Abstract
Despite significant efforts to control tuberculosis (TB), the disease remains a major global threat, with an estimated 8.6 million new cases and 1.3 million deaths in 2012 alone. Significant treatment challenges include HIV co-infection, the dramatic rise of multidrug-resistant TB and the vast reservoir of latently infected individuals, who will develop active disease years after the initial infection. The long duration of chemotherapy also remains a major barrier to effective large scale treatment of TB. Significant advances are being made in the development of shorter and effective TB drug regimens and there is growing evidence that host-directed and “non-antimicrobial” pathogen-directed therapies, could serve as novel approaches to enhance TB treatments. This review highlights the rationale for using these therapies and summarizes some of the progress in this field.
Keywords: Corticosteroids, efflux pump inhibitors, microenvironment, inflammation, TNF-α
INTRODUCTION
There were an estimated 8.6 million new cases of tuberculosis (TB), accounting for 1.3 million deaths in 2012 alone [1]. One third of world’s population is latently infected with Mycobacterium tuberculosis, of which 5% to 10% will develop active disease years after the initial infection [2]. HIV co-infection dramatically increases the risk of relapse to approximately 10% per year [3]. A recent study has demonstrated that TB is the main cause of death in HIV co-infected patients in Eastern Europe [4]. In 2012, there were an estimated 450,000 new cases of multidrug resistant TB (MDR), defined as disease with M. tuberculosis strains resistant to two of the first-line TB drugs (isoniazid and rifampin). Of these, ~10% are estimated to be extensively drug resistant (XDR) TB, defined as disease with MDR strains also resistant to any fluoroquinolone and to at least one of three injectable second-line TB drugs (capreomycin, kanamycin, and amikacin) [1]. It is estimated that a diseased individual can transmit M. tuberculosis to 10–15 close contacts over the course of a year. While significant advances are being made in developing shorter and effective TB drug regimens [5], there is growing evidence that host-directed and “non-antimicrobial” pathogen-directed therapies could serve as a novel approaches to shorten TB treatments [6, 7].
HOST MICROENVIRONMENT: FRIEND OR FOE?
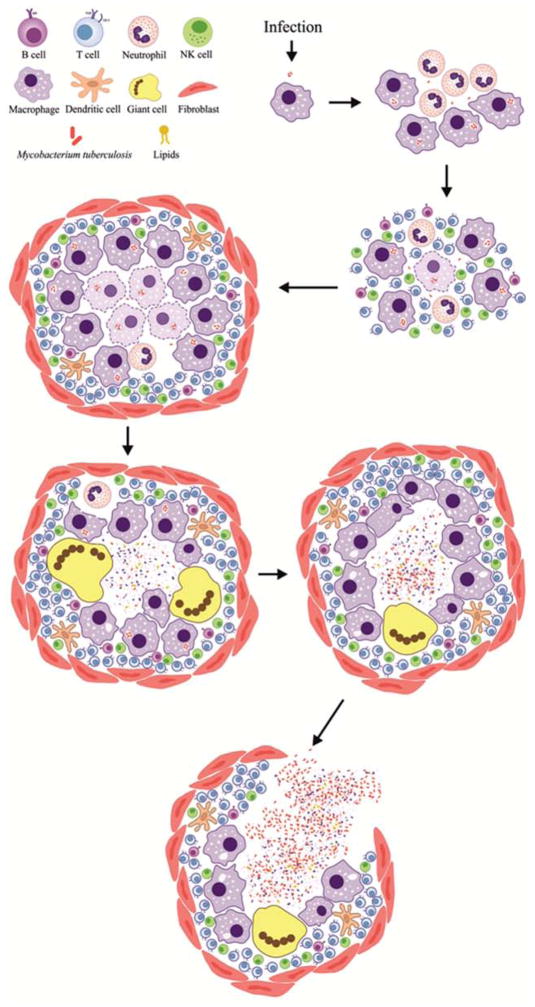
After deposition of M. tuberculosis in the alveoli via an airborne route, the bacteria are phagocytosed by alveolar macrophages and replicate within them. With the emergence of delayed-type hypersensitivity (DTH), infected macrophages in the interior of the granuloma are killed, and the periphery becomes fibrotic. The granuloma enlarges progressively to a macroscopic lesion with central areas of “caseous” necrosis. Occasionally, the necrotic TB granuloma bursts into an airway to form a cavitary lesion. This process releases a large number of bacteria directly into the airway and patients with cavitary TB are highly infectious (Fig. 1).
Fig. 1. Formation of cavitary TB lesions.
After deposition of M. tuberculosis in the alveoli via an airborne route, initial events include phagocytoses by alveolar macrophages, bacterial replication within macrophages, development of delayed-type hypersensitivity (DTH) and the formation of the TB granuloma. These initial microscopic TB granulomas are composed mainly of activated macrophages and lymphocytes and may often have multinucleated giant cells (also called Langhans giant cell) with nuclei arranged like a horseshoe. With the emergence of DTH, infected macrophages in the interior of the granuloma are killed, and the periphery becomes fibrotic due to the production of collagen by fibroblasts. The granuloma enlarges progressively to a macroscopic lesion with central areas of “caseous” necrosis, surrounded by activated macrophages, lymphocytes, and fibroblasts. Occasionally, the necrotic TB granuloma bursts into an airway to form a cavitary lesion. This process releases a large number of bacteria directly into the airway and patients with cavitary TB are highly infectious.
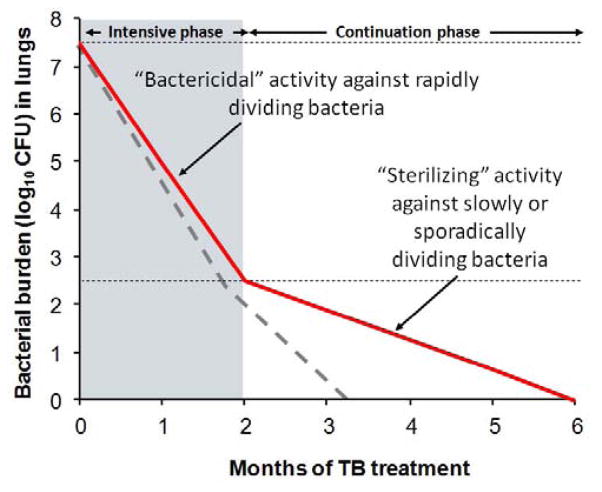
In the late 1940s, surgical resection of TB lesions became increasingly common. Several reports demonstrated that M. tuberculosis could be detected by microscopy in resected TB lesions months after patients on TB treatments had no detectable bacteria in their sputa [8]. Although M. tuberculosis could be cultured from some lesions, “closed lesions” lacking patent bronchial communication failed to yield bacilli. Vandiviere et al, showed that by extending the incubation period from the standard 8-weeks to 3–10 months, M. tuberculosis from “closed lesions” could be cultured [9]. Since isoniazid (a first-line TB drug) was known to readily penetrate lesions in human lungs, lack of drug access was not responsible for the survival of M. tuberculosis within the “closed lesions”. Using tissue samples from the lungs of TB patients, Haapanen et al., demonstrated that “closed lesions” are hypoxic and that hypoxia favors survival of dormant M. tuberculosis [10]. These classic studies suggest that the bactericidal activity of TB drugs is dependent on the physiological state of the bacteria and the host-microenvironment. Moreover, M. tuberculosis adapts to a quiescent physiological state, to successfully evade drugs (“persistence”) and host-immune responses (“dormancy”) for decades [11]. As the immune system falters, M. tuberculosis returns to replication mode, leading to relapse. It is therefore accepted that extended TB treatment is required to kill the dormant M. tuberculosis [8] (Fig. 2).
Fig. 2. Current model of TB treatment.
Rapid bacterial killing is observed during the initial intensive phase which typically requires 4-drugs. However, the rate of killing is slower during the continuous phase due to “dormant” or “persistent” bacteria which are slowly or sporadically multiplying and therefore not amenable to effective killing by TB drugs. By targeting “dormant” or “persistent” bacteria, adjunctive therapies could hasten bacterial clearance (dotted line) and help in development of shorter and effective TB treatments.
Host-directed therapies are well known and widely used to treat a variety of infections. Corticosteroids are recommended in the treatment of some forms of bacterial meningitis and have demonstrated a reduction in both mortality and neurological sequelae in adults [12, 13]. Similarly, adjunctive corticosteroids have been shown to prevent the development of hearing loss in children with Haemophilus influenzae type B mennigitis [14]. Corticosteroids are used as adjunctive therapy in patients with severe Pneumocystis pneumonia, an opportunistic infection known to cause life-threatening pulmonary inflammation in patients with advanced HIV [15]. Macrolides are known to have immunomodulatory properties, and long term azithromycin is recommended for patients with cystic fibrosis. Several studies have also demonstrated the efficacy of azithromycin in reducing the risk of acute exacerbations in patients with chronic obstructive pulmonary disease (COPD) [16]. Adjunctive low-dose corticosteroids are also recommended in the treatment of severe sepsis, as they reduce mortality [17].
CORTICOSTEROIDS
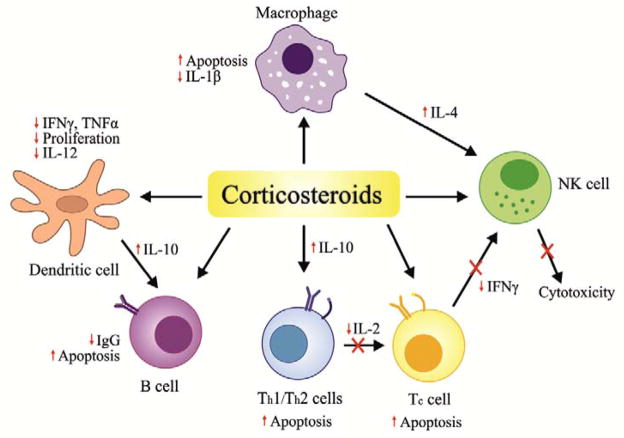
Corticosteroids diffuse across the cell membrane and bind to intracellular receptors to form a complex that translocates into the nucleus. This complex interacts with DNA, resulting in altered transcription of various corticosteroid-responsive genes [18]. Corticosteroid hormones have diverse physiological effects that directly or indirectly regulate several different cell types including T-cells, macrophages, eosinophils, neutrophils, mast-cells, endothelial and epithelial cells (Fig. 3). These actions are accomplished primarily through the modulation of inflammatory cytokines, as well as through induction of apoptosis [19], leading to a dramatic reduction in lymphocytes and accompanied by neutrophilic leukocytosis [20, 21].
Fig. 3. Mechanism of action of corticosteroids.
Corticosteroids diffuse across the cell membrane and bind to intracellular receptors that translocate into the nucleus. This complex interacts with DNA, resulting in altered transcription of various corticosteroid-responsive genes. Corticosteroids have diverse physiological effects and directly or indirectly regulate several different immune cell as shown here. These actions are primarily accomplished by modulating inflammatory cytokines as well as through induction of apoptosis.
Pulmonary TB
In the 1950’s corticosteroids were contraindicated as part of TB treatments [22]. However, this concept was rapidly discarded when it was found that corticosteroids in combination with effective chemotherapy proved to be useful in the treatment of TB patients [23]. In addition, adjunct therapy with prednisone (1–2 mg/kg/day) appeared to favorably influence the radiological lesions and hastened clinical improvement in children with severe TB (military TB, TB meningitis and pleural effusion) [24, 25]. Handley et al., demonstrated that the use of high doses of prednisone (15 mg/day for the initial 3 months and 30 mg/day during the final 3 months) in combination with isoniazid was beneficial in patients with advanced bilateral progressive cavitary pulmonary TB [26]. Similarly, the Research Committee of the Tuberculosis Society of Scotland published the first randomized controlled trial in adults comparing TB treatment versus TB treatment plus adjunctive prednisolone (20 mg/day for 3 months) [27]. The clinical improvement in the prednisolone group was hastened, especially in the acutely ill. No permanent side effects were observed in the prednisolone-treated patients. The general radiographic improvement was more rapid throughout the 12-month period in the prednisolone group, although the rate of cavity closure was not increased. Morris et al., studied whether this benefit was maintained in a 2-year follow-up study and demonstrated that the addition of prednisone during the early months of TB treatments not only led to more rapid clinical improvement initially, but also significantly greater radiographic resolution after 12 months [28]. Most of the initial trials utilizing high dose corticosteroids demonstrated an increased risk of side-effects [28–30]. However, subsequent studies using smaller doses of prednisone (1 mg/day) for 2–3 months also demonstrated remarkable clinical and radiological improvements [31]. More recently, Dooley et al, reviewed data from 11 randomized trials with adjunctive corticosteroids for pulmonary TB that included 1676 patients with different TB regimens (isoniazid, streptomycin, para-aminosalicylic acid and pyrazinamide) combined with different steroid regimens (prednisolone, ACTH and methylprednisolone). They concluded that overall, with adequate TB treatments, adjunctive corticosteroids did not affect the rate of culture conversion [32]. However, patients receiving adjunctive corticosteroids had a faster radiological response (except for resolution of cavities). No differences in mortality and chronic restrictive disease were noted amongst patients receiving adjunctive corticosteroids or TB treatments alone. Similarly, Smego et al., analyzed 11 randomized trials of adjunctive corticosteroid therapy in the treatment of pulmonary TB that included 1814 patients treated with different regimens: prednisone and prednisolone (mean dose of 31 mg with a range of 16–60 mg) and/or ACTH (mean daily dose 40 units) [33]. The mean duration of steroid therapy was 85 days (range 30–180 days). They found that adjuvant use of corticosteroids in TB treatments hastened the reduction of fever, improved radiological changes (parenchymal infiltrates and to a lesser extent cavities), enhanced weight gain and reduced the length of hospital stay. It should however be noted that only two of the 11 studies reviewed included regimens that contained rifampin [34, 35]. In one of these studies utilizing rifampin (530 patients in Chennai, India), corticosteroids had no significant effect on bacteriological or radiological response. Therefore, there is a need for new trials evaluating the effect of corticosteroids in modern-era, rifampin-based TB treatments.
Multiple studies have analyzed the role of corticosteroids in TB-HIV co-infection. Mayanja-Kizza et al., conducted a phase 2, randomized, double-blind, placebo-controlled trial in Uganda, to determine the safety and effectiveness of adjuvant prednisolone therapy in TB-HIV co-infected patients with CD4 ≥ 200 [36]. They found that short-term; high-dose prednisolone therapy (2.75 mg/kg/day) enhanced clearance of M. tuberculosis from the sputum, decreased levels of TNF-α at 1-month and caused a transient increase in HIV RNA levels which nonetheless receded back after discontinuation of prednisolone. However, the intervention worsened underlying hypertension, caused fluid retention and induced hyperglycemia. Meintjes et al., also reported the use of adjunctive corticosteroids in the setting of HIV-TB associated immune reconstitution inflammatory syndrome (TB-IRIS) [37]. They demonstrated that a 4-week treatment with adjunctive prednisone (1.5 mg/kg/day for 2 weeks followed by 0.75 mg/kg/day for 2 weeks) while receiving TB treatments, improved TB-IRIS symptoms and radiological findings. Adjunctive corticosteroids also led to a reduction in the duration of hospitalization and the number of outpatient therapeutic procedures. Serum concentrations of C-reactive protein, IL-6, IL-10, IL-12 p40, IP-10 and TNF-α were reduced in prednisone-treated patients at 2 and 4-weeks compared to baseline [38]. Another study has demonstrated similar results, with adjunctive corticosteroids favorably modifying the inflammatory profile of patients with TB-IRIS [39].
TB meningitis
Since immunopathology is a major component of central nervous system (CNS) TB, corticosteroids may improve clinical outcomes by reducing inflammation, vasculitis, necrosis and elevated intracranial pressure [40–42]. Several clinical studies have demonstrated that adjunctive use of corticosteroids decreases both morbidity and mortality in children [41, 43] and adults with CNS TB [44, 45]. A recent study utilizing rifampin and moxifloxacin based regimens with adjunctive corticosteroids to treat TB meningitis, demonstrated significantly lower 6-month mortality in patients receiving a regimen with high-dose versus standard dose rifampin [46]. While the benefits of corticosteroids are clear in HIV-negative patients with CNS TB, there has been some controversy about its use in patients with HIV-coinfection. In the study by Thwaites et al, the treatment effect of adjunctive dexamethasone was homogeneous across HIV subsets, and stratified subgroup analysis showed that though dexamethasone was associated with a reduction in the risk of death, the results were not statistically significant (P = 0.08) [44]. However, the 98 HIV-infected patients recruited in this trial were severely immunocompromised (median CD4 = 66), and none were treated with antiretroviral drugs. These patients had a higher case fatality rate than HIV-negative patients in the same trial, and although it was not possible to determine the cause of death, undiagnosed opportunistic infections were postulated to have been a factor. The authors therefore concluded that: a) that the numbers of HIV-infected patients were too small for them to conclusively determine the effect of adjunctive dexamathasone; b) but that adjunctive dexamethasone is safe and may be of benefit in this group of HIV-infected patients and; c) that these results should not be generalized to populations treated with antiretroviral drugs. It should be noted that most studies reported the use of dexamethasone or prednisolone, and there are few data from controlled trials comparing different regimens [47]. However, a small clinical trial in India demonstrated no statistically significant differences between dexamethasone and methylprednisolone [48]. Finally, recent data also suggest that the use of adjunctive corticosteroids based on host genotype may be even more beneficial. A study by Tobin et al. demonstrated that host genotype (single nucleotide polymorphism in the LTA4H promoter) was is associated with recruitment of inflammatory cell, patient survival and response to adjunctive anti-inflammatory therapies in patients with TB meningitis [49].
In addition to adjunctive use for the treatment of CNS TB, corticosteroids are also useful to treat paradoxical reactions – worsening of disease after initiation of TB treatments – often seen in patients with CNS TB [50, 51].
There have been some concerns about reduced diffusion of antibacterial agents into the subarachnoid space due to corticosteroid therapy. However, it has been demonstrated that equivalent cerebrospinal fluid (CSF) concentrations were achieved with TB drugs (isoniazid, rifampin, pyrazinamide and streptomycin) with and without adjuvant corticosteroid therapy [52]. Based on all of these data, most infectious diseases bodies recommend use of adjunctive corticosteroids for CNS TB [53, 54].
TB pleuritis and pericarditis
Corticosteroids are often used for the treatment of TB pleuritis and pericarditis, but evidence for this remains inconclusive. A prospective, double-blind, randomized study evaluated the role of adjunctive corticosteroids in the treatment of TB pleuritis. All patients received isoniazid, 300 mg/day; rifampin, 450 mg/day; ethambutol, 20 mg/kg/day for more than nine months and were randomly assigned to take either prednisolone 0.75 mg/kg/day orally or placebo for the initial treatment, which was tapered gradually for the next 2–3 months. Adjunctive use of corticosteroids led to earlier resolution of clinical symptoms (fever, chest pain, dyspnea) and hastened the absorption of the pleural fluid. No serious side effects were noted during the treatment in either group [55]. Other studies also suggest that symptoms resolve faster with the use of adjuvant corticosteroid therapy [32]. A trial in HIV-positive patients with TB pleural effusion demonstrated faster resolution of clinical symptoms and radiological findings with adjunctive corticosteroid treatment. However, it showed no significant difference in survival and a significant risk of Kaposi’s sarcoma in the corticosteroid group [56]. A Cochrane review concluded that there are insufficient data to support the use of adjunctive corticosteroids in patients with TB pleuritis [57]. Similarly, Hakim et al., have demonstrated reduced mortality rates and a faster clinical improvement in HIV-positive patients with TB pericarditis receiving prednisolone as adjunctive therapy [58]. While a Cochrane review of four randomized controlled trials demonstrated beneficial effects on mortality with the use of adjunctive corticosteroids in patients with TB pericarditis, these data are inconclusive due to the small size of the studies included in the trials [59]. In summary, overall, the clinical data is supportive of the use of adjunctive corticosteroids for TB pleuritis and pericarditis. However, a careful case-specific assessment must be performed to decide the risk and benefits of corticosteroids in these patients.
TUMOR NECROSIS FACTOR-ALPHA (TNF-α) INHIBITORS
TNF-α is a well studied cytokine and has a central role in the host responses to M. tuberculosis, typically manifested by the symptoms of fever, weight loss and sweats. This cytokine is produced predominantly by monocytes and macrophages, but can also be produced by other cells (mast-cells, endothelial cells, neuronal tissue, T and B lymphocytes, and natural killer cells) [60]. TNF-α promotes migration of immune cell to the site of infection, increases the sterilizing ability of M. tuberculosis-infected macrophages [61] and is critical in the formation and maintenance of the granuloma complex [62, 63]. Although the granuloma is believed to constitute a host defense mechanism, it may benefit the pathogen by presenting a physical barrier against both the immune response and the antimicrobial therapy [64]. Moreover, central necrosis of the granuloma, leading to caseation, is also driven by TNF-α, and may provide a unique microenvironment that promotes bacterial survival and persistence [65]. A recent review details the molecular mechanisms of TNF-α mediated tissue damage. Using a zebrafish model, this group has demonstrated that excessive levels of TNF-α induce mitochondrial reactive oxygen species (ROS) which induce programmed necrosis in infected macrophages, and that this pathway can be targeted with specific agents [66]. While TNF-α remains critical for controlling bacterial replication, data also demonstrate that M. tuberculosis hijack this pathway to facilitate their own survival within the TB granuloma [67]. Tobin et al., have demonstrated that both inadequate or excess TNF-α levels increase host susceptibility to mycobacteria. Moreover, host genotype has also been shown to be a critical determinant of damage due to acute inflammation in patients with TB meningitis [49].
These data suggest that host-directed therapies may also need to be tailored to the host genotype. Thalidomide is known to reduce TNF-α levels, and adjunctive use of thalidomide reduces leukocytosis, brain pathology, and improves survival in a rabbit model of TB meningitis [68]. Nonetheless, the use of thalidomide as a supplemental therapy for TB is limited by well-known side effects including teratogenicity and peripheral neuropathy. Moreover, a double-blind randomized study in children with TB meningitis did not find adjunctive thalidomide to be beneficial [69]. However, other analogs of thalidomide with greater efficacy and fewer side effects are being synthesized and may prove to be useful adjunctive agents [70, 71].
Newer TNF-α inhibitors such as etanercept, adalimumab, and pentoxifylline have also been investigated for use in TB, and have the potential to shorten treatments [65, 72–77]. This is plausible, as TNF-α levels increase shortly after initiation of TB treatments in humans [78], which leads to tissue destruction and creation of a microenvironment that could favor bacterial survival. In addition to limiting inflammation, adjuvant host-directed therapies may also have other beneficial effects. For example, decreased immunopathology and necrosis due to adjunctive TNF-α inhibition [65], may improve the penetration of TB drugs into the granuloma. It is also possible that by disrupting the microenvironment, host-directed therapies selectively promote the clearance of persistent bacteria [65]. This could be highly beneficial, as some of the best TB drugs, such as isoniazid, are effective only against actively dividing bacilli. A recent study in a mouse model that develops necrotic and hypoxic TB lesions [82, 83], demonstrated that addition of etanercept, a soluble TNF receptor fusion molecule (sTNFR), for the initial 6-weeks to standard TB treatment, accelerated bacterial clearance and reduced the rate of relapse [65]. These animal data are also supported by case reports and small clinical series that demonstrate that adjunctive use of TNF-α inhibitors with TB treatment are beneficial [77, 79–81]. Wallis et al, have shown that adjunctive treatment with etanercept reduced time to sputum culture conversion in HIV-positive patients, as well as improved weight gain, performance score, radiographic disease, cavity closure and estimated relapse [70, 76]. However, the major limitation of this study was the small sample size (16 patients) and these data need to be confirmed in a bigger clinical trial. It should also be noted that most of the TNF-α inhibitors reported above are expensive and administered as injectables. However, these data provide proof-of-concept that TNF-α inhibitors could help shorten TB treatments. Moreover, new oral TNF-α inhibitors such as tofacitinib and fostamatinib have been developed to treat chronic inflammatory diseases [84, 85] and may be better suited for resource limited settings.
Phosphodiesterase inhibitors (PDE-I) are another novel group of agents. The concentration of nucleotide 3′, 5′-cyclic adenosine monophosphate (cAMP) - a highly conserved second messenger signaling molecule involved in the regulation of many cellular processes [86] - within a cell is determined by the activity of two types of enzymes: adenylate cyclases, which synthesize cAMP, and phosphodiesterases, which break down the cyclic nucleotide [87]. Increases in intracellular cAMP levels can reduce the production of TNF-a but also other innate immune mediators in macrophages. Since PDE-I lead to an increase in cAMP levels, they inhibit inflammation leading to tissue protective effects. Interestingly, it has also been demonstrated that adenylate cyclases of M. tuberculosis can directly produce a cAMP burst within macrophages leading to CREB phosphorylation and TNF-a secretion that promote the survival of bacteria [67]. Hence, the host cAMP-mediated signaling pathways represent a promising target against M. tuberculosis. Indeed, recent data from several investigators have shown the benefit of PDE-I as adjunctive therapy during TB treatment in animal models [72–75]. Several PDE-I are orally bioavailable and many are FDA-approved (cilostazol, theophylline, roflumilast, tadalafil and sildenafil).
In summary, TNF-a inhibitors should be evaluated as host-directed adjunct therapies that could shorten TB treatments. However, since TNF-a inhibitors can reactivate latent TB infection [88–90], additional studies need to be performed to evaluate safety.
OTHER HOST-DIRECTED THERAPIES
Matrix metalloproteinases (MMPs) are endopeptidases responsible for degrading components of the extracellular matrix (ECM), such as collagen and proteoglycans. They play an important role in leukocyte migration, granuloma formation, tissue remodeling and in the development of cavitation [91–94]. Like TNF-a inhibitors, MMP inhibitors could reduce necrosis and prevent the creating of host-microenvironments favorable for bacterial survival. In addition, since cavitation is essential for the transmission of M. tuberculosis, MMP inhibitors could also be useful to reduce transmission of M. tuberculosis for diseased patients. Walker et al. have demonstrated that doxycycline inhibits MMP activity in vitro, and also decreases mycobacterial burden in guinea pigs, suggesting that adjunctive doxycycline treatments could be beneficial [95].
Decades before the availability of antibiotics, Niels Finsen proposed that sun exposure could be used to treat lupus vulgaris – a cutaneous form of TB. While the mechanism for this therapy was not know, Finsen was awarded the Nobel Prize in 1903 for this discovery [96]. However, recent studies are shedding light on how sunlight may help in fighting infections and stimulation or activation of vitamin D may be an important mechanism in fighting TB. Recently, several clinical studies have associated vitamin D deficiency with increased risk of TB [97–102]. One study has also demonstrated that TB-HIV co-infected patients with severe vitamin D deficiency had high levels of pro-inflammatory cytokines [39]. Moreover, in vitro data suggests that 1,25-dihydroxyvitamin D3 (active form of vitamin D) induces and modulates innate and adaptive antimicrobial responses and suppress proinflammatory cytokine responses [103–106]. Vitamin D3 acts through the VDR receptor – a transcription factor and part of the superfamily of steroid/thyroid hormone receptors – and up-regulates protective innate host responses [107, 108]. Vitamin D-mediated activity against M. tuberculosis is also dependent on the induction of antimicrobial peptides (AMPs) like LL-37 (also known as cathelicidin) [109, 110] which are produced by neutrophils, lung epithelial cells and monocytes [111]. While most clinical studies support the association of vitamin D with TB, adjunctive use of vitamin D with TB treatments is controvertial. In one study, HIV-negative TB patients treated with adjunctive vitamin D had a higher rate of sputum conversion and radiological improvement compared with TB treatment alone [112]. Another recent randomized longitudinal study demonstrated that vitamin D supplementation accelerated sputum smear conversion and suppressed proinflammatory cytokines [113]. However, other studies show no overall differences in sputum conversion time [114] or clinical outcomes [115]. It is possible that host genotype may play a role in responses to vitamin D supplementation. Martineau et al., demonstrated that vitamin D supplementation significantly hasten sputum culture conversion in participants with the tt genotype of the TaqI vitamin D receptor [114], supporting this hypothesis. Conversely, a more recent study by the same group demonstrated that vitamin D supplementation also modulated immune responses in patients with the Tt and TT genotypes [113]. Additional clinical studies need to be performed to determine the role of vitamin D as an adjunctive treatment for TB.
Therapies targeting several important host-pathways could also be potentially useful as adjunctive therapies against TB. Programmed Death-1 (PD-1) is a co-inhibitory receptor that is known to negatively regulate adaptive immune responses and crucial for the development of protective host responses against M. tuberculosis [116]. PD-1 deficient mice are highly susceptible to M. tuberculosis and mount high levels of proinflammatory cytokines (TNF-a, IL-1, IL-6, IL-17) in response to M. tuberculosis infection [117]. It should be noted that as opposed to an anticipated immunostimulatory effect, the mouse studies demonstrated huge detrimental effect in the PD-1 knockout mice. However, the situation here is analogous to TNF-a (where knockout mice also do poorly with M. tuberculosis infection), but TNF-a inhibition combined with TB treatments during the early phase is paradoxically beneficial. Therefore, similar to TNF-a inhibition, modulation of the PD-1 during early stages of TB treatments may help in reducing the exaggerated proinflammatory responses and accelerate bacterial clearance. BMS-936558, an injectable antibody that specifically blocks PD-1 is currently under clinical investigation against cancers [118] and could potentially be evaluated for TB treatments. Fibrosis constitutes an important part of TB pathogenesis and may limit the penetration of drugs and immune cells into the granuloma. In addition to increasing penetration into the diseases tissues, anti-fibrotic agents can also reduce inflammation [119, 120] which may further help with TB treatments. Finally, while not host-directed, Gold et al. have demonstrated that oxyphenbutazone, a nonsteroidal anti-inflammatory drug, gets hydroxylated under acidic conditions and in the presence of reactive nitrogen intermediates. The hydroxylated compound has activity against both actively dividing and “nonreplicating” M. tuberculosis and was also synergistic with several TB drugs [121].
EFFLUX PUMP INHIBITORS
Bacterial efflux pump inhibitors are novel “non-antimicrobial” agents that could shorten TB treatments and also allow effective treatment of drug resistant organisms. Multidrug efflux pumps are broadly recognized as major mediators of drug-resistance to many classes of antimicrobials and anti-cancer agents [122, 123]. Efflux occurs due to the activity of membrane transport proteins known as multidrug efflux systems (MES) [124]. Bacterial efflux pumps are of special interest as they mediate antibiotic tolerance in mycobacteria [7], which can be overcome with efflux pump inhibitors [125, 126]. Since antibiotic tolerance is a major factor in mycobacterial persistence, efflux pump inhibitors have the potential to significantly shorten TB treatments. Moreover, it has also been recently demonstrated that efflux pump induction is a general first step in the evolution of mycobacterial drug resistance [127] and therefore efflux pump inhibitors could also help in prevent in the development of multidrug resistant M. tuberculosis. Verapamil is a well known calcium-channel blockers and has been extensively used as an anti-hypertensive in the clinic. Studies in the mouse model of TB demonstrate that verapamil in combination with first-line drugs significantly reduces pulmonary bacterial burden [128].
Phenothiazines are another class of drugs that inhibit bacterial efflux pumps, and have ex vivo activity against both drug-susceptible and drug-resistant strains of M. tuberculosis [129, 130]. However, phenothaizines may have multiple mechanisms of action against M. tuberculosis. Phenothiazine analogs have been shown to directly inhibit NADH:menaquinone oxidoreductase activity of M. tuberculosis [131]. Furthermore, they may also enhance intracellular killing of M. tuberculosis inside the phagolysosome, by inhibiting host-cell calcium and potassium channels [132]. Indeed, thioridazine has been shown to be effective as salvage therapy in patients with multidrug resistant TB [133, 134]. Other agents include reserpine (ATP-dependent efflux pump inhibitor) that has been shown to enhance the efficacy of ciprofloxacin [135] and linezolid [136] against M. tuberculosis strains. Piperine, a component of black pepper, has potential immunomodulatory activity [137] and is also an efflux pump inhibitor of Rv1258c of M. tuberculosis [138]. The efficacy of rifampin was increased by 4- to 8-fold in the presence of piperine both in sensitive and rifampin resistant M. tuberculosis.
CONCLUSION
In summary, while significant advances have been made in developing shorter TB drug regimens, there is growing evidence that adjunctive use of host-directed and “non-antimicrobial” pathogen-directed therapies could further accelerate TB treatments. While several promising adjunctive therapies are being developed and evaluated in pre-clinical settings, well controlled clinical studies with adequate power are needed to evaluate promising therapeutics with a focus on those that are already approved for clinical use.
Acknowledgments
This study was funded by the NIH Director’s New Innovator Award OD006492 (S.K.J.). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Footnotes
Send Orders for Reprints to reprints@benthamscience.net
CONFLICT OF INTEREST
The authors do not have a commercial or other association that might pose a conflict of interest.
References
- 1.WHO. [Accessed: November 28, 2013];Global tuberculosis report. 2013 Available from: http://apps.who.int/iris/bitstream/10665/91355/1/9789241564656_eng.pdf.
- 2.Dye C, Scheele S, Dolin P, Pathania V, Raviglione MC. Consensus statement. Global burden of tuberculosis: estimated incidence, prevalence, and mortality by country. WHO Global Surveillance and Monitoring Project. JAMA. 1999;282(7):677–86. doi: 10.1001/jama.282.7.677. [DOI] [PubMed] [Google Scholar]
- 3.Stead WW. Pathogenesis of a first episode of chronic pulmonary tuberculosis in man: recrudescence of residuals of the primary infection or exogenous reinfection? Am Rev Respir Dis. 1967;95(5):729–45. doi: 10.1164/arrd.1967.95.5.729. [DOI] [PubMed] [Google Scholar]
- 4.Podlekareva DN, Panteleev AM, Grint D, et al. Short and long term mortality and causes of death in HIV/TB patients in Europe. Eur Respir J. 2013 doi: 10.1183/09031936.00138712. epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 5.Grosset JH, Singer TG, Bishai WR. New drugs for the treatment of tuberculosis: hope and reality. Int J Tuberc Lung Dis. 2012;16(8):1005–14. doi: 10.5588/ijtld.12.0277. [DOI] [PubMed] [Google Scholar]
- 6.Churchyard G, Onyebujoh P, Zumla A, et al. World Health Organization. WHO-Special Programme for Research and Training in Tropical Diseases. 2007. [Google Scholar]
- 7.Szumowski JD, Adams KN, Edelstein PH, Ramakrishnan L. Antimicrobial Efflux Pumps and Mycobacterium Tuberculosis Drug Tolerance: Evolutionary Considerations. Curr Top Microbiol Immunol. 2012 doi: 10.1007/82_2012_300. epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gomez JE, McKinney JD. M. tuberculosis persistence, latency, and drug tolerance. Tuberculosis (Edinb) 2004;84(1–2):29–44. doi: 10.1016/j.tube.2003.08.003. [DOI] [PubMed] [Google Scholar]
- 9.Vandiviere HM, Loring WE, Melvin I, Willis S. The treated pulmonary lesion and its tubercle bacillus. II. The death and resurrection. Am J Med Sci. 1956;232(1):30–7. doi: 10.1097/00000441-195607000-00006. passim. [DOI] [PubMed] [Google Scholar]
- 10.Haapanen JH, Kass I, Gensini G, Middlebrook G. Studies on the gaseous content of tuberculous cavities. Am Rev Respir Dis. 1959;80(1 Part 1):1–5. doi: 10.1164/arrd.1959.80.1P1.1. [DOI] [PubMed] [Google Scholar]
- 11.Lillebaek T, Dirksen A, Vynnycky E, et al. Stability of DNA patterns and evidence of Mycobacterium tuberculosis reactivation occurring decades after the initial infection. J Infect Dis. 2003;188(7):1032–9. doi: 10.1086/378240. [DOI] [PubMed] [Google Scholar]
- 12.de Gans J, van de Beek D. Dexamethasone in adults with bacterial meningitis. N Engl J Med. 2002;347(20):1549–56. doi: 10.1056/NEJMoa021334. [DOI] [PubMed] [Google Scholar]
- 13.van de Beek D, de Gans J, McIntyre P, Prasad K. Steroids in adults with acute bacterial meningitis: a systematic review. Lancet Infect Dis. 2004;4(3):139–43. doi: 10.1016/S1473-3099(04)00937-5. [DOI] [PubMed] [Google Scholar]
- 14.McIntyre PB, Berkey CS, King SM, et al. Dexamethasone as adjunctive therapy in bacterial meningitis. A meta-analysis of randomized clinical trials since 1988. JAMA. 1997;278(11):925–31. doi: 10.1001/jama.278.11.925. [DOI] [PubMed] [Google Scholar]
- 15.Gilroy SA, Bennett NJ. Pneumocystis pneumonia. Semin Respir Crit Care Med. 2011;32(6):775–82. doi: 10.1055/s-0031-1295725. [DOI] [PubMed] [Google Scholar]
- 16.Spagnolo P, Fabbri LM, Bush A. Long-term macrolide treatment for chronic respiratory disease. Eur Respir J. 2012;42(1):239–251. doi: 10.1183/09031936.00136712. [DOI] [PubMed] [Google Scholar]
- 17.Annane D. Corticosteroids for severe sepsis: an evidence-based guide for physicians. Ann Intensive Care. 2011;1(1):7. doi: 10.1186/2110-5820-1-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.De Bosscher K, Haegeman G, Elewaut D. Targeting inflammation using selective glucocorticoid receptor modulators. Curr Opin Pharmacol. 2010;10(4):497–504. doi: 10.1016/j.coph.2010.04.007. [DOI] [PubMed] [Google Scholar]
- 19.Meagher LC, Cousin JM, Seckl JR, Haslett C. Opposing effects of glucocorticoids on the rate of apoptosis in neutrophilic and eosinophilic granulocytes. J Immunol. 1996;156(11):4422–8. [PubMed] [Google Scholar]
- 20.Sternberg EM. Neural regulation of innate immunity: a coordinated nonspecific host response to pathogens. Nat Rev Immunol. 2006;6(4):318–28. doi: 10.1038/nri1810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Stahn C, Buttgereit F. Genomic and nongenomic effects of glucocorticoids. Nat Clin Pract Rheumatol. 2008;4(10):525–33. doi: 10.1038/ncprheum0898. [DOI] [PubMed] [Google Scholar]
- 22.Capon AW. ACTH, cortisone and tuberculosis. Can Med Assoc J. 1952;67(1):46–8. [PMC free article] [PubMed] [Google Scholar]
- 23.Reale M, Garaventa A, Paoli G, Costa G. Prednisone and prednisolone, anti-reactive elective drugs in pulmonary tuberculosis. Minerva Med. 1956;47(45):1764–73. [PubMed] [Google Scholar]
- 24.Heyman A, Levitzky E. Steroids in the treatment of childhood tuberculosis. J Med Soc N J. 1959;56(2):62–7. [PubMed] [Google Scholar]
- 25.Lavers KW, Roberts JC. The use of prednisone in primary tuberculosis in children. Tubercle. 1959;40:173–6. doi: 10.1016/s0041-3879(59)80036-2. [DOI] [PubMed] [Google Scholar]
- 26.Handley AE. The use of corticosteroids in combination with isonicotinic acid hydrazide in the treatment of advanced bilateral progressive cavitary pulmonary tuberculosis; second report with discussion. S Afr Med J. 1956;30(52):1250–1. [PubMed] [Google Scholar]
- 27.Horne NW. Prednisolone in treatment of pulmonary tuberculosis: a controlled trial. Final report to the Research Committee of the Tuberculosis Society of Scotland. Br Med J. 1960;2(5215):1751–6. doi: 10.1136/bmj.2.5215.1751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Morris D. Trial of Corticotrophin and Prednisone with Chemotherapy in Pulmonary Tuberculosis: A Two-Year Radiographic Follow-Up. Tubercle. 1963;44:484–6. doi: 10.1016/s0041-3879(63)80091-4. [DOI] [PubMed] [Google Scholar]
- 29.El-Dewi T, Bonstein H, Favez G. Comparative effects of prednisone and dexamethasone (associated with bacteriostatics) in the clinical course and plasma parameters in pulmonary tuberculosis patients. Schweiz Med Wochenschr. 1962;92:1398–403. [PubMed] [Google Scholar]
- 30.Bornstein PK, Nair KG, Epstein IG. Reduction of chronicity in pulmonary tuberculosis by prednisone. Am Rev Respir Dis. 1960;81:421–2. doi: 10.1164/arrd.1960.81.3.421. [DOI] [PubMed] [Google Scholar]
- 31.Chatterjee PK, Goswami PN, Chatterjee SC, Das SK. Prednisone in small doses in the treatment of tuberculosis. J Indian Med Assoc. 1958;31(6):233–8. [PubMed] [Google Scholar]
- 32.Dooley DP, Carpenter JL, Rademacher S. Adjunctive corticosteroid therapy for tuberculosis: a critical reappraisal of the literature. Clin Infect Dis. 1997;25(4):872–87. doi: 10.1086/515543. [DOI] [PubMed] [Google Scholar]
- 33.Smego RA, Ahmed N. A systematic review of the adjunctive use of systemic corticosteroids for pulmonary tuberculosis. Int J Tuberc Lung Dis. 2003;7(3):208–13. [PubMed] [Google Scholar]
- 34.Tuberculosis Research Centre. Study of chemotherapy regimens of 5 and 7 months’ duration and the role of corticosteroids in the treatment of sputum-positive patients with pulmonary tuberculosis in South India. Tubercle. 1983;64(2):73–91. doi: 10.1016/0041-3879(83)90032-6. [DOI] [PubMed] [Google Scholar]
- 35.Bilaceroglu S, Perim K, Buyuksirin M, Celikten E. Prednisolone: a beneficial and safe adjunct to antituberculosis treatment? A randomized controlled trial. Int J Tuberc Lung Dis. 1999;3(1):47–54. [PubMed] [Google Scholar]
- 36.Mayanja-Kizza H, Jones-Lopez E, Okwera A, et al. Immunoadjuvant prednisolone therapy for HIV-associated tuberculosis: a phase 2 clinical trial in Uganda. J Infect Dis. 2005;191(6):856–65. doi: 10.1086/427995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Meintjes G, Wilkinson RJ, Morroni C, et al. Randomized placebo-controlled trial of prednisone for paradoxical tuberculosis-associated immune reconstitution inflammatory syndrome. AIDS. 2010;24(15):2381–90. doi: 10.1097/QAD.0b013e32833dfc68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Meintjes G, Skolimowska KH, Wilkinson KA, et al. Corticosteroid-modulated immune activation in the tuberculosis immune reconstitution inflammatory syndrome. Am J Respir Crit Care Med. 2012;186(4):369–77. doi: 10.1164/rccm.201201-0094OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Conesa-Botella A, Meintjes G, Coussens AK, et al. Corticosteroid therapy, vitamin D status, and inflammatory cytokine profile in the HIV-tuberculosis immune reconstitution inflammatory syndrome. Clin Infect Dis. 2012;55(7):1004–11. doi: 10.1093/cid/cis577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Escobar JA, Belsey MA, Duenas A, Medina P. Mortality from tuberculous meningitis reduced by steroid therapy. Pediatrics. 1975;56(6):1050–5. [PubMed] [Google Scholar]
- 41.Schoeman JF, Van Zyl LE, Laubscher JA, Donald PR. Effect of corticosteroids on intracranial pressure, computed tomographic findings, and clinical outcome in young children with tuberculous meningitis. Pediatrics. 1997;99(2):226–31. doi: 10.1542/peds.99.2.226. [DOI] [PubMed] [Google Scholar]
- 42.Be NA, Kim KS, Bishai WR, Jain SK. Pathogenesis of central nervous system tuberculosis. Curr Mol Med. 2009;9(2):94–9. doi: 10.2174/156652409787581655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Girgis NI, Farid Z, Kilpatrick ME, Sultan Y, Mikhail IA. Dexamethasone adjunctive treatment for tuberculous meningitis. Pediatr Infect Dis J. 1991;10(3):179–83. doi: 10.1097/00006454-199103000-00002. [DOI] [PubMed] [Google Scholar]
- 44.Thwaites GE, Nguyen DB, Nguyen HD, et al. Dexamethasone for the treatment of tuberculous meningitis in adolescents and adults. N Engl J Med. 2004;351(17):1741–51. doi: 10.1056/NEJMoa040573. [DOI] [PubMed] [Google Scholar]
- 45.Torok ME, Nguyen DB, Tran TH, et al. Dexamethasone and long-term outcome of tuberculous meningitis in Vietnamese adults and adolescents. PLoS One. 2011;6(12):e27821. doi: 10.1371/journal.pone.0027821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ruslami R, Ganiem AR, Dian S, et al. Intensified regimen containing rifampicin and moxifloxacin for tuberculous meningitis: an open-label, randomised controlled phase 2 trial. Lancet Infect Dis. 2013;13(1):27–35. doi: 10.1016/S1473-3099(12)70264-5. [DOI] [PubMed] [Google Scholar]
- 47.Prasad K, Singh MB. Corticosteroids for managing tuberculous meningitis. Cochrane Database Syst Rev. 2008;(1):CD002244. doi: 10.1002/14651858.CD002244.pub3. [DOI] [PubMed] [Google Scholar]
- 48.Malhotra HS, Garg RK, Singh MK, Agarwal A, Verma R. Corticosteroids (dexamethasone versus intravenous methylprednisolone) in patients with tuberculous meningitis. Ann Trop Med Parasitol. 2009;103(7):625–34. doi: 10.1179/000349809X12502035776315. [DOI] [PubMed] [Google Scholar]
- 49.Tobin DM, Roca FJ, Oh SF, et al. Host genotype-specific therapies can optimize the inflammatory response to mycobacterial infections. Cell. 2012;148(3):434–46. doi: 10.1016/j.cell.2011.12.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Afghani B, Lieberman JM. Paradoxical enlargement or development of intracranial tuberculomas during therapy: case report and review. Clin Infect Dis. 1994;19(6):1092–9. doi: 10.1093/clinids/19.6.1092. [DOI] [PubMed] [Google Scholar]
- 51.Jain SK, Kwon P, Moss WJ. Management and outcomes of intracranial tuberculomas developing during antituberculous therapy: case report and review. Clin Pediatr (Phila) 2005;44(5):443–50. doi: 10.1177/000992280504400510. [DOI] [PubMed] [Google Scholar]
- 52.Kaojarern S, Supmonchai K, Phuapradit P, Mokkhavesa C, Krittiyanunt S. Effect of steroids on cerebrospinal fluid penetration of antituberculous drugs in tuberculous meningitis. Clin Pharmacol Ther. 1991;49(1):6–12. doi: 10.1038/clpt.1991.2. [DOI] [PubMed] [Google Scholar]
- 53.Thwaites G, Fisher M, Hemingway C, et al. British Infection Society guidelines for the diagnosis and treatment of tuberculosis of the central nervous system in adults and children. J Infect. 2009;59(3):167–87. doi: 10.1016/j.jinf.2009.06.011. [DOI] [PubMed] [Google Scholar]
- 54.Blumberg HM, Burman WJ, Chaisson RE, et al. American Thoracic Society/Centers for Disease Control and Prevention/Infectious Diseases Society of America: treatment of tuberculosis. Am J Respir Crit Care Med. 2003;167(4):603–62. doi: 10.1164/rccm.167.4.603. [DOI] [PubMed] [Google Scholar]
- 55.Lee CH, Wang WJ, Lan RS, Tsai YH, Chiang YC. Corticosteroids in the treatment of tuberculous pleurisy. A double-blind, placebo-controlled, randomized study. Chest. 1988;94(6):1256–9. doi: 10.1378/chest.94.6.1256. [DOI] [PubMed] [Google Scholar]
- 56.Elliott AM, Luzze H, Quigley MA, et al. A randomized, double-blind, placebo-controlled trial of the use of prednisolone as an adjunct to treatment in HIV-1-associated pleural tuberculosis. J Infect Dis. 2004;190(5):869–78. doi: 10.1086/422257. [DOI] [PubMed] [Google Scholar]
- 57.Engel ME, Matchaba PT, Volmink J. Corticosteroids for tuberculous pleurisy. Cochrane Database Syst Rev. 2007;(4):CD001876. doi: 10.1002/14651858.CD001876.pub2. [DOI] [PubMed] [Google Scholar]
- 58.Hakim JG, Ternouth I, Mushangi E, et al. Double blind randomised placebo controlled trial of adjunctive prednisolone in the treatment of effusive tuberculous pericarditis in HIV seropositive patients. Heart. 2000;84(2):183–8. doi: 10.1136/heart.84.2.183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Mayosi BM, Volmink JA, Commerford PJ. Interventions for treating tuberculous pericarditis. Cochrane Database Syst Rev. 2000;(2):CD000526. doi: 10.1002/14651858.CD000526. [DOI] [PubMed] [Google Scholar]
- 60.Mootoo A, Stylianou E, Arias MA, Reljic R. TNF-alpha in tuberculosis: a cytokine with a split personality. Inflamm Allergy Drug Targets. 2009;8(1):53–62. doi: 10.2174/187152809787582543. [DOI] [PubMed] [Google Scholar]
- 61.Keane J, Balcewicz-Sablinska MK, Remold HG, et al. Infection by Mycobacterium tuberculosis promotes human alveolar macrophage apoptosis. Infect Immun. 1997;65(1):298–304. doi: 10.1128/iai.65.1.298-304.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Mohan VP, Scanga CA, Yu K, et al. Effects of tumor necrosis factor alpha on host immune response in chronic persistent tuberculosis: possible role for limiting pathology. Infect Immun. 2001;69(3):1847–55. doi: 10.1128/IAI.69.3.1847-1855.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Flynn JL, Chan J. What’s good for the host is good for the bug. Trends Microbiol. 2005;13(3):98–102. doi: 10.1016/j.tim.2005.01.005. [DOI] [PubMed] [Google Scholar]
- 64.Ramakrishnan L. Revisiting the role of the granuloma in tuberculosis. Nat Rev Immunol. 2012;12(5):352–66. doi: 10.1038/nri3211. [DOI] [PubMed] [Google Scholar]
- 65.Skerry C, Harper J, Klunk M, Bishai WR, Jain SK. Adjunctive TNF inhibition with standard treatment enhances bacterial clearance in a murine model of necrotic TB granulomas. PLoS One. 2012;7(6):e39680. doi: 10.1371/journal.pone.0039680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Roca FJ, Ramakrishnan L. TNF dually mediates resistance and susceptibility to mycobacteria via mitochondrial reactive oxygen species. Cell. 2013;153(3):521–34. doi: 10.1016/j.cell.2013.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Agarwal N, Lamichhane G, Gupta R, Nolan S, Bishai WR. Cyclic AMP intoxication of macrophages by a Mycobacterium tuberculosis adenylate cyclase. Nature. 2009;460(7251):98–102. doi: 10.1038/nature08123. [DOI] [PubMed] [Google Scholar]
- 68.Tsenova L, Sokol K, Freedman VH, Kaplan G. A combination of thalidomide plus antibiotics protects rabbits from mycobacterial meningitis-associated death. J Infect Dis. 1998;177(6):1563–72. doi: 10.1086/515327. [DOI] [PubMed] [Google Scholar]
- 69.Schoeman JF, Springer P, van Rensburg AJ, et al. Adjunctive thalidomide therapy for childhood tuberculous meningitis: results of a randomized study. J Child Neurol. 2004;19(4):250–7. doi: 10.1177/088307380401900402. [DOI] [PubMed] [Google Scholar]
- 70.Guo S, Zhao J. Immunotherapy for tuberculosis: what’s the better choice? Front Biosci. 2012;17:2684–90. doi: 10.2741/4079. [DOI] [PubMed] [Google Scholar]
- 71.Tsenova L, Mangaliso B, Muller G, et al. Use of IMiD3, a thalidomide analog, as an adjunct to therapy for experimental tuberculous meningitis. Antimicrob Agents Chemother. 2002;46(6):1887–95. doi: 10.1128/AAC.46.6.1887-1895.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Koo MS, Manca C, Yang G, et al. Phosphodiesterase 4 inhibition reduces innate immunity and improves isoniazid clearance of Mycobacterium tuberculosis in the lungs of infected mice. PLoS One. 2011;6(2):e17091. doi: 10.1371/journal.pone.0017091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Maiga M, Agarwal N, Ammerman NC, et al. Successful Shortening of Tuberculosis Treatment Using Adjuvant Host-Directed Therapy with FDA-Approved Phosphodiesterase Inhibitors in the Mouse Model. PLoS One. 2012;7(2):e30749. doi: 10.1371/journal.pone.0030749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Subbian S, Tsenova L, O’Brien P, et al. Phosphodiesterase-4 inhibition combined with isoniazid treatment of rabbits with pulmonary tuberculosis reduces macrophage activation and lung pathology. Am J Pathol. 2011;179(1):289–301. doi: 10.1016/j.ajpath.2011.03.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Subbian S, Tsenova L, O’Brien P, et al. Phosphodiesterase-4 inhibition alters gene expression and improves isoniazid-mediated clearance of Mycobacterium tuberculosis in rabbit lungs. PLoS Pathog. 2011;7(9):e1002262. doi: 10.1371/journal.ppat.1002262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Wallis RS, Kyambadde P, Johnson JL, et al. A study of the safety, immunology, virology, and microbiology of adjunctive etanercept in HIV-1-associated tuberculosis. AIDS. 2004;18(2):257–64. doi: 10.1097/00002030-200401230-00015. [DOI] [PubMed] [Google Scholar]
- 77.Wallis RS, van Vuuren C, Potgieter S. Adalimumab treatment of life-threatening tuberculosis. Clin Infect Dis. 2009;48(10):1429–32. doi: 10.1086/598504. [DOI] [PubMed] [Google Scholar]
- 78.Bekker LG, Maartens G, Steyn L, Kaplan G. Selective increase in plasma tumor necrosis factor-alpha and concomitant clinical deterioration after initiating therapy in patients with severe tuberculosis. J Infect Dis. 1998;178(2):580–4. doi: 10.1086/517479. [DOI] [PubMed] [Google Scholar]
- 79.Wallis RS. Reconsidering adjuvant immunotherapy for tuberculosis. Clin Infect Dis. 2005;41(2):201–8. doi: 10.1086/430914. [DOI] [PubMed] [Google Scholar]
- 80.Blackmore TK, Manning L, Taylor WJ, Wallis RS. Therapeutic use of infliximab in tuberculosis to control severe paradoxical reaction of the brain and lymph nodes. Clin Infect Dis. 2008;47(10):e83–5. doi: 10.1086/592695. [DOI] [PubMed] [Google Scholar]
- 81.Wallis RS, Kyambadde P, Johnson JL, et al. A study of the safety, immunology, virology, and microbiology of adjunctive etanercept in HIV-1-associated tuberculosis. AIDS. 2004;18(2):257–264. doi: 10.1097/00002030-200401230-00015. [DOI] [PubMed] [Google Scholar]
- 82.Pan H, Yan BS, Rojas M, et al. Ipr1 gene mediates innate immunity to tuberculosis. Nature. 2005;434(7034):767–72. doi: 10.1038/nature03419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Harper J, Skerry C, Davis SL, et al. Mouse model of necrotic tuberculosis granulomas develops hypoxic lesions. J Infect Dis. 2012;205(4):595–602. doi: 10.1093/infdis/jir786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Kremer JM, Bloom BJ, Breedveld FC, et al. The safety and efficacy of a JAK inhibitor in patients with active rheumatoid arthritis: Results of a double-blind, placebo-controlled phase IIa trial of three dosage levels of CP-690,550 versus placebo. Arthritis Rheum. 2009;60(7):1895–905. doi: 10.1002/art.24567. [DOI] [PubMed] [Google Scholar]
- 85.Sweeny DJ, Li W, Clough J, et al. Metabolism of fostamatinib, the oral methylene phosphate prodrug of the spleen tyrosine kinase inhibitor R406 in humans: contribution of hepatic and gut bacterial processes to the overall biotransformation. Drug Metab Dispos. 2010;38(7):1166–76. doi: 10.1124/dmd.110.032151. [DOI] [PubMed] [Google Scholar]
- 86.Johannessen M, Moens U. Multisite phosphorylation of the cAMP response element-binding protein (CREB) by a diversity of protein kinases. Front Biosci. 2007;12:1814–32. doi: 10.2741/2190. [DOI] [PubMed] [Google Scholar]
- 87.Omori K, Kotera J. Overview of PDEs and their regulation. Circ Res. 2007;100(3):309–27. doi: 10.1161/01.RES.0000256354.95791.f1. [DOI] [PubMed] [Google Scholar]
- 88.Keane J, Gershon S, Wise RP, et al. Tuberculosis associated with infliximab, a tumor necrosis factor alpha-neutralizing agent. N Engl J Med. 2001;345(15):1098–104. doi: 10.1056/NEJMoa011110. [DOI] [PubMed] [Google Scholar]
- 89.Centers for Disease Control and Prevention (CDC). . Tuberculosis associated with blocking agents against tumor necrosis factor-alpha--California, 2002–2003. MMWR Morb Mortal Wkly Rep. 2004;53(30):683–6. [PubMed] [Google Scholar]
- 90.Wallis RS, Broder MS, Wong JY, Hanson ME, Beenhouwer DO. Granulomatous infectious diseases associated with tumor necrosis factor antagonists. Clin Infect Dis. 2004;38(9):1261–5. doi: 10.1086/383317. [DOI] [PubMed] [Google Scholar]
- 91.Izzo AA, Izzo LS, Kasimos J, Majka S. A matrix metalloproteinase inhibitor promotes granuloma formation during the early phase of Mycobacterium tuberculosis pulmonary infection. Tuberculosis (Edinb) 2004;84(6):387–96. doi: 10.1016/j.tube.2004.07.001. [DOI] [PubMed] [Google Scholar]
- 92.Taylor JL, Hattle JM, Dreitz SA, et al. Role for matrix metalloproteinase 9 in granuloma formation during pulmonary Mycobacterium tuberculosis infection. Infect Immun. 2006;74(11):6135–44. doi: 10.1128/IAI.02048-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Elkington PT, Friedland JS. Matrix metalloproteinases in destructive pulmonary pathology. Thorax. 2006;61(3):259–66. doi: 10.1136/thx.2005.051979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Green JA, Elkington PT, Pennington CJ, et al. Mycobacterium tuberculosis upregulates microglial matrix metalloproteinase-1 and -3 expression and secretion via NF-kappaB- and Activator Protein-1-dependent monocyte networks. J Immunol. 2010;184(11):6492–503. doi: 10.4049/jimmunol.0903811. [DOI] [PubMed] [Google Scholar]
- 95.Walker NF, Clark SO, Oni T, et al. Doxycycline and HIV infection suppress tuberculosis-induced matrix metalloproteinases. Am J Respir Crit Care Med. 2012;185(9):989–97. doi: 10.1164/rccm.201110-1769OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Zasloff M. Fighting infections with vitamin D. Nat Med. 2006;12(4):388–90. doi: 10.1038/nm0406-388. [DOI] [PubMed] [Google Scholar]
- 97.Chun RF, Adams JS, Hewison M. Immunomodulation by vitamin D: implications for TB. Expert Rev Clin Pharmacol. 2011;4(5):583–91. doi: 10.1586/ecp.11.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Gibney KB, MacGregor L, Leder K, et al. Vitamin D deficiency is associated with tuberculosis and latent tuberculosis infection in immigrants from sub-Saharan Africa. Clin Infect Dis. 2008;46(3):443–6. doi: 10.1086/525268. [DOI] [PubMed] [Google Scholar]
- 99.Williams B, Williams AJ, Anderson ST. Vitamin D deficiency and insufficiency in children with tuberculosis. Pediatr Infect Dis J. 2008;27(10):941–2. doi: 10.1097/INF.0b013e31817525df. [DOI] [PubMed] [Google Scholar]
- 100.Wejse C, Olesen R, Rabna P, et al. Serum 25-hydroxyvitamin D in a West African population of tuberculosis patients and unmatched healthy controls. Am J Clin Nutr. 2007;86(5):1376–83. doi: 10.1093/ajcn/86.5.1376. [DOI] [PubMed] [Google Scholar]
- 101.Ustianowski A, Shaffer R, Collin S, Wilkinson RJ, Davidson RN. Prevalence and associations of vitamin D deficiency in foreign-born persons with tuberculosis in London. J Infect. 2005;50(5):432–7. doi: 10.1016/j.jinf.2004.07.006. [DOI] [PubMed] [Google Scholar]
- 102.Sasidharan PK, Rajeev E, Vijayakumari V. Tuberculosis and vitamin D deficiency. J Assoc Physicians India. 2002;50:554–8. [PubMed] [Google Scholar]
- 103.Martineau AR, Wilkinson KA, Newton SM, et al. IFN-gamma- and TNF-independent vitamin D-inducible human suppression of mycobacteria: the role of cathelicidin LL-37. J Immunol. 2007;178(11):7190–8. doi: 10.4049/jimmunol.178.11.7190. [DOI] [PubMed] [Google Scholar]
- 104.Baeke F, Takiishi T, Korf H, Gysemans C, Mathieu C. Vitamin D: modulator of the immune system. Curr Opin Pharmacol. 2010;10(4):482–96. doi: 10.1016/j.coph.2010.04.001. [DOI] [PubMed] [Google Scholar]
- 105.Hewison M. Antibacterial effects of vitamin D. Nat Rev Endocrinol. 2011;7(6):337–45. doi: 10.1038/nrendo.2010.226. [DOI] [PubMed] [Google Scholar]
- 106.Chen S, Sims GP, Chen XX, Gu YY, Lipsky PE. Modulatory effects of 1,25-dihydroxyvitamin D3 on human B cell differentiation. J Immunol. 2007;179(3):1634–47. doi: 10.4049/jimmunol.179.3.1634. [DOI] [PubMed] [Google Scholar]
- 107.Rockett KA, Brookes R, Udalova I, et al. 1,25-Dihydroxyvitamin D3 induces nitric oxide synthase and suppresses growth of Mycobacterium tuberculosis in a human macrophage-like cell line. Infect Immun. 1998;66(11):5314–21. doi: 10.1128/iai.66.11.5314-5321.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Liu PT, Stenger S, Li H, et al. Toll-like receptor triggering of a vitamin D-mediated human antimicrobial response. Science. 2006;311(5768):1770–3. doi: 10.1126/science.1123933. [DOI] [PubMed] [Google Scholar]
- 109.Liu PT, Stenger S, Tang DH, Modlin RL. Cutting edge: vitamin D-mediated human antimicrobial activity against Mycobacterium tuberculosis is dependent on the induction of cathelicidin. J Immunol. 2007;179(4):2060–3. doi: 10.4049/jimmunol.179.4.2060. [DOI] [PubMed] [Google Scholar]
- 110.Di Nardo A, Vitiello A, Gallo RL. Cutting edge: mast cell antimicrobial activity is mediated by expression of cathelicidin antimicrobial peptide. J Immunol. 2003;170(5):2274–8. doi: 10.4049/jimmunol.170.5.2274. [DOI] [PubMed] [Google Scholar]
- 111.Agerberth B, Charo J, Werr J, et al. The human antimicrobial and chemotactic peptides LL-37 and alpha-defensins are expressed by specific lymphocyte and monocyte populations. Blood. 2000;96(9):3086–93. [PubMed] [Google Scholar]
- 112.Nursyam EW, Amin Z, Rumende CM. The effect of vitamin D as supplementary treatment in patients with moderately advanced pulmonary tuberculous lesion. Acta Med Indones. 2006;38(1):3–5. [PubMed] [Google Scholar]
- 113.Coussens AK, Wilkinson RJ, Hanifa Y, et al. Vitamin D accelerates resolution of inflammatory responses during tuberculosis treatment. Proc Natl Acad Sci U S A. 2012;109(38):15449–54. doi: 10.1073/pnas.1200072109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Martineau AR, Timms PM, Bothamley GH, et al. High-dose vitamin D(3) during intensive-phase antimicrobial treatment of pulmonary tuberculosis: a double-blind randomised controlled trial. Lancet. 2011;377(9761):242–50. doi: 10.1016/S0140-6736(10)61889-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Wejse C, Gomes VF, Rabna P, et al. Vitamin D as supplementary treatment for tuberculosis: a double-blind, randomized, placebo-controlled trial. Am J Respir Crit Care Med. 2009;179(9):843–50. doi: 10.1164/rccm.200804-567OC. [DOI] [PubMed] [Google Scholar]
- 116.Lazar-Molnar E, Chen B, Jacobs W, Nathenson S. Inhibition through PD-1 is crucial for protection against M. tuberculosis infection. J Immunol. 2011;186:58.1. (Meeting Abstract Supplement) [Google Scholar]
- 117.Lazar-Molnar E, Chen B, Sweeney KA, et al. Programmed death-1 (PD-1)-deficient mice are extraordinarily sensitive to tuberculosis. Proc Natl Acad Sci U S A. 2010;107(30):13402–7. doi: 10.1073/pnas.1007394107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Topalian SL, Hodi FS, Brahmer JR, et al. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N Engl J Med. 2012;366(26):2443–54. doi: 10.1056/NEJMoa1200690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Azuma A. Pirfenidone treatment of idiopathic pulmonary fibrosis. Ther Adv Respir Dis. 2012;6(2):107–14. doi: 10.1177/1753465812436663. [DOI] [PubMed] [Google Scholar]
- 120.Azuma A. Pirfenidone: antifibrotic agent for idiopathic pulmonary fibrosis. Expert Rev Respir Med. 2010;4(3):301–10. doi: 10.1586/ers.10.32. [DOI] [PubMed] [Google Scholar]
- 121.Gold B, Pingle M, Brickner SJ, et al. Nonsteroidal anti-inflammatory drug sensitizes Mycobacterium tuberculosis to endogenous and exogenous antimicrobials. Proc Natl Acad Sci U S A. 2012;109(40):16004–11. doi: 10.1073/pnas.1214188109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Moitra K, Lou H, Dean M. Multidrug efflux pumps and cancer stem cells: insights into multidrug resistance and therapeutic development. Clin Pharmacol Ther. 2011;89(4):491–502. doi: 10.1038/clpt.2011.14. [DOI] [PubMed] [Google Scholar]
- 123.Amaral L, Engi H, Viveiros M, Molnar J. Comparison of multidrug resistant efflux pumps of cancer and bacterial cells with respect to the same inhibitory agents. In Vivo. 2007;21(2):237–44. [PubMed] [Google Scholar]
- 124.Tegos GP, Haynes M, Strouse JJ, et al. Microbial efflux pump inhibition: tactics and strategies. Curr Pharm Des. 2011;17(13):1291–302. doi: 10.2174/138161211795703726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Adams KN, Takaki K, Connolly LE, et al. Drug tolerance in replicating mycobacteria mediated by a macrophage-induced efflux mechanism. Cell. 2011;145(1):39–53. doi: 10.1016/j.cell.2011.02.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Balganesh M, Dinesh N, Sharma S, et al. Efflux pumps of Mycobacterium tuberculosis play a significant role in antituberculosis activity of potential drug candidates. Antimicrob Agents Chemother. 2012;56(5):2643–51. doi: 10.1128/AAC.06003-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Schmalstieg AM, Srivastava S, Belkaya S, et al. The antibiotic resistance arrow of time: efflux pump induction is a general first step in the evolution of mycobacterial drug resistance. Antimicrob Agents Chemother. 2012;56(9):4806–15. doi: 10.1128/AAC.05546-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Louw GE, Warren RM, Gey van Pittius NC, et al. Rifampicin reduces susceptibility to ofloxacin in rifampicin-resistant Mycobacterium tuberculosis through efflux. Am J Respir Crit Care Med. 2011;184(2):269–76. doi: 10.1164/rccm.201011-1924OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Amaral L, Viveiros M. Why thioridazine in combination with antibiotics cures extensively drug-resistant Mycobacterium tuberculosis infections. Int J Antimicrob Agents. 2012;39(5):376–80. doi: 10.1016/j.ijantimicag.2012.01.012. [DOI] [PubMed] [Google Scholar]
- 130.Ordway D, Viveiros M, Leandro C, et al. Clinical concentrations of thioridazine kill intracellular multidrug-resistant Mycobacterium tuberculosis. Antimicrob Agents Chemother. 2003;47(3):917–22. doi: 10.1128/AAC.47.3.917-922.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Weinstein EA, Yano T, Li LS, et al. Inhibitors of type II NADH:menaquinone oxidoreductase represent a class of antitubercular drugs. Proc Natl Acad Sci U S A. 2005;102(12):4548–53. doi: 10.1073/pnas.0500469102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Martins M, Viveiros M, Couto I, Amaral L. Targeting human macrophages for enhanced killing of intracellular XDR-TB and MDR-TB. Int J Tuberc Lung Dis. 2009;13(5):569–73. [PubMed] [Google Scholar]
- 133.Udwadia ZF, Sen T, Pinto LM. Safety and efficacy of thioridazine as salvage therapy in Indian patients with XDR-TB. Recent Pat Antiinfect Drug Discov. 2011;6(2):88–91. doi: 10.2174/157489111796064614. [DOI] [PubMed] [Google Scholar]
- 134.Abbate E, Vescovo M, Natiello M, et al. Successful alternative treatment of extensively drug-resistant tuberculosis in Argentina with a combination of linezolid, moxifloxacin and thioridazine. J Antimicrob Chemother. 2012;67(2):473–7. doi: 10.1093/jac/dkr500. [DOI] [PubMed] [Google Scholar]
- 135.Huang T-S, Kunin CM, Wang H-M, et al. Inhibition of the Mycobacterium tuberculosis reserpine-sensitive efflux pump augments intracellular concentrations of ciprofloxacin and enhances susceptibility of some clinical isolates. Journal of the Formosan Medical Association. 2012 doi: 10.1016/j.jfma.2012.03.009. in press. [DOI] [PubMed] [Google Scholar]
- 136.Escribano I, Rodriguez C, Llorca B, et al. Importance of the efflux pump systems in the resistance of Mycobacterium tuberculosis to Fluoroquinolones and linezolid. Chemotherapy. 2007;53(6):397–401. doi: 10.1159/000109769. [DOI] [PubMed] [Google Scholar]
- 137.Sunila ES, Kuttan G. Immunomodulatory and antitumor activity of Piper longum Linn. and piperine. J Ethnopharmacol. 2004;90(2–3):339–46. doi: 10.1016/j.jep.2003.10.016. [DOI] [PubMed] [Google Scholar]
- 138.Sharma S, Kumar M, Nargotra A, Koul S, Khan IA. Piperine as an inhibitor of Rv1258c, a putative multidrug efflux pump of Mycobacterium tuberculosis. J Antimicrob Chemother. 2010;65(8):1694–701. doi: 10.1093/jac/dkq186. [DOI] [PubMed] [Google Scholar]