Abstract
Innate lymphoid cells (ILCs) have emerged recently as an important component of the immune system and the cell type that regulates mucosal immune responses and tissue homeostasis. Group 2 ILCs (ILC2s), a subset of ILCs, reside in various tissues and are characterized by their capacity to produce type 2 cytokines and tissue growth factors. These ILC2s play an important role in allergic immune responses by linking signals in the atmospheric environment to the immune system. Fungi are one of the major allergens associated with human asthma, and animal and in vitro models using the fungal allergens have provided significant information toward our understanding of the mechanisms of allergic disease. In mouse models of fungus-induced allergic airway inflammation, IL-33, IL-25, and TSLP are released by airway epithelial cells. Lung ILC2s that respond to these cytokines quickly produce a large quantity of type 2 cytokines, resulting in airway eosinophilia, mucus production, and airway hyperreactivity even in the absence of adaptive immune cells. Evidence also suggests that ILC2s interact with conventional immune cells, such as CD4+ T cells, and facilitate development of adaptive immune response and persistent airway inflammation. ILC2s are also present in respiratory mucosa in humans. Further investigations into the biology of ILC2s and their roles in the pathophysiology of allergic diseases will provide major conceptual advances in the field and may provide useful information toward development of new therapeutic strategies for patients.
Keywords: Allergy, Asthma, Cytokines, Fungi, Innate lymphoid cells
Introduction
After the initial discovery and characterization of Th1- and Th2-type CD4+ T cells approximately 25 years ago, T cells were thought to play a central role in regulation of immune responses and pathophysiology of diseases by producing a repertoire of cytokines.1 However, the recent discovery of innate lymphoid cells (ILCs) added another layer of complexity and provided a major shift in this paradigm.2 ILCs have emerged as important effector cells of the immune system that are involved in pathogen clearance, lymphoid organogenesis, tissue remodeling and immune pathology through production of distinct sets of cytokines and growth factors. These cells are derived from a common lymphoid progenitor, exhibit lymphoid morphology, but express no phenotypic markers of conventional immune cells.3
ILCs have been categorized into three groups, including group 1 ILCs (ILC1s), group 2 ILCs (ILC2s), and group 3 ILCs (ILC3s), based on their cytokine profiles and the transcription factors that are utilized for their development and function.4 ILC1s comprise IFN-γ-secreting ILCs that use transcription factor T-bet for lineage commitment. ILC2s comprise type 2 cytokine-producing ILCs that require transcription factor GATA3 for their development and function. ILC3s comprise IL-17- and/or IL-22-producing ILCs that are dependent on transcription factor RORγt for lineage specification. This article will focus on ILC2s and discuss their roles in allergic immune responses induced by fungal allergens. While major progress has also been made regarding the roles for ILC2s in viral infection and regulation of tissue homeostasis and metabolism, they are discussed elsewhere in detail5 and will be described only briefly in this article.
Fungi in allergic diseases and disease models
Allergic asthma is generally mediated by dysregulated production of Th2-type cytokines, such as IL-4, IL-5 and IL-13, although other cell types, such as Th17 cells, have also been implicated.6 The interactions between mucosal epithelia and innate and adaptive immune cells were recently proposed as the underlying causes of dysregulated production of Th2-type cytokines in allergic diseases.7 In particular, the epithelium-derived cytokines IL-33, IL-25, and thymic stromal lymphopoietin (TSLP) have emerged as potential links between environmental exposure to allergens and type 2 immune responses.8–11 For example, intraperitoneal injection of IL-33 in mice increases serum levels of IgE and Th2 cytokines and promotes airway eosinophilia and mucosal hyperplasia.12,13 Inhalation of IL-33 induces type 2 lung inflammation even in T cell- and B cell-deficient animals.14 TSLP activates DCs to polarize naïve T cells towards the pro-inflammatory Th2 cells that produce IL-4, IL-5, and IL-13 as well as TNF-α.15–17 Mice expressing TSLP in the lungs develop spontaneous airway inflammation with characteristics similar to human asthma.18
While a number of environmental factors are associated with asthma and allergic diseases, allergen exposure likely plays a key role in triggering and exacerbating asthma and allergy symptoms.19 In particular, exposure to airborne allergens derived from animals, arthropods, and molds is considered to be an important risk factor.20–22 In humans, an association between fungal exposure, in particular to Alternaria and Aspergillus, and asthma is recognized clinically and epidemiologically.23,24 Furthermore, severe asthma and life-threatening acute exacerbations of asthma have also been associated with increased airborne exposure to Alternaria.25–27 However, the molecular mechanisms involved in fungal exposure and development of allergic immune responses are not well understood. Fungi secrete or contain numerous biologically active substances, including chitin,28 β-glucan29 and proteases.30 Fungi likely contribute to the majority of protease activity in house dust samples.31 Certain fungal allergens are also proteases themselves, including Asp f 5, f 6 and f 11 from Aspergillus,23 suggesting that proteases may be a key link between fungal exposure and Th2-type immune responses. Indeed, the protease activities produced by fungi were sufficient32,33 and necessary31 for induction of robust allergic airway inflammation induced by fungal organisms. Moreover, a prototypic cysteine protease, such as papain, enhanced Th2-type sensitization to bystander antigens.34
The conventional animal model of Th2-type immunity and “allergy” involved intraperitoneal or subcutaneous sensitization of mice with a model antigen ovalbumin (OVA) and an alum adjuvant, followed by airway challenge with the OVA antigen. More recent models used natural allergens, such as extracts of fungi and authentic proteases, which were directly administered into the airways of naïve and/or sensitized animals. These changes in the experimental model systems as well as new discoveries in basic science facilitated a major paradigm shift in our understanding of the mechanisms of type 2 immune responses in the airways.
ILC2s
In the early 2000s, ILC2s were first described in mice as non-B/non-T cells that secrete IL-5 and IL-13 in response to IL-25.35,36 When mice were infected with the parasite Nippostrongylus brasiliensis or exposed to the fungus Aspergillus, increased expression of IL-25 in the gut and lung was observed.35,36 A subsequent study showed that these IL-25-responsive innate immune cells play important roles in N. brasiliensis worm expulsion.37 In 2010, ILC2s were isolated and characterized by several investigators, and they were independently named as natural helper cells, nuocytes, and innate helper 2 cells.38–40 Later a consensus report designated them as group 2 ILCs (ILC2s).4
ILC2s arise from the common lymphoid progenitors (CLPs) in the bone marrow and, like other ILCs, require the transcriptional inhibitor Id2 for their development.38 Id2 inhibits the activity of the E proteins, which are implicated in differentiation of B cells and T cells.41 The transcription factor promyelocytic leukemia zinc finger protein (PLZF) then mediates generation of an ILC precursor that gives rises to ILC1, ILC2 and ILC3 but not conventional natural killer (NK) cells.42 The transcription factor RORα is critical for further development of ILC2s from the Id2-dependent ILC precursor. Indeed, RORα-deficient “Staggerer” mice, which carry a spontaneous mutation in the Rora gene, show severely impaired expansion of ILC2s as well as cerebellar developmental defects43; the other ILC subsets are not affected.44 Mice that have received bone marrow from the “Staggerer” mice to circumvent their neurological defects have been used as a model for ILC2-deficient mice.45 While GATA3 is required for the generation of the ILC precursor, it is also required for maintenance and effector functions of ILC2s.46,47
ILC2s do not express conventional cell surface markers for T cells, B cells, NK cells, myeloid cells, and DCs; thus, they are designated lineage-negative (Lin−). Mouse ILC2s express ST2 (IL-33 receptor), CD127 (IL-7R α-chain), ICOS, CD117 (c-kit), Thy1, IL-17RB (IL-25 receptor), CD44 and CD25 (IL-2R α-chain); the expression levels of these molecules varies depending on the anatomical location and activation states of the cells.45 Mouse ILC2s are widely distributed in the tissues, including fat-associated lymphoid clusters (FALC), mesenteric and mediastinal lymph nodes, liver, spleen, intestine, bone marrow, visceral adipose tissue and lung. Thus, ILC2s appear to be critically positioned to maintain homeostasis by responding rapidly to environmental cues, including metabolic stress and nutrient intake, and poised to rapidly respond to damage or stress in mucosal tissues. Functionally, ILC2s are considered to be the counterpart of Th2-type CD4+ T cells. They characteristically produce type 2 cytokines, such as IL-5, IL-13 and IL-9, as well as certain growth factors, such as amphiregulin.48 Amphiregulin is a member of the epidermal growth factor (EGF) family that promotes epithelial cell growth.49
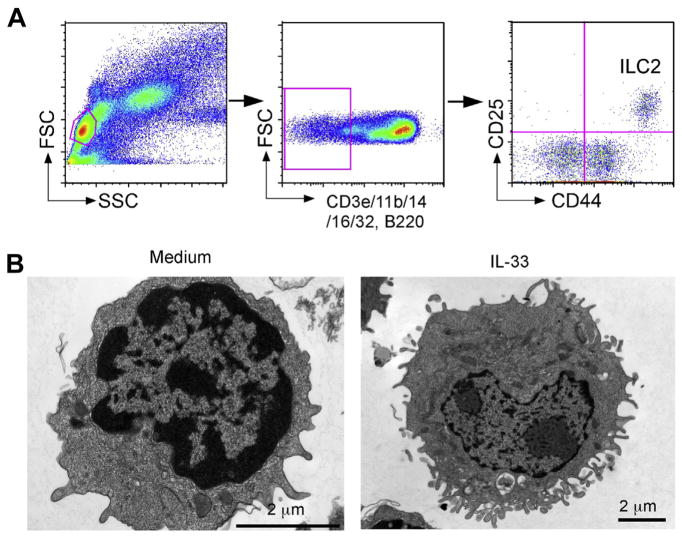
ILC2s normally reside in the lungs of naïve non-sensitized animals; these ILC2s are Lin− and generally express various cell surface markers, including CD117, CD122 (IL-2R β-chain), CD25, CD127, Ly5.2, Thy1, Sca-1, ST2, CD69, CD9, CD38, MHC class II, CD44 and ICOS.40,49–51 These cell markers have been used to identify and isolate ILC2s among the Lin− populations in the lung of naïve mice (Fig. 1A). Importantly, lung ILC2s are present in Rag2−/− mice (i.e. deficient in T cells) and ST2−/− mice (i.e. deficient in IL-33R), suggesting that they do not require T cell help or IL-33 for their development. In contrast, mice that are deficient in IL-2 receptor common γ-chain (cγ) or IL-7R α-chain lack mature ILC2s, consistent with their dependency on IL-7 for their development. Lung ILC2s are a rare cell population. In wild-type C57BL/6 mice, lung ILC2s represent only 0.25–1% of total live cells in the lung. ILC2s are located in collagen-rich regions close to the confluence of medium-sized blood vessels and airways, but not in alveolar areas of the lung.52 Resting lung ILC2s show morphology similar to that of resting lymphocytes, with no apparent intracellular granule structures50 (Fig. 1B). Once they are activated by cytokines such as IL-33, lung ILC2s increase in size and display pronounced endoplasmic reticulum and Golgi apparatus, suggesting that they are a highly-activated cell type.
Fig. 1.
Lung ILC2s respond vigorously to IL-33 and produce a large quantity of IL-5 and IL-13 in vitro. (A) Gating strategy and identification of ILC2s in lung single cell suspensions from naïve BALB/c mice. (B) Morphology of lung ILC2s. Lung ILC2s were cultured with medium alone or IL-33 and examined under electron microscopy. Original magnifications; 25,000× (medium alone, left) and 12,000× (IL-33, right).
Human ILC2s are typically Lin−, CD45+, CD127+, NKp44−, CD25+ and CD161+.49,53–55 Distinct expression of two prototypic Th2-type CD4+ T cell markers, namely chemoattractant receptor-homologous molecule expressed on Th2 lymphocytes (CRTH2) and IL-33 receptor ST2, is an unique feature of human ILC2s, and these molecules are useful to differentiate human ILC2s from other human ILCs.4 Human ILC2s are reportedly found in peripheral blood, lung, BAL fluid, nasal tissue, tonsil, gut and skin of normal healthy individuals.
Regulation and functions of ILC2s
Resting lung ILC2s show high mRNA expression levels of Gata3, Rora, Cd69, Il2ra, Il2rg, Il4ra, Il7r, Il17rb, Il1rl1, Il5, and Il13,40,51,52,56 consistent with transcriptional regulation of this cell type and its effector function. ELISPOT assays revealed IL-5-producing lung ILC2s when cultured without stimuli,52 suggesting constitutive (but minimal) production of IL-5 by resting ILC2s. Importantly, this constitutive expression of IL-5 by ILC2s may play an important role in regulating eosinophil homeostasis in various organs.52,57 Growing evidence also links ILC2s with metabolic homeostasis, obesity, and dietary stress.57–59 For example, deficiency of ILC2-derived IL-5 and IL-13 led to increased adiposity and insulin resistance.57 ILC2s also responded to the daily cycle of caloric intake, resulting in cytokine expression associated with circadian rhythms.52
ILC2s are activated by cytokines, such as IL-25, IL-33 and TSLP,38–40,53,54 which are derived from epithelial cells and certain immune cells. Activated lung ILC2s produce IL-4, IL-5, IL-9, IL-13 and GM-CSF.50,51,60 In particular, IL-5 and IL-13 protein are produced in large quantities by ILC2s, perhaps beyond the levels that are produced by Th2-type CD4+ T cells, making these cells a unique “factory” of cytokines.50 In in vitro culture systems, IL-33 activates lung ILC2s probably more potently than IL-25 to produce IL-5 and IL-13.50,51,61 In certain experiments, IL-25 and TSLP did not activate lung ILC2s by themselves, but they synergistically enhanced cytokine production by ILC2s in the presence of other cytokines, such as IL-33.51 IL-25 and IL-33 also promote expansion and/or migration of lung ILC2s, as intraperitoneal or intranasal administration of IL-25 or IL-33 increased ILC2 cell numbers in lung tissues and draining lymph nodes in vivo.40,62
Lung ILC2 activities can also be regulated by IL-2-family cytokines. In vitro, neither IL-2 nor IL-7 alone induces significant IL-5 and IL-13 production by ILC2s. However, these two cytokines synergistically enhance IL-33- and IL-25-induced proliferation and type 2 cytokine production by lung ILC2s.49–51 ILC2s were a dominant source of IL-9 in mice exposed to the protease papain.60 IL-9 produced by ILC2s may have an autocrine positive feedback effect on ILC2s since lung ILC2s cultured with IL-9 increased production of type 2 cytokines.60,63 Finally, TL1A, a TNF superfamily member, has also been reported to induce ILC2 cell expansion.64
Besides cytokines, lung ILC2s can be regulated by lipid mediators that are presumably generated during allergic inflammation by mast cells, eosinophils, and other inflammatory cells.65 In vitro, leukotriene D4 (LTD4) potently stimulates mouse lung ILC2s to produce not only IL-5 and IL-13 but also a large amount of IL-4; IL-4 is not generally produced by ILC2s stimulated with IL-33.66 Intra-nasal administration of LTD4 led to expansion of IL-5-producing ILC2s in the lung in vivo. Furthermore, prostaglandin D2 (PGD2) has also been shown to induce migration and functions of human ILC2s through the CRTH2 receptor.67
Roles of ILC2s in innate type 2 responses to fungi
Initial studies on ILC2s demonstrated their roles in innate immunity against a variety of infectious organisms. For example, ILC2s play critical roles in protective immunity against helminth infection,38–40 in influenza-induced lung inflammation and airway hyperreactivity (AHR),68 and in respiratory epithelial repair after influenza infection.49 Therefore, it is reasonable to speculate that ILC2s may involved in immune responses to fungal organisms or their products.
To investigate this hypothesis, Alternaria extract was administered once into the airways of naïve non-sensitized BALB/c mice.69 Th2 cytokine levels, including IL-5 and IL-13, were increased in as early as 6 h after Alternaria exposure. Interestingly, within 1 h after receiving Alternaria extract, IL-33 levels increased markedly in BAL fluids, preceding the increases in IL-5 and IL-13. The production of IL-5 and IL-13 in Alternaria–exposed mice was similar in Rag1−/− and wild-type mice, suggesting that these type 2 cytokine responses are independent of adaptive immunity. In contrast, Alternaria-induced increases in IL-5 and IL-13 were abolished in mice deficient in IL-33 receptor ST2. Altogether, these findings suggested that IL-33 likely mediates rapid production of Th2-type cytokines in the airway mucosa through an innate mechanism(s). Robust Alternaria-induced IL-33-dependent type 2 cytokine responses were also observed in other studies.70,71 Interestingly, among the various allergen extracts tested in these studies, only Alternaria induced significant IL-33 release into BAL fluids, suggesting that Alternaria has unique biological properties.
IL-33 was also involved in airway pathological changes in response to Alternaria when non-sensitized naïve mice were exposed repeatedly to Alternaria for a week.50 Mice exposed to Alternaria for 7 days demonstrated marked peribronchial infiltration of inflammatory cells, epithelial hyperplasia, and pronounced airway eosinophilia. The magnitude of airway eosinophilia in Rag1−/− mice was roughly comparable to that in the wild type BALB/c mice for up to 5 days; airway eosinophilia further increased at a later time point in the wild type but not in Rag1−/− mice. Airway eosinophilia on day 5 or day 7 was significantly inhibited by >80% in the ST2−/− mice as compared to the wild type mice. These findings suggested that airway type 2 immune responses to Alternaria exposure consist of at least two arms: an initial innate immune response and a subsequent involvement of adaptive immunity; both arms are IL-33 dependent.
Subsequent studies established that the lung ILC2 population is the cell type that mediates this rapid type 2 cytokine production and pathological changes in response to Alternaria allergens.50 In vitro culture of lung cells from naïve mice with IL-33 induced robust production of IL-5 and IL-13. Importantly, the lung cells that produced IL-5 did not express authentic markers for T cells (CD3, CD4, CD8), B cells (B220), macrophages (F4/80), mast cells/basophils (FcεRIα), or granulocytes (Gr-1), but they expressed Thy1.2, CD25 and CD44. When Lin−CD25+CD44hi lung cells were cultured with IL-33, they produced large quantities of IL-5 and IL-13. By FACS analysis, Lin−CD25+CD44hi cells highly expressed ST2, CD127 (IL7Rα), Sca-1, CD69, Thy1.2, CD9, CD38, and ICOS, consistent with published the characteristics of ILC2s.4 The lung ILC2 cell population was nearly absent in the lungs of Il7r−/− C57BL mice, suggesting a critical role for IL-7 to induce and/or maintain ILC2s.
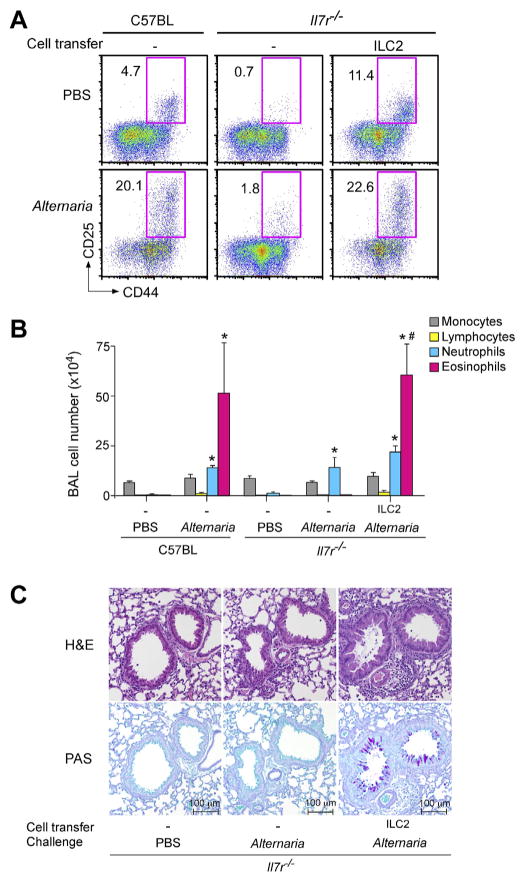
To directly examine the involvement of ILC2 cells in airway responses to Alternaria, a reconstitution approach was undertaken. When ILC2-deficient Il7r−/− mice received lung ILC2 cells from a donor, the ILC2 population was clearly detected in the lungs of the recipients, suggesting a successful homing (Fig. 2A). Importantly, Il7r−/− mice that were reconstituted with ILC2s and exposed to Alternaria extracts showed marked increases in eosinophil numbers and levels of IL-5 and IL-13 proteins (Fig. 2B). Pathologically, peribronchial infiltration of inflammatory cells, epithelial hyperplasia, and increased mucus production were observed in Il7r−/− mice reconstituted with ILC2s and exposed to Alternaria (Fig. 2C). Together, these results demonstrate the potent capacity of lung ILC2s in mediating IL-5 and IL-13 production, type-2 airway inflammation and pathological features of asthma upon exposure to Alternaria even in the absence of adaptive immune cells, such as T cells and B cells. Furthermore, in another study, a single intranasal exposure to Alternaria induced rapid production of CysLT that could activate ILC2s through CysLT1R, suggesting that an IL-33-independent pathway may also be involved.66 In mice that were already sensitized to ryegrass allergen, exposure to Alternaria enhanced proliferation and/or recruitment of both ILC2s and CD4+ T cells and promoted airway inflammation and pathology.72 The roles of ILC2s in innate type 2 responses were also demonstrated by mouse models using authentic proteases, such as papain and bromelain as surrogates of allergen proteases, as well as Aspergillus proteases.51,73
Fig. 2.
Lung ILC2s mediate eosinophilic airway inflammation and pathology in mice exposed to fungal allergen Alternaria. (A) ILC2s were isolated from the lungs of naïve C57BL/6 mice, and they were adoptively transferred to naive Il7r−/− mice, which are deficient in mature CD4+ T cells and ILCs. Mice were then exposed intranasally three times to PBS or Alternaria extract over 6 days. Transferred ILC2s are identified as Lin−CD25+CD44hi cells. The FACS plots were gated on Lin− cells. (B) Cell number and differentials in BAL fluids were determined. (C) Representative histology (upper panels: H&E staining, lower panels: PAS staining) of the mice as described in B.
Chitin is a polysaccharide constituent of fungi. Exposure of naïve mice to chitin particles induced expression of IL-25, IL-33 and TSLP, which activated lung ILC2s and induced IL-5 and IL-13 production and subsequent accumulation of eosinophils and alternatively activated macrophages.74 In the absence of all three epithelial-derived cytokines, ILC2s failed to produce IL-5 or IL-13. Interestingly, genetic ablation of ILC2s enhanced IL-1β, TNFα, and IL-23 expression, increased activation of IL-17A-producing γδT cells, and induced prolonged neutrophilic inflammation of the airways. Thus, chitin-elicited activation of ILC2s may promote type 2 immune response and airway eosinophilia while it may suppress γδT cell-mediated neutrophilic airway inflammation.
A major question remains how fungi and authentic proteases trigger production of epithelium-derived cytokines, such as IL-33. Allergen could be recognized by the immune system via three major mechanisms: 1) engagement of pattern recognition receptors, 2) molecular mimicry of TLR signaling complex molecules, and 3) proteolytic activity.75,76 In particular, TLR4 likely plays a critical role in type 2 immune responses to inhaled HDM allergens,77–79 low-dose LPS in the airways,80 and papain injected into skin.81 Furthermore, inhaled proteases promoted fibrinogen cleavage; the fibrinogen cleaved products in turn served as ligands for TLR4.82 IL-33, IL-25 and TSLP were produced by airway epithelial cells when they were cultured with Alternaria and proteases in vitro.69,83,84 IL-33 and TSLP were produced quickly in the lungs of naïve mice exposed to cysteine proteases, such as bromelain and papain.73 However, mice deficient in TLR4 or a protease-activated receptor 2 (PAR2) developed comparable levels of airway eosinophilia compared to WT mice. Importantly, upon exposure to proteases, uric acid (UA) was rapidly released into the airway lumen, and removal of this endogenous UA by uricase prevented type 2 immune responses.73 Conversely, exogenous UA promoted secretion of IL-33 by airway epithelial cells in vitro, and administration of UA into the airways of naïve animals induced extracellular release of IL-33, followed by activation of ILC2s. These findings provide mechanistic insights into the development of type 2 immunity to fungi and allergen proteases and suggest that an endogenous danger signaling molecules, such as UA, may play key roles in regulating type 2 immune responses in respiratory mucosa. Clearly, further studies are necessary to elucidate the molecular mechanisms involved in cytokine production by airway epithelial cells when they are exposed to allergens.
Role of ILC2s in chronic airway inflammation induced by fungi
ILC2s may interact with other immune cells and play roles in chronic airway inflammation. For example, ILC2 cell numbers were not maintained in Rag2−/− mice infected with helminths or challenged with the protease papain,39,60 suggesting that adaptive immune cells (presumably T cells) are required for ILC2 expansion, migration or survival. Naïve CD4+ T cells supported proliferation and type 2 cytokine production by ILC2s in an IL-2-dependent manner.85 Conversely, lung ILC2s enhanced CD4+ T cell proliferation and promoted production of type 2 cytokines in vitro.86 The interaction between ILC2s and CD4+ T cells likely involved the costimulatory molecule OX40L and IL-4, which was mainly derived from ILC2s. Nonetheless, ILC2-deficient mice effectively produced Th2 cell-mediated allergic airway inflammation in an OVA model, suggesting that ILC2s may promote the initiation of Th2 cell differentiation whereas they do not seem to be required for activation of established memory Th2 cells.87 ILC2s may also interact with other immune cells. A more recent study showed that activation and proliferation of lung ILC2s in mice exposed to the protease papain were reduced in mice that are deficient in IL-4, specifically in the basophil compartment.88 Furthermore, the initial description of ILC2s in FALC demonstrated that ILC2-derived IL-5 and IL-6 may promote IgA antibody production by B1 B cells.38 Thus, ILC2s and their products have the potential to interact with a variety of immune cells, including CD4+ T cells, basophils and B cells, and ILC2s may need to be considered as a key part of the immune cell network involved in allergic immune responses.
To investigate the immunological mechanisms in fungus-mediated chronic airway inflammation, mice were exposed to multiple airborne allergens, including the fungals allergen Alternaria and Aspergillus as well as HDM, for a prolonged period.89 Chronic and multiple exposures to these allergens induced a robust increase in BAL eosinophils, which peaked in 4 weeks; eosinophils made up approximately 70% of total BAL cells. Plasma concentrations of antigen-specific IgE and IgG1 antibodies continued to rise for at least 8 weeks. Increased lung levels of type 2 cytokines, including IL-4, IL-5, and IL-13, were also observed. Notably, a marked increase in the lung levels of IL-33, approximately 10-times more than a baseline level, was observed in 4 weeks. In this model, unlike the acute exposure models as described above, increases in BAL eosinophils and cytokines as well as in plasma IgE were abolished in Rag1−/− mice, suggesting a critical role for adaptive immunity. Nonetheless, the number of ILC2s increased by >2-fold when animals were exposed to allergens for 4 weeks, suggesting their potential involvement.
The functions of these ILC2s during chronic inflammation were examined more in detail by using cytokine reporter mice, including the IL-5 reporter IL-5+/venus mice and IL-13 reporter IL-13+/eGFP mice. In PBS-exposed mice, a small fraction of ILC2s expressed IL-5 or IL-13; no other cell populations within the Lin− population expressed these cytokines. Importantly, when mice were exposed to allergens, both the proportion and absolute number of IL-5- or IL-13-producing ILC2s increased by approximately 4-fold. Unlike ILC2s, IL-5 or IL-13 signals were undetectable within the CD3+ cell population in PBS-exposed animals. When mice were exposed to allergens, the prevalence of IL-5- or IL-13-positive cells increased dramatically; these cytokine-positive CD3+ T cells expressed CD4. The absolute number of type 2 cytokine-positive CD4+ T cells was approximately 5 times more than that of the cytokine-positive ILC2s.89 Similarly, during adaptive immune responses in mice exposed to HDM or OVA, both ILC2s and CD4+ T cells were sources of IL-5 and IL-13.62,90 Altogether, both ILC2s and CD4+ T cells likely contribute to the increased IL-5 and IL-13 production in mice after prolonged airway exposure to airborne allergens. However, the relative contributions of these cell types still need to be elucidated, considering that ILC2s might have a greater capacity to produce type 2 cytokines than CD4+ T cells on a per cell basis.38
Recent studies provide mechanistic insights as to how ILC2s might regulate adaptive immunity and chronic airway inflammation. For example, adoptive transfer of both ILC2 and CD4+ T cell populations, but not each population alone, into Il7ra−/− mice resulted in induction of a robust antigen-specific type 2-cytokine response and airway inflammation when mice were exposed to protease allergens, suggesting synergistic interactions between ILC2s and CD4+ T cells during development of adaptive immune responses.86 In another study, intranasal administration of papain stimulated both ILC2s and Th2 cells, causing allergic lung inflammation and elevated levels of IgE antibodies.91 This process was severely impaired in ILC2-deficient mice. Indeed, ILC2-derived IL-13, but not IL-4, was critical as it promoted migration of activated lung DCs into the draining lymph node where they primed naive T cells to differentiate into Th2 cells. Importantly, both papain-induced ILC2 activation and Th2 cell differentiation were dependent on IL-33.
ILC2s and IL-33 also played key roles in the persistence of airway remodeling and AHR induced by fungal and other allergens.92 Elimination of T cells in mice with allergen-induced chronic airway inflammation resulted in resolution of airway inflammation, but AHR or remodeling continued without T cells. Elimination of both T cells and ILC2 or blockade of IL-33 resulted in resolution of airway inflammation and AHR. Importantly, epithelial IL-33 activated ILC2s to produce IL-13, which in turn promoted production of IL-33 and ST2 expression by airway epithelial cells. Thus, during a chronic phase of airway inflammation, epithelial cells and ILC2s may form a positive feedback loop, resulting in continued airway remodeling and AHR even in the absence of antigens or adaptive immunity.
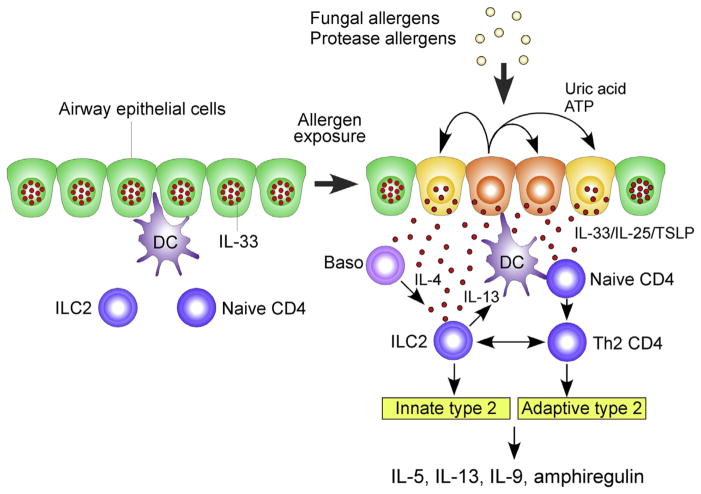
Finally, corticosteroid resistance is associated with persistent asthma and poses a major problem in the treatment of patients with severe asthma. In a mouse model of airway inflammation, in which mice were sensitized with OVA and then challenged with OVA, treatment with dexamethasone generally attenuated airway inflammation. In contrast, when mice were challenged with OVA plus IL-33, increased numbers of ILC2s and considerable production of type 2 cytokines persisted despite dexamethasone treatment.93 These steroid-resistant ILC2s are likely induced by TSLP that is produced during allergic inflammation of the airway and then activates STAT5 and Bcl-xL in ILC2s. Altogether, these studies suggest that involvement of ILC2s in allergic airway inflammation is unlikely to be limited to the innate immune response but extends to regulation of chronicity and persistence of airway inflammation induced by allergens. The current working model to describe the roles of ILC2s in fungus- and protease-induced type 2 airway immune responses is provided in Fig. 3.
Fig. 3.
Working model to describe the roles of ILC2s in type 2 immune response in the airway exposed to fungal and protease allergens. In the resting condition, IL-33 is stored in the nucleus of airway epithelial cells. Exposure to fungal or protease allergens induce the extracellular release of IL-33 and production of IL-25 and TSLP by the airway epithelium. Autocrine secretion of ATP and uric acid likely play a key role in regulating epithelial release of IL-33. IL-33, IL-25 and TSLP activate ILC2s to produce a large quantity of type 2 cytokines, such as IL-5, IL-13, IL-9, and amphiregulin. Basophil-derived IL-4 may facilitate ILC2 production of cytokines. ILC2-derived IL-13 enhances antigen uptake and migration of dendritic cells and promotes proliferation and differentiation of Th2-type CD4+ T cells. In addition, ILC2s and Th2-type CD4+ T cells may interact directly to sustain production of type 2 cytokines. Abbreviations: Baso, basophils; DC, dendritic cells; TSLP, thymic stromal lymphopoietin.
Concluding remarks
After identification and characterization of ILC2s in 2010,38–40 our knowledge of the biology of this novel cell type has expanded quickly. ILC2s are resident in various normal tissues. Although small in number, ILC2s likely play major roles in innate immunity and disease processes by producing large quantities of type 2 cytokines and tissue growth factors. In mice, ILC2s show both pathological and protective functions in virus- or allergen-induced allergic immune responses. Furthermore, ILC2s may also work with other immune cells, such as CD4+ T cells, DCs, and epithelial cells, and promote development and persistence of allergic airway inflammation.
However, the research in ILC2s is still in its infancy, and many questions remain to be addressed. For example, ILC2s contribute to the initiation and persistence of fungus-mediated allergic immune responses in mice. However, little is known about whether and how ILC2s are involved in the chronic and recurrent airway inflammation that is observed in human patients with asthma and other allergic diseases. The increased prevalence of ILC2s in nasal polyp tissues from patients with chronic rhinosinusitis55,94,95 and in peripheral blood specimens from patients with asthma96 suggest that ILC2s are likely involved. At the cellular level, the interactions between ILC2s and other immune and tissue cells such as mast cells and epithelial cells, as well the mechanisms involved in these interactions, need to be studied. Our knowledge of the processes involved in migration and tissue localization of ILC2s is also lacking. Finally, information is limited regarding the molecular mechanisms that explain how airway exposure to fungi and other allergens results in increased production and secretion of pro-type 2 cytokines, such as IL-33, leading to activation of ILC2s and other inflammatory cells in airway mucosa. Thus, a further understanding of the biology of ILC2s, their roles in disease status, and the molecular and cellular mechanisms involved in the activation and persistence of ILC2s will help us to better understand the mechanisms of asthma and other allergic airway diseases and to develop novel therapeutic options for these diseases.
Abbreviations
- AHR
airway hyperreactivity
- CLPs
common lymphoid progenitors
- cγ
common γ-chain
- CRTH2
chemoattractant receptor-homologous molecule expressed on Th2 lymphocytes
- FALC
fat-associated lymphoid cluster
- ILCs
innate lymphoid cells
- ILC1s
group 1 ILCs
- ILC2s
group 2 ILCs
- ILC3s
group 3 ILCs
- Lin−
lineage-negative
- LTD4
leukotriene D4
- NK
natural killer
- OVA
ovalbumin
- PGD2
prostaglandin D2
- PLZF
promyelocytic leukemia zinc finger
- TSLP
thymic stromal lymphopoietin
Footnotes
Conflict of interest
The author has no conflict of interest to declare.
References
- 1.O’Shea JJ, Paul WE. Mechanisms underlying lineage commitment and plasticity of helper CD4+ T cells. Science. 2010;327:1098–102. doi: 10.1126/science.1178334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Walker JA, Barlow JL, McKenzie AN. Innate lymphoid cells–how did we miss them? Nat Rev Immunol. 2013;13:75–87. doi: 10.1038/nri3349. [DOI] [PubMed] [Google Scholar]
- 3.Spits H, Di Santo JP. The expanding family of innate lymphoid cells: regulators and effectors of immunity and tissue remodeling. Nat Immunol. 2011;12:21–7. doi: 10.1038/ni.1962. [DOI] [PubMed] [Google Scholar]
- 4.Spits H, Artis D, Colonna M, Diefenbach A, Di Santo JP, Eberl G, et al. Innate lymphoid cells–a proposal for uniform nomenclature. Nat Rev Immunol. 2013;13:145–9. doi: 10.1038/nri3365. [DOI] [PubMed] [Google Scholar]
- 5.McKenzie AN, Spits H, Eberl G. Innate lymphoid cells in inflammation and immunity. Immunity. 2014;41:366–74. doi: 10.1016/j.immuni.2014.09.006. [DOI] [PubMed] [Google Scholar]
- 6.Halwani R, Al-Muhsen S, Hamid Q. T helper 17 cells in airway diseases: from laboratory bench to bedside. Chest. 2013;143:494–501. doi: 10.1378/chest.12-0598. [DOI] [PubMed] [Google Scholar]
- 7.Locksley RM. Asthma and allergic inflammation. Cell. 2010;140:777–83. doi: 10.1016/j.cell.2010.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Paul WE, Zhu J. How are T(H)2-type immune responses initiated and amplified? Nat Rev Immunol. 2010;10:225–35. doi: 10.1038/nri2735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wang YH, Liu YJ. Thymic stromal lymphopoietin, OX40-ligand, and interleukin-25 in allergic responses. Clin Exp Allergy. 2009;39:798–806. doi: 10.1111/j.1365-2222.2009.03241.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ziegler SF, Artis D. Sensing the outside world: TSLP regulates barrier immunity. Nat Immunol. 2010;11:289–93. doi: 10.1038/ni.1852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Saenz SA, Taylor BC, Artis D. Welcome to the neighborhood: epithelial cell-derived cytokines license innate and adaptive immune responses at mucosal sites. Immunol Rev. 2008;226:172–90. doi: 10.1111/j.1600-065X.2008.00713.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schmitz J, Owyang A, Oldham E, Song Y, Murphy E, McClanahan TK, et al. IL-33, an interleukin-1-like cytokine that signals via the IL-1 receptor-related protein ST2 and induces T helper type 2-associated cytokines. Immunity. 2005;23:479–90. doi: 10.1016/j.immuni.2005.09.015. [DOI] [PubMed] [Google Scholar]
- 13.Kondo Y, Yoshimoto T, Yasuda K, Futatsugi-Yumikura S, Morimoto M, Hayashi N, et al. Administration of IL-33 induces airway hyperresponsiveness and goblet cell hyperplasia in the lungs in the absence of adaptive immune system. Int Immunol. 2008;20:791–800. doi: 10.1093/intimm/dxn037. [DOI] [PubMed] [Google Scholar]
- 14.Oboki K, Ohno T, Kajiwara N, Arae K, Morita H, Ishii A, et al. IL-33 is a crucial amplifier of innate rather than acquired immunity. Proc Natl Acad Sci U S A. 2010;107:18581–6. doi: 10.1073/pnas.1003059107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hammad H, Lambrecht BN. Dendritic cells and epithelial cells: linking innate and adaptive immunity in asthma. Nat Rev Immunol. 2008;8:193–204. doi: 10.1038/nri2275. [DOI] [PubMed] [Google Scholar]
- 16.Ito T, Wang YH, Duramad O, Hori T, Delespesse GJ, Watanabe N, et al. TSLP-activated dendritic cells induce an inflammatory T helper type 2 cell response through OX40 ligand. J Exp Med. 2005;202:1213–23. doi: 10.1084/jem.20051135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang YH, Ito T, Homey B, Watanabe N, Martin R, Barnes CJ, et al. Maintenance and polarization of human TH2 central memory T cells by thymic stromal lymphopoietin-activated dendritic cells. Immunity. 2006;24:827–38. doi: 10.1016/j.immuni.2006.03.019. [DOI] [PubMed] [Google Scholar]
- 18.Zhou B, Comeau MR, De Smedt T, Liggitt HD, Dahl ME, Lewis DB, et al. Thymic stromal lymphopoietin as a key initiator of allergic airway inflammation in mice. Nat Immunol. 2005;6:1047–53. doi: 10.1038/ni1247. [DOI] [PubMed] [Google Scholar]
- 19.Langley SJ, Goldthorpe S, Craven M, Morris J, Woodcock A, Custovic A. Exposure and sensitization to indoor allergens: association with lung function, bronchial reactivity, and exhaled nitric oxide measures in asthma. J Allergy Clin Immunol. 2003;112:362–8. doi: 10.1067/mai.2003.1654. [DOI] [PubMed] [Google Scholar]
- 20.Sporik R, Holgate ST, Platts-Mills TA, Cogswell JJ. Exposure to house-dust mite allergen (Der p I) and the development of asthma in childhood. A prospective study. N Engl J Med. 1990;323:502–7. doi: 10.1056/NEJM199008233230802. [DOI] [PubMed] [Google Scholar]
- 21.Rosenstreich DL, Eggleston P, Kattan M, Baker D, Slavin RG, Gergen P, et al. The role of cockroach allergy and exposure to cockroach allergen in causing morbidity among inner-city children with asthma. N Engl J Med. 1997;336:1356–63. doi: 10.1056/NEJM199705083361904. [DOI] [PubMed] [Google Scholar]
- 22.Knutsen AP, Bush RK, Demain JG, Denning DW, Dixit A, Fairs A, et al. Fungi and allergic lower respiratory tract diseases. J Allergy Clin Immunol. 2012;129:280–91. doi: 10.1016/j.jaci.2011.12.970. [DOI] [PubMed] [Google Scholar]
- 23.Denning DW, O’Driscoll BR, Hogaboam CM, Bowyer P, Niven RM. The link between fungi and severe asthma: a summary of the evidence. Eur Respir J. 2006;27:615. doi: 10.1183/09031936.06.00074705. [DOI] [PubMed] [Google Scholar]
- 24.Bush RK, Prochnau JJ. Alternaria-induced asthma. J Allergy Clin Immunol. 2004;113:227–34. doi: 10.1016/j.jaci.2003.11.023. [DOI] [PubMed] [Google Scholar]
- 25.O’Hollaren MT, Yunginger JW, Offord KP, Somers MJ, O’Connell EJ, Ballard DJ, et al. Exposure to an aeroallergen as a possible precipitating factor in respiratory arrest in young patients with asthma. N Engl J Med. 1991;324:359–63. doi: 10.1056/NEJM199102073240602. [DOI] [PubMed] [Google Scholar]
- 26.Delfino RJ, Zeiger RS, Seltzer JM, Street DH, Matteucci RM, Anderson PR, et al. The effect of outdoor fungal spore concentrations on daily asthma severity. Environ Health Perspect. 1997;105:622–35. doi: 10.1289/ehp.97105622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Downs SH, Mitakakis TZ, Marks GB, Car NG, Belousova EG, Leuppi JD, et al. Clinical importance of Alternaria exposure in children. Am J Respir Crit Care Med. 2001;164:455–9. doi: 10.1164/ajrccm.164.3.2008042. [DOI] [PubMed] [Google Scholar]
- 28.Reese TA, Liang HE, Tager AM, Luster AD, Van Rooijen N, Voehringer D, et al. Chitin induces accumulation in tissue of innate immune cells associated with allergy. Nature. 2007;447:92–6. doi: 10.1038/nature05746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wan GH, Li CS, Guo SP, Rylander R, Lin RH. An airbone mold-derived product, beta-1,3-D-glucan, potentiates airway allergic responses. Eur J Immunol. 1999;29:2491–7. doi: 10.1002/(SICI)1521-4141(199908)29:08<2491::AID-IMMU2491>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- 30.Porter PC, Ongeri V, Luong A, Kheradmand F, Corry DB. Seeking common pathophysiology in asthma, atopy and sinusitis. Trends Immunol. 2011;32:43–9. doi: 10.1016/j.it.2010.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Porter P, Susarla SC, Polikepahad S, Qian Y, Hampton J, Kiss A, et al. Link between allergic asthma and airway mucosal infection suggested by proteinase-secreting household fungi. Mucosal Immunol. 2009;2:504–17. doi: 10.1038/mi.2009.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Comoy EE, Pestel J, Duez C, Stewart GA, Vendeville C, Fournier C, et al. The house dust mite allergen, Dermatophagoides pteronyssinus, promotes type 2 responses by modulating the balance between IL-4 and IFN-gamma. J Immunol. 1998;160:2456–62. [PubMed] [Google Scholar]
- 33.Kheradmand F, Kiss A, Xu J, Lee SH, Kolattukudy PE, Corry DB. A protease-activated pathway underlying Th cell type 2 activation and allergic lung disease. J Immunol. 2002;169:5904–11. doi: 10.4049/jimmunol.169.10.5904. [DOI] [PubMed] [Google Scholar]
- 34.Sokol CL, Barton GM, Farr AG, Medzhitov R. A mechanism for the initiation of allergen-induced T helper type 2 responses. Nat Immunol. 2008;9:310–8. doi: 10.1038/ni1558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fort MM, Cheung J, Yen D, Li J, Zurawski SM, Lo S, et al. IL-25 induces IL-4, IL-5, and IL-13 and Th2-associated pathologies in vivo. Immunity. 2001;15:985–95. doi: 10.1016/s1074-7613(01)00243-6. [DOI] [PubMed] [Google Scholar]
- 36.Hurst SD, Muchamuel T, Gorman DM, Gilbert JM, Clifford T, Kwan S, et al. New IL-17 family members promote Th1 or Th2 responses in the lung: in vivo function of the novel cytokine IL-25. J Immunol. 2002;169:443–53. doi: 10.4049/jimmunol.169.1.443. [DOI] [PubMed] [Google Scholar]
- 37.Fallon PG, Ballantyne SJ, Mangan NE, Barlow JL, Dasvarma A, Hewett DR, et al. Identification of an interleukin (IL)-25-dependent cell population that provides IL-4, IL-5, and IL-13 at the onset of helminth expulsion. J Exp Med. 2006;203:1105–16. doi: 10.1084/jem.20051615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Moro K, Yamada T, Tanabe M, Takeuchi T, Ikawa T, Kawamoto H, et al. Innate production of T(H)2 cytokines by adipose tissue-associated c-Kit(+)Sca-1(+) lymphoid cells. Nature. 2010;463:540–4. doi: 10.1038/nature08636. [DOI] [PubMed] [Google Scholar]
- 39.Neill DR, Wong SH, Bellosi A, Flynn RJ, Daly M, Langford TK, et al. Nuocytes represent a new innate effector leukocyte that mediates type-2 immunity. Nature. 2010;464:1367–70. doi: 10.1038/nature08900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Price AE, Liang HE, Sullivan BM, Reinhardt RL, Eisley CJ, Erle DJ, et al. Systemically dispersed innate IL-13-expressing cells in type 2 immunity. Proc Natl Acad Sci U S A. 2010;107:11489–94. doi: 10.1073/pnas.1003988107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kee BL. E and ID proteins branch out. Nat Rev Immunol. 2009;9:175–84. doi: 10.1038/nri2507. [DOI] [PubMed] [Google Scholar]
- 42.Constantinides MG, McDonald BD, Verhoef PA, Bendelac A. A committed precursor to innate lymphoid cells. Nature. 2014;508:397–401. doi: 10.1038/nature13047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hamilton BA, Frankel WN, Kerrebrock AW, Hawkins TL, FitzHugh W, Kusumi K, et al. Disruption of the nuclear hormone receptor RORalpha in staggerer mice. Nature. 1996;379:736–9. doi: 10.1038/379736a0. [DOI] [PubMed] [Google Scholar]
- 44.Wong SH, Walker JA, Jolin HE, Drynan LF, Hams E, Camelo A, et al. Transcription factor RORalpha is critical for nuocyte development. Nat Immunol. 2012;13:229–36. doi: 10.1038/ni.2208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Halim TY, MacLaren A, Romanish MT, Gold MJ, McNagny KM, Takei F. Retinoic-acid-receptor-related orphan nuclear receptor alpha is required for natural helper cell development and allergic inflammation. Immunity. 2012;37:463–74. doi: 10.1016/j.immuni.2012.06.012. [DOI] [PubMed] [Google Scholar]
- 46.Hoyler T, Klose CS, Souabni A, Turqueti-Neves A, Pfeifer D, Rawlins EL, et al. The transcription factor GATA-3 controls cell fate and maintenance of type 2 innate lymphoid cells. Immunity. 2012;37:634–48. doi: 10.1016/j.immuni.2012.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Furusawa J, Moro K, Motomura Y, Okamoto K, Zhu J, Takayanagi H, et al. Critical role of p38 and GATA3 in natural helper cell function. J Immunol. 2013;191:1818–26. doi: 10.4049/jimmunol.1300379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Martinez-Gonzalez I, Steer CA, Takei F. Lung ILC2s link innate and adaptive responses in allergic inflammation. Trends Immunol. 2015;36:189–95. doi: 10.1016/j.it.2015.01.005. [DOI] [PubMed] [Google Scholar]
- 49.Monticelli LA, Sonnenberg GF, Abt MC, Alenghat T, Ziegler CG, Doering TA, et al. Innate lymphoid cells promote lung-tissue homeostasis after infection with influenza virus. Nat Immunol. 2011;12:1045–54. doi: 10.1031/ni.2131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bartemes KR, Iijima K, Kobayashi T, Kephart GM, McKenzie AN, Kita H. IL-33-responsive lineage- CD25+ CD44(hi) lymphoid cells mediate innate type 2 immunity and allergic inflammation in the lungs. J Immunol. 2012;188:1503–13. doi: 10.4049/jimmunol.1102832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Halim TY, Krauss RH, Sun AC, Takei F. Lung natural helper cells are a critical source of Th2 cell-type cytokines in protease allergen-induced airway inflammation. Immunity. 2012;36:451–63. doi: 10.1016/j.immuni.2011.12.020. [DOI] [PubMed] [Google Scholar]
- 52.Nussbaum JC, Van Dyken SJ, von Moltke J, Cheng LE, Mohapatra A, Molofsky AB, et al. Type 2 innate lymphoid cells control eosinophil homeostasis. Nature. 2013;502:245–8. doi: 10.1038/nature12526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kim BS, Siracusa MC, Saenz SA, Noti M, Monticelli LA, Sonnenberg GF, et al. TSLP elicits IL-33-independent innate lymphoid cell responses to promote skin inflammation. Sci Transl Med. 2013;5:170ra16. doi: 10.1126/scitranslmed.3005374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Mjosberg J, Bernink J, Golebski K, Karrich JJ, Peters CP, Blom B, et al. The transcription factor GATA3 is essential for the function of human type 2 innate lymphoid cells. Immunity. 2012;37:649–59. doi: 10.1016/j.immuni.2012.08.015. [DOI] [PubMed] [Google Scholar]
- 55.Mjosberg JM, Trifari S, Crellin NK, Peters CP, van Drunen CM, Piet B, et al. Human IL-25- and IL-33-responsive type 2 innate lymphoid cells are defined by expression of CRTH2 and CD161. Nat Immunol. 2011;12:1055–62. doi: 10.1038/ni.2104. [DOI] [PubMed] [Google Scholar]
- 56.Ikutani M, Yanagibashi T, Ogasawara M, Tsuneyama K, Yamamoto S, Hattori Y, et al. Identification of innate IL-5-producing cells and their role in lung eosinophil regulation and antitumor immunity. J Immunol. 2012;188:703–13. doi: 10.4049/jimmunol.1101270. [DOI] [PubMed] [Google Scholar]
- 57.Molofsky AB, Nussbaum JC, Liang HE, Van Dyken SJ, Cheng LE, Mohapatra A, et al. Innate lymphoid type 2 cells sustain visceral adipose tissue eosinophils and alternatively activated macrophages. J Exp Med. 2013;210:535–49. doi: 10.1084/jem.20121964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hams E, Locksley RM, McKenzie AN, Fallon PG. Cutting edge: IL-25 elicits innate lymphoid type 2 and type II NKT cells that regulate obesity in mice. J Immunol. 2013;191:5349–53. doi: 10.4049/jimmunol.1301176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Stanya KJ, Jacobi D, Liu S, Bhargava P, Dai L, Gangl MR, et al. Direct control of hepatic glucose production by interleukin-13 in mice. J Clin Invest. 2013;123:261–71. doi: 10.1172/JCI64941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wilhelm C, Hirota K, Stieglitz B, Van Snick J, Tolaini M, Lahl K, et al. An IL-9 fate reporter demonstrates the induction of an innate IL-9 response in lung inflammation. Nat Immunol. 2011;12:1071–7. doi: 10.1038/ni.2133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Barlow JL, Peel S, Fox J, Panova V, Hardman CS, Camelo A, et al. IL-33 is more potent than IL-25 in provoking IL-13-producing nuocytes (type 2 innate lymphoid cells) and airway contraction. J Allergy Clin Immunol. 2013;132:933–41. doi: 10.1016/j.jaci.2013.05.012. [DOI] [PubMed] [Google Scholar]
- 62.Barlow JL, Bellosi A, Hardman CS, Drynan LF, Wong SH, Cruickshank JP, et al. Innate IL-13-producing nuocytes arise during allergic lung inflammation and contribute to airways hyperreactivity. J Allergy Clin Immunol. 2012;129:191–8. e1–4. doi: 10.1016/j.jaci.2011.09.041. [DOI] [PubMed] [Google Scholar]
- 63.Turner JE, Morrison PJ, Wilhelm C, Wilson M, Ahlfors H, Renauld JC, et al. IL-9-mediated survival of type 2 innate lymphoid cells promotes damage control in helminth-induced lung inflammation. J Exp Med. 2013;210:2951–65. doi: 10.1084/jem.20130071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Yu X, Pappu R, Ramirez-Carrozzi V, Ota N, Caplazi P, Zhang J, et al. TNF superfamily member TL1A elicits type 2 innate lymphoid cells at mucosal barriers. Mucosal Immunol. 2014;7:730–40. doi: 10.1038/mi.2013.92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Boyce JA. Mast cells and eicosanoid mediators: a system of reciprocal paracrine and autocrine regulation. Immunol Rev. 2007;217:168–85. doi: 10.1111/j.1600-065X.2007.00512.x. [DOI] [PubMed] [Google Scholar]
- 66.Doherty TA, Khorram N, Lund S, Mehta AK, Croft M, Broide DH. Lung type 2 innate lymphoid cells express cysteinyl leukotriene receptor 1, which regulates TH2 cytokine production. J Allergy Clin Immunol. 2013;132:205–13. doi: 10.1016/j.jaci.2013.03.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Chang JE, Doherty TA, Baum R, Broide D. Prostaglandin D2 regulates human type 2 innate lymphoid cell chemotaxis. J Allergy Clin Immunol. 2014;133:899–901. e3. doi: 10.1016/j.jaci.2013.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Chang YJ, Kim HY, Albacker LA, Baumgarth N, McKenzie AN, Smith DE, et al. Innate lymphoid cells mediate influenza-induced airway hyper-reactivity independently of adaptive immunity. Nat Immunol. 2011;12:631–8. doi: 10.1038/ni.2045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kouzaki H, Iijima K, Kobayashi T, O’Grady SM, Kita H. The danger signal, extracellular ATP, is a sensor for an airborne allergen and triggers IL-33 release and innate Th2-type responses. J Immunol. 2011;186:4375–87. doi: 10.4049/jimmunol.1003020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Doherty TA, Khorram N, Chang JE, Kim HK, Rosenthal P, Croft M, et al. STAT6 regulates natural helper cell proliferation during lung inflammation initiated by Alternaria. Am J Physiol Lung Cell Mol Physiol. 2012;303:L577–88. doi: 10.1152/ajplung.00174.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Snelgrove RJ, Gregory LG, Peiro T, Akthar S, Campbell GA, Walker SA, et al. Alternaria-derived serine protease activity drives IL-33-mediated asthma exacerbations. J Allergy Clin Immunol. 2014;134:583–592.e6. doi: 10.1016/j.jaci.2014.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Kim HK, Lund S, Baum R, Rosenthal P, Khorram N, Doherty TA. Innate type 2 response to Alternaria extract enhances ryegrass-induced lung inflammation. Int Arch Allergy Immunol. 2014;163:92–105. doi: 10.1159/000356341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Hara K, Iijima K, Elias MK, Seno S, Tojima I, Kobayashi T, et al. Airway uric acid is a sensor of inhaled protease allergens and initiates type 2 immune responses in respiratory mucosa. J Immunol. 2014;192:4032–42. doi: 10.4049/jimmunol.1400110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Van Dyken SJ, Mohapatra A, Nussbaum JC, Molofsky AB, Thornton EE, Ziegler SF, et al. Chitin activates parallel immune modules that direct distinct inflammatory responses via innate lymphoid type 2 and gammadelta T cells. Immunity. 2014;40:414–24. doi: 10.1016/j.immuni.2014.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Karp CL. Guilt by intimate association: what makes an allergen an allergen? J Allergy Clin Immunol. 2010;125:955–60. doi: 10.1016/j.jaci.2010.03.002. quiz 961–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Wills-Karp M, Nathan A, Page K, Karp CL. New insights into innate immune mechanisms underlying allergenicity. Mucosal Immunol. 2010;3:104–10. doi: 10.1038/mi.2009.138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Kool M, van Loo G, Waelput W, De Prijck S, Muskens F, Sze M, et al. The ubiquitin-editing protein A20 prevents dendritic cell activation, recognition of apoptotic cells, and systemic autoimmunity. Immunity. 2011;35:82–96. doi: 10.1016/j.immuni.2011.05.013. [DOI] [PubMed] [Google Scholar]
- 78.Hammad H, Chieppa M, Perros F, Willart MA, Germain RN, Lambrecht BN. House dust mite allergen induces asthma via Toll-like receptor 4 triggering of airway structural cells. Nat Med. 2009;15:410–6. doi: 10.1038/nm.1946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Trompette A, Divanovic S, Visintin A, Blanchard C, Hegde RS, Madan R, et al. Allergenicity resulting from functional mimicry of a Toll-like receptor complex protein. Nature. 2009;457:585–8. doi: 10.1038/nature07548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Eisenbarth SC, Piggott DA, Huleatt JW, Visintin I, Herrick CA, Bottomly K. Lipopolysaccharide-enhanced, toll-like receptor 4-dependent T helper cell type 2 responses to inhaled antigen. J Exp Med. 2002;196:1645–51. doi: 10.1084/jem.20021340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Tang H, Cao W, Kasturi SP, Ravindran R, Nakaya HI, Kundu K, et al. The T helper type 2 response to cysteine proteases requires dendritic cell-basophil cooperation via ROS-mediated signaling. Nat Immunol. 2010;11:608–17. doi: 10.1038/ni.1883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Millien VO, Lu W, Shaw J, Yuan X, Mak G, Roberts L, et al. Cleavage of fibrinogen by proteinases elicits allergic responses through Toll-like receptor 4. Science. 2013;341:792–6. doi: 10.1126/science.1240342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Kouzaki H, O’Grady SM, Lawrence CB, Kita H. Proteases induce production of thymic stromal lymphopoietin by airway epithelial cells through protease-activated receptor-2. J Immunol. 2009;183:1427–34. doi: 10.4049/jimmunol.0900904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Kouzaki H, Tojima I, Kita H, Shimizu T. Transcription of interleukin-25 and extracellular release of the protein is regulated by allergen proteases in airway epithelial cells. Am J Respir Cell Mol Biol. 2013;49:741–50. doi: 10.1165/rcmb.2012-0304OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Mirchandani AS, Besnard AG, Yip E, Scott C, Bain CC, Cerovic V, et al. Type 2 innate lymphoid cells drive CD4+ Th2 cell responses. J Immunol. 2014;192:2442–8. doi: 10.4049/jimmunol.1300974. [DOI] [PubMed] [Google Scholar]
- 86.Drake LY, Iijima K, Kita H. Group 2 innate lymphoid cells and CD4+ T cells cooperate to mediate type 2 immune response in mice. Allergy. 2014;69:1300–7. doi: 10.1111/all.12446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Gold MJ, Antignano F, Halim TY, Hirota JA, Blanchet MR, Zaph C, et al. Group 2 innate lymphoid cells facilitate sensitization to local, but not systemic, TH2-inducing allergen exposures. J Allergy Clin Immunol. 2014;133:1142–8. doi: 10.1016/j.jaci.2014.02.033. [DOI] [PubMed] [Google Scholar]
- 88.Motomura Y, Morita H, Moro K, Nakae S, Artis D, Endo TA, et al. Basophil-derived interleukin-4 controls the function of natural helper cells, a member of ILC2s, in lung inflammation. Immunity. 2014;40:758–71. doi: 10.1016/j.immuni.2014.04.013. [DOI] [PubMed] [Google Scholar]
- 89.Iijima K, Kobayashi T, Hara K, Kephart GM, Ziegler SF, McKenzie AN, et al. IL-33 and thymic stromal lymphopoietin mediate immune pathology in response to chronic airborne allergen exposure. J Immunol. 2014;193:1549–59. doi: 10.4049/jimmunol.1302984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Klein Wolterink RG, Kleinjan A, van Nimwegen M, Bergen I, de Bruijn M, Levani Y, et al. Pulmonary innate lymphoid cells are major producers of IL-5 and IL-13 in murine models of allergic asthma. Eur J Immunol. 2012;42:1106–16. doi: 10.1002/eji.201142018. [DOI] [PubMed] [Google Scholar]
- 91.Halim TY, Steer CA, Matha L, Gold MJ, Martinez-Gonzalez I, McNagny KM, et al. Group 2 innate lymphoid cells are critical for the initiation of adaptive T helper 2 cell-mediated allergic lung inflammation. Immunity. 2014;40:425–35. doi: 10.1016/j.immuni.2014.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Christianson CA, Goplen NP, Zafar I, Irvin C, Good JT, Jr, Rollins DR, et al. Persistence of asthma requires multiple feedback circuits involving type 2 innate lymphoid cells and IL-33. J Allergy Clin Immunol. 2015 doi: 10.1016/j.jaci.2014.11.037. http://dx.doi.org/10.1016/j.jaci.2014.11.037. [DOI] [PMC free article] [PubMed]
- 93.Kabata H, Moro K, Fukunaga K, Suzuki Y, Miyata J, Masaki K, et al. Thymic stromal lymphopoietin induces corticosteroid resistance in natural helper cells during airway inflammation. Nat Commun. 2013;4:2675. doi: 10.1038/ncomms3675. [DOI] [PubMed] [Google Scholar]
- 94.Shaw JL, Fakhri S, Citardi MJ, Porter PC, Corry DB, Kheradmand F, et al. IL-33-responsive innate lymphoid cells are an important source of IL-13 in chronic rhinosinusitis with nasal polyps. Am J Respir Crit Care Med. 2013;188:432–9. doi: 10.1164/rccm.201212-2227OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Walford HH, Lund SJ, Baum RE, White AA, Bergeron CM, Husseman J, et al. Increased ILC2s in the eosinophilic nasal polyp endotype are associated with corticosteroid responsiveness. Clin Immunol. 2014;155:126–35. doi: 10.1016/j.clim.2014.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Bartemes KR, Kephart GM, Fox SJ, Kita H. Enhanced innate type 2 immune response in peripheral blood from patients with asthma. J Allergy Clin Immunol. 2014;134:671–8. e4. doi: 10.1016/j.jaci.2014.06.024. [DOI] [PMC free article] [PubMed] [Google Scholar]