Abstract
This brief review resolves a number of persistent conflicts regarding the location and characteristics of the mesencephalic locomotor region (MLR), which has in the past been described as not locomotion-specific and is more likely the pedunculopontine nucleus (PPN). The parameters of stimulation used to elicit changes in posture and locomotion we now know are ideally suited to match the intrinsic membrane properties of PPN neurons. The physiology of these cells is important not only because it is a major element of the reticular activating system (RAS), but also because it is a novel target for the treatment of gait and postural deficits in Parkinson’s disease (PD). The discussion explains many of the effects reported following deep brain stimulation (DBS) of the PPN by different groups, and provides guidelines for the determination of long-term assessment and effects of PPN DBS. A greater understanding of the physiology of the target nuclei within the brainstem and basal ganglia, amassed over the past decades, has enabled increasingly better patient outcomes from DBS for movement disorders. Despite these improvements, there remains a great opportunity for further understanding of the mechanisms through which DBS has its effects and for further development of appropriate technology to effect these treatments. We review the scientific basis for one of the newest targets, the PPN, in the treatment of PD and other movement disorders, and address the needs for further investigation.
Keywords: Reticular Activating System, Pedunculopontine nucleus, Deep Brain Stimulation, Calcium Channels
Pedunculopontine cell physiology
The pedunculopontine nucleus (PPN) has become a target for deep brain stimulation (DBS) for the treatment of movement and postural disorders in human subjects. Such use was predicted almost 30 years ago based on animal studies using stimulation in the region of the PPN, which at the time was thought to be in the mesencephalic locomotor region (MLR) (Garcia-Rill 1986; 1991; 1997). The MLR was originally described as a region that, when stimulated with increasing current amplitudes using long duration (0.5–1.0 msec) pulses at 40–60 Hz in the precollicular-postmamillary transected, weight-suspended cat, would induce controlled locomotion on a treadmill (Shik et al. 1966). The effective site was localized to the lateral cuneiform nucleus, dorsal to the lateral aspect of the superior cerebellar peduncle. The same year, the PPN was reported to be a target of descending basal ganglia projections (Nauta and Mehler 1966). Years later, we had been studying the electrophysiology of striatal neurons (Garcia-Rill et al. 1979), before becoming interested in the PPN (Garcia-Rill et al. 1981; 1983). We began a series of studies to determine if the PPN and the MLR were indeed the same or different structures. There had been very little investigation into the cellular substrate for the MLR since its discovery. We were the first to use neuroactive agents injected into the region of the PPN to induce locomotion (Garcia-Rill et al. 1985). We also recorded from locomotion-related PPN neurons, and some cells were active in relation to left-right alternation, while others were related to the duration of the stepping episode (Garcia-Rill and Skinner 1988). However, we were always puzzled why, in order to induce locomotion, a) lateral, but not medial, cuneiform nucleus stimulation was required, b) ramping up of the current was required, c) stimulation at 40–60 Hz, but not higher or lower, was required, and d) long duration pulses were required. These specific and puzzling requirements have been fairly well resolved and need to be considered when carrying out PPN DBS.
Our early studies used the term MLR because we were recording or injecting agents into a region that we had first physiologically identified to induce locomotion. However, we soon became convinced that the optimal sites for inducing locomotion were within the PPN (Garcia-Rill et al. 1987). We began to question the interpretation that the MLR was a “locomotion-specific” area, providing considerable evidence showing that the region being stimulated instead was a “rhythmogenic” region that was part of the reticular activating system (RAS) over the next 15 years (Garcia-Rill 1991; 1997; Garcia-Rill and Skinner 1988; 1991; Garcia-Rill et al. 1996; Skinner and Garcia-Rill 1990; 1994; Reese et al. 1995). Unfortunately, this distinction is still blurred by those not familiar with complex literature published many years ago. Modern researchers still pay homage to the MLR, which, as will become clear, can now be retired.
Localization
The PPN was identified as a cholinergic cell group many years ago (see e.g. Mesulam et al. 1983), but recent studies have established that this nucleus is composed of mostly non-overlapping populations of cholinergic, glutamatergic and GABAergic neurons (Wang and Morales 2009). The best way to visualize this cell group is in sagittal sections, which reveal its wedge shape extending from the dorsolateral to the ventrolateral mesopontine region. The posterior pars compacta is located immediately dorsal to the lateral aspect of the superior cerebellar peduncle, precisely in one of the optimal sites for inducing locomotion at low threshold (Garcia-Rill et al. 1987). As the nucleus extends anteroventrally, it mixes with the cerebellar peduncle and ends at the posterior edge of the substantia nigra. We reconstructed this nucleus in three dimensions in both rodents and humans (Garcia-Rill et al. 1987, 1996; Karson et al. 1991). The optimal sites for inducing locomotion were never located in the medial part of the cuneiform nucleus, but rather in the lateral part, in which the PPN pars compacta is embedded (Garcia-Rill et al. 1987; 1996). Later, others localized their stimulation sites to the cuneiform nucleus dorsal to the brachium conjunctivum (superior cerebellar peduncle), but did not label for PPN cells in the stimulated animals (Takakusaki et al. 2003). This led to the erroneous localization of the MLR dorsal to the superior cerebellar peduncle, and the PPN within, but not dorsal to the brachium conjunctivum. However, cholinergic PPN neurons are evident well dorsal to the brachium conjunctivum, in the region most authors report positive effects on locomotion (Garcia-Rill et al. 1987; Garcia-Rill and Skinner 1988; Wang and Morales 2009). Stimulation at more ventral sites typically did not induce stepping, but rather changes in muscle tone (e.g. Takakusaki et al. 2003), an effect that may have been due to suddenly switching on the stimulus (see below). We should also note that stimulation of more medial regions such as the laterodorsal tegmental nucleus, the medial partner of the PPN, or anteriorly in the region of the substantia nigra, does not induce locomotion (Garcia-Rill 1991; Garcia-Rill and Skinner 1991; Skinner and Garcia-Rill 1990; 1994; Reese et al. 1995). This explains why stimulation of only lateral, but not medial, cuneiform, in which the PPN is embedded, produces reliable stepping on a treadmill.
As far as the human PPN is concerned, a very useful schematic of the PPN and surrounding structures in relation to different anatomic levels was provided recently (Alam et al. 2011). Figure 1 shows two three-dimensional reconstructions of the human PPN in relation to adjacent structures. At the top is a lateral view of sagittal sections of the human posterior midbrain and pons. The fourth ventricle is at the top, basis pontis is at the bottom, and the inferior and superior colliculi appear at the top right. Each neuron in the PPN and other nuclei is denoted by a 100 µM sphere, with PPN cells in red, while substantia nigra neurons are shown in green. The use of the spheres provides an indication of the minimum spread of the dendritic tree of individual cells, which is typically in the order of 200 µM. This provides a more accurate picture of the relationships between cell groups in the posterior midbrain (Garcia-Rill and Skinner 1991). Note that the anterior end of the PPN is embedded in the posterior end of the substantia nigra. As the PPN spans posteriorly, its most medial edge overlaps with the lateral border of the locus coeruleus, with scattered cholinergic cells located within the boundaries of the locus coeruleus. At the lateral edge of the locus coeruleus, these cells overlap with cholinergic neurons of the laterodorsal tegmental nucleus. This medial cholinergic partner of the PPN does not modulate sleep-wake cycles and its functions remain unclear. In more medial sections deeper in the view, the cells from the locus coeruleus are shown in purple, and more medially still, the cells of the laterodorsal tegmental nucleus are shown in yellow. Given the intermingling of cells, it is clear that the most effective location for specifically stimulating the PPN is in the posterior end, within the pars compacta (denoted by a circle). The bottom reconstruction in Figure 1 is from a different brain viewed from the medial side. Sagittal sections along the midline show the fourth ventricle dorsally, the basis pontis ventrally, and the inferior and superior colluculi to the left and dorsally. Note the central canal dividing the colliculi from the tegmentum in the most medial section. Neurons of the laterodorsal tegmental nucleus are shown as yellow spheres, and those of the locus coeruleus are again in purple, while PPN cells in red are evident more laterally and deepest in the image. Substantia nigra neurons are in green, showing that the medial subtsantia nigra is well separated from the more dorsal laterodorsal tegmental nucleus and locus coeruleus. Note that the pars compacta of the PPN in humans is also dorsal/posterior to the superior cerebellar peduncle and immediately ventral/anterior to the inferior colliculus (denoted as a circle). The PPN does not range very far caudally beyond the level of the inferior colliculus. Stimulation of sites caudally to the PPN would engage the peribrachial region that has a number of cell groups related to pain and micturition (the so-called “pontine micturition center” is located caudally to PPN in the peribrachial region) (Garcia-Rill et al. 1988, 1991; Reese et al. 1995).
Figure 1. The human PPN in relation to adjacent structures. Top image.
Sagittal sections from a human brain in which cholinergic cells from the PPN and laterodorsal tegmental nuclei were immunocytochemically labeled with choline acetyltransferase antibody, and locus coeruleus and substantia nigra neurons were labeled with tyrosine hydroxylase antibody. Each neuron in each section was rendered as a 100 µM sphere. This view is from the lateral aspect of the pons and midbrain showing at top the fourth ventricle (IV), at bottom the basis pontis, and at the top right, the inferior and superior colliculi (IC, SC). The PPN appears as a cluster of red neurons with the densest collection at the posterior edge, the pars compacta (denoted as a circle). Anterior to the PPN is the subtantia nigra with green cells. More medially and deep in the view are the puprple cells denoting the locus coeruleus and the yellow cells denoting the laterodorsal tegmental nucleus. Bottom image. Sagittal sections from another human brain labeled similarly to the top image. The first section along the midline shows the fourth ventricle dorsally (IV), the basis pontis ventrally, and the inferior and superior colluculi to the left and dorsally (IC, SC). Note the central canal dividing the colliculi from the tegmentum in this section. Neurons of the laterodorsal tegmental nucleus are shown as yellow spheres, and those of the locus coeruleus are again in purple, while PPN cells in red are evident more laterally and deepest in the image with the pars compacta denoted as a circle. Substantia nigra neurons are in green, showing the medial substantia nigra to be well separated from the more dorsal laterodorsal tegmental nucleus and locus coeruleus.
Stimulation at 40–60 Hz
Figure 2A shows the effects of delivering gradually increasing current steps in single patch clamped PPN neurons (Simon et al. 2010). We found that virtually all PPN cells increased firing frequency and then plateaued at 40–60 Hz (Fig. 2B), regardless of neurotransmitter or electrophysiological type (Fig. 2C). That is, these neurons could not be induced to fire any faster than 40–60 Hz, regardless of the level of current delivered intracellularly. This property was further investigated to determine the mechanism responsible for such a unique characteristic by recording patch clamped neurons in the presence of synaptic blockers (to prevent afferent signals) and tetrodotoxin (to prevent action potential generation). In our investigation of the intrinsic properties of these cells, we applied current steps to attempt to drive high threshold channels, but the steps were unable to depolarize the membrane sufficiently to maintain depolarization (Fig. 3A), probably due to the activation of potassium channels by the sudden depolarization (Kezunovic et al. 2011). However, when we applied ramps to slowly depolarize the membrane to activate high threshold channels, we were able to elicit membrane oscillations in the beta and gamma band range (Fig. 3B). We found that all PPN neurons, regardless of transmitter or electrophysiological type, manifested voltage-dependent, high threshold P/Q- and N-type calcium channels that mediated the plateau in maximal membrane oscillatory activity at 40–60 Hz (Kezunovic et al. 2011). That is, the presence of these calcium channels determines the maximal oscillatory frequency displayed by ALL of these cells. We used specific calcium channel blockers (especially ω-agatoxin for P/Q-type calcium channels) to identify these as the main channels responsible for beta/gamma band oscillations. We should note that cells outside of the PPN do not show similar properties, making the cellular boundaries of the nucleus specific to this kind of activity. The presence of a frequency plateau in PPN cells explains the requirement to stimulate this region at 40–60 Hz to optimally induce locomotion.
Figure 2. Gamma band activity in whole-cell recorded PPN cells.
A. Increasing steps of current (increase of 30 pA per step, each step was 500 ms in duration delivered every 2.5 sec) caused cells to fire action potentials at increasingly higher frequencies. This cell fired maximally at 54 Hz, i.e within gamma band range. B. Graph showing the firing frequency of ten cells at each current step (small bullets) and of the average of the 50 recorded cells (large red bullets, R2 = 0.994). The average maximal firing frequency was at the 180 pA current step, where cells fired at the average rate of 50 ± 16 Hz for all cells. C. Graph showing the firing frequency of each cell at the 180 pA current step, divided according to cell type (black dots). The average firing frequency of each cell type is also shown (red bullets). Type 1 neurons fired significantly faster than type II or III cells, but type II and III neurons did not fire action potentials significantly faster than one another. The average maximal firing frequency for type I, II, and III neurons were: 58 ± 15 Hz, 45 ± 15 Hz, and 46 ± 16 Hz, respectively.
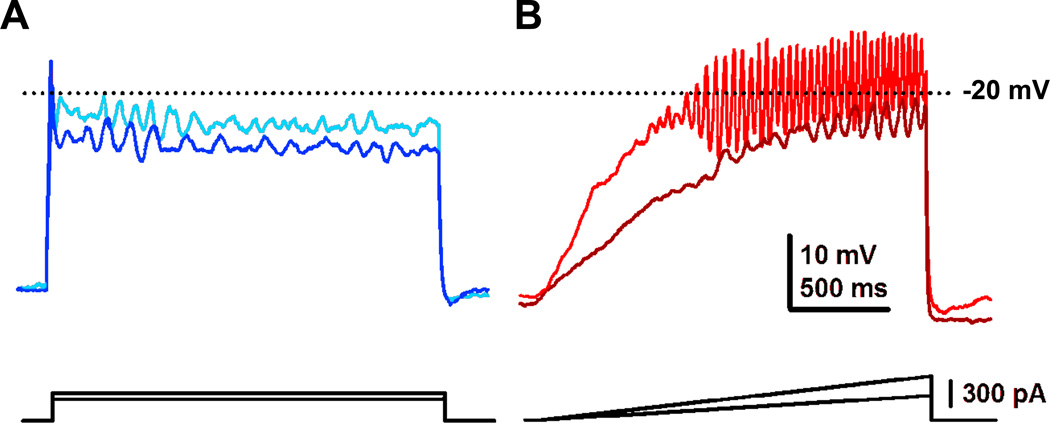
Figure 3. Effects of steps vs ramps in eliciting gamma band oscillations in PPN neurons.
A. Current clamp recording of a PPN neuron to which were applied current steps of increasing amplitude (dark blue trace represents the response to lower amplitude square current while light blue represents the response to higher amplitude square current pulses). Note that the membrane potential failed to be maintained and repolarized below the window for high threshold, voltage-dependent calcium channels. B. Recordings in the same neuron but using ramps of increasing amplitude (brown trace represents the response to lower amplitude current ramp while red represents the response to higher amplitude current ramp). Note that the membrane potential could be gradually increased to induce membrane oscillations that could be maintained within the window for activation of high threshold calcium channels (around −20 mV in the soma, but much lower in the dendrites). Further studies showed that these were P/Q-type voltage-dependent calcium channels (Kezunovic et al. 2011).
Ramping up current
Early studies revealed that suddenly switching on the current at previously determined threshold levels elicited only decrements in muscle tone instead of stepping (Garcia-Rill et al. 1986; 1995; Takakusaki et al. 2003). Pulses delivered to the PPN had to be increased gradually until locomotion was induced, and only then was continuous stepping evident (Garcia-Rill et al. 1986; 1991; Reese et al. 1995). We referred to this characteristic as “recruiting” locomotion. Single cells in the PPN also need to be “recruited” to fire at gamma band frequencies. Figure 3 shows the effects of using sudden current steps (Fig. 3A) vs current ramps (Fig. 3B) to induce membrane oscillations. The requirement to use ramps to elicit oscillations helps explain why current has to be gradually increased to depolarize PPN neurons slowly, i.e. to avoid activating potassium channels, and thus induce stepping. Moreover, current ramps can depolarize membrane potential with a similar time course, as previously observed during temporal summation of EPSPs during gamma band stimulation (Pedroarena and Llinas 2001). Sudden application of high amplitude currents would activate potassium channels and fail to sufficiently depolarize neurons. This would instead lead to inactivation of PPN neurons, explaining the decrement in muscle tone evident with sudden onset stimulation. This explains the need for ramping up current, instead of suddenly switching it on, in order to “recruit” stepping. Once stepping was initiated, current levels were decreased slightly in order to maintain stepping, as if a lower frequency was required for maintaining than initiating locomotion (Garcia-Rill 1991; Garcia-Rill & Skinner 1991; Garcia-Rill et al. 2004).
Long duration pulses
We hypothesize that the need to use long duration pulses is related to the high threshold calcium channels present in PPN neurons. We used calcium imaging to visualize ramp-activated calcium channels in PPN cells (Hyde et al. 2013). Figure 4 shows that voltage-dependent high threshold P/Q-type calcium channels are located in the dendrites of PPN neurons. This explains the need to apply high levels of depolarization of the cell body in order to ultimately depolarize the dendrites sufficiently to activate the high threshold calcium channels and promote gamma band oscillations. Long duration pulses, along with ramping up current levels, would be effective methods for activating dendritic calcium channels that would in turn allow PPN neurons to fire maximally at gamma band frequencies.
Figure 4. Localization of calcium channels in the dendrites of PPN neurons.
These studies used a ratiometric calcium imaging method designed to detect calcium flux at high speeds (Hyde et al. 2013). A. Records of ramp-elicited calcium oscillations recorded simultaneously with both electrical recording (black record) and high speed fluorescence imaging (red in the soma, blue in one dendrite). The oscillations in the somatic fluorescence recording (red record) closely matched those in the electrical record. The blue dendritic record also followed the electrical record, but with more variation. Note that the electrical and fluorescence changes were eliminated by the addition of Cd2+ or a P/Q-type calcium channel blocker (Hyde et al. 2013). B. Cross-correlation of the electrical record (black record) and the somatic fluorescence record (red record). The inset shows a view of calcium flux in the patched neuron. Calibration bar 25 µm.
Is gamma band activity in the RAS different from that in the cortex?
A common question is, does gamma band activity in the PPN differ from that in the cortex? In other words, does RAS gamma band activity drive cortical gamma band EEG? There is undeniable evidence that mesopontine gamma band activity is essential to maintaining wakefulness, that the RAS drives cortical EEG rhythms, and that cells in the RAS fire in relation to gamma band activity. That is, cortical gamma band activity is normally interleaved with subcortical gamma band activity. The earliest studies on the RAS showed that acute transections of the midbrain anterior to the PPN led to the manifestation of only high amplitude, low frequency EEG activity in the cortex, but acute transections posterior to the PPN led to low voltage fast activity in the cortex (Lindsley et al. 1949). That is, even after such disruptive lesions, the fact that the PPN was connected to the forebrain allowed the manifestation of “waking-type” EEG in the cortex, while the hypothalamus and basal forebrain were unable to maintain such activity in the absence of the RAS. Later studies showed that chronic transections anterior to the RAS did allow low voltage, fast activity to occur in the cortex after several days to weeks in the “isolated forebrain” (Villablanca 1962). These studies showed that the distributed control of fast activity by thalamic and subthalamic structures could recover in the absence of input from the RAS. But, in the acute transection model, the presence of intact ascending projections from the RAS was sufficient to induce cortical fast activity when by itself the cortex was incapable of producing such activity. Further chronic injury studies determined that the isolated forebrain could generate wake-sleep-like EEG patterns and exhibit arousal to olfactory stimuli, suggesting “waking can occur independently in both forebrain and brainstem” (Villablanca 2004).
However, in an intact brain, gamma band activity in the RAS is intrinsically linked to cortical EEG gamma band activity. Steriade’s group has shown that PPN thalamic projecting neurons in vivo manifested firing properties relative to ponto-geniculo-occipital (PGO) wave generation and EEG (Steriade et al. 1990). Some of these neurons showed low rates of spontaneous firing (<10 Hz), but most had high rates of tonic firing in the beta/gamma range (20–80 Hz). Stimulation of the PPN potentiated the appearance of fast (20–40 Hz) oscillations in the cortical EEG, outlasting stimulation by 10 to 20 seconds (Steriade et al. 1991). More recent studies have shown that cholinergic PPN neurons modulate cortical gamma band activity in vivo (Mena-Segovia et al. 2008). Even in lightly anesthetized animals, PPN cells fired rhythmically during cortical slow oscillations but enhanced nested gamma oscillations (30–60 Hz). Recordings of PPN neurons in humans during DBS showed that these cells fire in the beta-gamma band range, while neurons dorsal to the PPN fired at slower rates in the awake patient (Shimamoto et al. 2010). Local field potentials of spontaneous activity increase to the beta range as recording electrodes enter the PPN in the human (Shimamoto et al. 2010; Weinberger et al. 2008). While activity in the absence of stimulation or movement is most abundant in the alpha range in the human PPN, higher frequency activity in the beta range is evident in relation to passive and self-paced movements (Shimamoto et al. 2010; Tsang et al. 2010; Weinberger et al. 2008).
Why is the PPN involved in posture and locomotion?
The PPN has long been known to be part of the RAS in charge of sleep-wake control (Steriade and McCarley 1990). The fact that the PPN is involved in sleep-wake control and arousal led us to conclude that the “MLR” was not a locomotion-specific region, but rather a phenomenon elicited when activating a region with very specific “rhythmogenic” characteristics. The RAS is a phylogenetically conserved system that modulates fight-or-flight responses. During waking, man’s ability to detect predator or prey is essential to survival. Under these circumstances, it is not surprising that the RAS can modulate muscle tone and locomotion. This system is thus intrinsically linked to control of the motor system in order to optimize attack or escape. During rapid eye movement (REM) sleep, while we are in a vulnerable state, atonia keeps us from acting out our dreams. In fact, only our diaphragm and eye muscles appear to be acting out dream content. Therefore, during waking and REM sleep, two states modulated by the PPN, the RAS can modulate muscle tone and locomotion via the same reticulospinal systems. For example, in a standing individual, there is tonic activation of anti-gravity, mainly extensor, muscles (the same ones inhibited in the atonia of REM sleep). Before the first step can be taken, there must be flexion of the leg, therefore, there must be a release, or extensor inhibition, from this postural extensor bias. It should be noted that the first sign of stepping from a standing position will always be extensor inhibition to unlock the knees, followed by flexion. Interestingly, the startle response, which is manifested as a rapid response to a supra-maximal auditory stimulus, is basically a flexor response, placing the body in a "ready" position. This response shifts the standing individual from extensor to flexor bias, as if going from a standing position (extended) to a "ready" position (flexed). The startle response is composed of a short latency activation of muscle activity (the "ready" condition) that occurs too fast for RAS modulation, followed by a brief inhibition (the "reset" state), then a long latency activation (the "go" condition). The brief, intermediate latency inhibition is thought to be part of the modulation of the startle response by the PPN, and may represent a "resetting" of motor programs which allow the subsequent selection of response strategies, the triggering of attack or escape movements (Garcia-Rill et al. 1996). The complexity of these electromyographic responses unfortunately is missing when the startle response is measured by whole-body or segmental/reflex movement.
There appear to be multiple descending pathways via which the PPN modulates postural and locomotor control. In the oral pontine reticular formation there is a REM sleep-inducing region, the SubCoeruleus dorsalis (SubCD) nucleus, which can be activated by cholinergic agonists (arising from cholinergic projections from the PPN) to induce paradoxical sleep with atonia (Baghdoyan et al. 1984). Lesions of this region produce an animal exhibiting REM sleep without atonia (Sanford et al. 1994). Presumably, outputs from the PPN and/or SubCD activate reticulospinal systems that lead to profound hyperpolarization of motoneurons, which is the mechanism responsible for the atonia of REM sleep (Chase and Morales 1994). This pathway may also be involved in the startle reflex inhibition of extensors (Davis 1984). On the other hand, cholinergic projections from the PPN to the medioventral medulla appear to elicit increases in locomotion. Presumably, outputs from this region activate reticulospinal systems that lead to the triggering of spinal pattern generators to induce stepping (Garcia-Rill et al. 1991; 2004; Reese et al. 1995). Electrical stimulation of the pontine and medullary reticular formation is known to induce decreased muscle tone at some sites, while producing stepping movements at other sites. This suggests the presence of a heterogeneous, distributed system of reticulospinal motor control. The required parameters of stimulation for eliciting these differing effects are important such that instantaneous, high frequency trains (similar to high frequency bursting activity in the range of PGO burst neurons that may drive the atonia of REM sleep) trigger pathways which lead to decreased muscle tone, while lower frequency tonic stimulation lead gradually to the “recruitment” of locomotor movements (Reese et al. 1995). Therefore, given the extensive evidence, it is to be expected that the PPN should modulate both posture and locomotion.
Implications for DBS
The considerable attention paid to this system over the years ultimately led to the use of DBS to treat the postural and locomotor deficits induced by Parkinson’s disease (PD). Early studies focused on patients with PD who presented severe axial symptoms and gait disorders not responsive to L-Dopa treatment (Mazzone et al. 2005). Some attempts used ventriculography and intracranial recordings to verify the stimulation sites, while later studies modified the electrode trajectories and visualization methods for assessing location (Mazzone et al. 2008; 2009; Zrinzo et al. 2008). In terms of the effects of PPN DBS on PD symptoms, the results are diverse. Ferraye et al (2010) found that bilateral PPN stimulation at 15 Hz and 25 Hz improved freezing of gait and decreased falls. Most patients (4 out of 5) showed moderate or significant improvements in gait. Moro et al (2010) used unilateral stimulation at 50 Hz and 70 Hz, and showed improvements in falls and motor scores. Stefani et al (2007, 2013) used PPN stimulation at 10 Hz and 25 Hz, with some improvements in motor scores but a significant improvement in sleep patterns and modest improvement in gait. Alessandro et al (2010) using 25 Hz stimulation showed no motor improvements but significant amelioration in sleep scores and executive function. Thevanasathan et al (2010, 2012) showed that stimulation at 20–35 Hz improved reaction time and fall scores, and that gait freezing was significantly improved, particularly with bilateral stimulation. The latter study is one of the few that used double-blind analysis and established that bilateral stimulation was more effective than unilateral.
In general, these results taken together suggest that stimulation, especially bilaterally, at mid-range frequencies (25–50 Hz) improved gait scores and prevented falls. It is likely that the differences across the results of different groups are due to the selection of patients, of surgical approach, of method of verification of stimulation site, of parameters of stimulation, and of measures of end result. In addition, a number of specific issues remain to be clarified before this therapy can be used consistently across groups, namely, a) current practice in DBS is to stimulate using 50–100 µsec pulses, which may preferentially be activating intrinsic axons and fibers of passage rather than neurons; b) current practice in DBS is to use frequencies >60 Hz, which, when applied to PPN, may tend to inactivate these neurons by depolarization block [In reports of DBS of the PPN, only some studies have used stimulation at 40–60 Hz, to good effect (Mazzone et al. 2008; 2009; Moro et al. 2010)]; and c) the practice of applying continuous DBS throughout the day may not result in creating a continuous facilitation, and it is difficult to extrapolate from animal studies using periodic stimulation to the potential effects of continuous DBS in the human. One potentially fortuitous effect of continuous DBS is that the RAS is a system that responds well to novelty but quickly habituates to repetitive or continuous input. This may be the reason why PPN DBS does not produce obviously untoward arousal effects, and may in the end help to stabilize RAS output when subjected to persistent, appropriate (optimal frequency range for activation) stimulation. This may be why improvements in sleep architecture are also seen. Overall, better localization of stimulating leads in the human requires consultation with studies of three-dimensional reconstruction of the PPN (Garcia-Rill et al. 1995). This would help establish the optimal stimulation sites for the region. While some groups have been able to confirm stimulation sites postmortem as being within the PPN (Hazrati et al. 2012), it is not at all clear that all groups are stimulating within the PPN, and some are likely to be stimulating at the edges or outside the nucleus. Problems with dysaesthesias due to activation of nearby pathways such as the medial lemniscus (Moro et al. 2010) can be avoided by using somatosensory evoked potential monitoring to avoid this pathway (Insola et al. 2014). In addition, the use of DBS electrodes of >1 mm in diameter may produce undesired effects (Mazzone et al. 2011; Aviles-Olmos et al. 2011). As the field matures to allow more exact localization and determination of effects of stimulation, PPN DBS may prove to be a more widely used, viable and effective strategy for the treatment of at least some of the postural and locomotor deficits in PD and other movement disorders. For now, this treatment option is far from becoming clinical routine.
Suggestions for the future
A critical consideration that must be addressed is the dimension of stimulating electrodes used for DBS. The diameter of the electrodes used in animal experiments is in the order of a few microns to 100 µm wires, an order of magnitude smaller than electrodes currently available for DBS in the human (>1 mm). The PPN is far too small to be stimulated effectively, and without lesioning, using such electrodes. Manufacturers need to create thinner electrodes for DBS in such regions. The preceding discussion on the duration and frequency of stimulation suggests that, if the purpose is to activate the PPN, then long duration (~200 msec) and low frequency (40–60 Hz) pulses should be used. On the other hand, if the purpose is to inactivate the PPN, then short duration (<1 msec) and high frequency (>80 Hz) pulses would be more effective. An additional concern when considering stimulation using long duration (>200 msec) pulses is the potential damage that could be induced by such stimuli by localized heating. Overall, the parameters of stimulation currently used by most groups suggest that DBS of the PPN is decreasing PPN activity. An ideal electrode would be of fine diameter and would also incorporate electrical and/or chemical monitoring technology that would allow the option of adjustments to DBS stimulation parameters based on measured PPN or nearby activity. Given that findings in PD patients suggest that the PPN is overactive in PD (Teo et al. 1997, 1998), such stimulation may result in dampening PPN activity, thus having a palliative effect.
Another issue that must be considered is that DBS is being applied to the RAS. As such, the effects on sleep-wake cycles need to be determined. For example, is PPN DBS disturbing or correcting the sleep dysregulation in PD? PD patients manifest decreased slow wave sleep, increased REM sleep, and increased vigilance, including exaggerated reflexes (reviewed in Garcia-Rill et al. 1997). It should be standard practice to determine the effects of PPN DBS on sleep-wake cycles by performing sleep studies before and after implantation. One study performed sleep measures and found that PPN DBS improved not only nighttime sleep, but also daytime sleepiness (Peppe et al. 2012). If the sleep-wake parameters are being normalized, then the procedure can be determined to have had a positive outcome as far as sleep-wake control is concerned. In PD patients followed longitudinally, delta and theta power increased while higher frequencies, including gamma, decreased, and these changes correlated with cognitive decline (Olde-Dubbelink et al. 2012). A potentially fruitful endeavor is to determine the effects of PPN DBS on gamma band power in PD. Determining the effects of PPN DBS on gamma band power and on gamma band maintenance would be very important for assessing the beneficial effects of PPN stimulation on higher functions. A number of neurological and psychiatric disorders are also characterized by interrupted or decreased gamma band activity (Garcia-Rill et al. 2014). Aberrant gamma band activity and coherence during cognitive tasks or attentional load have been reported in schizophrenic and bipolar disorder patients (Flynn et al. 2008; Ozerdem et al. 2011; Spencer et al. 2003; Symond et al. 2005; Ulhaas and Singer 2010). It may be possible to use DBS, in cases resistant to pharmacotherapy, to attempt to normalize gamma band activity in these disorders. Such an approach in these disorders would be more prudent if it can be shown that gamma band power is normalized in PD after PPN DBS.
It should be remembered that the PPN contributes to the manifestation of high frequency activity (beta and gamma band) in the cortical EEG (Steriade et al. 1991). Studies need to be performed to determine the effects of short- and long-term PPN DBS on cortical high frequency EEG activity. There is some information that PPN DBS may improve cognitive function (Tyckoki et al. 2011), and that low frequency stimulation (5–30 Hz) may improve executive and higher functions (Stefani et al. 2013), but this issue needs further elucidation. After all, lesions of the PPN disturb attention, executive function and working memory (Winn 2008). Therefore, it should be expected that PPN DBS will affect higher functions. In addition, the PPN has been proposed to participate in the process of preconscious awareness (Garcia-Rill et al. 2013; Urbano et al. 2012). The effects of PPN DBS need to be studied for potential modulation of this essential survival mechanism. Studies, perhaps using priming or distractors, to assess distractibility are called for in order to properly assess the full effects of this therapy.
Another consideration is whether or not PPN DBS is having a benign effect because it is providing compensation for an abnormal signal that the PPN was emitting, i.e. overactive output in PD, or replacing information being provided by the PPN that was previously read as misinformation by its targets. That is, does the DBS, either by lesion of the PPN due to the large electrode or by providing high frequency activation to the remaining cells in the region, somehow rebalance the system that was disturbed by the degeneration of substantia nigra neurons in PD? In such a case, it is possible that continuous DBS provides a persistent signal, regulating and stabilizing the circuits at physiological (gamma band) frequencies.
At present, the ameliorative effects that PPN DBS, when performed with the highest standards, has on locomotor and postural function far reasonably outweigh the potential side effects when the surgical approach minimizes the potential for bleeding and stimulation within the PPN is achieved. Nevertheless, this area requires much additional research in order to optimize treatment, and results from studies with long follow-ups certainly are needed.
Acknowledgments
This work was supported by NIH award R01 NS020246, and by core facilities of the Center for Translational Neuroscience supported by NIH awards P20 GM103425 and P30 GM110702 to Dr. Garcia-Rill. In addition, this work was supported by grants from FONCYT-Agencia Nacional de Promoción Científica y Tecnológica; BID 1728 OC.AR. PICT-2012-1769 (to Dr. Urbano).
Footnotes
Conflict of interest: The authors declare no conflict of interest.
Nomenclature of the PPN has always been inconsistent. When we began work in the region in 1979, some atlases used the contraction “PPT”, others “PPTg”, and others “PPN”, for nucleus tegmenti pedunculopontinus. We chose the “PPN” contraction for this terminology because the term “tegmental” is superfluous. The main descriptor, “pedunculo-pontine”, pinpoints the location to the body (tegmentum) of the pons near the peduncle, making the term “tegmental’ unnecessary. In addition, some workers use the contraction “T” for tegmental, while others use “Tg”, adding to the variability in this term. Since there is no other “pedunculopontine” nucleus in the brain, again, we consider the term “tegmental” unnecessary. For example, the “laterodorsal tegmental nucleus” does require the “tegmental” since there is a laterodorsal “thalamic” nucleus. Most investigators contract the laterodorsal tegmental nucleus to “LDT”, but never “LDTg”. The inconsistencies remain.
References
- Alam M, Schwabe K, Krauss JK. The pedunculopontine nucleus area: critical evaluation of interspecies differences relevant for its use as a target for deep brain stimulation. Brain. 2011;134:11–23. doi: 10.1093/brain/awq322. [DOI] [PubMed] [Google Scholar]
- Alessandro S, Ceravolo R, Brusa L, Pierantozzi M, Costa A, Galati S, Placidi F, Romigi A, Iani C, Marzetti F, Peppe A. Non-motor functions in parkinsonian patients implamted in the pedunculopontine nucleus: Focus on sleep and cognitive problems. J Neurol Sci. 2010;289:44–48. doi: 10.1016/j.jns.2009.08.017. [DOI] [PubMed] [Google Scholar]
- Aviles-Olmos I, Foltynie T, Panicker J, Cowie D, Limousin P, Hariz M, Fowler CJ, Zrinzo L. Urinary incontinence following deep brain stimulation of the pedunculopontine nucleus. Acta Neurochir (Wien) 2011;153(12):2357–2360. doi: 10.1007/s00701-011-1155-6. [DOI] [PubMed] [Google Scholar]
- Baghdoyan HA, Rodrigo-Angulo ML, McCarley RW, Hobson JA. Site-specific enhancement and suppression of desynchronized sleep signs following cholinergic stimulation of three brainstem regions. Brain Res. 1984;306:39–52. doi: 10.1016/0006-8993(84)90354-8. [DOI] [PubMed] [Google Scholar]
- Chase MH, Morales FR. The control of motoneurons during sleep. In: Kryger MH, Roth T, Dement WC, editors. Principles and Practice of Sleep Medicine. London: WB Saunders; 1994. pp. 163–176. [Google Scholar]
- Davis M. The mammalian startle response. In: Eaton RC, editor. Neural Mechanisms of Stratle Behavior. New York: Plenum Press; 1984. pp. 287–342. [Google Scholar]
- Ferraye MU, Debu B, Fraix V, Goetz L, Ardouin C, Yelnik J, et al. Effects of pedunculopontine nucleus area stimulation on gait disorders in Parkinson's disease. Brain. 2010;133:205–214. doi: 10.1093/brain/awp229. [DOI] [PubMed] [Google Scholar]
- Flynn G, Alexander D, Harris A, Whitford T, Wong W, Galletly C, et al. Increased absolute magnitude of gamma synchrony in first-episode psychosis. Schizophr Res. 2008;105:262–271. doi: 10.1016/j.schres.2008.05.029. [DOI] [PubMed] [Google Scholar]
- Garcia-Rill E. The basal ganglia and the locomotor regions. Brain Res Rev. 1986;11:47–63. [PubMed] [Google Scholar]
- Garcia-Rill E. The pedunculopontine nucleus. Prog Neurobiol. 1991;36:363–389. doi: 10.1016/0301-0082(91)90016-t. [DOI] [PubMed] [Google Scholar]
- Garcia-Rill E. Disorders of the Reticular Activating System. Med Hypoth. 1997;49:379–387. doi: 10.1016/s0306-9877(97)90083-9. [DOI] [PubMed] [Google Scholar]
- Garcia-Rill E, Biedermann JA, Chambers T, Skinner RD, Mrak RE, Husain M, Karson CN. Mesopontine neurons in schizophrenia. Neuroscience. 1995;66:321–335. doi: 10.1016/0306-4522(94)00564-l. [DOI] [PubMed] [Google Scholar]
- Garcia-Rill E, Hull CD, Levine MS, Buchwald NA. The spontaneous firing patterns of forebrain neurons. IV. Effects of bilateral and unilateral frontal cortical ablations on firing of caudate, globus pallidus and thalamic neurons. Brain Res. 1979;165:23–36. doi: 10.1016/0006-8993(79)90041-6. [DOI] [PubMed] [Google Scholar]
- Garcia-Rill E, Reese NB, Skinner RD. Arousal and locomotion: from schizophrenia to narcolepsy. In: Holstege G, Saper CB, editors. The Emotional Motor System. Prog Brain Res. Vol. 107. 1996. pp. 417–434. [DOI] [PubMed] [Google Scholar]
- Garcia-Rill E, Skinner RD. Modulation of rhythmic function in the posterior midbrain. Neuroscience. 1988;17:639–654. doi: 10.1016/0306-4522(88)90295-3. [DOI] [PubMed] [Google Scholar]
- Garcia-Rill E, Skinner RD. Modulation of rhythmic functions by the brainstem. In: Shimamura M, Grillner S, Edgerton VR, editors. Neurobiology of Human Locomotion. Tokyo: Japan Sci. Soc. Press; 1991. pp. 137–158. [Google Scholar]
- Garcia-Rill E, Skinner RD, Fitzgerald JA. Chemical activation of the mesencephalic locomotor region. Brain Res. 1985;330:43–54. doi: 10.1016/0006-8993(85)90006-x. [DOI] [PubMed] [Google Scholar]
- Garcia-Rill E, Skinner RD, Gilmore SA. Pallidal projections to the mesencephalic locomotor region (MLR) in the cat. Amer J Anat. 1981;161:311–322. doi: 10.1002/aja.1001610305. [DOI] [PubMed] [Google Scholar]
- Garcia-Rill E, Skinner RD, Fitzgerald JA. Activity in the mesencephalic locomotor region (MLR) during locomotion. Exp Neurol. 1983;82:609–622. doi: 10.1016/0014-4886(83)90084-5. [DOI] [PubMed] [Google Scholar]
- Garcia-Rill E, Houser CR, Skinner RD, Smith W, Woodward DJ. Locomotion-inducing sites in the vicinity of the pedunculopontine nucleus. Brain Res Bull. 1987;18:731–738. doi: 10.1016/0361-9230(87)90208-5. [DOI] [PubMed] [Google Scholar]
- Garcia-Rill E, Homma Y, Skinner RD. Arousal mechanisms related to posture and movement. I. Descending modulation. In: Mori S, Stuart DG, Wiesendanger M, editors. Brain Mechanisms for the Integration of Posture and Movement, Prog Brain Res. Vol. 143. 2004. pp. 283–290. [PubMed] [Google Scholar]
- Garcia-Rill E, Kezunovic N, D’Onofrio S, Luster B, Hyde J, Bisagno V, Urbano FJ. Gamma band activity in the RAS- intracellular mechanisms. Exp Brain Res. 2014 doi: 10.1007/s00221-013-3794-8. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Rill E, Skinner RD. Modulation of rhythmic functions by the brainstem. In: Shimamura M, Grillner S, Edgerton V, editors. Neurobiological Basis of Human Locomotion. Tokyo: Japan Scientific Societies Press; 1991. pp. 137–158. [Google Scholar]
- Hazrati LN, Wong JC, Hamani C, Lozano AM, Poon YY, Dostrovsky JO, Hutchison WD, Zadikoff C, Moro E. Clinicopathological study in progressive pupranuclear palsy with pedunculopontine stimulation. Mov Disord. 2012;27:1304–1307. doi: 10.1002/mds.25123. [DOI] [PubMed] [Google Scholar]
- Hyde J, Kezunovic N, Urbano FJ, Garcia-Rill E. Spatiotemporal properties of high speed calcium oscillations in the pedunculopontine nucleus. J Appl Physiol. 2013;115(1985):1402–1414. doi: 10.1152/japplphysiol.00762.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Insola A, Padua L, Mazzone P, Scarnati E, Valeriani M. Low and high-frequency somatosensory evoked potentials recorded from the human pedunculopontine nucleus. Clin Neurophysiol. 2014 doi: 10.1016/j.clinph.2013.12.112. In Press. [DOI] [PubMed] [Google Scholar]
- Karson DH, Garcia-Rill E, Biedermann JA, Mrak RE, Husain M, Skinner RD. The brain stem reticular formation in schizophrenia. Psychiat Res. 1991;40:31–48. doi: 10.1016/0925-4927(91)90027-n. [DOI] [PubMed] [Google Scholar]
- Kezunovic N, Urbano FJ, Simon C, Hyde J, Smith K, Garcia-Rill E. Mechanism behind gamma band activity in the pedunculopontine nucleus (PPN) Eur J Neurosci. 2011;34(3):404–415. doi: 10.1111/j.1460-9568.2011.07766.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindsley DB, Bowden JW, magoun HW. Effect upon the EEG of acute injury to the brain stem activating system. Electroenceph Clin Neurophysiol. 1949;1:475–486. [PubMed] [Google Scholar]
- Mazzone PSP, Lozano A, Sposato S, Scarnati E, Stefani A. Proceedings of 14th Meeting of the World Society of Stereotactic and Functional Neurosurgery (WSSFN) Bologna: Monduzzi; 2005. Brain stimulation and movement disorders: where we going? [Google Scholar]
- Mazzone P, Insola A, Sposato S, Scarnati E. The deep brain stimulation of the pedunculopontine tegmental nucleus. Neuromodulation. 2009;12:191–204. doi: 10.1111/j.1525-1403.2009.00214.x. [DOI] [PubMed] [Google Scholar]
- Mazzone P, Sposato S, Insola A, Dilazzaro V, Scarnati E. Stereotactic surgery of nucleus tegmenti pedunculopontine [corrected] Br J Neurosurg. 2008;22(Suppl 1):S33–S40. doi: 10.1080/02688690802448327. [DOI] [PubMed] [Google Scholar]
- Mazzone P, Scarnati E, Garcia-Rill E. Commentary: The pedunculopontine nucleus: clinical experience, basic questions and future directions. J Neural Trans. 2011;118:1391–1396. doi: 10.1007/s00702-010-0530-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mena-Segovia J, Sims HM, Magill PJ, Bolam JP. Cholinergic brainstem neurons modulate cortical gamma band activity during slow oscillations. J Physiol (Lond) 2008;586:2947–2960. doi: 10.1113/jphysiol.2008.153874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mesulam MM, Mufson EJ, Wainer BH, Levey AI. Central cholinergic pathways in the rat: an overview based on an alternative nomenclature (Ch1-Ch6) Neuroscience. 1983;10:1185–1201. doi: 10.1016/0306-4522(83)90108-2. [DOI] [PubMed] [Google Scholar]
- Moro E, Hamani C, Poon YY, Al-Khairallah T, Dostrovsky JO, Hutchison WD, Lozano AM. Unilateral pedunculopontine stimulation improves falls in Parkinson's disease. Brain. 2010;133:215–224. doi: 10.1093/brain/awp261. [DOI] [PubMed] [Google Scholar]
- Nauta WJH, Mehler WR. Projections of the lentiform nucleus in the monkey. Brain Res. 1966;1:3–42. doi: 10.1016/0006-8993(66)90103-x. [DOI] [PubMed] [Google Scholar]
- Olde-Dubbelink KTE, Stoffers D, Deijen JB, Twisk JWR, Stam CJ, Berendse HW. Cognitive decline in Parkinson’s disease is associated with slowing of resting-state brain activity: a longitudinal study. Neurobiol Aging. 2012;34(2):408–418. doi: 10.1016/j.neurobiolaging.2012.02.029. [DOI] [PubMed] [Google Scholar]
- Ozerdem A, Guntenkin B, Atagun I, Turp B, Basar E. Reduced long distance gamma (28–48 Hz) coherence in euthymic patients with bipolar disorder. J Affect Disord. 2011;132:325–332. doi: 10.1016/j.jad.2011.02.028. [DOI] [PubMed] [Google Scholar]
- Pedroarena C, Llinas RR. Interactions of synaptic and intrinsic electroresponsiveness determine corticothalamic activation dynamics. Thalamus & Rel Sys. 2001;1(1):3–14. [Google Scholar]
- Peppe A, Pierantozzi M, Baiamonte V, Moschella V, Caltagirone C, Stanzione P, Stefani A. Deep brain stimulation of pedunculopontine tegmental nucleus: role of sleep modulation in advanced Parkinson disease patients- one-year follow-up. Sleep. 2012;35:1637–1642. doi: 10.5665/sleep.2234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reese NB, Garcia-Rill E, Skinner RD. The pedunculopontine nucleus-auditory input, arousal and pathophysiology. Prog Neurobiol. 1995;47:105–133. doi: 10.1016/0301-0082(95)00023-o. [DOI] [PubMed] [Google Scholar]
- Sanford LD, Morrison AR, Mann GL, Harris JS, Yoo L, Ross RJ. Sleep patterning and behaviour in cats with pontine lesions creating REM without atonia. J Sleep Res. 1994;3:233–240. doi: 10.1111/j.1365-2869.1994.tb00136.x. [DOI] [PubMed] [Google Scholar]
- Shik ML, Severin FV, Orlovskii GN. Control of walking and running by means of electric stimulation of the midbrain. Biofizika. 1966;11:659–666. [PubMed] [Google Scholar]
- Shimamoto SA, Larson PS, Ostrem JL, Glass GA, Turner RS, Starr PA. Physiological identification of the human pedunculopontine nucleus. J Neurol Neurosurg Psychiat. 2010;81:80–86. doi: 10.1136/jnnp.2009.179069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon C, Kezunovic N, Ye M, Hyde J, Hayar A, Williams DK, Garcia-Rill E. Gamma band unit and population responses in the pedunculopontine nucleus. J Neurophysiol. 2010;104:463–474. doi: 10.1152/jn.00242.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skinner RD, Garcia-Rill E. Brainstem modulation of rhythmic functions and behaviors. In: Klemm WR, Vertes RP, editors. Brainstem Mechanisms of Behavior. New York: John Wiley & Sons; 1990. pp. 419–445. [Google Scholar]
- Skinner RD, Garcia-Rill E. Mesolimbic interactions with mesopontine modulation of locomotion. In: Kalivas P, Barnes C, editors. Limbic Motor Circuits and Neuropsychiatry. New York: CRC Press; 1994. pp. 155–191. [Google Scholar]
- Spencer KM, Nestor PG, Niznikiewicz MA, Salisbury DF, Shelton ME, McCarley RW. Abnormal neural synchrony in schizophrenia. J Neurosci. 1990;23:7407–7411. doi: 10.1523/JNEUROSCI.23-19-07407.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stefani A, Lozano AM, Peppe A, Stanzione P, Galati, Troppei D, Pierantozzi M, Brusa L, Scarnati E, Mazzone P. Bilateral deep brain stimulation of the pedunculopontine and subthalamic nuclei in severe Parkinson’s disease. Brain. 2007;130:1596–1607. doi: 10.1093/brain/awl346. [DOI] [PubMed] [Google Scholar]
- Stefani A, Peppe A, Galati S, Stampanoni Basso M, D’Angelo V, Pierantozzi M. The serendipity case of the pedunculopontine nucleus low-frequency barin stimulation: chasing a gait response, finding sleep, and cognitive improvement. Frontiers Neurol. 4:68. doi: 10.3389/fneur.2013.00068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steriade M, McCarley RW. Brainstem Control of Wakefulness and Sleep. New York: Plenum Press; 1990. [Google Scholar]
- Steriade M, Paré D, Datta S, Oakson G, Curro Dossi R. Different cellular types in mesopontine cholinergic nuclei related to ponto-geniculo-occipital waves. J Neurosci. 1990;10:2560–2579. doi: 10.1523/JNEUROSCI.10-08-02560.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steriade M, Curro Dossi R, Paré D, Oakson G. Fast oscillations (20–40 Hz) in thalamocortical systems and their potentiation by mesopontine cholinergic nuclei in the cat. Proc Natl Acad Sci USA. 1991;88:4396–4400. doi: 10.1073/pnas.88.10.4396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Symond MP, Harris AW, Gordon E, Williams LM. Gamma synchrony” in first- episode schizophrenia: a disorder of temporal connectivity? Am J Psychiatry. 2005;162:459–465. doi: 10.1176/appi.ajp.162.3.459. [DOI] [PubMed] [Google Scholar]
- Takakusaki K, Habaguchi T, Ohtinata-Sugimoto J, Saitoh K, Sakamoto T. Basal ganglia efferents to the brainstem centers controlling postural muscle tone and locomotion: a new concept for understanding motor disorders in basal ganglia dysfunction. Neuroscience. 2003;119:293–308. doi: 10.1016/s0306-4522(03)00095-2. [DOI] [PubMed] [Google Scholar]
- Teo C, Rasco L, Al-Mefty K, Skinner RD, Garcia-Rill E. Decreased habituation of midlatency auditory evoked responses in Parkinson's disease. Mov Dis. 1997;12:655–664. doi: 10.1002/mds.870120506. [DOI] [PubMed] [Google Scholar]
- Teo C, Rasco L, Skinner RD, Garcia-Rill E. Disinhibition of the sleep-state dependent P1 potential in Parkinson's disease-improvement after pallidotomy. Sleep Res Online. 1998;1:62–70. [PubMed] [Google Scholar]
- Thevanasathan W, Silburn PA, Brooker H, Coyne TJ, Kahn S, Gill SS, Aziz TZ, Brown P. The impact of low-frequency stimulation of the pedunculopontine nucleus region on reaction time in parkinsonism. J neurol Neurosurg Psychiat. 2010;81:1099–1104. doi: 10.1136/jnnp.2009.189324. [DOI] [PubMed] [Google Scholar]
- Thevanasathan W, Cole MH, Grapel CL, Hyam JA, Jenkinson N, Brittain JS, Coyne TJ, Silburn PA, Aziz TZ, Kerr G, Brown P. A spatiotemporal analysis of gait freezing and the impact of pedunculopontine nucleus stimulation. Brain. 2012;135:1446–1454. doi: 10.1093/brain/aws039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsang EW, Hamani C, Moro E, Mazzella F, Poon YY, Lozano AM, Chen R. Involvement of the human pedunculopontine nucleus region in voluntary movements. Neurology. 2010;75:950–959. doi: 10.1212/WNL.0b013e3181f25b35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urbano FJ, Kezunovic N, Hyde J, Simon C, Beck P, Garcia-Rill E. Gamma band activity in the reticular activating system. Front Neurol. 2012;3:6. doi: 10.3389/fneur.2012.00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villablanca J. Electroencephalogram in the permanently isolated forebrain of the cat. Science. 1962;138:44–46. doi: 10.1126/science.138.3536.44. [DOI] [PubMed] [Google Scholar]
- Villablanca J. Counterpointing the functional role of the forebrain and of the brainstem in the control of the sleep-waking system. J Sleep Res. 2004;13:179–208. doi: 10.1111/j.1365-2869.2004.00412.x. [DOI] [PubMed] [Google Scholar]
- Wang HL, Morales M. Pedunculopontine and laterodorsal tegmental nuclei contain distinct populations of cholinergic, glutamatergic and GABAergic neurons in the rat. Eur J Neurosci. 2009;29:340–358. doi: 10.1111/j.1460-9568.2008.06576.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinberger M, Hamani C, Hutchison WD, Moro E, Lozano AM, Dostrovsky JO. Pedunculopontine nucleus microelectrode recordings in movement disorder patients. Exp Brain Res. 188:165–174. doi: 10.1007/s00221-008-1349-1. [DOI] [PubMed] [Google Scholar]
- Winn P. Experimental studies of pedunculopontine functions: are they motor, sensory or integrative? Parkinsonism Relat Disord. 2008;14(S2):S194198. doi: 10.1016/j.parkreldis.2008.04.030. [DOI] [PubMed] [Google Scholar]
- Zrinzo L, Zrinzo LV, Tisch S, Limousin PD, Yousry TA, Afshar F, Hariz MI. Stereotactic localization of the human pedunculopontine nucleus: atlas-based coordinates and validation of a magnetic resonance imaging protocol for direct localization. Brain. 2008;131(Pt 6):1588–1598. doi: 10.1093/brain/awn075. [DOI] [PubMed] [Google Scholar]