1990;184:185–189. doi: 10.1016/0014-2999(90)90681-u. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Wang GJ, Begleiter H, Hitzemann R, Pappas N, Burr G, et al. Regional brain metabolic response to loraze-pam in subjects at risk for alcoholism. Alcohol Clin Exp Res. 1995;19:510–516. doi: 10.1111/j.1530-0277.1995.tb01539.x. [DOI] [PubMed] [Google Scholar]
- Weiner JL, Valenzuela CF. Ethanol modulation of GABAergic transmission: the view from the slice. Pharmacol Ther. 2006;111:533–554. doi: 10.1016/j.pharmthera.2005.11.002. [DOI] [PubMed] [Google Scholar]