Abstract
Background
The goal of the authors is to restore fine motor control and sensation for high-arm amputees. They developed a regenerative peripheral nerve interface with the aim of attaining closed loop neural control by integrating directly with the amputee's residual motor and sensory peripheral nerves. PEDOT, poly(3,4-ethylenedioxythiophene), has both electrical and ionic conduction characteristics. This hybrid character could help bridge the salutatory conduction of the nervous system to an electrode. The purpose of this study was to determine whether electrodes polymerized with PEDOT have improved ability to both record and stimulate peripheral nerve action potentials.
Methods
Impedance spectroscopy and cyclic voltammetry were performed on electrodes before and after polymerization to measure electrode impedance and charge capacity. Both recording needle and bipolar stimulating electrodes were polymerized with PEDOT. Plain and PEDOT electrodes were tested using rat (n = 18) in situ nerve conduction studies. The peroneal nerve was stimulated using a bipolar electrode at multiple locations along the nerve. Action potentials were measured in the extensor digitorum longus muscle.
Results
Bench testing showed PEDOT electrodes had a higher charge capacity and lower impedance than plain electrodes, indicating significantly improved electrode fidelity. Nerve conduction testing indicated a significant reduction in the stimulus threshold for both PEDOT recording and PEDOT stimulatory electrodes when compared with plain electrodes, indicating an increase in sensitivity.
Conclusions
PEDOT electrochemical polymerization improves electrode fidelity. Electrodes that have been electropolymerized with PEDOT show improved sensitivity when recording or stimulating action potentials at the tissue–electrode interface.
Technological advances in body armor have proved dramatically effective in reducing torso injuries and deaths among the armed services but have also resulted in a sharp increase in young amputees.1 One in 190 Americans has an amputated limb, and over 185,000 new amputations are performed every year.2 Advances in the field of neuroprosthetics have led to five-fingered designs, achieving as many as 20 degrees of freedom.3,4 The development of shape memory alloy–actuated prosthetic hands allows for more compact, lighter, and easier-to-manufacture prosthetics.5,6
Despite these advances in robotic prosthetic arms, the tissue–prosthetic interface remains a persistent problem.7 High charge density (C/m2) at the interface leads to chronic macrophage and fibroblast response with biofouling, leading to signal degradation over time.8 Chronic, closed loop control of a prosthesis requires both precision and sensitivity when recording or stimulating action potentials at the tissue–electrode interface. Interface charge density can be reduced by increasing electrode charge capacity and decreasing electrical impedance.9 High charge capacity, which is a measurement of charge transfer efficiency, facilitates more efficient recording and stimulation of action potentials at a lower charge density at the biotic–abiotic interface.10
A variety of electrically conductive polymers exist with high charge capacity and low electrical impedance. The use of one such polymer, poly-(3,4-ethylenedioxythiophene), PEDOT, has been explored by several groups for coating electrodes that are implanted in the central nervous system for long periods of time.11–13 They have shown good short-term improvement in recording fidelity for up to 6 weeks following implantation.11–13 Oxygen and sulfur substitutions along the carbon backbone of the PEDOT molecule produce an insulated resonance pathway for ionic conduction along the molecular backbone. This ionic conduction helps bridge the differences between electrical conduction of the electrode and the ionic conduction of nerve impulses. It is also responsible for the high charge capacity of PEDOT.14 There are a number of other polymers with similar properties that may be helpful in a peripheral nerve interface, including poly(pyrrole) and poly(5,6-dimethoxyindole-2-carboxylic acid).15,16 Unlike PEDOT, these polymers lose their conductive properties after serial and chronic stimulation. In addition, there are novel polymers that are melanin derived that also may be helpful in peripheral nerve interfaces, but these remain expensive and difficult to polymerize in significant quantities.17 Carbon nanotubes represent another area of intense interest, but their biocompatibility remains questionable.18,19
The purpose of this study was to determine whether electrodes polymerized with the electrically conductive polymer PEDOT have an improved ability to both record and stimulate neural signals in the peripheral nervous system. Experimental groups of plain stainless-steel electrodes and PEDOT-polymerized electrodes were compared for electrical/ionic conduction characteristics and for their performance during in situ nerve conduction studies. Bench tests included impedance spectroscopy and cyclic voltammetry to measure electrode impedance and charge capacity. These measurements allowed comparisons of electrode fidelity and signal precision. Acute in situ nerve conduction studies were then performed on normal rats. Once again, the independent experimental variable was whether or not the electrode was polymerized with PEDOT.14 Separate nerve conduction studies compared electrode type for sensitivity as recording electrodes and as stimulating electrodes.
METHODS
Electrode Electropolymerization
Two common styles of electrodes were studied. The 30-gauge needle electrode was used as a recording electrode, and the bipolar hook electrode was used for nerve stimulation. The needle electrodes were electrically polymerized with ethylenedioxythiophene using the dopant poly(sodium styrene sulfonate).20 These electrodes (Grass 30 gauge, Grass Tech, West Warwick, R.I. ) were rinsed in methanol and placed in a plating cell containing the aqueous monomer solution, ethylenedioxythiophene, and polyanionic dopant poly(sodium styrene sulfonate) in 0.1 M phosphate buffered saline (pH 7.0). A galvanostatic current of 50 μA was applied to the electrode and the monomer solution for 900 seconds (Figs. 1 and 2). The stimulating electrodes were bipolar stainless-steel hook electrodes (Harvard Apparatus, Holliston, Mass.). Biotectix, LLC (Boston, Mass.) electropolymerized these electrodes (n = 6) with PEDOT (trade name BT-DOT).
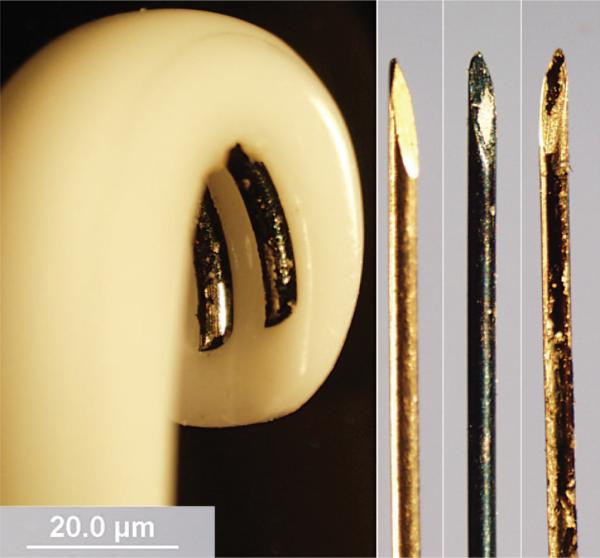
Fig. 1.
(Left to right) PEDOT stimulating electrode, plain recording electrode, PEDOT recording electrode, and PEDOT recording electrode with delamination. On the left is an electropolymerized bipolar stimulating electrode. PEDOT polymerizes as a fluffy, black coating on the metal portion of the electrode. Pictures of both the stimulating electrode and rightmost recording electrode were taken after performing a nerve conduction study. Delamination of PEDOT is present across both electrode surfaces.
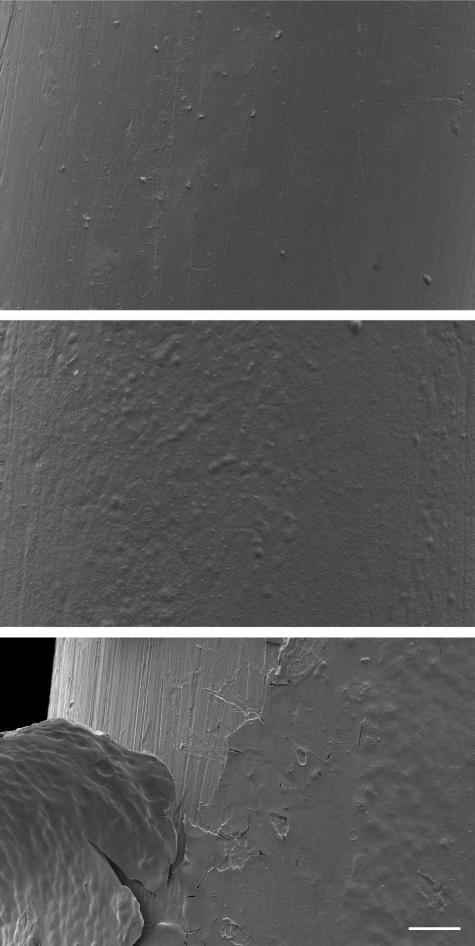
Fig. 2.
Scanning electron microscope images of needle electrodes taken at 500× magnification. (Above) Plain electrode, (center) PEDOT polymerized electrode, and (below) PEDOT polymerized electrode with delamination after heavy usage; the scale bar is 30 μm.
Cyclic voltammetry and impedance spectroscopy tests were conducted on the electrodes before and after polymerization with PEDOT.21 A three-electrode testing cell included (1) a platinum foil as the counter electrode, (2) a saturated calomel Ag/AgCl2 electrode as the reference electrode, and (3) the polymerized electrode as the working electrode. Cyclic voltammetry determines electrical charge transfer capacity. For cyclic voltammetry, a scan rate of 10 mVs−1 was used, and the potential on the working electrode was swept between −1 and +1 V in comparison with a single calomel electrode. This limit was wide enough to include reversible redox reactions yet narrow enough to avoid overoxidation and remain within the water window.22 An identical three-cell chamber with a platinum counter electrode and a saturated calomel Ag/AgCl2 reference electrode was used for measuring impedance. The real and imaginary components of the impedance were measured as a function of frequency from 1 to 10,000 Hz (Fig. 3).
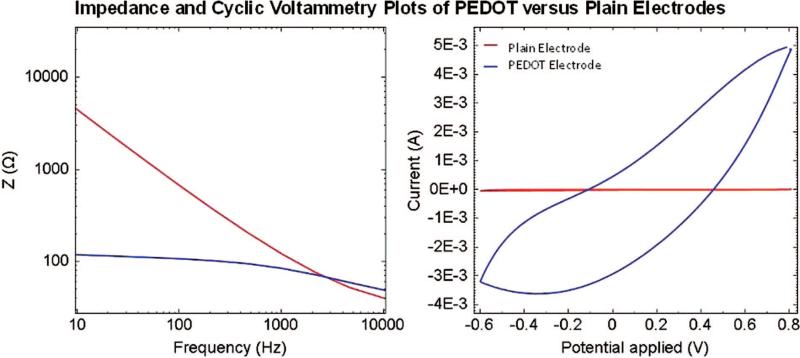
Fig. 3.
Cyclic voltammetry (right) and impedance spectroscopy plot of PEDOT-polymerized (blue) and plain (red) electrodes. The area under each plot of the cyclic voltammetry plot is the electrode's charge capacity. The charge capacity of the PEDOT-polymerized electrode is much larger than the plain electrode. The impedance of the PEDOT-polymerized electrode is significantly lower than the impedance of the plain electrode from 10 to 1000 Hz.
Animal Model
All procedures were approved by the University of Michigan Committee on Use and Care of Animals and were in strict accordance with the National Research Council's Guide for the Care and Use of Laboratory Animals.23 Rats were dosed appropriately with the analgesic buprenorphine hydrochloric acid (0.05 mg/kg) followed by anesthesia with sodium pentobarbital (50 mg/kg). The peripheral nerve signal recording study examined male, F344 rats (n = 12; Charles River, Wilmington, Mass.) weighing between 374 and 410 g, and the electrical stimulation study compared properties in male, F344 rats (n = 6) weighing between 332 and 352 g each.
Nerve Conduction Recording Study
The recording characteristics of needle electrodes were assessed with nerve conduction studies. These studies were conducted using a Teca Synegy System (Viasys Healthcare, Madison, Wis.) with a 50 to 60-Hz notch filter and cutoff frequencies between 3 and 10 kHz. During testing, the peroneal nerve was dissected free from sciatic notch to entrance into the lateral compartment. The tibialis anterior and extensor digitorum longus muscles were exposed through a small skin incision. The experimental recording, needle electrode was placed in the belly of the extensor digitorum longus muscle. Indirect muscle stimulation was provided with a single galvanostatic square wave applied on the peroneal nerve using a bipolar stainless-steel hook electrode. The order and location for stimulation included the fibular head, then 2 to 3 cm proximal to the fibular head (midperoneal), and lastly, 5 to 6 cm proximal to the fibular head (sciatic notch; Fig. 4). For each rat, a nerve conduction study was performed with either a plain recording needle electrode (n = 7) or a PEDOT-polymerized recording needle electrode (n = 7). The ground electrode was placed between the second and third toes of the ipsilateral foot, and the reference electrode was placed in the tendon of the anterior tibialis muscle. The ground and reference electrodes were plain needle electrodes, and the stimulation electrode was a plain bipolar stainless-steel hook electrode.
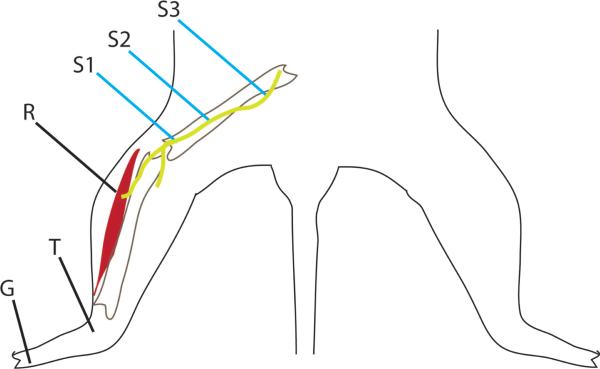
Fig. 4.
Dorsal drawing of a rat shows nerve conduction setup for recording study. The recording electrode (R) is either plain or PEDOT-polymerized. The stimulating electrode (shown in blue) is a bipolar electrode and is moved increasing proximal during testing. Testing is done at the three locations indicated: the fibular head (S1), midperoneal (S2), and the sciatic notch (S3). The ground (G) electrode is in the foot between the second and third toes. A reference electrode (T) is placed in the tendon of the tibialis anterior.
Nerve conduction studies record change in electrical voltages within a muscle when the nerve that innervates it is electrically stimulated. Three test protocols allow measurement of the following electrophysiology characteristics: stimulus threshold, chronaxie, and compound muscle action potential maximal amplitude. The test for stimulus threshold measures the minimum stimulatory current required to elicit a detectable action potential. Stimulus threshold was measured by increasing the amplitude of pulses applied with pulse duration of 1 ms until an action potential was recorded in the extensor digitorum longus muscle. Second, to determine chronaxie, the identified minimum stimulatory current was then doubled, and the pulse duration was increased from 0.01 ms until an action potential was again detected. Lastly, the nerve was stimulated with 0.1-ms pulses of increasing amperage until the maximal action potential amplitude response was reached. Using this compound muscle action, potential maximal amplitude response, latency, and conduction velocity were measured. The set of three characterization tests were then repeated with the stimulation applied at a second (more proximal nerve location) and possibly repeated again with stimulation at a third nerve location. PEDOT-coated electrodes were not reused on separate animals in recording studies.
Nerve Conduction Stimulating Study
The stimulating characteristics of the bipolar hook electrodes were also assessed in the rat with nerve conduction studies. One plain and one PEDOT-polymerized bipolar electrode were sequentially tested on alternate legs for each of six rats. The order for plain or an experimental PEDOT-coated bipolar electrode testing was randomly assigned. The peroneal nerve and muscles of the anterior compartment of one lower leg were surgically exposed. A stainless-steel needle electrode was placed in the belly of the extensor digitorum longus muscle as the recording electrode. A stainless-steel needle ground and references electrodes were placed in the distal extensor digitorum longus muscle tendon and between the toes. Stimulations were applied to the peroneal nerve in two locations: above the fibular head and 2 to 3 cm proximal to the fibular head (midperoneal). Stimulation was with a potentiostatic square wave applied to the peroneal nerve. Stimulus threshold, chronaxie, maximal response amplitude, latency, and conduction velocity were recorded.
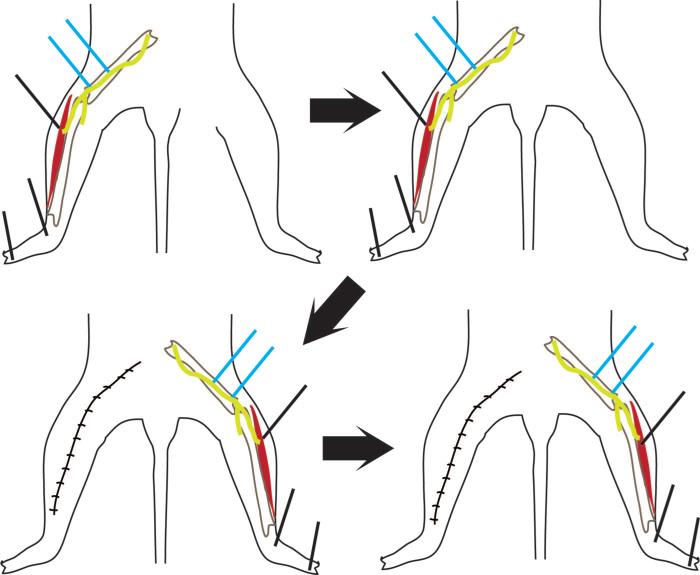
After 30 minutes of rest, testing was repeated on the same leg of the same rat with the same electrode. When testing was completed on the first leg, the contralateral peroneal nerve and lower leg muscles were exposed. Nerve conduction studies were then completed on this leg using identical electrode placements, but the alternate experimental type (plain or PEDOT) hook electrode was used for stimulation. Each animal was tested with a PEDOT-polymerized and plain hook electrode (Fig. 5). PEDOT-coated hook electrodes were not reused on multiple rats during the stimulation study.
Fig. 5.
Dorsal drawings of a rat show the nerve conduction setup for stimulation studies. Stimulation is at two locations on the peroneal nerve (indicated in blue). Each animal was tested on the left leg first (above, left and above, right). The left leg was then sutured closed and the right leg exposed (below, left and below, right). Between these steps, there was a 30-minute resting period, and the electrodes were removed. Stimulating electrodes for each rat consisted of one plain and one polymerized with PEDOT. The type of stimulating electrode used on the left side was randomly assigned.
Statistical Methods
Data were analyzed using SPSS v17 (Chicago, Ill.). Statistical significance for data of the recording electrode study was determined using a non-parametric Kruskal-Wallis one-way analysis of variance test. Repeated measure analysis of variance was used to determine the reliability between runs 1 and 2 for the plain and PEDOT-polymerized stimulating electrode measurements. This repeated measures test was also used to determine statistical differences between measurements for the plain and PEDOT-polymerized stimulation electrodes, as each electrode type was tested in the right or left leg of one rat. A Levene's test for equality of variance was applied to needle and hook electrode data for the plain and PEDOT. Significance was set a priori at α less than or equal to 0.05.
RESULTS
Characterization of Plain and PEDOT-Polymerized Needle Electrodes as Recording Electrodes
PEDOT polymerization deposited a dark and evenly distributed coating of PEDOT that covered the entire active portion of the needle electrode. The PEDOT coating remained uniform throughout ex vivo bench testing. During in situ testing, the PEDOT coating on the needle electrodes began to flake off if the electrode was repositioned into the muscle several times. Fresh electrodes were used for each rat (recording study) or each rat leg (stimulating study).
The mean impedances for recording needle electrodes at increasing frequencies from 10 to 10,000 Hz are summarized in Table 1. The PEDOT-polymerized electrodes had significantly lower electrical impedance when compared with plain electrodes at the identified frequencies between 10 to 10,000 Hz (p < 0.001). Charge capacity for PEDOT-polymerized needle electrodes was significantly higher (4.2 ± 1.7 mC/cm2) than plain needle electrodes (0.21 ± 0.14 mC/cm2, p < 0.001). The increase in charge capacity and lower impedance of PEDOT-polymerized electrodes indicate that PEDOT increased needle electrode fidelity.
Table 1.
Impedance Spectroscopy Data for Needle (Recording) Electrodes
| Plain (before Polymerization) (n = 7) | PEDOT (after Polymerization) (n = 7) | |
|---|---|---|
| Impedance at 10 Hz (kΩ) | 1.2 ± 0.7 | 0.11 ± 0.01* p < 0.001 |
| Impedance at 102 Hz (kΩ) | 0.73 ± 0.18 | 0.10 ± 0.05* p < 0.001 |
| Impedance at 103 Hz (kΩ) | 0.25 ± 0.11 | 0.07 ± 0.03* p < 0.001 |
| Impedance at 104 Hz (kΩ) | 0.021 ± 0.04 | 0.03 ± 0.01 |
Results are expressed mean ± SD.
Significantly different from plain; α was set a priori at p < 0.05.
In Situ Recording Study
The acute, in situ performance characteristics of the plain and PEDOT-coated recording needle electrodes varied significantly in this study. The nerve conduction study stimulus threshold was significantly lower for PEDOT-polymerized recording electrodes when compared with plain electrodes. This was true for PEDOT-polymerized recording electrodes when the peroneal nerve was stimulated at the fibular (p = 0.009), midperoneal (p = 0.005), and sciatic notch (p = 0.005). The lower stimulus threshold indicates that the PEDOT-polymerized electrode had greater electrode sensitivity. In addition, statistical variance for the stimulus threshold was significantly smaller for PEDOT-polymerized electrodes, indicating that the stimulus threshold occurred within a tighter range of values. This decreased variance was significant at two of three locations tested, including at the midperoneal level (p = 0.042) and the sciatic notch (p = 0.039). Less variance is important for both signal predictability and stability over time. Other nerve conduction study values, such as chronaxie, maximal compound muscle action potential amplitude, compound muscle action potential duration, and latency, were very similar across both groups, as would be expected (Table 2). These similar values indicate that the nerve was uninjured and that they conduct action potentials similarly whether the electrode for recording was a plain or PEDOT-polymerized needle. Latency increased equally; as the stimulating electrode was moved proximally, the distance between the recording and stimulating electrode increased. Thus, electropolymerizing recording electrodes with PEDOT showed increased electrode sensitivity during nerve conduction studies. PEDOT allowed us to record pulses of lower current with greater reliability.
Table 2.
Results from the Nerve Conduction Study (Recording)*
| Plain |
PEDOT |
|||||
|---|---|---|---|---|---|---|
| Fibular Head | Midperoneal | Sciatic | Fibular Head | Midperoneal | Sciatic | |
| Stimulus threshold (mA) | 0.19 ± 0.12 | 0.14 ± 0.05 | 0.21 ± 0.12 | 0.055 ± 0.00 †p = 0.009 |
0.051 ± 0.02 †p = 0.005 |
0.05 ± 0.024 †p = 0.005 |
| Chronaxie (ms) | 0.05 ± 0.0 | 0.05 ± 0.0 | 0.058 ± 0.02 | 0.05 ± 0.0 | 0.046 ± 0.01 | 0.05 ± 0.0 |
| CMAP amplitude (mV) | 19.6 ± 3.7 | 19.4 ± 4.8 | 19.83 ± 10.56 | 37.3 ± 22 | 23.4 ± 11.9 | 19.15 ± 11.66 |
| CMAP duration (ms) | 4.78 ± 1.1 | 7.4 ± 5.3 | 6.50 ± 4.02 | 12.90 ± 4.9 | 7.36 ± 5.1 | 5.95 ± 2.25 |
| Latency (ms) | 0.98 ± 0.16 | 1.21 ± 0.16 | 1.37 ± 0.16 | 1.16 ± 0.25 | 1.20 ± 0.16 | 1.25 ± 0.18 |
CMAP, compound muscle action potential.
Results indicate mean ± SD. Chronaxie is the minimal stimulus duration at twice the stimulus threshold amplitude.
Significant (p < 0.05) differences from plain by stimulating location on the nerve.
In Situ Stimulation Study
Bipolar hook electrodes remained coated with PEDOT throughout each in situ testing. The mean stimulus threshold during nerve conduction studies was lower for PEDOT-polymerized stimulating hook electrodes, reaching significance on three of four possible comparisons (Table 3). This indicates that the PEDOT-polymerized stimulating electrodes were able to more efficiently depolarize the stimulated nerve. Stimulus threshold variance was significantly lower for PEDOT-polymerized stimulating electrodes. This decreased variance was significant for the fibular head stimulation location during both run 1 and run 2 (p = 0.44 and p = 0.016, respectively). Variance was also significantly lower at the midperoneal level during run 1 (p = 0.001). The results from the in situ stimulation study were similar to the results found during the recording electrode study. Repeated measure analysis of variance between run 1 and 2 for stimulus threshold showed high reliability (r = 0.89 at the fibular head and r = 0.73 at the midperoneal). This very good reliability attests to the reliability of our testing procedures. Other measured values, amplitude, signal duration, and latency, were all similar across testing groups as expected. Chronaxie was also very similar across both nerve conduction groups during stimulus testing and at each stimulation site. Electropolymerizing stimulating electrodes with PEDOT showed increased electrode sensitivity during nerve conduction studies. PEDOT allowed us to stimulate at lower currents and with greater reliability.
Table 3.
Results from the Nerve Conduction Study (Stimulating)*
| Plain |
PEDOT |
|||||||
|---|---|---|---|---|---|---|---|---|
| Fibular Head |
Midperoneal |
Fibular Head |
Midperoneal |
|||||
| Run 1 | Run 2 | Run 1 | Run 2 | Run 1 | Run 2 | Run 1 | Run 2 | |
| Stimulus threshold (mV) | 485.1 ± 212 | 370.5 ± 195 | 508.5 ± 207.62 | 391.83 ± 166.82 | 200.3 ± 69 †p = 0.024 | 104.3 ± 45 †p = 0.010 |
228.67 ± 31.6 †p = 0.035 |
130.33 ± 48.2 p = 0.056 |
| Chronaxie (ms) | 0.05 ± 0.019 | 0.05 ± 0.017 | 0.04 ± 0.02 | 0.04 ± 0.01 | 0.05 ± 0.035 | 0.05 ± 0.026 | 0.06 ± 0.05 | 0.05 ± 0.03 |
| Amplitude (mV) | 18.3 ± 10.5 | 22.9 ± 8.9 | 17.1 ± 11.6 | 19.9 ± 10.9 | 21.7 ± 11 | 20.7 ± 8.9 | 20.13 ± 13 | 15.71 ± 8.6 |
| Duration (ms) | 8.05 ± 1.8 | 11.40 ± 4.7 | 8.41 ± 1.6 | 10.7 ± 4.13 | 8.20 ± 3.9 | 9.61 ± 4.6 | 9.38 ± 5.1 | 9.65 ± 4.9 |
| Latency (ms) | 1.74 ± 0.24 | 1.85 ± 0.34 | 1.81 ± 0.16 | 1.9 ± 0.32 | 1.80 ± 0.21 | 1.81 ± 0.32 | 1.89 ± 0.25 | 1.97 ± 0.4 |
Results indicate mean ± SD.
Significantly different from plain and corresponding run number (p < 0.05).
Significantly smaller variance than plain and corresponding run number (p < 0.05).
DISCUSSION
PEDOT polymerization of recording electrodes greatly improved the electrical characteristics of the tested electrodes. After PEDOT polymerization, needle electrode impedance decreased an average of one to two orders of magnitude. In addition, the charge capacity for PEDOT-polymerized electrodes was also one to two orders of magnitude higher than for that of plain electrodes alone. These electrical characteristics of PEDOT-polymerized electrodes correspond with previous research.24,25 The increased electrode fidelity allows increased signal discrimination of low-amplitude electrical signals. In addition, this conduction helps bridge the differences between the ionic conduction of nerve impulses and electrical conduction of the electrode.14
The reduced stimulus threshold for PEDOT-polymerized electrodes is likely an effect of both increased electrode charge capacity and decreased impedance of the polymerized electrode. The use of PEDOT polymerization allows recording of detectable signals from fewer simultaneously firing motor units. The lower stimulus threshold of the PEDOT-polymerized stimulating electrodes is due to greater recruitment of peroneal axons for a given applied potential. Both of these results have important implications for the design of a neuroprosthetics interface. One of the primary cited reasons for neuroprosthetic interface failure is charge density at the site of stimulation.26–29 The lower stimulus threshold of PEDOT-polymerized electrodes allows for threshold level stimulation at lower levels of current, which has potential not only in improving recording sensitivity and fidelity but also in decreasing interface scarring and biofouling. This is also significant for the recording of efferent motor signals; a higher stimulus threshold is indicative of an increased ability to detect electrical potentials.
The decrease in standard deviation between the plain and PEDOT-polymerized stimulatory and recording electrodes threshold potential has important consequences for neuroprosthetics design as well. The decreased standard deviation is a measure of a more predictable response to a given stimulus. True neuroprosthetic sensory feedback requires fine discrimination of graded responses; therefore, this increased predictability is very important.
The electrochemical polymerization method used in this study has the advantage of both excellent electrical characteristics and high biocompatibility but also relies on electrostatic adhesion to the electrode surface. This electrostatic adhesion is vulnerable to mechanical delamination. Although some mechanical delamination did occur in all electrodes used during nerve conduction testing, this delamination was not sufficient to significantly degrade electrode performance but would present a potential problem for chronic applications. Other chemical polymerization procedures result in covalent bonding to the electrode substrate and show high PEDOT adhesion and retention out to 16 months. PEDOT's structure is similar to biological compounds such as melanin,30,31 and the biocompatibility of PEDOT has been well established for acute implantation25 The development of a neuroprosthetics interface, however, requires stability and biocompatibility for upward of 70 years; PEDOT biocompatibility over this timeframe is still unknown.
The needle and bipolar electrodes utilized in this study were chosen based on our research group's previous experience utilizing these electrodes during acute nerve conduction testing14,32 Our methodology with these electrodes is well established and highly replicable. Implantation of parylene film–based microelectrodes into our neural interface is a current direction for our group. Parylene film–based electrodes have the advantage of high biocompatibility, flexibility, and biocompatibilbilty.33 PEDOT polymerization of the active sites on these microelectrodes would likely be highly advantageous.
Although not significantly different, there was a consistent decrease in stimulus threshold from run 1 to run 2 of the stimulation study. Spinal potentiation due to plateau potentials in the spinal cord is one explanation for this result.34 Spinal potentiation can occur because although stimulation was orthodromic, nerve depolarization from the bipolar electrode causes depolarization in both directions along the nerve. The repeated stimulation of the peroneal nerve during testing might have caused slight depolarization of the nerve above resting potential.
CONCLUSIONS
We demonstrate that when needle electrodes are electrochemically polymerized with PEDOT, the recording fidelity is significantly better due to increased charge capacity and decreased impedance. Our recording needle electrodes when coated with PEDOT showed significantly lower impedance at all frequencies from 10 to 1000 Hz. In addition, the electrode charge capacity of PEDOT-coated needle electrodes was also significantly higher. These electrical characteristics of PEDOT-polymerized electrodes correspond with previous research showing that PEDOT lowered impedance and increased electrode charge capacity.24,25 We also demonstrate that bipolar and needle electrodes electrochemically polymerized with PEDOT require significantly less current to stimulate and record neural signals during in situ nerve conduction testing. The addition of PEDOT to bioelectrical interfaces allows signal reception over a greater range of signal strength and signal administration at a lower relative percent of maximum. We conclude that PEDOT electrochemical polymerization improves electrode fidelity and sensitivity.
ACKNOWLEDGMENTS
This work was supported by the Department of Defense Multidisciplinary University Research Initiative (MURI) program administered by the Army Research Office under grant W911NF0610218. The authors thank Jana Feldkamp, Katie Ewing, and other members of the Neuromuscular Laboratory for technical assistance and discussion. They also thank Sarah Richardson-Burns for her assistance in polymerizing the electrodes. Scanning electron microscopy imaging was performed in the Microscopy and Image-analysis Laboratory at the University of Michigan. The views expressed in this work are those of the authors and do not necessarily reflect official Army policy.
Footnotes
Presented at the Biomedical Engineering Society 2010 Annual Meeting, in Austin, Texas, October 6 through 9, 2010.
Disclosure: The authors have no commercial or financial disclosures.
REFERENCES
- 1.Gawande A. Casualties of war: Military care for the wounded from Iraq and Afghanistan. N Engl J Med. 2004;351:2471–2475. doi: 10.1056/NEJMp048317. [DOI] [PubMed] [Google Scholar]
- 2.Ziegler-Graham K, MacKenzie EJ, Ephraim PL, et al. Estimating the prevalence of limb loss in the United States: 2005 to 2050. Arch Phys Med Rehabil. 2008;89:422–429. doi: 10.1016/j.apmr.2007.11.005. [DOI] [PubMed] [Google Scholar]
- 3.Hijjawi JB, Kuiken TA, Lipschutz RD, et al. Improved myoelectric prosthesis control accomplished using multiple nerve transfers. Plast Reconstr Surg. 2006;118:1573–1578. doi: 10.1097/01.prs.0000242487.62487.fb. [DOI] [PubMed] [Google Scholar]
- 4.Kuiken TA, Li G, Lock BA, et al. Targeted muscle reinnervation for real-time myoelectric control of multifunction artificial arms. JAMA. 2009;301:619–628. doi: 10.1001/jama.2009.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.De Laurentis KJ, Mavroidis C. Mechanical design of a shape memory alloy actuated prosthetic hand. Technol Health Care. 2002;10:91–106. [PubMed] [Google Scholar]
- 6.Lai JC, Schoen MP, Perez G, et al. Prosthetic devices: Challenges and implications of robotic implants and biological interfaces. Proc Inst Mech Eng H. 2007;221:173–183. doi: 10.1243/09544119JEIM210. [DOI] [PubMed] [Google Scholar]
- 7.Kipke DR, Shain W, Buzsaki G, et al. Advanced neuro-technologies for chronic neural interfaces: New horizons and clinical opportunities. J Neurosci. 2008;28:11830–11838. doi: 10.1523/JNEUROSCI.3879-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Polikov VS, Tresco PA, Reichert WM. Response of brain tissue to chronically implanted neural electrodes. J Neurosci Methods. 2005;148:1–18. doi: 10.1016/j.jneumeth.2005.08.015. [DOI] [PubMed] [Google Scholar]
- 9.Cogan SF. Neural stimulation and recording electrodes. Annu Rev Biomed Eng. 2008;10:275–309. doi: 10.1146/annurev.bioeng.10.061807.160518. [DOI] [PubMed] [Google Scholar]
- 10.Hodson S, Wigham C. The permeability of rabbit and human corneal endothelium. J Physiol. 1983;342:409–419. doi: 10.1113/jphysiol.1983.sp014859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ludwig KA, Uram JD, Yang J, et al. Chronic neural recordings using silicon microelectrode arrays electrochemically deposited with a poly(3,4-ethylenedioxythiophene) (PEDOT) film. J Neural Eng. 2006;3:59–70. doi: 10.1088/1741-2560/3/1/007. [DOI] [PubMed] [Google Scholar]
- 12.Luo X, Weaver CL, Zhou DD, et al. Highly stable carbon nanotube doped poly(3,4-ethylenedioxythiophene) for chronic neural stimulation. Biomaterials. 2011;32:5551–5557. doi: 10.1016/j.biomaterials.2011.04.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Venkatraman S, Hendricks J, King ZA, et al. In vitro and in vivo evaluation of PEDOT microelectrodes for neural stimulation and recording. IEEE Trans Neural Syst Rehabil Eng. 2011;19:307–316. doi: 10.1109/TNSRE.2011.2109399. [DOI] [PubMed] [Google Scholar]
- 14.Egeland BM, Urbanchek MG, Peramo A, et al. In vivo electrical conductivity across critical nerve gaps using poly(3,4-ethylenedioxythiophene)-coated neural interfaces. Plast Reconstr Surg. 2010;126:1865–1873. doi: 10.1097/PRS.0b013e3181f61848. [DOI] [PubMed] [Google Scholar]
- 15.Abidian MR, Martin DC. Experimental and theoretical characterization of implantable neural microelectrodes modified with conducting polymer nanotubes. Biomaterials. 2008;29:1273–1283. doi: 10.1016/j.biomaterials.2007.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Povlich LK, Le J, Kin J, et al. Poly(5,6-dimethoxyindole-2-carboxylic acid) (PDMICA): A melanin-like polymer with unique electrochromic and structural properties. Macromolecules. 2010;43:3770–3774. [Google Scholar]
- 17.Ambrico M, Ambrico PF, Cardone A, et al. Melanin layer on silicon: An attractive structure for a possible exploitation in bio-polymer based metal-insulator-silicon devices. Adv Mater. 2011;23:3332–3336. doi: 10.1002/adma.201101358. [DOI] [PubMed] [Google Scholar]
- 18.Aschberger K, Johnston HJ, Stone V, et al. Review of carbon nanotubes toxicity and exposure: Appraisal of human health risk assessment based on open literature. Crit Rev Toxicol. 2010;40:759–790. doi: 10.3109/10408444.2010.506638. [DOI] [PubMed] [Google Scholar]
- 19.Cui HF, Vashist SK, Al-Rubeaan K, et al. Interfacing carbon nanotubes with living mammalian cells and cytotoxicity issues. Chem Res Toxicol. 2010;23:1131–1147. doi: 10.1021/tx100050h. [DOI] [PubMed] [Google Scholar]
- 20.Cui XT, Zhou DD. Poly (3,4-ethylenedioxythiophene) for chronic neural stimulation. IEEE Trans Neural Syst Rehabil Eng. 2007;15:502–508. doi: 10.1109/TNSRE.2007.909811. [DOI] [PubMed] [Google Scholar]
- 21.King ZA, Shaw CM, Spanninga SA, et al. Structural, chemical and electrochemical characterization of poly(3,4-ethylenedioxythiophene) (PEDOT) prepared with various counter-ions and heat treatments. Polymer (Guildf.) 2011;52:1302–1308. doi: 10.1016/j.polymer.2011.01.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cui X, Lee VA, Raphael Y, et al. Surface modification of neural recording electrodes with conducting polymer/biomolecule blends. J Biomed Mater Res. 2001;56:261–272. doi: 10.1002/1097-4636(200108)56:2<261::aid-jbm1094>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- 23.Institute of Laboratory Animals Resources . National Research Council’s Guide for the Care and Use of Laboratory Animals. 7th ed. National Academy Press; Washington, D.C.: 1996. [Google Scholar]
- 24.Boretius T, Schuettler M, Stieglitz T. On the stability of poly-ethylenedioxythiopene as coating material for active neural implants. Artif Organs. 2011;35:245–248. doi: 10.1111/j.1525-1594.2011.01210.x. [DOI] [PubMed] [Google Scholar]
- 25.Richardson-Burns SM, Hendricks JL, Foster B, et al. Polymerization of the conducting polymer poly(3,4-ethylenedioxythiophene) (PEDOT) around living neural cells. Biomaterials. 2007;28:1539–1552. doi: 10.1016/j.biomaterials.2006.11.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Egeland B, Urbanchek MG, Abidian M, et al. A tissue-based bioelectrical interface has reduced impedance compared to copper wire and nerve. Plast Reconstr Surg. 2009;123:26. [Google Scholar]
- 27.Halpern JM, Cullins MJ, Chiel HJ, et al. Chronic in vivo nerve electrical recordings of Aplysia californica using a boron-doped polycrystalline diamond electrode. Diamond Relat Mater. 2010;19:178–181. [Google Scholar]
- 28.Leuthardt EC, Schalk G, Moran D, et al. The emerging world of motor neuroprosthetics: A neurosurgical perspective. Neurosurgery. 2006;59:1–14. doi: 10.1227/01.NEU.0000221506.06947.AC. [DOI] [PubMed] [Google Scholar]
- 29.Otto KJ, Johnson MD, Kipke DR. Voltage pulses change neural interface properties and improve unit recordings with chronically implanted microelectrodes. IEEE Trans Biomed Eng. 2006;53:333–340. doi: 10.1109/TBME.2005.862530. [DOI] [PubMed] [Google Scholar]
- 30.Luo SC, Mohamed AE, Tansil NC, et al. Poly(3,4-ethylenedioxythiophene) (PEDOT) nanobiointerfaces: Thin, ultrasmooth, and functionalized PEDOT films with in vitro and in vivo bio-compatibility. Langmuir. 2008;24:8071–8077. doi: 10.1021/la800333g. [DOI] [PubMed] [Google Scholar]
- 31.Martin DC. Organic electronics: Polymers manipulate cells. Nat Mater. 2007;6:626–627. doi: 10.1038/nmat1992. [DOI] [PubMed] [Google Scholar]
- 32.Frost CM, Urbanchek MG, Egeland BM, et al. Development of a biosynthetic “living interface” with severed peripheral nerve. Plast Reconstr Surg. 2009;12:6S. [Google Scholar]
- 33.Seymour JP, Elkasabi YM, Chen HY, et al. The insulation performance of reactive parylene films in implantable electronic devices. Biomaterials. 2009;30:6158–6167. doi: 10.1016/j.biomaterials.2009.07.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lagerquist O, Collins DF. Influence of stimulus pulse width on M-waves, H-reflexes, and torque during tetanic low-intensity neuromuscular stimulation. Muscle Nerve. 2010;42:886–893. doi: 10.1002/mus.21762. [DOI] [PubMed] [Google Scholar]