Abstract
Chronic systemic inflammation is a hallmark feature of obesity and type 2 diabetes. Both resident and recruited islet macrophages contribute to the proinflammatory milieu of the diabetic islet. However, macrophages also appear to be critical for β-cell formation during development and support β-cell replication in experimental models of pancreas regeneration. In light of these findings, perhaps macrophages in the islet need to be viewed more as a fulcrum where deleterious inflammatory activation is balanced with beneficial tissue repair processes. Undoubtedly, defining the factors that contribute to the ontogeny, heterogeneity, and functionality of macrophages in normal, diseased, and regenerating islets will be necessary to determine whether that fulcrum can be moved to preserve functional β-cell mass in persons with diabetes. The intent of this review is to introduce the reader to emerging concepts of islet macrophage biology that may challenge the perception that macrophage accumulation in islets is merely a pathological feature of type 2 diabetes.
Macrophages are an integral component of the pancreatic islet that appear during embryonic development and persist well into adulthood. Historically, interest in islet macrophage biology has been largely confined to understanding the role of macrophages in type 1 diabetes (T1D), where these cells are effectors in the autoimmune process (1–3), or in islet transplantation where macrophage recruitment and activation often results in islet graft rejection (4–6). However, interest in islet macrophages has been rejuvenated recently because the islet has emerged as a site of sterile inflammation in obesity and type 2 diabetes (T2D) (7–10).
The pathogenesis of islet inflammation in T2D is a complex process, minimally involving immune cell infiltration, cytokine production, β-cell apoptosis, amyloid deposition, and islet fibrosis (8). Islet macrophages are beginning to take center stage as being significant regulators of islet inflammation in T2D (11–13). Pathology studies have documented increased macrophage infiltration in islets from humans with T2D (14–16) and from preclinical rodent models of obesity and T2D (14, 17–19). Additional studies have shown that glucolipotoxicity, endotoxemia, and islet amyloid deposits induce a proinflammatory activation state in islet macrophages that can add to the cytokine-rich islet milieu in T2D (18, 20–24). Collectively, these findings suggest that islet macrophages contribute significantly to the pathophysiology of T2D.
On the other hand, macrophages are required for normal β-cell development during embryogenesis (25). Macrophages are also necessary to support β-cell replication in some experimental rodent models of pancreas regeneration (26, 27). These findings indicate that macrophages can be beneficial to the islet in certain contexts and suggest that trophic factors produced by islet macrophages could possibly be exploited to facilitate regenerative therapies aimed at restoring functional β-cell mass in T2D.
The purpose of this review is to provide an update on recent findings in the T2D literature that underscore a renewed interest in islet macrophage biology. The discussion is framed in a more general context of macrophage biology in order to highlight important questions about the heterogeneity, ontogeny, and function of islet macrophages that need to be addressed if we are to truly understand the contribution of these cells in healthy, diseased, and regenerating islets. Understanding these foundational aspects of islet macrophage biology will likely prove to be essential for designing novel therapies aimed at either retarding islet inflammation or increasing functional β-cell mass in T2D.
Tissue Macrophages and M1/M2 Polarization
Tissue macrophages exhibit a broad range of physiological functions that range from immune surveillance and host defense to tissue remodeling and repair. To accomplish these distinct feats, macrophages integrate a variety of activation cues in situ. In vitro studies using cultured macrophages and in vivo studies in mice have provided key information about how these signals are integrated into diverse functions. To describe the apparent functional plasticity that is inherit to these cells, immunologists have tended to classify macrophages as being either “classically activated” M1 macrophages or “alternatively activated” M2 macrophages based on activation stimuli, gene and surface marker expression, and cytokine production (28, 29). To begin to appreciate the functional plasticity of islet macrophages, it is necessary to understand this nomenclature, its application to metabolic research, and its limitations.
Classically activated M1 macrophages produce proinflammatory cytokines (eg, IL-6, IL-1β, IL-12, and IL-23) (30). M1 macrophages are also cytotoxic and produce copious amounts of nitric oxide and reactivate oxygen species necessary for pathogen clearance. M1 macrophages can present antigens and activate T lymphocytes to link the innate immune system to adaptive immunity. Interferon-γ, TNF-α, and microbial products (eg, lipopolysaccharides [LPSs]) are prototypical factors that drive the M1 phenotype via activation of nuclear factor-κB signaling (30).
Alternatively activated M2 macrophages represent a continuum of phenotypically related macrophages that, in general, antagonize the actions of M1 macrophages (30–33). M2 macrophages play a key role in resolving inflammation by limiting the activation of adaptive immunity, promoting angiogenesis, and facilitating tissue repair (30, 33). M2 macrophages can be further classified into 4 subtypes (M2a, M2b, M2c, and M2d) based largely on their in vitro activation triggers, gene expression profiles, and functions. Th2 cytokines IL-4 and IL-13 activate M2a macrophages that can be identified by the increased expression of scavenger and phagocytic receptors. M2a macrophages secrete profibrotic and trophic factors, including fibronectin, IGF, and TGFβ. Immune complexes, Toll-like receptor (TLR) ligands, and IL-1 receptor antagonist activate M2b macrophages. M2b macrophages are a bit of an enigma in that they produce both proinflammatory (IL-1β, IL-6, and TNF-α), and antiinflammatory (IL-10) cytokines. M2c macrophages are activated by IL-10 and glucocorticoids and express high level of mer tyrosine kinase (MerTK), a phagocytic receptor that is critical for the removal of apoptotic cells (34). Finally, TLR ligands and adenosine A2A receptor agonists induce a unique M2d macrophage population that produces IL-10 and vascular endothelial growth factor (VEGF) (35). Notably, M2d macrophages have been proposed to be the result of adenosine-dependent “switch” from an inflammatory M1 phenotype into an angiogenic phenotype (35, 36).
Although the M1/M2 classification of macrophages is useful for understanding divergent macrophage responses in vivo, this classification has some shortcomings. M1 and M2 macrophages represent polar ends of a much wider activation spectrum, so this classification system is likely to be an oversimplification of the real phenotypic diversity demonstrated by tissue macrophages. Immunologists have openly debated whether a cataloging system based on well-controlled in vitro experiments is appropriate for describing the phenotypic and functional diversity of tissue macrophages because these macrophages are in reality exposed to complex and dynamic microenvironments in vivo (29, 37, 38). The Immunological Genome Project (ImmGen) has greatly extended our understanding of the transcriptome of murine macrophages (39–41), and these unbiased datasets fuel skepticism as to whether the M1/M2 classification can be extrapolated to macrophages in situ. On one hand, macrophages from a variety of mouse tissues have conserved gene expression signatures that can be used to distinguish them from functionally related dendritic cells (42, 43). At the same time, it is clear from these data that the tissue microenvironment influences the gene profiles of macrophages, with most tissue macrophages displaying transcriptomes that do not neatly stratify into M1 or M2 classifications (42). Indeed, a recent study demonstrated that bone marrow-derived macrophages exposed to an artificial T2D milieu (high glucose, high insulin, and palmitate) in vitro exhibited a “metabolically activated” phenotype similar to that of macrophages from obese fat yet highly distinct from bone marrow-derived macrophages induced by classic M1 stimuli (LPS and interferon-γ) (44). Not surprisingly, epigenetic and proteomic studies also indicate that the tissue macrophages differ from cultured macrophages in terms of both chromatin architecture (45–47) and membrane proteomes (48, 49). As new studies continue to expand our knowledge of tissue macrophages in health and disease, one challenge will be to agree upon whether a unifying nomenclature to classify tissue macrophages based on ontogeny, gene expression profiles, immunophenotype, and function is necessary or even possible.
Despite inherit limitations, the M1/M2 nomenclature has frequently appeared in the metabolism literature during the past decade, particularly in the context of obesity-induced adipose tissue inflammation. Adipose tissue macrophages (ATMs) were among the first tissue macrophages to be studied for their putative involvement in obesity-induced insulin resistance and T2D (50, 51). We now know that ATMs are heterogeneous in both mice and humans (52–54).
In lean mice and metabolically healthy humans, most ATMs that reside between adipocytes in visceral fat are phenotypically similar to alternatively activated M2 macrophages (55–57). M2 ATMs are identified by high expression of scavenger receptors (eg, macrophage galactose-type C-type lectin 1 [Mgl1/CD301a] and mannose receptor [CD206] in mice) (57, 58). In lean, metabolically healthy adipose tissue, resident ATMs primarily express antiinflammatory genes indicative of M2 macrophages, including Il10 and Il1ra (55, 60). Notably, IL-10 from murine M2 ATMs has been shown to promote insulin action in adipocytes (55). In mice, the M2 phenotype of ATMs is largely regulated by peroxisome proliferator-activated receptor-γ (61–64) and IL-4/signal transducers and activators of transcription (STAT) signaling (65, 66).
In response to diet- and genetic-induced obesity in mice, bone marrow-derived macrophages that express CD11c accumulate within visceral fat (perigonadal fat) and also express proinflammatory molecules typical of activated M1 macrophages (eg, TNF-α, IL-1β, and IL-6) (50, 51, 55, 67, 68). Recruitment of CD11c+ M1 ATMs in obesity is dependent upon the chemokine receptor chemokine (C-C motif) receptor 2 (50, 55, 69, 70). Adipose tissue lipolysis has been shown to be a potent stimulus for ATM recruitment (71), and microscopy studies suggest that CD11c+ macrophages are recruited into “crown-like structures” in visceral fat in an attempt to encapsulate dead or dysfunctional adipocytes during obesity (72, 73). Multiple studies have demonstrated that infiltration of CD11c+ M1 ATMs into visceral fat is associated with local and systemic insulin resistance (55, 67, 74–76), and ablation of CD11c+ ATMs in obese mice improves insulin sensitivity (77–79). Collectively, these and other findings have led to the prevailing view that CD11c+ M1 ATMs initially infiltrate adipose tissue in response to tissue damage. However, the unresolved activation of these proinflammatory macrophages likely exacerbates tissue injury, and this is manifested as adipose tissue inflammation, insulin resistance, and fibrosis (80, 81).
Lumeng et al (55) proposed the “phenotypic switch” model to describe how M1 ATM infiltration into visceral fat promotes obesity-induced inflammation and insulin resistance. This model proposes that accumulation of M1 ATMs with obesity induces an imbalance between the number of proinflammatory and antiinflammatory ATMs thereby skewing the balance towards a proinflammatory milieu that favors chronic adipose tissue inflammation. Chronic inflammation then exacerbates adipocyte insulin resistance and dysfunction, which contributes to dyslipidemia, systemic insulin resistance, and development of T2D (55). At present, it is unclear whether this M2-to-M1 phenotypic switch paradigm can or should be applied more broadly to describe the activation of macrophage populations in other key metabolic tissues (eg, liver Kupffer cells, brain microglia, and islet macrophages) during obesity and T2D. Nonetheless, this paradigm of macrophage activation in obese adipose tissue as well as the M1/M2 classification systems provides a logical framework from which to begin describing islet macrophage activation and function during obesity and T2D.
Islet Macrophage Heterogeneity in T2D
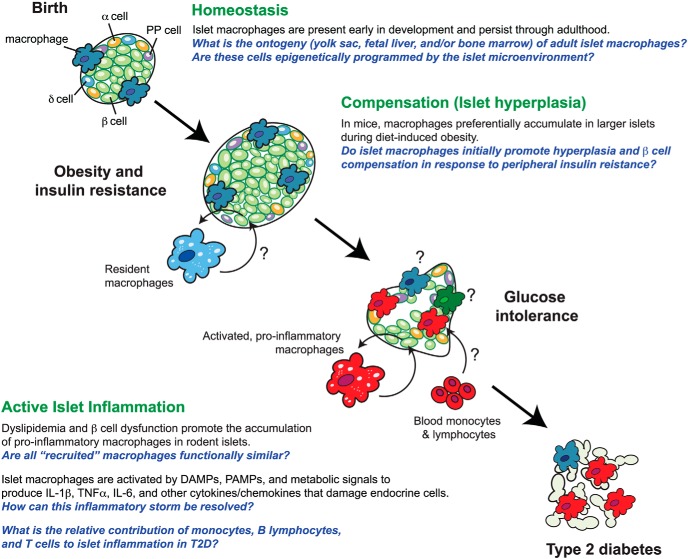
The first indication that macrophages may contribute to islet inflammation in T2D came from histological studies documenting the accumulation of CD68+ macrophages in the islets of T2D patients (14–16) and in islets from rodent models (14, 17–19). The immunophenotype of resident and infiltrating macrophages in mouse models of T2D and islets from human donors with T2D is starting to be unveiled (Table 1), which is critical for understanding the biology of these cells in T2D. In mice, resident islet macrophages coexpress F4/80 and CD11b, and F4/80+ CD11b+ cells also uniformly express CD11c at steady state (23, 24). Islet-associated macrophages in human cadaveric islets have also been shown to coexpress CD11b and CD11c (82). Eguchi et al (18) reported 2 distinct populations of macrophages (CD11b+ Ly-6C+ and CD11b+ Ly-6CNeg) in islets obtained from C57BL/6 perfused with ethyl palmitate to model hyperlipidemia, as well as db/db and KK-Ay mice. Dyslipidemia and lipotoxicity were associated with a quantitative increase in islet-associated CD11b+ Ly-6C+ macrophages that expressed higher levels of proinflammatory genes (Il1β and Tnfα) in these models (18). By contrast, antiinflammatory genes (Il10) were elevated in CD11b+ Ly-6CNeg macrophages (18). Selective depletion of the CD11b+ Ly-6C+ population with clodronate increased β-cell function and improved glucose tolerance in db/db and KK-Ay mice (18). In a second study, a resident CD68+F4/80+ population and a recruited CD68+F4/80neg population was described in db/db islets (83). Both populations express TLR4 and could be activated by LPS, a signature of M1 macrophages (84); however, CD68+F4/80+ in diabetic islets also expressed the scavenger receptor CD206 (83), suggesting a M2 phenotype. These initial findings indicate that islet macrophages may be quite heterogeneous both immunophenotypically and functionally. Defining the phenotypic heterogeneity and plasticity of islet macrophages, and determining how the distribution and activation of these cells may change with disease progression, are just some of the important questions that will need to be addressed if we are to further our understanding of how macrophages contribute to islet inflammation in T2D (Figure 1).
Table 1.
Markers Used to Identify Islet-Associated Macrophages in Preclinical Rodent Models of T2D and in Specimens From Human Donors
| Markers | T2D Model(s) | Notes | Reference |
|---|---|---|---|
| Rodent islet studies | |||
| CD68 (IHC) | Goto-Kakizaki (GK) rats | Accumulation of CD68+ macrophages in islets correlated with hyperglycemia and islet fibrosis | 17 |
| MHC class II (IHC) | |||
| CD11b (IHC) | C57BL/6 mice, high-fat diet-induced obesity db/db mice | Accumulation of CD11b+ cells was associated with islet size during diet-induced obesity | 14 |
| CD68 (IHC) | Goto-Kakizaki (GK) rats | ||
| F4/80 (IHC) | C57BL/6 mice, ethyl palmitate-infused db/db mice | CD11b+Ly-6C+ macrophages accumulated in T2D islets and expressed higher levels of M1 genes (Il1b and Tnfa) | 18 |
| CD11b (IF/FC) Ly-6C (FC) |
KK-Ay mice | ||
| CD68 (IHC) | ZDF rats | CB1R and NLRP3 were highly coexpressed with CD68 in pancreatic islets | 19 |
| F4/80 (FC) | db/db mice | db/db islets contained a mix of F4/80negCD68+CD11c+, F4/80+ CD68+CD11c+CD206+, and F4/80+CD68+CD206+ macrophages | 83 |
| CD68 (FC) | |||
| CD11c (FC) | |||
| CD206 (FC) | |||
| Ly-6C (FC) | |||
| F4/80 (FC) | hIAPPTg mice, high-fat diet-induced obesity | CD11b, CD11c, and Ly-6C expression was higher on islet macrophages (F4/80+CD11b+) from hIAPPTg mice | 24 |
| CD11b (FC) | |||
| CD11c (FC) | |||
| Ly-6C (FC) | |||
| Human islet studies | |||
| CD68 (IHC) | Pancreas sections from nondiabetic (n = 7) and T2D (n = 9) donors | 14 | |
| CD163 (IHC) | |||
| HLA-2 (IHC) | |||
| CD68 (IHC) | Pancreas sections from nondiabetic (n = 16) and T2D (n = 15) donors | 15 | |
| CD68 (IHC/IF) | Pancreas sections from nondiabetic (n = 20) and T2D donors without (n = 20) or with (n = 26) amyloid deposition | Frequency of CD68+iNOS+ macrophages per islet positively correlated with amyloid deposition in T2D samples | 16 |
| CD163 (IHC/IF) | |||
| CD204 (IF) | |||
| iNOS (IF) | |||
| CD11b (FC) | Isolated islets from nondiabetic (n = 15–16) and T2D (n = 12–13) donors | Macrophages coexpressed CD11b and CD11c; frequency (%CD45 leukocytes) of CD11b+ and CD11c+ cells was not different between nondiabetic and T2D islets | 82 |
| CD11c (FC) |
The method of detection is indicated in parenthesis. FC, flow cytometry; IF, immunofluorescence microscopy; IHC, immunohistochemistry.
Figure 1.
Islet-associated macrophages in the progression of T2D: the knowns and unknowns. The pathogenesis of T2D is a complex process, but islet macrophages are emerging as significant regulators of the islet inflammatory milieu at various nodes during the progression of T2D. Although a number of studies highlighted in this review have provided key insight into islet macrophage biology, several important questions remain. Refer to the text for additional discussion.
Islet Macrophages and Regulation of Intraislet Cytokine Levels: IL-1β as an Example
It is becoming increasingly evident that cytokines (eg, TNF-α, IL-1β, IL-6, and IL-18) and chemokines (eg, monocyte chemoattractant protein-1/chemokine [C-C motif] ligand (CCL)2, macrophage inflammatory protein 1α/CCL3, and chemokine [C-X-C motif] ligand 1) within the islet contribute to localized inflammation and islet dysfunction in T2D. Of these, the relationship between IL-1β and β-cell dysfunction has been extensively studied, and IL-1β signaling has recently emerged as a therapeutic target for T2D (85). β-Cells express high levels of the IL-1β receptor (IL-1βR1) (86). At very low concentrations (0.01–0.02 ng/mL), IL-1β can stimulate β-cell proliferation and glucose-stimulated insulin secretion in cultured human and rat islets (87, 88). However, at higher concentrations, IL-1β is highly cytotoxic to islets (89–91). New data indicate that resident and recruited islet macrophages integrate several signals to increase intra-islet IL-1β concentrations which in turn debilitates β-cells in both the prediabetic state and during overt T2D.
IL-1β expression, maturation, and secretion is tightly regulated (92). Most cells do not constitutively express Il1β. Rather, proinflammatory stimuli that activate nuclear factor-κB, including TLR ligands and TNF-α, induce transcription of Il1β, resulting in the accumulation of the inactive proform of IL-1β (93). This process is referred to as “priming.” Importantly, TLR ligands, specifically endotoxin and high-mobility group box 1, are systemically elevated in obese rodents (94) as well as in persons with T2D (95–97). High-mobility group box 1 is also increased in diabetic islets (98). These findings suggest that islet IL-1β production may be primed by local and systemic factors in T2D.
Maturation of pro-IL-1β and subsequent release of bioactive IL-1β is dependent upon assembly of inflammasomes. Inflammasomes consist of multiple self-oligomerizing scaffold proteins that provide a molecular platform for the caspase-1 mediated cleavage of pro-IL-1β (99). Pathogen-associated molecular patterns (PAMPs), damage-associated molecular patterns (DAMPs), and environmental stimuli “trigger” inflammasome assembly for host defense (92). A number of inflammasomes with varying composition have been described (92). The nucleotide oligomerization domain-like receptor protein 3 (NLRP3) inflammasome appears to be significant for metabolic inflammation (22, 100–102). NLRP3 consists of the NLRP3 scaffold, the apoptosis-associated speck-like protein adapter, and caspase-1 (92). Notably, Nlrp3- and Asc-knockout mice are protected from islet dysfunction and β-cell apoptosis induced by chronic exposure to a 60% high-fat diet (103), suggesting that the NLRP3 inflammasome contributes to obesity-induced T2D pathology. Related to islet inflammation, the NLRP3 inflammasome can also be triggered by extracellular ATP released from damaged cells (104), saturated free fatty acids (76, 101), endocannabinoids (19), hyperglycemia (105), and human islet amyloid polypeptide (hIAPP) (20).
Although β-cells are known to be capable of secreting IL-1β in response to stress, new studies have demonstrated that islet macrophages are the dominant producers of IL-1β in inflamed, diabetic islets. Resident islet macrophages express TLR2, TLR4, and TLR6 and respond to TLR ligands by increasing Il1β, Il6, and Tnfα expression (23, 84). Notably, the induction of these inflammatory cytokines was significantly higher in purified macrophages when compared with nonmacrophages in the same murine islets (23). Conditioned media from bone marrow-derived macrophages treated with either TLR2 or TLR4 ligands was also sufficient to reduce Pdx1, Ins1, and Ins2 expression and impair glucose-stimulated insulin secretion in mouse islets (23). Importantly, depleting macrophages from isolated human islets with clodronate reduced TLR-stimulated IL-1β secretion (23). Collectively, these findings show that TLR2/4 signaling in resident islet macrophages contribute to the priming of the islet inflammasome in both mice and man.
Recent studies have also identified a number of inflammasome triggers in T2D that promote IL-1β secretion from resident and recruited islet macrophages including islet amyloid and endocannabinoids. IAPP is secreted from β-cells, and extracellular aggregation of IAPP leads to amyloid deposition, which is associated with islet pathology in T2D (106, 107). Kamata et al (16) recently reported that islet amyloid deposition in islets from human donors with T2D was strongly correlated with the accumulation of CD68+ macrophages expressing the M1 activation marker inducible nitric oxide synthase (iNOS). It has been known for some time that macrophages can phagocytose islet amyloid (108), but only recently has it been shown that islet amyloid deposits elicit a proinflammatory immune response from murine macrophages (20). Using bone marrow-derived macrophages as a model system, Masters et al (20) demonstrated that oligomers of human IAPP activate the NLRP3 inflammasome. Notably, inflammasome activation required phagocytosis of IAPP but not thioredoxin-interacting protein (20). The latter finding is noteworthy because thioredoxin-interacting protein has been implicated as a key mediator between hyperglycemia and inflammasome activation/IL-1β production in β-cells (105). Additional studies also showed that culturing islets in the presence of hIAPP resulted in the activation of caspase-1 and accumulation of IL-1β in resident islet macrophages but not in β-cells (24). Similarly, depleting resident islet macrophages with clodronate attenuated the hIAPP-induced expression of proinflammatory genes (24). Collectively, these findings support the idea that islet amyloid is a specific inducer of islet macrophage inflammasomes and a potent trigger for islet inflammation.
Because amyloid deposits do not naturally form in mice islets, transgenic mice expressing hIAPP (hIAPPTg mice) were developed to study the role of islet amyloid in the pathogenesis of T2D (24, 109, 110). In two independent strains, transgenic expression of hIAPP alone was not sufficient to result in a T2D phenotype. However, when combined with diet- or genetic- induced obesity and insulin resistance, hIAPPTg mice manifest amyloid deposition, impaired insulin secretion, and other pathologies reminiscent of T2D (24, 109, 110). Islet macrophages (F4/80+CD11b+) from hIAPPTg mice expressed higher mRNA levels of Nlrp3, Il1b, and Itgam (CD11b) than macrophages from nontransgenic islets (24). Importantly, the expression of these genes was significantly lower (Nlrp3) or nearly undetectable (Il1β) in the nonmacrophage (CD11b−) fractions purified from the same islets (24). Flow cytometry analysis further revealed that islet macrophages from obese hIAPPTg mice had higher cell surface expression of CD11b, CD11c, and Ly-6C (24). Notably, Ly-6C is a marker for inflammatory monocytes in mice (111), and Ly-6CHigh monocytes have been shown to participate in proinflammatory responses in other inflamed tissues (112, 113). Although, the study by Westwell-Roper et al (24) did not completely resolve the relative contribution of Ly-6CHigh monocytes vs resident macrophages, it is likely that recruitment of monocytes contributes to the pool of F4/80+CD11b+ macrophages that drive the pathophysiology of islet inflammation induced by amyloid. Regardless, administering recombinant IL-1 receptor antagonist (anakinra) to obese hIAPPTg mice limits islet inflammation and restores glucose tolerance (114), thereby providing strong in vivo evidence that macrophage-derived inflammasome activation and IL-1β are effectors of amyloid-induced islet inflammation and β-cell dysfunction.
Amyloid is not the only NLRP3 activating signal in diabetic islets. In diabetic Zucker diabetic fatty (ZDF) rats, M1 polarized macrophages infiltrate islets and express components of the NLRP3 inflammasome (19). Depletion of macrophages in ZDF rats only marginally improves peripheral insulin resistance, but significantly improves glucose tolerance and measures of β-cell function (19). The endocannabinoid system is activated by stress, and obesity induces chronic activation of the peripheral endocannabinoid system (115). Remarkably, systemically blocking endocannabinoid signaling by using a cannabinoid receptor (CB1R) inverse agonist or small interfering RNA-mediated knockdown of CB1R in macrophages not only ameliorated islet macrophage infiltration and islet inflammation, but also improved β-cell survival and function in ZDF rats (19). Collectively, these studies underscore the key role that islet macrophages play in the regulation of intraislet IL-1β production, islet inflammation, and β-cell dysfunction in T2D.
Islet Macrophages, VEGF-A, and Islet Fibrosis
Islets are highly vascularized, which is essential for coupling insulin secretion to glucose regulation. Members of the VEGF family play a critical role in angiogenesis and vascularization, and VEGF-A, in particular, is highly expressed by β-cells (116, 117). VEGF-A deficiency in β-cells impairs islet vascularization, resulting in insufficient insulin secretion and glucose intolerance in mice (118–120). VEGF-A signaling is also required for revascularization of transplanted islets (118, 121). However, excess VEGF leads to vascular abnormalities that appear to be deleterious to islet function in the setting of obesity and T2D. Elevated VEGF levels are associated with expansion of endothelial cells and fibrosis in islets from rodent models of obesity and T2D (17, 122–125). Indeed, sustained overexpression of VEGF-A from β-cells in transgenic mice not only results in hypervascularization of islets but also leads to age-dependent loss of β-cell mass and development of glucose intolerance (125). VEGF-overexpressing islets showed progressive increases in macrophage accumulation, collagen deposition, and expression of proinflammatory mediators (eg, Il1β and Tnfα) (125). These findings suggest that VEGF-A, proinflammatory islet macrophages, and endothelial cells may conspire to promote islet fibrosis and impair insulin secretion in T2D.
Using a doxycycline-inducible transgenic mouse to raise intraislet VEGF-A levels, Brissova et al (126) recently confirmed that VEGF-A production in β-cells is sufficient to increase intraislet endothelial cells, reduce β-cell mass, and impair glucose tolerance in mice. These investigators further showed that normalization of VEGF-A levels after doxycycline withdrawal promoted β-cell replication and restored euglycemia (126). Importantly, β-cell replication that accompanied VEGF-A withdrawal required infiltration of bone marrow-derived macrophages that had a mixed M1:M2 phenotype (126). At present, it is unclear whether VEGF-A itself, endothelial cell activation, or some combination thereof directs islet macrophage recruitment and dictates whether newly arrived macrophage will enter into either a profibrotic program or provide trophic support for β-cell regeneration. Nonetheless, these observations demonstrate the plasticity of recruited islet macrophages, and suggest that the islet microenvironment itself likely determines whether infiltrating macrophages injure β-cells or benefit their survival.
Macrophages in Pancreas Development and Endocrine Cell Differentiation
As described earlier, tissue macrophages have pleotropic functions that extend beyond being proinflammatory meditators. Macrophages are present in nearly all organs during embryonic development (127), and they are believed to be the primary phagocytes that clear apoptotic cells during morphogenesis (128, 129). Tissue macrophages also produce a range of growth and angiogenic factors that are necessary for embryonic development (130). In mice, fetal macrophages from multiple tissues have gene expression profiles that are highly comparable with alternatively activated M2 macrophages (127) or proangiogenic tumor-associated macrophages (131). Mice homozygous for the osteopetrosis spontaneous mutation (op/op mice) lack macrophages due to the inability to produce functional colony-stimulating factor (CSF)-1 (132). Consequently, op/op mice exhibit a number of developmental abnormalities, including skeletal, endocrine, and neurological defects. These observations suggest an essential role for alternatively activated M2 macrophages in normal organogenesis.
To date, the phenotype of pancreatic macrophages during embryonic development is largely undefined, but several observations underscore the important roles that macrophages may play in the formation of the endocrine pancreas. In the mouse, leukocytes can be detected by immunofluorescence in the pancreatic buds, duodenal loop, and stomach at embryonic day (E) 12.5, with differentiated CD11b+ F4/80+ macrophages detected in the developing pancreas by E14.5 (133). During this window of embryonic development (E12.5–E14.5), the ventral and dorsal pancreatic buds are fusing as a result of gut tube rotation, and the developing pancreas is initiating “secondary transition” (134). Secondary transition is a key time for differentiation of all endocrine and exocrine cell types. The number of macrophages as well as CD115+ monocytes near insulin+ β-cells progressively increases from E14.5 to E17.5 (133). This developmental window corresponds to the timing of a wave of β-cell proliferation and islet formation in the mouse. Notably, culturing embryonic (E12.5) mouse pancreatic explants with CSF-1 induced a 3-fold increase in the number of macrophages and significantly increased the number of insulin- and glucagon-producing cells (133). Conversely, β-cell mass and insulin production are reduced in op/op mice due to the combined effects of reduced fetal β-cell proliferation and decreased size of individual β-cells (25). Although overall islet morphology is perturbed by macrophage deficiency in op/op mice, postnatal α-cell mass remains unaffected, suggesting that macrophages are dispensable for the normal patterning of α-cells (25). It is unclear whether the development and function of δ and PP cells are altered in op/op mice. Collectively, these observations indicate that embryonic macrophages in the pancreas play a key role in supporting β-cell expansion and differentiation during secondary transition, which could have significant ramifications on functional β-cell mass and regulation of glucose homeostasis well into adulthood.
The effects of macrophages on the development of endocrine cells in the pancreas may also extend to the formation of pancreatic ducts. From the secondary transition to birth in mice, the pancreatic epithelium extends and branches significantly, thereby giving rise to a unique 3-dimensional, tree-like structure of tubules or ducts (135). One study identified CSF-expressing epithelial cells within human embryonic pancreata with a number of mesenchymal cells expressing macrophage markers near these ductal cells during weeks 6–12 of development (136). Though defects in pancreatic ductal formation have not yet been reported in op/op mice, macrophages do appear to be required for ductal morphogenesis during mammary gland development. Ductal branching and formation of terminal end buds is perturbed in the mammary glands of female op/op mice (137, 138). Subsequently, op/op dams fail to adequately lactate and support viable pups (137). Because both formation of mammary and pancreatic ducts require trophic stimulation and extensive remodeling of epithelial cells, it stands to reason that CSF-dependent macrophages may be engaged in the patterning and branching of pancreatic ducts during development. Proper patterning of pancreatic ducts is not only necessary for the structural organization of the pancreas, but also may be required for maintaining unique niches of progenitor cells capable of differentiating into cells of both endocrine and exocrine lineages (135). Using embryonic pancreas transplant models and organ cultures, Mussar et al (139) recently demonstrated that M2 macrophages interact with epithelial cells to attenuate proliferation and promote differentiation of ductal progenitor cells towards endocrine cells in mice. The basic helix-loop-helix transcription factor Neurogenin-3 (Ngn3) is considered to be the master regulator that controls differentiation of pancreatic endocrine progenitors into the principle constituent cells of the islet (140). Mouse models with structurally malformed pancreatic ducts show reduced Ngn3 expression and reduced endocrine cell mass (141, 142). Therefore, because macrophage-deficient op/op mice clearly manifest defects in postnatal insulin production, it would be interesting to further investigate the effects of pancreatic macrophages on branching morphogenesis of ductal epithelial cells, regulation of Ngn3 expression, and differentiation of ductal progenitor cells into endocrine lineages during embryonic development.
Macrophages and β-Cell Regeneration in the Adult
Both T1D and T2D ultimately manifest as β-cell insufficiency. Restoring functional β-cell mass in adults is challenging on many fronts. Self-renewal of existing β-cells is thought to be the primary source of new β-cells in the adult rodent islet (143, 144), yet this proliferative capacity declines progressively with age (145). Human β-cells are thought to be particularly long-lived, because they exhibit histological features of senescence (146). Moreover, the shortage of donor pancreata and inherit immunological difficulties of islet transplantation have spurred increased emphasis on identifying alternative sources for new functional β-cells that can be used to retain or increase functional β-cell mass in people with diabetes. Pancreatic progenitor cells, induced pluripotent stem cells (iPCs), reprogrammed adult exocrine cells, and transdifferentiated cells have all been shown to have some capacity to restore the β-cell compartment in mouse models of diabetes (147–149). Admittedly, there is both promise and controversy surrounding these cell-based approaches, which is beyond the scope of this review. Regardless of the source of newly formed β-cells, exploiting tissue macrophages for regenerative medicine is becoming increasingly in vogue for other diseases (150–152). Here, two recent studies are highlighted that suggest that pancreatic macrophages may have utility as facilitators of β-cell regeneration in vivo.
Pancreatic ductal ligation (PDL) is a surgical pancreatic injury model often used to evaluate the regenerative potential of β-cells and ductal progenitors in rodents. In PDL, the main duct connecting the duodenum to the pancreas is surgically closed, resulting in apoptosis of acinar tissue throughout the pancreatic tail (153). PDL induces mononuclear cell infiltration and localized inflammation, as evident by increased mRNA levels of cytokines (Tnfα, Ilβ, Il10, and Il6 among others) (154). Notably, Xiao et al (27) reported that depleting pancreatic macrophages with clodronate significantly attenuated β-cell replication after PDL. A recent paper by Van Gassen et al (155) provided a comprehensive description of the dynamics of myeloid cell infiltration in the pancreas of mice after PDL. Naïve pancreatic macrophages were identified as being CD11b+Ly-6GnegSiglecFnegF4/80+ cells with two distinct expression levels of major histocompatibility complex (MHC) class II molecules: MHC-IIlow and MHC-IIhigh155. At steady state, the ratio of MHC-IIhigh to MHC-IIlow macrophages was approximately 2:1. Within the first 24 hours after PDL, Ly-6CHigh monocytes, eosinophils, and neutrophils appeared in the injured pancreas (155). This was followed (d 1–3 after PDL) by a proliferative expansion of MHC-IIlow macrophages expressing M2 markers that were highly angiogenic (155). Others have also shown that CD206+ M2 macrophages purified from the regenerating PDL tail express increased levels of Tgfb1 and Egf (epidermal growth factor [EGF]) and that M2 macrophages induce β-cell proliferation via TGF-β receptor 1- and EGF receptor-dependent signaling pathways (27). Importantly, M1 macrophages (F4/80+CD206neg cells) failed to induce β-cell proliferation (27), and PDL-induced β-cell proliferation in situ does not appear to require recruitment of Ly-6C+ monocytes (155). Collectively, these findings indicate that pancreatic macrophages with an M2 signature can produce trophic and angiogenic factors that support β-cell replication, even within the context of a largely proinflammatory tissue microenvironment.
Using an elegant model of diphtheria toxin-induced cell ablation, a study by Criscimanna et al (26) demonstrated that macrophages infiltrate the pancreas in response to both endocrine and exocrine cell apoptosis. The infiltrating cells initially expressed cytokines reminiscent of M1 macrophages (IL-6, TNF-α) but later expressed 2 hallmarks of an M2 activation state: IL-10 and CD206. Macrophages were required to promote β-cell proliferation in situ when diphtheria toxin was removed and the islets were allowed to regenerate (26). One fascinating experiment in this paper demonstrated that bone marrow-derived macrophages exposed to apoptotic islet cells in vitro could induce β-cell differentiation from endothelial cells in embryonic pancreatic explants (E11.5). Importantly, the converse was also true in that macrophages cultured with apoptotic ancinar cells induced exocrine tissue differentiation (26). These findings led the authors to conclude that macrophages can be entrained by what they “eat” to create lineage-specific niches for pancreatic cell regeneration. Although expanding our knowledge of the molecular events underpinning these observations is necessary, these two studies collectively demonstrate that pancreatic macrophages can support β-cell proliferation after injury and point towards the potential use of macrophages as facilitators of β-cell regenerative therapies for T1D and T2D (Figure 2).
Figure 2.
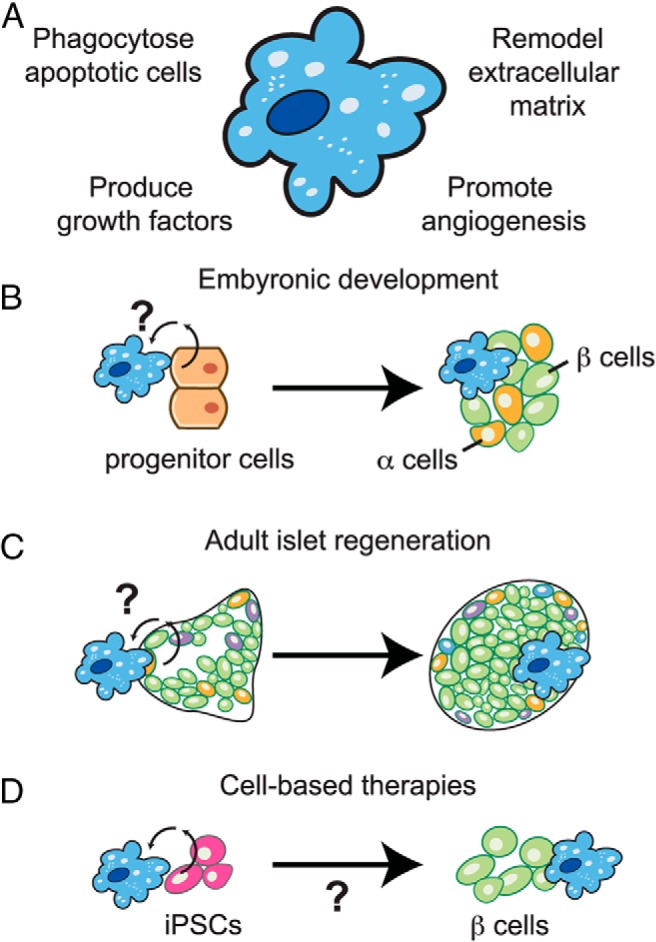
Can islet-associated macrophages be beneficial? A, Some established functions of alternatively activated M2 macrophages that may be beneficial to islet biology. B, Pancreatic macrophages are required for β-cell development during embryogenesis, yet how macrophages and endocrine progenitor cells interface during development is unclear. C, Based on PDL models, a subset of pancreatic macrophages can promote islet vascularization and β-cell regeneration. Identifying the mechanisms whereby macrophages participate in this process may lead to novel therapies to promote adult β-cell regeneration in T2D. D, Given that macrophages play a key role in embryonic patterning of the endocrine compartment, it is plausible that macrophages could be exploited to promote expansion of iPCs and/or induction of β-cell phenotypes for emerging cell-based therapies.
Islet Macrophages in Compensatory Islet Hyperplasia
Compensatory islet hyperplasia is a physiological response to increasing β-cell workload. Pregnancy, peripheral insulin resistance (through either diet-induced obesity or more directly via insulin receptor antagonism), and partial pancreatectomy increase functional demand on β-cells, and all have been reported to stimulate β-cell proliferation and increase β-cell mass in vivo (154, 156–159). Although the signals that induce β-cell replication in these settings are likely to act directly on the β-cell (eg, hyperglycemia or prolactin in pregnancy), it is conceivable that resident islet macrophages may provide angiogenic (as in islet transplantation) or trophic support (as in development and PDL) to the growing islet. Consistent with this, Ehses et al (14) reported that macrophages preferentially accumulate in larger islets during diet-induced obesity in mice. Islet macrophages may also remodel the extracellular matrix to facilitate islet expansion, which would be analogous to how tumor-associated macrophages support tumor growth and malignancies (160, 161). Previous studies suggest that ATMs support angiogenesis and physiological expansion of adipose tissue in obesity (80, 162). Whether and how resident islet macrophages support β-cell replication during compensatory islet hyperplasia has not been rigorously examined. However, this question should be prioritized in order to determine whether mechanisms that are intrinsic to islet macrophages during these physiological processes might be viable modalities for maintaining functional β-cell mass in people with T2D.
Origin of Pancreatic and Islet Macrophages
The functional heterogeneity and plasticity of macrophages depends, in part, on their ontology (163). Given the evidence that islet and pancreatic macrophages appear to be heterogeneous in terms of their in vivo functions, understanding the origin of these cells is growing in importance. It has long been held that tissue macrophages are derived from hematopoietic stem cells (HSCs) in the bone marrow. However, recent lineage tracing and fate mapping studies in mice have revealed that macrophages with distinct lineages and ontogeny populate many tissues during development (164–166). During primitive hematopoiesis in mice (E6.5–E8.5), progenitors from the extraembryonic yolk sac give rise to tissue macrophages and red blood cells (167). Yolk sac-derived macrophages in mice embryos are F4/80High, and these macrophages do not require the transcription factor Myb, a master regulator of HSCs, for their development or survival (168). At E8.5–E10, the developing aorta-gonad-mesonephros generates definitive HSCs capable of giving rise to all immune cell lineages (167). At around E10.5, HSCs populate the liver, and the liver then becomes the site of hematopoiesis during the rest of embryonic development. Only after birth do HSCs within the bone marrow transition into the primary source of progenitors for myelopoiesis in the immune system. A series of elegant studies have shown that tissues may be differentially populated with macrophages of diverse origin. For example, microglia are almost exclusively yolk sac derived (169, 170), whereas Kupffer cells in the liver are a heterogeneous mixture of both yolk sac- and embryonic HSC-derived cells (171). One surprising conclusion from these and other studies is that diverse pools of tissue macrophages can coexist and be maintained by homeostatic proliferation throughout adulthood with varying degrees of contribution from bone marrow-derived monocytes (169, 172, 173).
Macrophages are a constituent of the embryonic pancreas. Most macrophages in the developing pancreas are F4/80High and do not require Myb (168). These findings suggest that pancreatic macrophages are predominantly of yolk-sac origin, similar to microglia. F4/80High yolk sac-derived macrophages express colony stimulating factor receptor 1, the receptor for CSF-1 (168). Islet morphogenesis is abnormal and proliferative expansion of β-cell mass is disrupted in op/op mice (25), and these findings suggest that yolk sac-derived, CSF-1/CSFR1-dependent macrophages have beneficial effects on the islet during development. However, there was a small percent of pancreatic macrophages that are absent in Myb-deficient mice (168), suggesting that embryonic hematopoiesis contributes to the total population of macrophages in the developing pancreas. Additional studies aimed at fully defining the developmental ontogeny of islet macrophages may add to our understanding of the relative contribution of yolk sac- vs HSC-derived islet macrophages to islet inflammation and β-cell function throughout adulthood. Moreover, such studies may also shed new insights into the developmental origins of T2D, because both yolk sac- and embryonic HSC-derived macrophage populations are likely to be subjected to epigenetic reprogramming during embryogenesis, especially if the nutritional plane of the fetus is altered.
After birth and throughout adulthood, the bone marrow is the principle site of hematopoiesis. Myelopoiesis generates at least two, and possibly three, functionally distinct populations of circulating monocytes that can ultimately differentiate into tissue macrophages (111, 174–176). Short-lived, “classical” monocytes (CD14High CD16Neg CX3CR1Low CCR2High in man; Ly-6CHigh CX3CR1Low CCR2High in mice) are the predominant circulating monocyte population. Classical monocytes can produce inflammatory cytokines in response to a variety of PAMPs and DAMPs, and these monocytes selectively extravasate into inflamed tissues. Classical monocytes are often referred to as “inflammatory” monocytes because their activation contributes to local and systemic inflammation. However, classical monocytes can either differentiate into proinflammatory M1 macrophages or antiinflammatory M2 macrophages, depending up on the tissue context. “Nonclassical” monocytes (CD14+ CD16High CX3CR1High CCR2Low in man; Ly-6CLowCX3CR1High CCR2Low in mice) are long-lived patrolling leukocytes that extravasate into tissues under homeostatic conditions and during the resolution phase of inflammation to facilitate tissue repair. Nonclassical monocytes are largely thought to differentiate into M2 polarized macrophages. A third “intermediate” population of monocytes (CD14High CD16+ CX3CR1High CCR2Mid CCR5+) has been described as a biomarker in patients with chronic inflammatory diseases such as congestive heart failure (177) and rheumatoid arthritis (178). Human intermediate monocytes express the chemokine receptor CCR5 and can produce inflammatory cytokines, including TNF-α upon activation (178). However, the biological significance of these cells in inflammation and macrophage biology is not well known.
At present, the relative contribution of monocyte pools to the accumulation of macrophages in the inflamed and regenerating pancreas is not fully established. However, several lines of evidence suggest that monocyte recruitment likely contributes to macrophage infiltration into islets during obesity and T2D. First, monocytosis is clinical feature of obesity and T2D (179–183) and also a characteristic of rodent models (184–186). Recent studies have demonstrated that obesity and hyperglycemia promote myelopoiesis in mice, thereby resulting in an expansion in the pool of circulating Ly-6CHigh monocytes (184, 185). Notably, monocytes from mice with high-fat diet-induced obesity are predisposed to acquire a proinflammatory phenotype upon differentiation into macrophages (186, 187). Second, a number of monocyte chemokines are also induced in β-cell lines and pancreatic islets upon exposure to obesity or glucolipotoxicity (14, 188–190), and transgenic overexpression of CCL2 in β-cells promotes monocytosis and myeloid cell infiltration into islets in mice (191, 192). In the ethyl palmitate infusion model of glucolipotoxicity, β-cell secretion of CCL2 is elevated in a TLR4/Myd88-dependent manner, thereby promoting the recruitment of CD11b+Ly-6C+ macrophages to islets (18). Because Ly-6CHigh monocytes are CCR2+, these results suggest that the CCL2/CCR2 chemokine/receptor axis is involved in recruitment of inflammatory macrophages to the islet during lipotoxicity. Engagement of the CCL2/CCR2 axis in islet inflammation is not entirely surprising given that the same axis is involved in both recruitment of proinflammatory macrophages to visceral adipose tissue in obesity (50, 55, 69, 70) and foam cell formation in atherosclerosis (193–195). Collectively, these studies suggest that hyperglycemia- and/or hyperlipidemia-induced monocytosis and engagement of the CCL2/CCR2 axis by emerging monocytes is likely a key node in the accumulation of proinflammatory macrophages, islet inflammation, and β-cell dysfunction during obesity and T2D. Clearly, therapeutic interventions aimed at controlling monocytosis, particularly mobilization of inflammatory monocytes, should be examined as such therapies may be beneficial on multiple levels for persons with obesity and T2D (eg, improving peripheral insulin sensitivity, promoting β-cell survival, and attenuating atherogenesis).
Conclusions
Macrophages are key constituents of nearly every mammalian tissue. Tissue macrophages play many critical roles within the innate immune system, but also are involved in a vast array of functions including embryonic development and tissue repair. The studies highlighted in this review emphasize the emerging heterogeneity and functional plasticity of islet macrophages.
Although the current literature indicates that islet macrophages can be recruited to and activated in islets during obesity and T2D, this field is in its infancy. Many studies now clearly show that islet macrophages integrate signals from DAMPs (eg, β-cell death, hIAPP), PAMPs (eg, endotoxins), and metabolic cues (eg, glucolipoxocity) into a proinflammatory response that contributes to islet inflammation that promotes β-cell dysfunction and death. However, the integration of signals between macrophages, lymphocytes, and β-cells in the islet in T2D should also be considered. Emerging studies indicate that B and T lymphocytes accumulate in human islets from T2D donors (82), and lymphocytes from individuals with T2D display limited autoreactivity to β-cell antigens (196, 197). Islet macrophages have the capacity to present islet antigens (198), so these recent findings call into question whether cross talk between innate immune cells and adaptive/humeral immunity contributes to islet failure in T2D.
Although not stressed in this review, there is also emerging data that activated islet macrophages drive hyperglycemia in T2D by altering α-cell function. For example, IL-6 produced by TLR2/4-activated macrophages appears to induce glucagon secretion from α-cells and promote hyperglycemia in mice models (23, 59). These data suggest that we must consider the collective influence of macrophage-derived cytokines on all endocrine cells if islet inflammation is to be effectively targeted to restore functional endocrine cell mass and manage hyperglycemia in obesity and T2D.
Several reports also challenge the perception that macrophage accumulation in islets is purely a pathological feature of T2D. Rather, macrophages may be beneficial to islet physiology, particularly during development and pancreatic regeneration. Macrophages may also facilitate islet remodeling to accommodate β-cell expansion, and this process is often observed in mice models of pregnancy and in acute compensatory responses to obesity-induced insulin resistance. Clarifying these features of islet macrophages may improve therapeutic strategies aimed at expanding functional β-cell mass, including the use of iPCs. Ultimately, we may one day be able to harness the reparative and trophic features inherit to some islet macrophages to maintain or ultimately restore functional β-cell mass in diabetic islets.
In summary, rather than assume that islet macrophages are merely a pathological feature of diseased islets, it may be better to view islet macrophages as a fulcrum between deleterious islet inflammation and beneficial islet repair. In some settings, islet macrophages are activated and shifted towards a deleterious proinflammatory phenotype that promotes islet pathophysiology in obesity and T2D. In other settings, these cells can be shifted toward beneficial phenotypes that promote β-cell regeneration. Undoubtedly, defining the factors that contribute to the ontogeny, heterogeneity, and functionality of macrophages in normal, diseased, and regenerating islets will be necessary to determine whether, how, and by how much that fulcrum can be moved to preserve functional β-cell mass in persons with obesity and T2D.
Acknowledgments
I thank Dr Teresa L. Mastracci, Indiana University School of Medicine, for helpful discussions, and Dr John Spence, Indiana University School of Medicine, for proof reading this manuscript. I also thank the reviewers and editors of Molecular Endocrinology for their helpful comments. Thomas Weinzerl, Office of Visual Media at Indiana University School of Medicine, contributed to the illustrations in this review.
This work was supported by National Institutes of Health Grant DK100515.
Disclosure Summary: The author has nothing to disclose.
Footnotes
- ATMs
- adipose tissue macrophages
- CB1R
- cannabinoid CB1 receptor
- CCL
- chemokine [C-C motif] ligand
- CCR
- chemokine [C-C motif] receptor 2
- CSF
- colony-stimulating factor
- DAMP
- damage-associated molecular pattern
- E
- embryonic day
- EGF
- epidermal growth factor
- hIAPP
- human islet amyloid polypeptide
- HSC
- hematopoietic stem cell
- IL-1βR1
- IL-1β receptor
- iNOS
- inducible nitric oxide synthase
- iPC
- induced pluripotent stem cells
- LPS
- lipopolysaccharide
- Ngn3
- Neurogenin-3
- NLRP3
- NOD-like receptor protein 3
- PAMP
- pathogen-associated molecular pattern
- PDL
- pancreatic ductal ligation
- T1D
- type 1 diabetes
- T2D
- type 2 diabetes
- TLR
- Toll-like receptor
- VEGF
- vascular endothelial growth factor
- ZDF
- Zucker diabetic fatty.
References
- 1. Appels B, Burkart V, Kantwerk-Funke G, Funda J, Kolb-Bachofen V, Kolb H. Spontaneous cytotoxicity of macrophages against pancreatic islet cells. J Immunol. 1989;142:3803–3808. [PubMed] [Google Scholar]
- 2. Kolb H, Burkart V, Appels B, et al. Essential contribution of macrophages to islet cell destruction in vivo and in vitro. J Autoimmun. 1990;3(suppl 1):117–120. [DOI] [PubMed] [Google Scholar]
- 3. Burkart V, Kolb H. Macrophages in islet destruction in autoimmune diabetes mellitus. Immunobiology. 1996;195:601–613. [DOI] [PubMed] [Google Scholar]
- 4. Kaufman DB, Platt JL, Rabe FL, Dunn DL, Bach FH, Sutherland DE. Differential roles of Mac-1+ cells, and CD4+ and CD8+ T lymphocytes in primary nonfunction and classic rejection of islet allografts. J Exp Med. 1990;172:291–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Wu GS, Korsgren O, Zhang JG, Song ZS, Van Rooijen N, Tibell A. Role of macrophages and natural killer cells in the rejection of pig islet xenografts in mice. Transplant Proc. 2000;32:1069. [DOI] [PubMed] [Google Scholar]
- 6. Yi S, Hawthorne WJ, Lehnert AM, et al. T cell-activated macrophages are capable of both recognition and rejection of pancreatic islet xenografts. J Immunol. 2003;170:2750–2758. [DOI] [PubMed] [Google Scholar]
- 7. Donath MY, Ehses JA, Maedler K, et al. Mechanisms of β-cell death in type 2 diabetes. Diabetes. 2005;54(suppl 2):S108–S113. [DOI] [PubMed] [Google Scholar]
- 8. Donath MY, Böni-Schnetzler M, Ellingsgaard H, Ehses JA. Islet inflammation impairs the pancreatic β-cell in type 2 diabetes. Physiology. 2009;24:325–331. [DOI] [PubMed] [Google Scholar]
- 9. Dinarello CA, Donath MY, Mandrup-Poulsen T. Role of IL-1β in type 2 diabetes. Curr Opin Endocrinol Diabetes Obes. 2010;17:314–321. [DOI] [PubMed] [Google Scholar]
- 10. Odegaard JI, Chawla A. Connecting type 1 and type 2 diabetes through innate immunity. Cold Spring Harb Perspect Med. 2012;2:a007724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Ehses JA, Böni-Schnetzler M, Faulenbach M, Donath MY. Macrophages, cytokines and β-cell death in Type 2 diabetes. Biochem Soc Trans. 2008;36:340–342. [DOI] [PubMed] [Google Scholar]
- 12. Donath MY. Targeting inflammation in the treatment of type 2 diabetes. Diabetes Obes Metab. 2013;15(suppl 3):193–196. [DOI] [PubMed] [Google Scholar]
- 13. Eguchi K, Manabe I. Macrophages and islet inflammation in type 2 diabetes. Diabetes Obes Metab. 2013;15(suppl 3):152–158. [DOI] [PubMed] [Google Scholar]
- 14. Ehses JA, Perren A, Eppler E, et al. Increased number of islet-associated macrophages in type 2 diabetes. Diabetes. 2007;56:2356–2370. [DOI] [PubMed] [Google Scholar]
- 15. Richardson SJ, Willcox A, Bone AJ, Foulis AK, Morgan NG. Islet-associated macrophages in type 2 diabetes. Diabetologia. 2009;52:1686–1688. [DOI] [PubMed] [Google Scholar]
- 16. Kamata K, Mizukami H, Inaba W, et al. Islet amyloid with macrophage migration correlates with augmented β-cell deficits in type 2 diabetic patients. Amyloid. 2014;21:191–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Homo-Delarche F, Calderari S, Irminger JC, et al. Islet inflammation and fibrosis in a spontaneous model of type 2 diabetes, the GK rat. Diabetes. 2006;55:1625–1633. [DOI] [PubMed] [Google Scholar]
- 18. Eguchi K, Manabe I, Oishi-Tanaka Y, et al. Saturated fatty acid and TLR signaling link β cell dysfunction and islet inflammation. Cell Metab. 2012;15:518–533. [DOI] [PubMed] [Google Scholar]
- 19. Jourdan T, Godlewski G, Cinar R, et al. Activation of the Nlrp3 inflammasome in infiltrating macrophages by endocannabinoids mediates β cell loss in type 2 diabetes. Nat Med. 2013;19:1132–1140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Masters SL, Dunne A, Subramanian SL, et al. Activation of the NLRP3 inflammasome by islet amyloid polypeptide provides a mechanism for enhanced IL-1β in type 2 diabetes. Nat Immunol. 2010;11:897–904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Westwell-Roper C, Dai DL, Soukhatcheva G, et al. IL-1 blockade attenuates islet amyloid polypeptide-induced proinflammatory cytokine release and pancreatic islet graft dysfunction. J Immunol. 2011;187:2755–2765. [DOI] [PubMed] [Google Scholar]
- 22. Lee HM, Kim JJ, Kim HJ, Shong M, Ku BJ, Jo EK. Upregulated NLRP3 inflammasome activation in patients with type 2 diabetes. Diabetes. 2013;62:194–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Nackiewicz D, Dan M, He W, et al. TLR2/6 and TLR4-activated macrophages contribute to islet inflammation and impair β cell insulin gene expression via IL-1 and IL-6. Diabetologia. 2014;57:1645–1654. [DOI] [PubMed] [Google Scholar]
- 24. Westwell-Roper CY, Ehses JA, Verchere CB. Resident macrophages mediate islet amyloid polypeptide-induced islet IL-1β production and β-cell dysfunction. Diabetes. 2014;63:1698–1711. [DOI] [PubMed] [Google Scholar]
- 25. Banaei-Bouchareb L, Gouon-Evans V, Samara-Boustani D, et al. Insulin cell mass is altered in Csf1op/Csf1op macrophage-deficient mice. J Leukoc Biol. 2004;76:359–367. [DOI] [PubMed] [Google Scholar]
- 26. Criscimanna A, Coudriet GM, Gittes GK, Piganelli JD, Esni F. Activated macrophages create lineage-specific microenvironments for pancreatic acinar- and β-cell regeneration in mice. Gastroenterology 2014;147:1106–1118 e1111. [DOI] [PubMed] [Google Scholar]
- 27. Xiao X, Gaffar I, Guo P, et al. M2 macrophages promote β-cell proliferation by up-regulation of SMAD7. Proc Natl Acad Sci USA. 2014;111:E1211–E1220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Mantovani A, Sozzani S, Locati M, Allavena P, Sica A. Macrophage polarization: tumor-associated macrophages as a paradigm for polarized M2 mononuclear phagocytes. Trends Immunol. 2002;23:549–555. [DOI] [PubMed] [Google Scholar]
- 29. Martinez FO, Gordon S. The M1 and M2 paradigm of macrophage activation: time for reassessment. F1000Prime Rep. 2014;6:13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Liu G, Yang H. Modulation of macrophage activation and programming in immunity. J Cell Physiol. 2013;228:502–512. [DOI] [PubMed] [Google Scholar]
- 31. Mosser DM. The many faces of macrophage activation. J Leukoc Biol. 2003;73:209–212. [DOI] [PubMed] [Google Scholar]
- 32. Mosser DM, Edwards JP. Exploring the full spectrum of macrophage activation. Nat Rev Immunol. 2008;8:958–969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Colin S, Chinetti-Gbaguidi G, Staels B. Macrophage phenotypes in atherosclerosis. Immunol Rev. 2014;262:153–166. [DOI] [PubMed] [Google Scholar]
- 34. Zizzo G, Hilliard BA, Monestier M, Cohen PL. Efficient clearance of early apoptotic cells by human macrophages requires M2c polarization and MerTK induction. J Immunol. 2012;189:3508–3520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Grinberg S, Hasko G, Wu D, Leibovich SJ. Suppression of PLCβ2 by endotoxin plays a role in the adenosine A(2A) receptor-mediated switch of macrophages from an inflammatory to an angiogenic phenotype. Am J Pathol. 2009;175:2439–2453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Ferrante CJ, Leibovich SJ. Regulation of macrophage polarization and wound healing. Adv Wound Care (New Rochelle). 2012;1:10–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Stout RD, Watkins SK, Suttles J. Functional plasticity of macrophages: in situ reprogramming of tumor-associated macrophages. J Leukoc Biol. 2009;86:1105–1109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Murray PJ, Allen JE, Biswas SK, et al. Macrophage activation and polarization: nomenclature and experimental guidelines. Immunity. 2014;41:14–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Heng TS, Painter MW, Immunological Genome Project Consortium. The Immunological Genome Project: networks of gene expression in immune cells. Nat Immunol. 2008;9:1091–1094. [DOI] [PubMed] [Google Scholar]
- 40. Gazit R, Garrison BS, Rao TN, et al. Transcriptome analysis identifies regulators of hematopoietic stem and progenitor cells. Stem Cell Rep. 2013;1:266–280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Jojic V, Shay T, Sylvia K, et al. Identification of transcriptional regulators in the mouse immune system. Nat Immunol. 2013;14:633–643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Gautier EL, Shay T, Miller J, et al. Gene-expression profiles and transcriptional regulatory pathways that underlie the identity and diversity of mouse tissue macrophages. Nat Immunol. 2012;13:1118–1128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Miller JC, Brown BD, Shay T, et al. Deciphering the transcriptional network of the dendritic cell lineage. Nat Immunol. 2012;13:888–899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Kratz M, Coats BR, Hisert KB, et al. Metabolic dysfunction drives a mechanistically distinct proinflammatory phenotype in adipose tissue macrophages. Cell Metab. 2014;20:614–625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Ishii M, Wen H, Corsa CA, et al. Epigenetic regulation of the alternatively activated macrophage phenotype. Blood. 2009;114:3244–3254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Mullican SE, Gaddis CA, Alenghat T, et al. Histone deacetylase 3 is an epigenomic brake in macrophage alternative activation. Genes Dev. 2011;25:2480–2488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Kittan NA, Allen RM, Dhaliwal A, et al. Cytokine induced phenotypic and epigenetic signatures are key to establishing specific macrophage phenotypes. PLoS One. 2013;8:e78045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Bailey MJ, Lacey DC, de Kok BV, Veith PD, Reynolds EC, Hamilton JA. Extracellular proteomes of M-CSF (CSF-1) and GM-CSF-dependent macrophages. Immunol Cell Biol. 2011;89:283–293. [DOI] [PubMed] [Google Scholar]
- 49. Becker L, Liu NC, Averill MM, et al. Unique proteomic signatures distinguish macrophages and dendritic cells. PLoS One. 2012;7:e33297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Weisberg SP, McCann D, Desai M, Rosenbaum M, Leibel RL, Ferrante AW., Jr Obesity is associated with macrophage accumulation in adipose tissue. J Clin Invest. 2003;112:1796–1808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Xu H, Barnes GT, Yang Q, et al. Chronic inflammation in fat plays a crucial role in the development of obesity-related insulin resistance. J Clin Invest. 2003;112:1821–1830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Zeyda M, Stulnig TM. Adipose tissue macrophages. Immunol Lett. 2007;112:61–67. [DOI] [PubMed] [Google Scholar]
- 53. Morris DL, Singer K, Lumeng CN. Adipose tissue macrophages: phenotypic plasticity and diversity in lean and obese states. Curr Opin Clin Nutr Metab Care. 2011;14:341–346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Kraakman MJ, Murphy AJ, Jandeleit-Dahm K, Kammoun HL. Macrophage polarization in obesity and type 2 diabetes: weighing down our understanding of macrophage function? Front Immunol. 2014;5:470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Lumeng CN, Bodzin JL, Saltiel AR. Obesity induces a phenotypic switch in adipose tissue macrophage polarization. J Clin Invest. 2007;117:175–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Strissel KJ, Stancheva Z, Miyoshi H, et al. Adipocyte death, adipose tissue remodeling, and obesity complications. Diabetes. 2007;56:2910–2918. [DOI] [PubMed] [Google Scholar]
- 57. Shaul ME, Bennett G, Strissel KJ, Greenberg AS, Obin MS. Dynamic, M2-like remodeling phenotypes of CD11c+ adipose tissue macrophages during high-fat diet-induced obesity in mice. Diabetes. 2010;59:1171–1181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Westcott DJ, Delproposto JB, Geletka LM, et al. MGL1 promotes adipose tissue inflammation and insulin resistance by regulating 7/4hi monocytes in obesity. J Exp Med. 2009;206:3143–3156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Chow SZ. Glycoprotein 130 receptor signaling mediates alpha-cell dysfunction in a rodent model of type 2 diabetes. Diabetes. 2014;63:2984–2995. [DOI] [PubMed] [Google Scholar]
- 60. Dayer JM, Chicheportiche R, Juge-Aubry C, Meier C. Adipose tissue has anti-inflammatory properties: focus on IL-1 receptor antagonist (IL-1Ra). Ann NY Acad Sci. 2006;1069:444–453. [DOI] [PubMed] [Google Scholar]
- 61. Charo IF. Macrophage polarization and insulin resistance: PPARγ in control. Cell Metab. 2007;6:96–98. [DOI] [PubMed] [Google Scholar]
- 62. Odegaard JI, Ricardo-Gonzalez RR, Goforth MH, et al. Macrophage-specific PPARγ controls alternative activation and improves insulin resistance. Nature. 2007;447:1116–1120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Guri AJ, Hontecillas R, Ferrer G, et al. Loss of PPAR γ in immune cells impairs the ability of abscisic acid to improve insulin sensitivity by suppressing monocyte chemoattractant protein-1 expression and macrophage infiltration into white adipose tissue. J Nutr Biochem. 2008;19:216–228. [DOI] [PubMed] [Google Scholar]
- 64. Kang K, Reilly SM, Karabacak V, et al. Adipocyte-derived Th2 cytokines and myeloid PPARδ regulate macrophage polarization and insulin sensitivity. Cell Metab. 2008;7:485–495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Vats D, Mukundan L, Odegaard JI, et al. Oxidative metabolism and PGC-1β attenuate macrophage-mediated inflammation. Cell Metab. 2006;4:13–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Qiu Y, Nguyen KD, Odegaard JI, et al. Eosinophils and type 2 cytokine signaling in macrophages orchestrate development of functional beige fat. Cell. 2014;157:1292–1308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Lumeng CN, Deyoung SM, Bodzin JL, Saltiel AR. Increased inflammatory properties of adipose tissue macrophages recruited during diet-induced obesity. Diabetes. 2007;56:16–23. [DOI] [PubMed] [Google Scholar]
- 68. Lumeng CN, DelProposto JB, Westcott DJ, Saltiel AR. Phenotypic switching of adipose tissue macrophages with obesity is generated by spatiotemporal differences in macrophage subtypes. Diabetes. 2008;57:3239–3246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Weisberg SP, Hunter D, Huber R, et al. CCR2 modulates inflammatory and metabolic effects of high-fat feeding. J Clin Invest. 2005;116:115–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Tamura Y, Sugimoto M, Murayama T, et al. Inhibition of CCR2 ameliorates insulin resistance and hepatic steatosis in db/db mice. Arterioscler Thromb Vasc Biol. 2008;28:2195–2201. [DOI] [PubMed] [Google Scholar]
- 71. Kosteli A, Sugaru E, Haemmerle G, et al. Weight loss and lipolysis promote a dynamic immune response in murine adipose tissue. J Clin Invest. 2010;120:3466–3479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Cinti S, Mitchell G, Barbatelli G, et al. Adipocyte death defines macrophage localization and function in adipose tissue of obese mice and humans. J Lipid Res. 2005;46:2347–2355. [DOI] [PubMed] [Google Scholar]
- 73. Murano I, Barbatelli G, Parisani V, et al. Dead adipocytes, detected as crown-like structures (CLS), are prevalent in visceral fat depots of genetically obese mice. J Lipid Res. 2008;49:1562–1568. [DOI] [PubMed] [Google Scholar]
- 74. Lumeng CN, Deyoung SM, Saltiel AR. Macrophages block insulin action in adipocytes by altering expression of signaling and glucose transport proteins. Am J Physiol Endocrinol Metab. 2007;292:E166–E174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Wentworth JM, Naselli G, Brown WA, et al. Pro-inflammatory CD11c+CD206+ adipose tissue macrophages are associated with insulin resistance in human obesity. Diabetes. 2010;59:1648–1656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Vandanmagsar B, Youm YH, Ravussin A, et al. The NLRP3 inflammasome instigates obesity-induced inflammation and insulin resistance. Nat Med. 2011;17:179–188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Patsouris D, Li PP, Thapar D, Chapman J, Olefsky JM, Neels JG. Ablation of CD11c-positive cells normalizes insulin sensitivity in obese insulin resistant animals. Cell Metab. 2008;8:301–309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Wu H, Perrard XD, Wang Q, et al. CD11c expression in adipose tissue and blood and its role in diet-induced obesity. Arterioscler Thromb Vasc Biol. 2009;30:186–192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Cho KW, Morris DL, DelProposto JL, et al. An MHC II-dependent activation loop between adipose tissue macrophages and CD4(+) T cells controls obesity-induced inflammation. Cell Rep. 2014;9:605–617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Sun K, Kusminski CM, Scherer PE. Adipose tissue remodeling and obesity. J Clin Invest. 2011;121:2094–2101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Cildir G, Akıncılar SC, Tergaonkar V. Chronic adipose tissue inflammation: all immune cells on the stage. Trends Mol Med. 2013;19:487–500. [DOI] [PubMed] [Google Scholar]
- 82. Butcher MJ, Hallinger D, Garcia E, et al. Association of proinflammatory cytokines and islet resident leucocytes with islet dysfunction in type 2 diabetes. Diabetologia. 2014;57:491–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Cucak H, Grunnet LG, Rosendahl A. Accumulation of M1-like macrophages in type 2 diabetic islets is followed by a systemic shift in macrophage polarization. J Leukoc Biol. 2014;95:149–160. [DOI] [PubMed] [Google Scholar]
- 84. Cucak H, Mayer C, Tonnesen M, Thomsen LH, Grunnet LG, Rosendahl A. Macrophage contact dependent and independent TLR4 mechanisms induce β-cell dysfunction and apoptosis in a mouse model of type 2 diabetes. PLoS One. 2014;9:e90685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Donath MY. Targeting inflammation in the treatment of type 2 diabetes: time to start. Nat Rev Drug Discov. 2014;13:465–476. [DOI] [PubMed] [Google Scholar]
- 86. Böni-Schnetzler M, Boller S, Debray S, et al. Free fatty acids induce a proinflammatory response in islets via the abundantly expressed interleukin-1 receptor I. Endocrinology. 2009;150:5218–5229. [DOI] [PubMed] [Google Scholar]
- 87. Maedler K, Schumann DM, Sauter N, et al. Low concentration of interleukin-1β induces FLICE-inhibitory protein-mediated β-cell proliferation in human pancreatic islets. Diabetes. 2006;55:2713–2722. [DOI] [PubMed] [Google Scholar]
- 88. Arous C, Ferreira PG, Dermitzakis ET, Halban PA. Short term exposure of β cells to low concentrations of interleukin-1β improves insulin secretion through focal adhesion and actin remodeling and regulation of gene expression. J Biol Chem. 2015;290:6653–6669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Bendtzen K, Mandrup-Poulsen T, Nerup J, Nielsen JH, Dinarello CA, Svenson M. Cytotoxicity of human pI 7 interleukin-1 for pancreatic islets of Langerhans. Science. 1986;232:1545–1547. [DOI] [PubMed] [Google Scholar]
- 90. Mandrup-Poulsen T, Bendtzen K, Nerup J, Egeberg J, Nielsen JH. Mechanisms of pancreatic islet cell destruction. Dose-dependent cytotoxic effect of soluble blood mononuclear cell mediators on isolated islets of Langerhans. Allergy. 1986;41:250–259. [DOI] [PubMed] [Google Scholar]
- 91. Loweth AC, Williams GT, James RF, Scarpello JH, Morgan NG. Human islets of Langerhans express Fas ligand and undergo apoptosis in response to interleukin-1β and Fas ligation. Diabetes. 1998;47:727–732. [DOI] [PubMed] [Google Scholar]
- 92. Schroder K, Tschopp J. The inflammasomes. Cell. 2010;140:821–832. [DOI] [PubMed] [Google Scholar]
- 93. Hiscott J, Marois J, Garoufalis J, et al. Characterization of a functional NF-κ B site in the human interleukin 1 β promoter: evidence for a positive autoregulatory loop. Mol Cell Biol. 1993;13:6231–6240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Cani PD, Amar J, Iglesias MA, et al. Metabolic endotoxemia initiates obesity and insulin resistance. Diabetes. 2007;56:1761–1772. [DOI] [PubMed] [Google Scholar]
- 95. Creely SJ, McTernan PG, Kusminski CM, et al. Lipopolysaccharide activates an innate immune system response in human adipose tissue in obesity and type 2 diabetes. Am J Physiol Endocrinol Metab. 2007;292:E740–E747. [DOI] [PubMed] [Google Scholar]
- 96. Dasu MR, Devaraj S, Park S, Jialal I. Increased toll-like receptor (TLR) activation and TLR ligands in recently diagnosed type 2 diabetic subjects. Diabetes Care. 2010;33:861–868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Guzman-Ruiz R, Ortega F, Rodriguez A, et al. Alarmin high-mobility group B1 (HMGB1) is regulated in human adipocytes in insulin resistance and influences insulin secretion in β-cells. Int J Obes (Lond). 2014;38:1545–1554. [DOI] [PubMed] [Google Scholar]
- 98. Li M, Song L, Gao X, Chang W, Qin X. Toll-like receptor 4 on islet β cells senses expression changes in high-mobility group box 1 and contributes to the initiation of type 1 diabetes. Exp Mol Med. 2012;44:260–267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Martinon F, Burns K, Tschopp J. The inflammasome: a molecular platform triggering activation of inflammatory caspases and processing of proIL-β. Mol Cell. 2002;10:417–426. [DOI] [PubMed] [Google Scholar]
- 100. Schroder K, Zhou R, Tschopp J. The NLRP3 inflammasome: a sensor for metabolic danger? Science. 2010;327:296–300. [DOI] [PubMed] [Google Scholar]
- 101. Wen H, Gris D, Lei Y, et al. Fatty acid-induced NLRP3-ASC inflammasome activation interferes with insulin signaling. Nat Immunol. 2011;12:408–415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Westwell-Roper C, Nackiewicz D, Dan M, Ehses JA. Toll-like receptors and NLRP3 as central regulators of pancreatic islet inflammation in type 2 diabetes. Immunol Cell Biol. 2014;92:314–323. [DOI] [PubMed] [Google Scholar]
- 103. Youm YH, Adijiang A, Vandanmagsar B, Burk D, Ravussin A, Dixit VD. Elimination of the NLRP3-ASC inflammasome protects against chronic obesity-induced pancreatic damage. Endocrinology. 2011;152:4039–4045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Mariathasan S, Weiss DS, Newton K, et al. Cryopyrin activates the inflammasome in response to toxins and ATP. Nature. 2006;440:228–232. [DOI] [PubMed] [Google Scholar]
- 105. Zhou R, Tardivel A, Thorens B, Choi I, Tschopp J. Thioredoxin-interacting protein links oxidative stress to inflammasome activation. Nat Immunol. 2010;11:136–140. [DOI] [PubMed] [Google Scholar]
- 106. Kahn SE, Andrikopoulos S, Verchere CB. Islet amyloid: a long-recognized but underappreciated pathological feature of type 2 diabetes. Diabetes. 1999;48:241–253. [DOI] [PubMed] [Google Scholar]
- 107. Jaikaran ET, Clark A. Islet amyloid and type 2 diabetes: from molecular misfolding to islet pathophysiology. Biochim Biophys Acta. 2001;1537:179–203. [DOI] [PubMed] [Google Scholar]
- 108. Badman MK, Pryce RA, Chargé SB, Morris JF, Clark A. Fibrillar islet amyloid polypeptide (amylin) is internalised by macrophages but resists proteolytic degradation. Cell Tissue Res. 1998;291:285–294. [DOI] [PubMed] [Google Scholar]
- 109. Soeller WC, Janson J, Hart SE, et al. Islet amyloid-associated diabetes in obese A(vy)/a mice expressing human islet amyloid polypeptide. Diabetes. 1998;47:743–750. [DOI] [PubMed] [Google Scholar]
- 110. Hull RL, Andrikopoulos S, Verchere CB, et al. Increased dietary fat promotes islet amyloid formation and β-cell secretory dysfunction in a transgenic mouse model of islet amyloid. Diabetes. 2003;52:372–379. [DOI] [PubMed] [Google Scholar]
- 111. Woollard KJ, Geissmann F. Monocytes in atherosclerosis: subsets and functions. Nat Rev Cardiol. 2010;7:77–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. King IL, Dickendesher TL, Segal BM. Circulating Ly-6C+ myeloid precursors migrate to the CNS and play a pathogenic role during autoimmune demyelinating disease. Blood. 2009;113:3190–3197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Zigmond E, Varol C, Farache J, et al. Ly6C hi monocytes in the inflamed colon give rise to proinflammatory effector cells and migratory antigen-presenting cells. Immunity. 2012;37:1076–1090. [DOI] [PubMed] [Google Scholar]
- 114. Westwell-Roper CY, Chehroudi CA, Denroche HC, Courtade JA, Ehses JA, Verchere CB. IL-1 mediates amyloid-associated islet dysfunction and inflammation in human islet amyloid polypeptide transgenic mice. Diabetologia. 2015;58:575–585. [DOI] [PubMed] [Google Scholar]
- 115. Di Marzo V. The endocannabinoid system in obesity and type 2 diabetes. Diabetologia. 2008;51:1356–1367. [DOI] [PubMed] [Google Scholar]
- 116. Christofori G, Naik P, Hanahan D. Vascular endothelial growth factor and its receptors, flt-1 and flk-1, are expressed in normal pancreatic islets and throughout islet cell tumorigenesis. Mol Endocrinol. 1995;9:1760–1770. [DOI] [PubMed] [Google Scholar]
- 117. Inoue M, Hager JH, Ferrara N, Gerber HP, Hanahan D. VEGF-A has a critical, nonredundant role in angiogenic switching and pancreatic β cell carcinogenesis. Cancer Cell. 2002;1:193–202. [DOI] [PubMed] [Google Scholar]
- 118. Brissova M, Shostak A, Shiota M, et al. Pancreatic islet production of vascular endothelial growth factor–a is essential for islet vascularization, revascularization, and function. Diabetes. 2006;55:2974–2985. [DOI] [PubMed] [Google Scholar]
- 119. Iwashita N, Uchida T, Choi JB, et al. Impaired insulin secretion in vivo but enhanced insulin secretion from isolated islets in pancreatic β cell-specific vascular endothelial growth factor-A knock-out mice. Diabetologia. 2007;50:380–389. [DOI] [PubMed] [Google Scholar]
- 120. Jabs N, Franklin I, Brenner MB, et al. Reduced insulin secretion and content in VEGF-a deficient mouse pancreatic islets. Exp Clin Endocrinol Diabetes. 2008;116(suppl 1):S46–S49. [DOI] [PubMed] [Google Scholar]
- 121. Christoffersson G, Vagesjo E, Vandooren J, et al. VEGF-A recruits a proangiogenic MMP-9-delivering neutrophil subset that induces angiogenesis in transplanted hypoxic tissue. Blood. 2012;120:4653–4662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Nakamura M, Kitamura H, Konishi S, et al. The endocrine pancreas of spontaneously diabetic db/db mice: microangiopathy as revealed by transmission electron microscopy. Diabetes Res Clin Pract. 1995;30:89–100. [DOI] [PubMed] [Google Scholar]
- 123. Ko SH, Kwon HS, Kim SR, et al. Ramipril treatment suppresses islet fibrosis in Otsuka Long-Evans Tokushima fatty rats. Biochem Biophys Res Commun. 2004;316:114–122. [DOI] [PubMed] [Google Scholar]
- 124. Li X, Zhang L, Meshinchi S, et al. Islet microvasculature in islet hyperplasia and failure in a model of type 2 diabetes. Diabetes. 2006;55:2965–2973. [DOI] [PubMed] [Google Scholar]
- 125. Agudo J, Ayuso E, Jimenez V, et al. Vascular endothelial growth factor-mediated islet hypervascularization and inflammation contribute to progressive reduction of β-cell mass. Diabetes. 2012;61:2851–2861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Brissova M, Aamodt K, Brahmachary P, et al. Islet microenvironment, modulated by vascular endothelial growth factor-A signaling, promotes β cell regeneration. Cell Metab. 2014;19:498–511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Rae F, Woods K, Sasmono T, et al. Characterisation and trophic functions of murine embryonic macrophages based upon the use of a Csf1r-EGFP transgene reporter. Dev Biol. 2007;308:232–246. [DOI] [PubMed] [Google Scholar]
- 128. Lang RA, Bishop JM. Macrophages are required for cell death and tissue remodeling in the developing mouse eye. Cell. 1993;74:453–462. [DOI] [PubMed] [Google Scholar]
- 129. Marín-Teva JL, Dusart I, Colin C, Gervais A, van Rooijen N, Mallat M. Microglia promote the death of developing Purkinje cells. Neuron. 2004;41:535–547. [DOI] [PubMed] [Google Scholar]
- 130. Jones CV, Ricardo SD. Macrophages and CSF-1: implications for development and beyond. Organogenesis. 2013;9:249–260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. Ojalvo LS, King W, Cox D, Pollard JW. High-density gene expression analysis of tumor-associated macrophages from mouse mammary tumors. Am J Pathol. 2009;174:1048–1064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. Wiktor-Jedrzejczak W, Bartocci A, Ferrante AW, Jr., et al. Total absence of colony-stimulating factor 1 in the macrophage-deficient osteopetrotic (op/op) mouse. Proc Natl Acad Sci USA. 1990;87:4828–4832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. Geutskens SB, Otonkoski T, Pulkkinen MA, Drexhage HA, Leenen PJ. Macrophages in the murine pancreas and their involvement in fetal endocrine development in vitro. J Leukoc Biol. 2005;78:845–852. [DOI] [PubMed] [Google Scholar]
- 134. Gittes GK. Developmental biology of the pancreas: a comprehensive review. Dev Biol. 2009;326:4–35. [DOI] [PubMed] [Google Scholar]
- 135. Kim SK, MacDonald RJ. Signaling and transcriptional control of pancreatic organogenesis. Curr Opin Genet Dev. 2002;12:540–547. [DOI] [PubMed] [Google Scholar]
- 136. Banaei-Bouchareb L, Peuchmaur M, Czernichow P, Polak M. A transient microenvironment loaded mainly with macrophages in the early developing human pancreas. J Endocrinol. 2006;188:467–480. [DOI] [PubMed] [Google Scholar]
- 137. Pollard JW, Hennighausen L. Colony stimulating factor 1 is required for mammary gland development during pregnancy. Proc Natl Acad Sci USA. 1994;91:9312–9316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138. Gouon-Evans V, Rothenberg ME, Pollard JW. Postnatal mammary gland development requires macrophages and eosinophils. Development. 2000;127:2269–2282. [DOI] [PubMed] [Google Scholar]
- 139. Mussar K, Tucker A, McLennan L, et al. Macrophage/epithelium cross-talk regulates cell cycle progression and migration in pancreatic progenitors. PLoS One. 2014;9:e89492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140. Gradwohl G, Dierich A, LeMeur M, Guillemot F. neurogenin3 is required for the development of the four endocrine cell lineages of the pancreas. Proc Natl Acad Sci USA. 2000;97:1607–1611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141. Jacquemin P, Durviaux SM, Jensen J, et al. Transcription factor hepatocyte nuclear factor 6 regulates pancreatic endocrine cell differentiation and controls expression of the proendocrine gene ngn3. Mol Cell Biol. 2000;20:4445–4454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142. Zhang H, Ables ET, Pope CF, et al. Multiple, temporal-specific roles for HNF6 in pancreatic endocrine and ductal differentiation. Mech Dev. 2009;126:958–973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143. Dor Y, Brown J, Martinez OI, Melton DA. Adult pancreatic β-cells are formed by self-duplication rather than stem-cell differentiation. Nature. 2004;429:41–46. [DOI] [PubMed] [Google Scholar]
- 144. Teta M, Rankin MM, Long SY, Stein GM, Kushner JA. Growth and regeneration of adult β cells does not involve specialized progenitors. Dev Cell. 2007;12:817–826. [DOI] [PubMed] [Google Scholar]
- 145. Kushner JA. The role of aging upon β cell turnover. J Clin Invest. 2013;123:990–995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146. Cnop M, Hughes SJ, Igoillo-Esteve M, et al. The long lifespan and low turnover of human islet β cells estimated by mathematical modelling of lipofuscin accumulation. Diabetologia. 2010;53:321–330. [DOI] [PubMed] [Google Scholar]
- 147. Soggia A, Hoarau E, Bechetoille C, Simon MT, Heinis M, Duvillie B. Cell-based therapy of diabetes: what are the new sources of β cells? Diabetes Metab. 2011;37:371–375. [DOI] [PubMed] [Google Scholar]
- 148. Bouwens L, Houbracken I, Mfopou JK. The use of stem cells for pancreatic regeneration in diabetes mellitus. Nat Rev Endocrinol. 2013;9:598–606. [DOI] [PubMed] [Google Scholar]
- 149. Gioviale MC, Bellavia M, Damiano G, Lo Monte AI. Beyond islet transplantation in diabetes cell therapy: from embryonic stem cells to transdifferentiation of adult cells. Transplant Proc. 2013;45:2019–2024. [DOI] [PubMed] [Google Scholar]
- 150. Santini MP, Rosenthal N. Myocardial regenerative properties of macrophage populations and stem cells. J Cardiovasc Transl Res. 2012;5:700–712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151. Miron VE. Dissecting the damaging versus regenerative roles of CNS macrophages: implications for the use of immunomodulatory therapeutics. Regen Med. 2013;8:673–676. [DOI] [PubMed] [Google Scholar]
- 152. Brown BN, Sicari BM, Badylak SF. Rethinking regenerative medicine: a macrophage-centered approach. Front Immunol. 2014;5:510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153. Wang RN, Klöppel G, Bouwens L. Duct- to islet-cell differentiation and islet growth in the pancreas of duct-ligated adult rats. Diabetologia. 1995;38:1405–1411. [DOI] [PubMed] [Google Scholar]
- 154. Xiao X, Wiersch J, El-Gohary Y, et al. TGFβ receptor signaling is essential for inflammation-induced but not β-cell workload-induced β-cell proliferation. Diabetes. 2013;62:1217–1226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155. Van Gassen N, Van Overmeire E, Leuckx G, et al. Macrophage dynamics are regulated by local macrophage proliferation and monocyte recruitment in injured pancreas. Eur J Immunol. 2015;45:1482–1493. [DOI] [PubMed] [Google Scholar]
- 156. Sorenson RL, Brelje TC. Adaptation of islets of Langerhans to pregnancy: β-cell growth, enhanced insulin secretion and the role of lactogenic hormones. Horm Metab Res. 1997;29:301–307. [DOI] [PubMed] [Google Scholar]
- 157. Sone H, Kagawa Y. Pancreatic β cell senescence contributes to the pathogenesis of type 2 diabetes in high-fat diet-induced diabetic mice. Diabetologia. 2005;48:58–67. [DOI] [PubMed] [Google Scholar]
- 158. Yi P, Park JS, Melton DA. Betatrophin: a hormone that controls pancreatic β cell proliferation. Cell. 2013;153:747–758. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 159. Jiao Y, Le Lay J, Yu M, Naji A, Kaestner KH. Elevated mouse hepatic betatrophin expression does not increase human β-cell replication in the transplant setting. Diabetes. 2014;63:1283–1288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160. Lewis CE, Pollard JW. Distinct role of macrophages in different tumor microenvironments. Cancer Res. 2006;66:605–612. [DOI] [PubMed] [Google Scholar]
- 161. Siveen KS, Kuttan G. Role of macrophages in tumour progression. Immunol Lett. 2009;123:97–102. [DOI] [PubMed] [Google Scholar]
- 162. Suganami T, Ogawa Y. Adipose tissue macrophages: their role in adipose tissue remodeling. J Leukoc Biol. 2010;88:33–39. [DOI] [PubMed] [Google Scholar]
- 163. Van Overmeire E, Laoui D, Keirsse J, Van Ginderachter JA, Sarukhan A. Mechanisms driving macrophage diversity and specialization in distinct tumor microenvironments and parallelisms with other tissues. Front Immunol. 2014;5:127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164. Bird L. Macrophages: yolky beginnings. Nat Rev Immunol. 2012;12:322–323. [DOI] [PubMed] [Google Scholar]
- 165. Epelman S, Lavine KJ, Randolph GJ. Origin and functions of tissue macrophages. Immunity. 2014;41:21–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166. Gentek R, Molawi K, Sieweke MH. Tissue macrophage identity and self-renewal. Immunol Rev. 2014;262:56–73. [DOI] [PubMed] [Google Scholar]
- 167. Samokhvalov IM, Gomez Perdiguero E, Chorro L, et al. Deconvoluting the ontogeny of hematopoietic stem cells. Cell Mol Life Sci. 2014;71:957–978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168. Schulz C, Gomez Perdiguero E, Chorro L, et al. A lineage of myeloid cells independent of Myb and hematopoietic stem cells. Science. 2012;336:86–90. [DOI] [PubMed] [Google Scholar]
- 169. Alliot F, Godin I, Pessac B. Microglia derive from progenitors, originating from the yolk sac, and which proliferate in the brain. Brain Res Dev Brain Res. 1999;117:145–152. [DOI] [PubMed] [Google Scholar]
- 170. Ginhoux F, Greter M, Leboeuf M, et al. Fate mapping analysis reveals that adult microglia derive from primitive macrophages. Science. 2010;330:841–845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171. Yona S, Kim KW, Wolf Y, et al. Fate mapping reveals origins and dynamics of monocytes and tissue macrophages under homeostasis. Immunity. 2013;38:79–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172. Hashimoto D, Chow A, Noizat C, et al. Tissue-resident macrophages self-maintain locally throughout adult life with minimal contribution from circulating monocytes. Immunity. 2013;38:792–804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173. Haase J, Weyer U, Immig K, et al. Local proliferation of macrophages in adipose tissue during obesity-induced inflammation. Diabetologia. 2014;57:562–571. [DOI] [PubMed] [Google Scholar]
- 174. Auffray C, Fogg D, Garfa M, et al. Monitoring of blood vessels and tissues by a population of monocytes with patrolling behavior. Science. 2007;317:666–670. [DOI] [PubMed] [Google Scholar]
- 175. De Kleer I, Willems F, Lambrecht B, Goriely S. Ontogeny of myeloid cells. Front Immunol. 2014;5:423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176. Yang J, Zhang L, Yu C, Yang XF, Wang H. Monocyte and macrophage differentiation: circulation inflammatory monocyte as biomarker for inflammatory diseases. Biomark Res. 2014;2:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177. Barisione C, Garibaldi S, Ghigliotti G, et al. CD14CD16 monocyte subset levels in heart failure patients. Dis Markers. 2010;28:115–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178. Rossol M, Kraus S, Pierer M, Baerwald C, Wagner U. The CD14(bright) CD16+ monocyte subset is expanded in rheumatoid arthritis and promotes expansion of the Th17 cell population. Arthritis Rheum. 2012;64:671–677. [DOI] [PubMed] [Google Scholar]
- 179. Kullo IJ, Hensrud DD, Allison TG. Comparison of numbers of circulating blood monocytes in men grouped by body mass index (<25, 25 to <30, > or =30). Am J Cardiol. 2002;89:1441–1443. [DOI] [PubMed] [Google Scholar]
- 180. Poitou C, Dalmas E, Renovato M, et al. CD14dimCD16+ and CD14+CD16+ monocytes in obesity and during weight loss: relationships with fat mass and subclinical atherosclerosis. Arterioscler Thromb Vasc Biol. 2011;31:2322–2330. [DOI] [PubMed] [Google Scholar]
- 181. Yang M, Gan H, Shen Q, Tang W, Du X, Chen D. Proinflammatory CD14+CD16+ monocytes are associated with microinflammation in patients with type 2 diabetes mellitus and diabetic nephropathy uremia. Inflammation. 2012;35:388–396. [DOI] [PubMed] [Google Scholar]
- 182. Krinninger P, Ensenauer R, Ehlers K, et al. Peripheral monocytes of obese women display increased chemokine receptor expression and migration capacity. J Clin Endocrinol Metab. 2014;99:2500–2509. [DOI] [PubMed] [Google Scholar]
- 183. Terasawa T, Aso Y, Omori K, Fukushima M, Momobayashi A, Inukai T. Bezafibrate, a peroxisome proliferator-activated receptor α agonist, decreases circulating CD14CD16 monocytes in patients with type 2 diabetes. Transl Res. 2015;165:336–345. [DOI] [PubMed] [Google Scholar]
- 184. Nagareddy PR, Murphy AJ, Stirzaker RA, et al. Hyperglycemia promotes myelopoiesis and impairs the resolution of atherosclerosis. Cell Metab. 2013;17:695–708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185. Nagareddy PR, Kraakman M, Masters SL, et al. Adipose tissue macrophages promote myelopoiesis and monocytosis in obesity. Cell Metab. 2014;19:821–835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186. Singer K, DelProposto J, Morris DL, et al. Diet-induced obesity promotes myelopoiesis in hematopoietic stem cells. Mol Metab. 2014;3:664–675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187. Gallagher KA, Joshi A, Carson WF, et al. Epigenetic changes in bone marrow progenitor cells influence the inflammatory phenotype and alter wound healing in type 2 diabetes. Diabetes. 2015;64:1420–1430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188. Igoillo-Esteve M, Marselli L, Cunha DA, et al. Palmitate induces a pro-inflammatory response in human pancreatic islets that mimics CCL2 expression by β cells in type 2 diabetes. Diabetologia. 2010;53:1395–1405. [DOI] [PubMed] [Google Scholar]
- 189. Li J, Chen L, Zhang Y, et al. TLR4 is required for the obesity-induced pancreatic β cell dysfunction. Acta Biochim Biophys Sin (Shanghai). 2013;45:1030–1038. [DOI] [PubMed] [Google Scholar]
- 190. Meier DT, Morcos M, Samarasekera T, Zraika S, Hull RL, Kahn SE. Islet amyloid formation is an important determinant for inducing islet inflammation in high-fat-fed human IAPP transgenic mice. Diabetologia. 2014;57:1884–1888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191. Grewal IS, Rutledge BJ, Fiorillo JA, et al. Transgenic monocyte chemoattractant protein-1 (MCP-1) in pancreatic islets produces monocyte-rich insulitis without diabetes: abrogation by a second transgene expressing systemic MCP-1. J Immunol. 1997;159:401–408. [PubMed] [Google Scholar]
- 192. Martin AP, Rankin S, Pitchford S, Charo IF, Furtado GC, Lira SA. Increased expression of CCL2 in insulin-producing cells of transgenic mice promotes mobilization of myeloid cells from the bone marrow, marked insulitis, and diabetes. Diabetes. 2008;57:3025–3033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193. Boring L, Gosling J, Cleary M, Charo IF. Decreased lesion formation in CCR2−/− mice reveals a role for chemokines in the initiation of atherosclerosis. Nature. 1998;394:894–897. [DOI] [PubMed] [Google Scholar]
- 194. Tacke F, Alvarez D, Kaplan TJ, et al. Monocyte subsets differentially employ CCR2, CCR5, and CX3CR1 to accumulate within atherosclerotic plaques. J Clin Invest. 2007;117:185–194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195. Saederup N, Chan L, Lira SA, Charo IF. Fractalkine deficiency markedly reduces macrophage accumulation and atherosclerotic lesion formation in CCR2−/− mice: evidence for independent chemokine functions in atherogenesis. Circulation. 2008;117:1642–1648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196. Brooks-Worrell BM, Boyko EJ, Palmer JP. Impact of islet autoimmunity on the progressive β-cell functional decline in type 2 diabetes. Diabetes Care. 2014;37:3286–3293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197. Sarikonda G, Pettus J, Phatak S, et al. CD8 T-cell reactivity to islet antigens is unique to type 1 while CD4 T-cell reactivity exists in both type 1 and type 2 diabetes. J Autoimmun. 2014;50:77–82. [DOI] [PubMed] [Google Scholar]
- 198. Calderon B, Carrero JA, Unanue ER. The central role of antigen presentation in islets of Langerhans in autoimmune diabetes. Curr Opin Immunol. 2014;26:32–40. [DOI] [PMC free article] [PubMed] [Google Scholar]