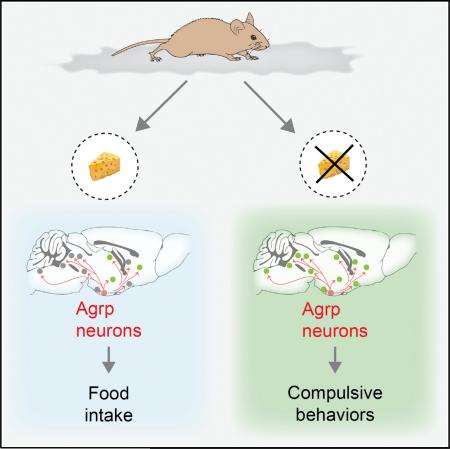
SUMMARY
The nervous system evolved to coordinate flexible goal-directed behaviors by integrating interoceptive and sensory information. Hypothalamic Agrp neurons are known to be crucial for feeding behavior. Here, however, we show that these neurons also orchestrate other complex behaviors in adult mice. Activation of Agrp neurons in the absence of food triggers foraging and repetitive behaviors, which are reverted by food consumption. These stereotypic behaviors that are triggered by Agrp neurons are coupled with decreased anxiety. NPY5 receptor signaling is necessary to mediate the repetitive behaviors after Agrp neuron activation while having minor effects on feeding. Thus, we have unmasked a functional role for Agrp neurons in controlling repetitive behaviors mediated, at least in part, by neuropeptidergic signaling. The findings reveal a new set of behaviors coupled to the energy homeostasis circuit and suggest potential therapeutic avenues for diseases with stereotypic behaviors.
Graphical Abstract
INTRODUCTION
Neural circuits are responsible for organizing and regulating flexible goal-oriented behaviors by integrating sensory and interoceptive information. The observation that mice can perform complex dynamic computations similar to humans (Kheifets and Gallistel, 2012) supports the view that brain mechanisms involved in complex goal-oriented behaviors rely on phylogenetically primitive neural circuits.
Homeostatic functions—for example, food intake—are adaptive responses that allow successful survival of the individual in the environment. The hypothalamus is an ancient brain region present in all vertebrates that is critical for the regulation of homeostatic functions, including energy balance, sexual behavior, sleep, and thirst. For more than 20 years, hypothalamic neurons that produce NPY, Agrp, and GABA have been thought to be involved in the promotion of hunger (Hahn et al., 1998; Horvath et al., 1992; Horvath et al., 1997). Neuropeptide injections in the brain elicit robust increases in food intake (Clark et al., 1984; Ollmann et al., 1997; Rossi et al., 1998; Stanley et al., 1986), and food deprivation increases the activity of these neurons (Hahn et al., 1998; Liu et al., 2012; Takahashi and Cone, 2005; Yang et al., 2011). Acute (Gropp et al., 2005; Luquet et al., 2005), but not chronic (Xu et al., 2005), ablation of Agrp neurons leads to cessation of feeding and, ultimately, death (Luquet et al., 2005). Conversely, acute activation of these neurons induces robust feeding (Aponte et al., 2011; Krashes et al., 2011). The neural circuits involved in the regulation of hunger by Agrp neurons seem to involve several brain nuclei (Atasoy et al., 2012; Betley et al., 2013; Wu et al., 2012). Agrp neurons have a broad projection field (Broberger et al., 1998) with important developmental characteristics as well (Dietrich et al., 2012; Grove et al., 2001). It is, therefore, intuitive to postulate that Agrp neurons orchestrate complex behavioral and physiological changes that encompass hunger rather than just food intake. This hypothesis gains momentum when neuropsychiatric conditions with strong homeostatic components are considered (e.g., anorexia nervosa). For instance, anorexia nervosa is a state of severe negative energy balance, in which brain circuits controlling feeding may be involved in the development of cognitive impairments of this disorder.
Here, we tested these assumptions by performing analysis of mouse behavior under conditions of Agrp neuron activation. Our results uncover a fundamental role for Agrp neuron activation in promoting repetitive/stereotypic behaviors in mice, unmasking a previously unsuspected role for these hypothalamic neurons.
RESULTS
Hunger-Related Behaviors
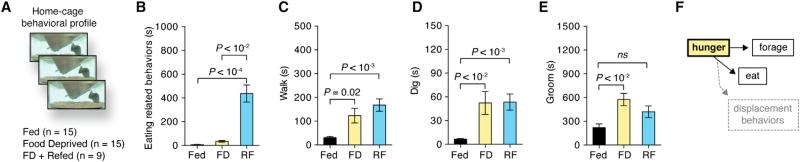
We first determined the effects of food deprivation, a physiological state of elevated Agrp neuronal activity (Hahn et al., 1998; Takahashi and Cone, 2005), on behavior. We used software-assisted characterization of mouse home-cage behaviors (Adamah-Biassi et al., 2013; Jhuang et al., 2010; Kyzar et al., 2012) to assess different aspects of the behavioral repertoire that occurs during hunger (Figure 1A). We studied fed, food deprived (FD), and food-deprived mice that were re-fed (RF). We divided our analysis into three large groups of behaviors: (1) consummatory responses represented by eating-related behaviors (e.g., time spent in the eating zone and chewing); (2) appetitive behaviors (forage-related behaviors, e.g., digging and walking); and (3) displacement behaviors (e.g., grooming). As expected, fed and FD animals did not engage in eating-related behaviors when food was not presented in the home cage, an effect promptly reverted in re-fed animals (Figure 1B). Food deprivation stimulated forage-related behaviors, an effect that persisted in the re-fed group (Figures 1C and 1D). Because our analyses lasted for 1 hr after the introduction of food to mice, our data indicate that the mechanisms involved in foraging behaviors during food deprivation are slowly switched off by satiety and not acutely by immediate presentation of food. Food deprivation also exacerbated grooming behavior (Figure 1E). In such conditions, grooming has been considered a displacement behavior (Barnett, 1956), a substitute of consummatory eating. Re-feeding acutely attenuated grooming (Figure 1E), reinforcing that displacement behaviors, such as grooming, manifest when animals lack the consummatory response. Thus, hunger promotes foraging (appetitive), eating (consummatory), and grooming (displacement) behaviors in mice (Figure 1F). Because the activation of Agrp neurons promotes hunger in sated mice (Aponte et al., 2011; Krashes et al., 2009; Krashes et al., 2011), we next asked what aspects of the behavior repertoire promoted by food deprivation may be induced by acute activation of the Agrp neurons.
Figure 1. Home-Cage Behaviors in Food-Deprived and Re-Fed Mice.
(A) Mouse behaviors in the home cage of fed (black bars), food-deprived (yellow bars), and re-fed (blue bars) mice.
(B–E) Time spent in (B) eating-related behaviors, (C) walking, (D) digging, and (E) grooming.
(F) Behaviors elicited by hunger states.
Error bars represent mean ± SEM. p values represent Holm-Sidak's multiple comparisons test.
Acute Agrp Neuronal Activation
Agrp neurons have a broad projection field (Broberger et al., 1998), which extends to a wide range of subcortical areas (Figure 2A). This complex connectivity indicates that Agrp neurons have the capability to modulate a broad range of behaviors using multiple parallel circuits. In a previous study, we showed that Agrp neurons influence motivational states not related to feeding—for example, responses to cocaine (Dietrich et al., 2012). As an underlying mechanism, our data indicated that Agrp neurons have a developmental effect on dopamine cell function. These data reinforce the notion that animal models with altered Agrp neuronal activity during development are not suitable for the study of their acute role in the adult (Dietrich et al., 2012). Here, to examine the acute effects of Agrp neurons on adult animal behavior, we utilized animal models that allowed activation of Agrp neurons in a rapid, reliable, and reproducible manner.
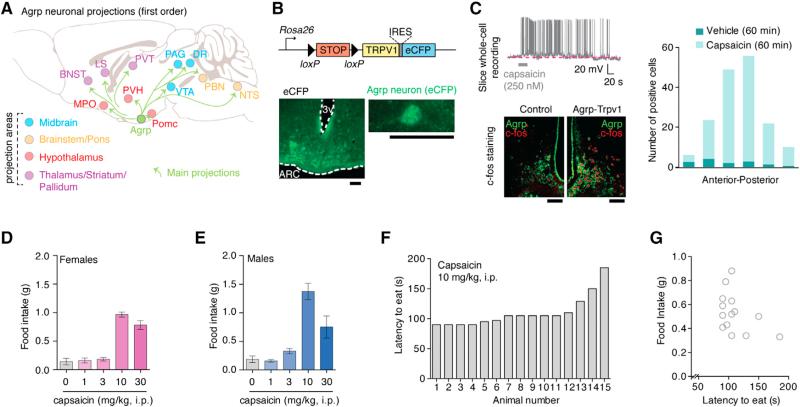
Figure 2. Trpv1 Channels in Agrp Neurons Allow Acute Control of Neuronal Activity.
(A) Main projection from Agrp neurons.
(B) Reporter Trpv1 mice and CFP staining in the arcuate nucleus of Agrp-Trvp1 mice.
(C) Whole-cell recording of an Agrp-Trpv1 neuron and c-fos staining in Agrp-Trpv1-HA reporter mice 60 min after capsaicin injection (10 mg.kg, i.p.).
(D and E) Food intake in (D) female Agrp-Trpv1 and in (E) male mice.
(F) Latency to eat in female Agrp-Trpv1 mice.
(G) Correlation between latency and food intake.
Error bars represent mean ± SEM. Scale bars, 50 μm. See also Figure S1 and Movie S1.
Several techniques have been developed to acutely manipulate neuronal function in vivo. Optogenetics (Aponte et al., 2011) and chemical genetics using designer receptors exclusively activated by designer drugs (DREADDs) (Krashes et al., 2011) have been used to study the effects of Agrp neuron activity on the feeding behavior of adult mice. Optogenetics provide good time resolution with early onset of feeding behavior (Aponte et al., 2011); however, it requires the insertion of a light source deep into the brain, which adds a bias when analyzing complex behaviors. On the other hand, DREADD can be used to activate Agrp neurons by peripherally injecting receptor-ligand with robust induction of food intake (Krashes et al., 2011) but with more coarse kinetics (Rogan and Roth, 2011). We used transgenic mice that conditionally express Trpv1 in Cre-expressing cells (Arenkiel et al., 2008; Güler et al., 2012) (R26-LSL-Trpv1; Figure 2B) to selectively introduce Trpv1 in Agrp neurons. By backcrossing these mice (R26-LSL-Trpv1) to a Trpv1 knockout background and then to Agrp-Cre mice, we generated animals that express Trpv1 exclusively in the Agrp neurons (hereafter, Agrp-Trpv1 mice; Figures 2B and S1). We performed a series of control experiments to confirm that expression of Trpv1 was restricted to Agrp neurons in the arcuate nucleus and not in off-target cells (Figure S1 and Experimental Procedures). Trpv1 is a cation channel that is activated by the exogenous agonist capsaicin (Caterina et al., 1997) in a rapid and reversible manner (Güler et al., 2012). Slice whole-cell recordings showed that capsaicin increased the firing rate of Agrp neurons (Figure 2C). The analysis of c-fos expression in Agrp neurons after capsaicin injection (i.p.) in Agrp-Trpv1 mice revealed that most Agrp neurons throughout the arcuate nucleus were activated in these transgenic mice (Figure 2C). Capsaicin injection of Agrp-Trpv1 mice led to increased food intake in both female (Figure 2D) and male mice (Figure 2E and Movie S1). Notably, the amount of food consumed by the activation of Agrp neurons in our studies was of similar magnitude as that observed when these cells were activated by optogenetics or DREADDs (Aponte et al., 2011; Krashes et al., 2011). The latency to eat in Agrp-Trvp1 mice was faster (mean = 110.1 s [95% CI = 95.5–124.6], n = 15 mice) compared to these other techniques (Aponte et al., 2011; Krashes et al., 2011) (Figures 2F and 2G). Thus, this animal model enabled us to rapidly and reliably activate Agrp neurons by peripheral injection of capsaicin and explore their role on behaviors.
Repertoire of Home-Cage Behaviors
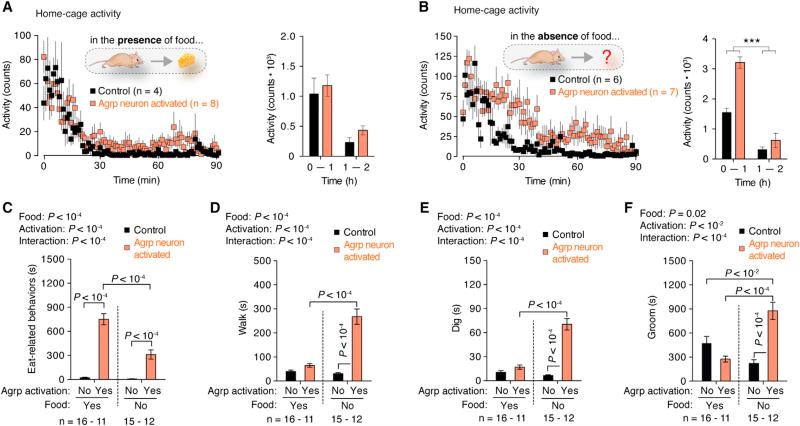
To screen for broad changes in behavior after Agrp neuron activation, we investigated changes in home-cage behaviors in the presence or absence of food in sated mice (Figure 3). In the presence of food, activation of Agrp neurons did not statistically change ambulatory activity (Figure 3A), while it evoked feeding in all Agrp-Trvp1 mice tested. Conversely, when food was removed, Agrp neuron activation increased activity levels (Figure 3B). To dissect these behavioral changes, we characterized mouse behaviors in their home cages upon activation of the Agrp neurons, similarly to what we did in FD mice (Figure 1). These experiments were performed in sated mice provided with food or with an empty food container. In all Agrp-Trpv1 mice tested in this paradigm, when food was present in their home cage, injection of capsaicin evoked robust food intake (data not shown). As expected, consummatory aspects of feeding, as measured by eating-related behaviors, were greatly enhanced by Agrp neuron activation (Figure 3C). Interestingly, activation of Agrp neurons in sated mice in the absence of food also led to increases in eating-related behaviors (e.g., interaction with the empty food container and chew bedding material; Figure 3C). The persistence of these behaviors indicates a degree of repetitiveness and stereotypy in the behavior repertoire of Agrp neuron activated animals in the absence of food.
Figure 3. Home-Cage Behavior Analysis of Agrp Neuronal Activated Mice.
(A) Activity in the home cage with food provided.
(B) Activity with no food provided.
(C) Eat-related behaviors.
(D) Time walking.
(E) Time digging.
(F) Time grooming.
Symbols and bars represent mean ± SEM. Statistical data derived from two-way ANOVA and Holm-Sidak's multiple comparisons test. See also Figure S2.
Forage-related behaviors were increased in Agrp-neuron-activated mice in the absence of food, an effect that was almost completely reverted in the presence of food (Figures 3D and 3E). Grooming also increased after treatment of Agrp-Trpv1 mice with capsaicin in the absence of food but decreased when animals were provided food (Figure 3F). Grooming is considered a displacement behavior to attenuate the appetitive response (forage) in the absence of the stimulus (food). When manifested in excess, grooming has also been related to obsessive-compulsive behaviors in mice (Ahmari et al., 2013; Burguière et al., 2013), similar to digging (Karvat and Kimchi, 2012). Thus, our findings indicate that, in addition to appetitive and consummatory aspects of hunger, the activation of Agrp neurons in Agrp-Trpv1 mice is sufficient to drive repetitive/stereotypic behaviors, an unsuspected role for these hypothalamic neurons. To corroborate these findings, we expressed hM3Dq in Agrp neurons by injecting Agrp-Cre mice with a recombinant AAV vector carrying a cre-dependent coding sequence (rAAV-FLEX- hM3Dq-mCherry). The activation of Agrp neurons by peripheral injection of the receptor ligand, clozapine-N-oxide (CNO, 0.3 mg/kg, i.p), led to similar results as observed in Agrp-Trpv1 mice injected with capsaicin (Figure S2) but with a delayed response, consistent with the slow effect of hM3Dq in stimulating neuronal activity (Krashes et al., 2011; Rogan and Roth, 2011). Altogether, we conclude that activation of the Agrp neurons resembles many, but not all, aspects of food deprivation. Our findings place interoceptive regions of the mammalian brain, such as the arcuate nucleus of the hypothalamus, as crucial mediators of repetitive and stereotypic behaviors (Figures 3C and 3F). Thus, we set out to investigate these behavioral responses in greater detail.
Agrp Neurons Trigger Repetitive Behaviors
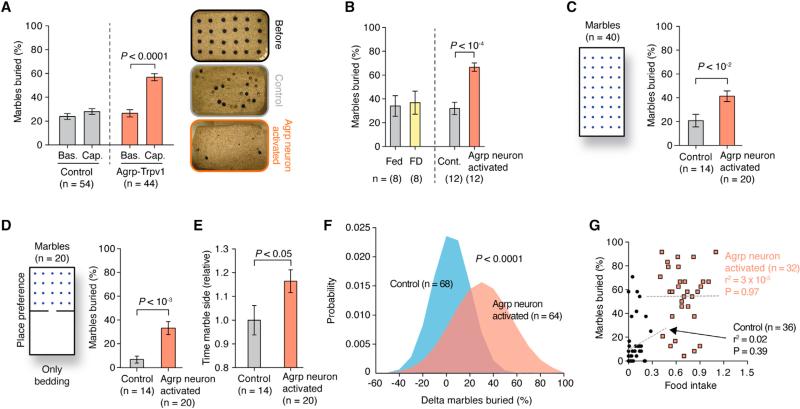
To further evaluate the extent to which the activation of Agrp neurons can engage mice in repetitive behaviors, we tested Agrp-Trpv1 mice in the marble-burying test (Deacon, 2006; Gyertyán, 1995; Witkin, 2008). The activation of Agrp neurons led to a robust increase in the number of marbles buried by males (Figure 4A and Movies S2 and S3) and females (data not shown), an effect that was, at least, in the same order of magnitude as mouse models of obsessive-compulsive disorders (Amodeo et al., 2012). Because food deprivation increases digging and grooming in the absence of food (Figure 1), which can also be considered repetitive behaviors (Ahmari et al., 2013; Burguière et al., 2013; Karvat and Kimchi, 2012), we tested food-deprived mice in parallel to Agrp-neuron-activated mice in the marble-burying test. We did not find statistical differences in the number of marbles buried after food deprivation (Figure 4B). To further test whether the increase in marble-burying behavior was due to repetitiveness, we performed a modified marble-burying test. We assessed mice in a larger cage with 40 marbles, which decreases the overall number of marbles buried and increases exploratory behavior. We found similar data in this modified version of the marble-burying test, with activation of Agrp neurons increasing the number of marbles buried (Figure 4C) while decreasing total activity during the test (control = 42.96 ± 3.12 m [n = 14], Agrp-Trpv1 = 32.27 ± 2.05 m [n = 20, mean ± SEM]; p = 0.004, two-tailed Mann-Whitney test), likely due to the extended time that mice spent burying marbles rather than exploring the arena. To test whether chronic negative energy balance impacts Agrp neuron activation responses, we placed animals on a 20% calorie-restricted regimen for 4 weeks and then tested them. Similar to the ad libitum fed animals (Figure 4), the activation of Agrp neurons by capsaicin increased marble-burying behavior in calorie-restricted mice (Figure S3). These results, together with the data gained in sated mice, argue for the importance of Agrp neuronal activity rather than metabolic state per se as a controller of stereotypic behaviors.
Figure 4. Repetitive Behaviors after Agrp Neuron Activation.
(A) Marbles buried after Agrp neuron activation.
(B) Marble buried in fed, food-deprived (FD), control, and Agrp-neuron-activated mice.
(C) Marble buried in the modified marble-burying test.
(D) Marble buried in the modified place-preference test.
(E) Time animals spent in the marble side relative to control animals.
(F) Normal distribution fitted to pooled experimental data (delta marbles buried [capsaicin injection – baseline]). p value was calculated using unpaired t test with Welch's correction.
(G) Linear regression analysis correlating marble-burying behavior and food intake. Each data point represents one mouse. Female mice were used in this study.
Error bars represent mean ± SEM, and p values were calculated using t test. See also Figure S3 and Movies S2 and S3.
To further investigate whether the increase in marble burying was due to a goal-oriented repetitive behavior (to bury marbles) (Gyertyán, 1995; Londei et al., 1998; Thomas et al., 2009), we performed a place preference test (Figure 4D). Marbles were distributed on only one side of the cage, and bedding was present on both sides. Agrp-Trvp1 mice that received capsaicin buried a much larger number of marbles (Figure 4D) and spent ~16% more time on the marble side of the chamber (Figure 4E) than control mice. Notably, even with only half of the cage covered with marbles (Figure 4D), the number of marbles buried did not differ from the previous experiment (Figure 4C) in Agrp-Trpv1 mice injected with capsaicin )full cage = 41.25% ± 4.55% [n = 20]; half cage = 33.00% ± 5.45% [n = 20, mean ± SEM]; p = 0.183, two-tailed Mann-Whitney test) but decreased in the control group (full cage = 20.89% ± 5.25% [n = 14]; half cage = 6.78% ± 2.80% [n = 14, mean ± SEM]; p = 0.01, two-tailed Mann-Whitney test], indicating that the activation of Agrp neurons directs the animal's behavior toward repetitive, stereotypic responses when food is not available.
We hypothesized that, if Agrp neuron-mediated feeding and repetitive behaviors are a result of the same brain circuit, then these two behaviors should be correlated. We took advantage of the marble-burying behavior to test repetitive responses in mice. Frequency distribution histograms show a shift to the right in the number of marbles buried in Agrp-neuron-activated mice (Figure 4F), highlighting the idea that these behavioral changes are variable and affect differently subpopulations of mice. Linear regression analysis of individual responses did not show a correlation between marble-burying and feeding behaviors (Figure 4G), suggesting that the brain circuits that drive these behaviors by Agrp neurons are distinct and not completely overlapping.
Agrp Neuron Activation Decreases Anxiety
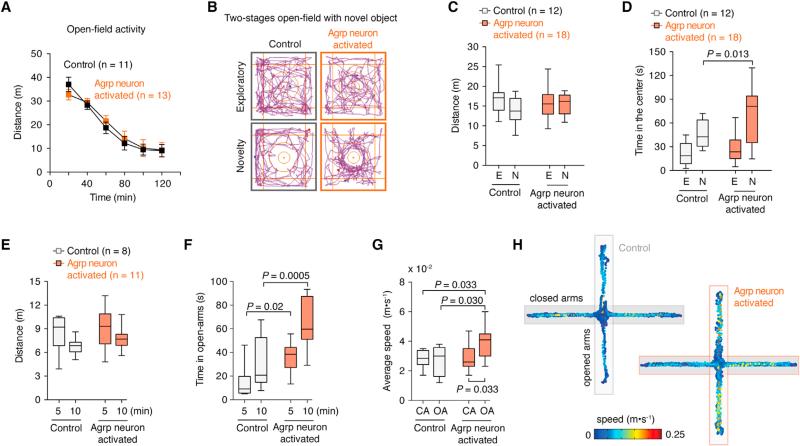
It is possible that changes in repetitive and stereotypic behaviors observed after Agrp neuron activation are due to increased anxiety. It is expected that treatments that increase anxiety levels will also increase repetitive/stereotypic responses in mice. Hunger is an unpleasant physiological state. Thus, it is possible that the promotion of hunger by activation of Agrp neurons generates an anxiogenic state in mice that leads to repetitive behaviors, as described above. To test anxiety-related behaviors, we performed a series of tests. First, we placed mice in a novel open-field exploratory test following activation of Agrp neurons by capsaicin. We did not find significant changes in total activity (Figure 5A) or time that animals explored the center of the arena (data not shown). We then put mice in a two-stage open-field test, in which a novel object is added to the center of the arena to induce novelty exploration and anxiety (Dietrich et al., 2012). In this test, activation of Agrp neurons increased the time that animals spent exploring the object (Figures 5B and 5D), but not total activity (Figure 5C). This indicates a decrease in anxiety levels compared to control mice. Next, we assessed mice in the zero- and plus-maze apparatuses, in which anxiety-related behaviors inversely correlate with the time that animals spend in the open arms. In both tests, we did not observe significant changes in activity levels between groups (Figures 5E and S4 and Movies S4 and S5), but we found that the activation of Agrp neurons increased the time in the open arms (Figures 5F and S4). Intriguingly, Agrp-neuron-activated mice accelerated once in the open arms (Figures 5G-H), perhaps due to changes in risk assessment. This hypothesis needs further investigation. Overall, the data show that activation of Agrp neurons in mice leads to repetitive behaviors that are not due to increases in anxiety levels. Conversely, the activation of Agrp neurons is anxiolytic in several behavior tests.
Figure 5. Activation of Agrp Neurons Decreases Anxiety-Related Behaviors.
(A) Activity in the open field. Data points represent mean ± SEM.
(B) Two-stage open-field test.
(C) Total distance traveled in the two-stage open-field test.
(D) Time spent in the center of the open field.
(E) Distance traveled by mice in the plus-maze test.
(F) Time animals spent in the open arms.
(G) Average speed of mice in the close arms (CA) and open arms (OA) of the apparatus.
(H) Representative tracking data.
See also Figure S4 and Movies S4 and S5. Box and whiskers represent median ± min/max values. p values were calculated using two-way ANOVA with repeated-measures followed by Holm-Sidak's multiple comparisons test.
Alleviation of Behaviors by Y5 Receptor Antagonist
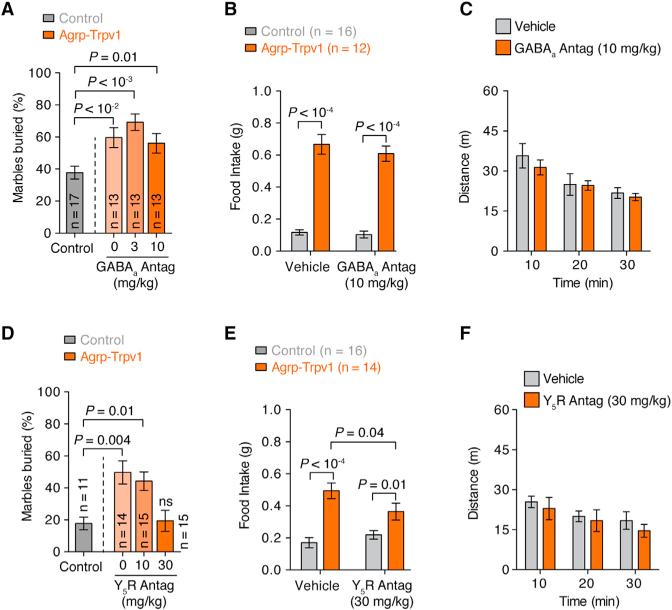
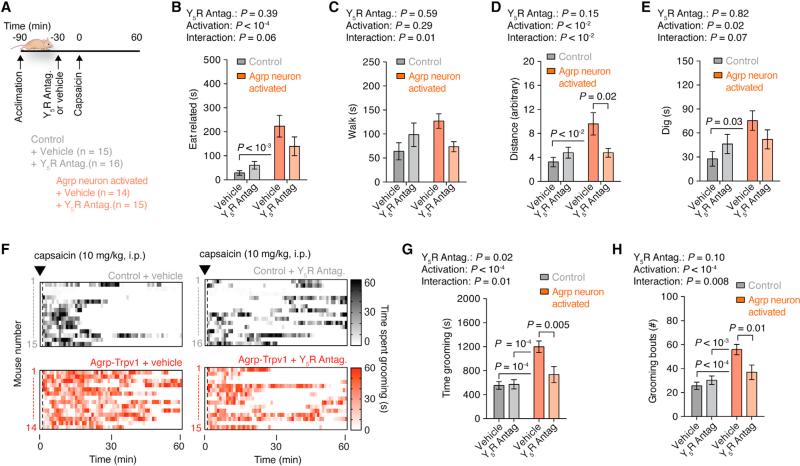
Agrp neurons have been shown to induce voracious food intake after acute activation due to NPY and GABA release (Aponte et al., 2011; Krashes et al., 2013). Because animal models in which GABA and/or NPY signaling is removed from Agrp neurons have developmental consequences (Atasoy et al., 2012; Dietrich et al., 2012), we examined whether pharmacological blockage of these signaling pathways would prevent repetitive behaviors after Agrp neuron activation. Systemic injection of a GABAA receptor antagonist was unable to reverse the induction of marble-burying behavior (Figure 6A) and food intake (Figure 6B) in Agrp-Trpv1 mice injected with capsaicin. NPY from the arcuate nucleus seems to signal mostly through NPY1 and NPY5 receptors, with overlapping expression and function (Atasoy et al., 2012; Gerald et al., 1996; Kanatani et al., 2000; Pedrazzini et al., 1998; Wolak et al., 2003). We have shown an anatomical link between the lateral hypothalamic orexin/hypocretin neurons and the arcuate nucleus NPY/Agrp cells (Horvath et al., 1999). Neuropeptides released by orexin/hypocretin neurons promote feeding, an effect that we showed to be diminished by administration of a NPY5 receptor antagonist (Dube et al., 2000). These previous observations together with the translatability of NPY5 receptor antagonists (Erondu et al., 2006) led us to interrogate the role of NPY5 receptor signaling in behavioral changes mediated by Agrp neuron activation. Systemic injection of a NPY5 receptor antagonist before activation of Agrp neurons was sufficient to block the increase in marble-burying behavior (Figure 6D) while slightly decreasing food intake (Figure 6E). Neither GABAA receptor nor NPY5 receptor antagonists altered locomotor activity in an open field at the maximum dose used in this study (Figures 6C and 6F). These results indicate that NPY5 receptor signaling is necessary for the repetitive behaviors induced by the activation of Agrp neurons. To further evaluate the participation of NPY5 receptor signaling in the behavior repertoire of mice after Agrp neuronal activation, we scrutinized mouse behavior in the home cage. We treated mice with the NPY5 receptor blocker before activating Agrp neurons by capsaicin in Agrp-Trpv1 mice (Figure 7A). While activation of Agrp neurons increased eating-related (Figure 7B) and foraging-related behaviors (Figures 7C–7E), blockage of NPY5 receptor signaling attenuated all of these behavioral responses with no effects in control mice (Figures 7B–7E). Remarkably, the effects of Agrp neuron activation on grooming were completely reverted by systemic injection of NPY5 receptor blocker (Figures 7F–7H), similar to the effects reported in the marble-burying experiment (Figure 6D). Thus, we found that activation of Agrp neurons leads to repetitive behaviors, a behavioral phenotype that is completely reverted by NPY5 receptor blockade. Notably, treatment of control mice with a NPY5 receptor antagonist did not significantly alter baseline behaviors, but only behaviors driven by Agrp neuron activation. Because feeding response is not fully reverted by blocking NPY5 receptor signaling (Figure 6E) and because repetitive and feeding responses are not correlated behaviors (Figure 4G), our data provide further support for the idea that different Agrp neuronal subpopulations promote food intake versus repetitive/stereotypic behaviors (Figure S5).
Figure 6. Effects of GABAA or NPY5 Receptors Blockade in Agrp-Neuron-Activated Mice.
(A) Effect of the GABAA receptor blocker, bicuculline, in the marble-burying test after activation of Agrp neurons.
(B) Effect of bicuculline on food intake.
(C) Effect of bicuculline on locomotor activity.
(D) Similar to A but using the NPY5 receptor antagonist (CGP71683 hydrochloride).
(E) Similar to B using CGP71683.
(F) Similar to C using CGP71683.
Error bars represent mean ± SEM. p values were calculated using one-way ANOVA in A and D and two-way ANOVA with repeated-measures in B, C, E, and F followed by Holm-Sidak's multiple comparisons test.
Figure 7. NPY5 Receptor Signaling Is Necessary for Agrp-Neuron-Mediated Behaviors.
(A) Protocol to record home-cage behaviors using CGP71683 (30 mg/kg, i.p).
(B) Time spent in eating-related behaviors.
(C) Time spent walking.
(D) Total traveled distance.
(E) Time spent digging.
(F) Raster plots showing grooming behavior in individual mouse.
(G) Time spent grooming.
(H) Grooming bouts.
Error bars represent mean ± SEM. p values were calculated using two-way ANOVA followed by Holm-Sidak's multiple comparisons test and are reported in the panels. See also Figure S5.
DISCUSSION
The hypothalamus integrates hormonal and ascending neural inputs that bring information from the periphery (Chaudhri et al., 2006; Coll et al., 2007; Dietrich and Horvath, 2009; Lam et al., 2005). Our findings highlight the importance of Agrp neurons in mediating the effect of the peripheral environment on complex brain functions and behaviors. Our results identified the hypothalamic Agrp neurons as initiators of stereotypic behaviors in mice. These behaviors were triggered when the vast majority of Agrp neurons were simultaneously activated. Some aspects of the stereotypic behaviors induced by chemical genetic activation of Agrp neurons were not seen in food-deprived animals or calorie-restricted mice. These observations suggest that different subpopulations of Agrp neurons subserve different functions, and it is likely that their activity patterns are not synchronized and are under differential input control. The fact that some behavioral shifts induced by Agrp neuronal activation can while others cannot be suppressed by a NPY5 receptor blocker further argue for the segregation of function of different subpopulations of Agrp cells. Thus, it is anticipated that an intricate and highly complex input organization and efferent connectivity of various sub-populations of Agrp neurons exists to support predictable and dynamic behavioral and autonomic adaptations to the changing environment (Figure S5).
Our results unmasked a previously unsuspected role for the hypothalamic hunger-promoting neurons in controlling repetitive, stereotypic behaviors in mice. Also, we showed that the activation of Agrp neurons decreases anxiety levels in several tests in mice. Because the hypothalamus is an evolutionarily conserved brain region, it is likely that these results are relevant to higher-order organisms, including humans. A recent report reinforces this view by providing evidence that mice are capable of estimating probabilities and calculating risks to make behavioral adjustments in dynamic environments analogous to humans (Kheifets and Gallistel, 2012). This supports the argument that brain mechanisms involved in complex behaviors are phylogenetically preserved. It is relevant to note, however, that our behavior tests were performed in animals in isolation, and not in a social context. It will be important to study whether these neurons also participate in social behaviors. Additionally, it remains to be tested whether the role of Agrp neurons in feeding and/or repetitive/stereotypic behaviors are influenced by the social context. At present, these studies are extremely challenging to perform in mice (Anderson and Perona, 2014). With the advent of technology and emerging tools to analyze animal behavior, future studies dissecting the role of Agrp neurons (as well as other brain circuits) on behaviors in social settings are of utmost relevance and priority for our understanding of brain function.
Our data also suggest that these ancient brain regions play a role in psychiatric conditions. Specifically, misalignments between environmental cues (peripheral tissue function) and hypothalamic circuits may lead to maladaptive behaviors, including those associated with psychiatric and neurological disorders. Regarding the latter, we suggest that our results have implications for the etiology of anorexia nervosa. Patients suffering from this condition avoid ingesting calories despite the fact that they have elevated activity and a higher physiological state of hunger.
Because hunger signals activate Agrp neurons (Hahn et al., 1998; Liu et al., 2012; Takahashi and Cone, 2005; Yang et al., 2011), we postulate that, in individuals with a vulnerability to develop anorexia nervosa, Agrp neurons may respond to negative energy balance cues in an exacerbated manner and lead to repetitive and compulsive behaviors (Halmi et al., 2003; Matsunaga et al., 1999; Thiel et al., 1995). Future studies are needed to interrogate whether inert differences in Agrp neuronal excitability exist between vulnerable and invulnerable individuals. From this perspective, it is of interest to note that patients with anorexia nervosa have elevated circulating blood levels of Agrp compared to controls (Merle et al., 2011; Moriya et al., 2006) and that Agrp levels are associated with cognitive rigidity in these patients (Sarrar et al., 2011). Because NPY5 receptor antagonists have been tested in humans (Erondu et al., 2006) and we found it to reverse many Agrp activation-triggered stereotypic behaviors, we suggest that human clinical trials with safe compounds can be initiated for addressing the behavioral aspects of anorexia nervosa as well as other neuropsychiatric diseases with both homeostatic and behavioral components.
EXPERIMENTAL PROCEDURES
Mice
All mice used in the experiments were 2–6 months old from both genders. We did not observe differences in the responses of males and females to capsaicin. Agrp-Trpv1 mice were: AgrpCreTm/+::Trpv1−/−::R26-LSL-Trpv1Gt/+; control animals were either Agrp-Trpv1 mice injected with vehicle (3.3% Tween 80 in saline) or Trpv1−/−:R26-LSL-Trpv1Gt/+ mice injected with capsaicin. All animals were littermates (Agrp neuron activated and controls) in the experiments. We did not observe any differences between the two control groups, and therefore, throughout the manuscript we referred to them as “controls.” The following mouse lines were used in this study: Agrptm1(cre)Lowl/J, Gt(ROSA)26Sortm1(Trpv1,ECFP)Mde/J, Trpv1tm1Jul/J, Rpl22tm1.1Psam/J, Tg(Npy-MAPT/Sapphire)1Rck/J. All animals were kept in temperature- and humidity-controlled rooms, in a 12/12 hr light/dark cycle, with lights on from 7:00 AM–7:00 PM. Food and water were provided ad libitum unless otherwise stated. All procedures were approved by IACUC (Yale University).
Immunohistochemistry
Mice were deeply anesthetized and perfused with 0.9% saline containing heparin followed by freshly prepared fixative (paraformaldehyde 4%, picric acid 15%, in PB 0.1M [pH = 7.4]). Brains were post-fixed overnight in fixative. Coronal brain sections (50 μm) were washed several times in PB 0.1M (pH = 7.4) and pre-incubated with Triton X-100 for 30 min. Sections were then washed several times and blocked with 2% normal goat serum and incubated with chicken anti-GFP (1:8,000, 4°C, 48 hr; ABCAM), rabbit anti-cfos (1:20,000 at 4°C for 48 hr; Oncogene), and/or mouse anti-HA (1:1,000 dilution at RT for 24 hr; Covance). After, sections were extensively washed and incubated with secondary fluorescent Alexa antibodies (1:500). Sections were mounted, coverslipped, and visualized by a Zeiss microscope or an Olympus Confocal microscope.
Drugs
Drugs used were: capsaicin (3.33% Tween-80 in PBS; from Sigma), Bicuculline methiodide (in saline; from Sigma), and CGP71683 hydrochloride (in 5% DMSO, 5% Tween-80 in water; from Tocris). All drugs were injected in a volume of 10 ml/kg of body weight intraperitoneally (i.p.).
Food Intake
For the capsaicin dose-response experiment, mice were acclimated to metabolic chambers (TSE Systems) before recordings. Mice received vehicle or capsaicin (3, 10, and 30 mg/kg, i.p.), and food intake was automatically recorded (see Movie S1). Alternatively, food intake was manually recorded in single-housed mice. Bedding was changed 24 hr before the experiment, and animals were acclimated for at least 1 week with a minimum quantity of food in the cage to alleviate spillage. On the day of the experiments, food was removed 1 hr before the test and food intake was recorded before and 1 hr after capsaicin injection.
Electrophysiology
Four-week-old Agrp-Cretm/+::Trpv1−/−::R26-LSL-Trpv1Gt/+::NpyGFPTg/+ mice were killed at the beginning of the light cycle, and the arcuate nucleus was sliced into 250 μm slices, containing GFP cells. After stabilization in ACSF, slices were transferred to the recording chamber and perfused with ACSF. Basal firing rate was recorded for at least 5 min. The slice was then incubated with a pulse of capsaicin (0.25 μM), followed by a washout. Whole-cell current-clamp recording was performed using low-resistance (3–4 MΩ) pipettes. The composition of the pipette solution was as follows (in mM): K-gluconate125, MgCl2 2, HEPES 10, EGTA 1.1, Mg-ATP 4, and Na2-phosphocreatin 10, Na2-GTP 0.5 (pH 7.3) with KOH. The composition of the bath solution was as follows (in mM): NaCl 124, KCl 3, CaCl2 2, MgCl2 2, NaH2PO4 1.23, glucose 2.5, sucrose 7.5, NaHCO3 26. After a gigaohm (GΩ) seal and whole-cell access were achieved, membrane potential and action potentials were recorded under current clamp at 0 pA. All data were sampled at 3–10 kHz and filtered at 1–3 kHz. Electrophysiological data were analyzed with Axo-graph 4.9.
Home-Cage Behavior
Four-month-old Agrp-Trpv1 or control female mice were singly housed in their normal home cage 11 days prior to the start of the first behavioral study. Animals were acclimated to handling for 1 week before experiments. The day preceding the behavioral analysis, the mice were given fresh bedding. For a 1 hr acclimation period, cages were placed in front of the cameras of the HomeCageScan system (CleverSys, Reston, VA) and were backlit by IR light panels. Mice were injected with either 10 mg/kg capsaicin or vehicle and recorded for 1 hr. Food was removed for the acclimation period as well as the analysis period for groups reported as “no food.” Mice in the fasted study were fasted for 16 hr prior to the experiment, and the re-fed group was given food at the time of injection. The NPY5 receptor blocker (CGP71683 hydrochloride, 30 mg/kg, i.p.) was given to the animals 30 min prior to capsaicin injection. Videos were analyzed with the HomeCageScan software (v3.00).
Marble-Burying Test
Marble-burying test was as described (Deacon, 2006) with modifications. Mice were tested (baseline) and randomized to groups. Capsaicin (10 mg/kg, i.p.) was injected immediately before test. Drugs were injected 20 (for bicuculline) or 30 min (for CGP71683 hydrochloride) before capsaicin. Modified marble-burying test was performed in a rat cage containing 40 evenly distributed marbles. Place preference was performed in the same rat cage divided using a separator with an open door. Marble side contained 20 marbles. All studies were performed in cages containing 5 cm of corn-based animal bedding.
Calorie Restriction
Female mice (9 weeks old) were housed two-by-two to avoid chronic stress due to social isolation. We have used the balanced NIH-41 diet (3.34 kcal/g, protein 16.9%, fat 12.5%, fiber 3.8%, nitrogen-free extract 53.6%, vitamins, minerals) to avoid malnourishment during calorie restriction due to insufficient nutrient levels. Mice received 20% less calories than their ad libitum food intake baseline measurements. The marble-burying test was performed on the last days of the study (a baseline was recorded without injection, and on the next day mice were tested after capsaicin injection). We used the modified marble-burying test with a rat cage containing 40 marbles (as described above).
DREADD Experiment
Recombinant rAAV5-Ef1a-DIO-hm3D(Gq)-mcherry virus (500 nl from UNC Viral Core) was injected bilaterally into the arcuate nucleus of Agrp-Cre male mice (AP = 1.40 mm; DV = −5.90 mm; L = ± 0.30 mm). Animals were allowed to recover for 3 weeks. All mice were singly housed in their normal home cage 3 weeks prior to the start of the first home-cage behavioral study. Two days preceding the behavioral analysis, the mice were given fresh bedding. Home-cage behaviors were analyzed as above. Mice were injected (i.p.) with either 0.3 mg/kg CNO (n = 7) or saline (n = 4) and recorded during 2 hr with no food available. Mice were later tested for feeding response and showed robust induction of food intake after CNO injection (data not shown). Infection was confirmed by visualizing mCherry in the arcuate nucleus. Clozapine N-oxide (CNO) was from Enzo Life Science.
Locomotor Activity
Mice were allowed to explore a novel environment (a rat cage, 45 × 24 × 20 cm) for 120 min after capsaicin injection. To test the side effects of the receptor blockers in locomotor activity, animals received an injection of bicuculline methiodide (10 mg/kg, i.p) or vehicle (PBS) 20 min before experiment. CGP71683 hydrochloride (30 mg/kg, i.p) or vehicle (5% DMSO, 5% tween-80 in water) were injected 30 min prior to the experiment. Male mice were used in these experiments (n = 25, 3–4 months old) and were allowed to explore the apparatus for 30 min. The experiment was performed under dim light during the light cycle.
Two-Stages Open-Field Test
The apparatus consists of a Plexiglas open-field (37 × 37 × 37 cm). Mice were first put in the open field for 5 min (“exploratory stage”). Immediately after, mice were returned to their home cages for 2 min. A new object (a cylinder of 5 cm radius and 10 cm high) was placed in the center of the arena. Mice were then returned to the open field for an additional 5 min (“novelty stage”). The room was illuminated with infrared lights and dim red light.
Elevated Plus Maze and Zero Maze
The plus maze consisted of four elevated arms (40 cm from the floor, 25 cm long, and 5.2 cm wide) arranged at right angles. Two opposite arms were enclosed by 15-cm high walls, and the other two were open (no walls). Male control (n = 8) and Agrp-Trpv1 (n = 11) mice (3–4 months old) were placed on the 5 × 5 cm center section and allowed to explore the apparatus. The zero maze consisted of an elevated circular platform with two opposite quadrants enclosed and two open, allowing uninterrupted exploration. The apparatus has a 50 cm diameter, 5 cm lane width, 15 cm wall height, and 40 cm elevation (from Stoeling, #68016). Capsaicin (10 mg/ kg, i.p.) was injected immediately before the experiments. Experiments were performed during the night cycle of the animals using infrared illumination and dim red light. Mice were recorded for 10 min and tracked using Any-Maze (Stoelting).
Statistical Analysis
Matlab R2009a, PASW Statistics 18.0, and Prism 6.0 were used to analyze data and plot figures. When homogeneity was assumed, a parametric analysis of variance test was used. The student's t test was used to compare two groups. One-, two-way, or two-way with repeated measures ANOVA were used as the other tests unless stated otherwise. When significant, a multiple comparisons post hoc test was used (Holm-Sidak's test). When homogeneity was not assumed, the Kruskal-Wallis nonparametric ANOVA was selected for multiple statistical comparisons. The Mann-Whitney U test was used to determine significance between groups. Statistical data are provided in the figures. p < 0.05 was considered statistically significant.
Supplementary Material
Highlights.
Agrp neuron activation leads to foraging and displacement behaviors
Agrp neurons promote stereotypic behavioral responses
Activation of Agrp neurons decreases anxiety levels
Y5R signaling is necessary for Agrp neuron-mediated stereotypic behaviors
ACKNOWLEDGMENTS
We thank Zhong-Wu Liu for electrophysiological recordings. We thank Marya Shanabrough for assistance. T.L.H. was supported by NIH (DP1 DK006850, R01AG040236, and P01NS062686), the American Diabetes Association, the Helmholtz Society (ICEMED), and by Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq: 401476/2012-0, Brazil). M.O.D. received support from Brain and Behavior Research Foundation, NCATS (UL1 TR000142), and CNPq (487096/2013-4, Brazil). M.R.Z. was partially supported by a Science Without Borders fellowship from CNPq/Brazil.
Footnotes
AUTHOR CONTRIBUTIONS
M.O.D., M.R.Z., and J.B. performed the experiments. All authors designed, analyzed, and interpreted data. M.O.D. and T.L.H. wrote the manuscript.
SUPPLEMENTAL INFORMATION
Supplemental Information includes five figures and five movies and can be found with this article online at http://dx.doi.org/10.1016/j.cell.2015.02.024.
REFERENCES
- Adamah-Biassi EB, Stepien I, Hudson RL, Dubocovich ML. Automated video analysis system reveals distinct diurnal behaviors in C57BL/ 6 and C3H/HeN mice. Behav. Brain Res. 2013;243:306–312. doi: 10.1016/j.bbr.2013.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmari SE, Spellman T, Douglass NL, Kheirbek MA, Simpson HB, Deisseroth K, Gordon JA, Hen R. Repeated cortico-striatal stimulation generates persistent OCD-like behavior. Science. 2013;340:1234–1239. doi: 10.1126/science.1234733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amodeo DA, Jones JH, Sweeney JA, Ragozzino ME. Differences in BTBR T+ tf/J and C57BL/6J mice on probabilistic reversal learning and stereotyped behaviors. Behav. Brain Res. 2012;227:64–72. doi: 10.1016/j.bbr.2011.10.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson DJ, Perona P. Toward a science of computational ethology. Neuron. 2014;84:18–31. doi: 10.1016/j.neuron.2014.09.005. [DOI] [PubMed] [Google Scholar]
- Aponte Y, Atasoy D, Sternson SM. AGRP neurons are sufficient to orchestrate feeding behavior rapidly and without training. Nat. Neurosci. 2011;14:351–355. doi: 10.1038/nn.2739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arenkiel BR, Klein ME, Davison IG, Katz LC, Ehlers MD. Genetic control of neuronal activity in mice conditionally expressing TRPV1. Nat. Methods. 2008;5:299–302. doi: 10.1038/nmeth.1190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atasoy D, Betley JN, Su HH, Sternson SM. Deconstruction of a neural circuit for hunger. Nature. 2012;488:172–177. doi: 10.1038/nature11270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnett SA. Behaviour Components in the Feeding of Wild and Laboratory Rats. Behaviour. 1956;9:24–43. [Google Scholar]
- Betley JN, Cao ZF, Ritola KD, Sternson SM. Parallel, redundant circuit organization for homeostatic control of feeding behavior. Cell. 2013;155:1337–1350. doi: 10.1016/j.cell.2013.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broberger C, Johansen J, Johansson C, Schalling M, Hökfelt T. The neuropeptide Y/agouti gene-related protein (AGRP) brain circuitry in normal, anorectic, and monosodium glutamate-treated mice. Proc. Natl. Acad. Sci. USA. 1998;95:15043–15048. doi: 10.1073/pnas.95.25.15043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burguière E, Monteiro P, Feng G, Graybiel AM. Optogenetic stimulation of lateral orbitofronto-striatal pathway suppresses compulsive behaviors. Science. 2013;340:1243–1246. doi: 10.1126/science.1232380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caterina MJ, Schumacher MA, Tominaga M, Rosen TA, Levine JD, Julius D. The capsaicin receptor: a heat-activated ion channel in the pain pathway. Nature. 1997;389:816–824. doi: 10.1038/39807. [DOI] [PubMed] [Google Scholar]
- Chaudhri O, Small C, Bloom S. Gastrointestinal hormones regulating appetite. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2006;361:1187–1209. doi: 10.1098/rstb.2006.1856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark JT, Kalra PS, Crowley WR, Kalra SP. Neuropeptide Y and human pancreatic polypeptide stimulate feeding behavior in rats. Endocrinology. 1984;115:427–429. doi: 10.1210/endo-115-1-427. [DOI] [PubMed] [Google Scholar]
- Coll AP, Farooqi IS, O'Rahilly S. The hormonal control of food intake. Cell. 2007;129:251–262. doi: 10.1016/j.cell.2007.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deacon RM. Digging and marble burying in mice: simple methods for in vivo identification of biological impacts. Nat. Protoc. 2006;1:122–124. doi: 10.1038/nprot.2006.20. [DOI] [PubMed] [Google Scholar]
- Dietrich MO, Horvath TL. Feeding signals and brain circuitry. Eur. J. Neurosci. 2009;30:1688–1696. doi: 10.1111/j.1460-9568.2009.06963.x. [DOI] [PubMed] [Google Scholar]
- Dietrich MO, Bober J, Ferreira JG, Tellez LA, Mineur YS, Souza DO, Gao XB, Picciotto MR, Araújo I, Liu ZW, Horvath TL. AgRP neurons regulate development of dopamine neuronal plasticity and nonfood-associated behaviors. Nat. Neurosci. 2012;15:1108–1110. doi: 10.1038/nn.3147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dube MG, Horvath TL, Kalra PS, Kalra SP. Evidence of NPY Y5 receptor involvement in food intake elicited by orexin A in sated rats. Peptides. 2000;21:1557–1560. doi: 10.1016/s0196-9781(00)00311-9. [DOI] [PubMed] [Google Scholar]
- Erondu N, Gantz I, Musser B, Suryawanshi S, Mallick M, Addy C, Cote J, Bray G, Fujioka K, Bays H, et al. Neuropeptide Y5 receptor antagonism does not induce clinically meaningful weight loss in overweight and obese adults. Cell Metab. 2006;4:275–282. doi: 10.1016/j.cmet.2006.08.002. [DOI] [PubMed] [Google Scholar]
- Gerald C, Walker MW, Criscione L, Gustafson EL, Batzl-Hartmann C, Smith KE, Vaysse P, Durkin MM, Laz TM, Linemeyer DL, et al. A receptor subtype involved in neuropeptide-Y-induced food intake. Nature. 1996;382:168–171. doi: 10.1038/382168a0. [DOI] [PubMed] [Google Scholar]
- Gropp E, Shanabrough M, Borok E, Xu AW, Janoschek R, Buch T, Plum L, Balthasar N, Hampel B, Waisman A, et al. Agouti-related peptide-expressing neurons are mandatory for feeding. Nat. Neurosci. 2005;8:1289–1291. doi: 10.1038/nn1548. [DOI] [PubMed] [Google Scholar]
- Grove KL, Brogan RS, Smith MS. Novel expression of neuro-peptide Y (NPY) mRNA in hypothalamic regions during development: region-specific effects of maternal deprivation on NPY and Agouti-related protein mRNA. Endocrinology. 2001;142:4771–4776. doi: 10.1210/endo.142.11.8498. [DOI] [PubMed] [Google Scholar]
- Güler AD, Rainwater A, Parker JG, Jones GL, Argilli E, Arenkiel BR, Ehlers MD, Bonci A, Zweifel LS, Palmiter RD. Transient activation of specific neurons in mice by selective expression of the capsaicin receptor. Nat. Commun. 2012;3:746. doi: 10.1038/ncomms1749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gyertyán I. Analysis of the marble burying response: marbles serve to measure digging rather than evoke burying. Behav. Pharmacol. 1995;6:24–31. [PubMed] [Google Scholar]
- Hahn TM, Breininger JF, Baskin DG, Schwartz MW. Coexpression of Agrp and NPY in fasting-activated hypothalamic neurons. Nat. Neurosci. 1998;1:271–272. doi: 10.1038/1082. [DOI] [PubMed] [Google Scholar]
- Halmi KA, Sunday SR, Klump KL, Strober M, Leckman JF, Fichter M, Kaplan A, Woodside B, Treasure J, Berrettini WH, et al. Obsessions and compulsions in anorexia nervosa subtypes. Int. J. Eat. Disord. 2003;33:308–319. doi: 10.1002/eat.10138. [DOI] [PubMed] [Google Scholar]
- Horvath TL, Naftolin F, Kalra SP, Leranth C. Neuropeptide-Y innervation of beta-endorphin-containing cells in the rat mediobasal hypothalamus: a light and electron microscopic double immunostaining analysis. Endocrinology. 1992;131:2461–2467. doi: 10.1210/endo.131.5.1425443. [DOI] [PubMed] [Google Scholar]
- Horvath TL, Bechmann I, Naftolin F, Kalra SP, Leranth C. Heterogeneity in the neuropeptide Y-containing neurons of the rat arcuate nucleus: GABAergic and non-GABAergic subpopulations. Brain Res. 1997;756:283–286. doi: 10.1016/s0006-8993(97)00184-4. [DOI] [PubMed] [Google Scholar]
- Horvath TL, Diano S, van den Pol AN. Synaptic interaction between hypocretin (orexin) and neuropeptide Y cells in the rodent and primate hypothalamus: a novel circuit implicated in metabolic and endocrine regulations. J. Neurosci. 1999;19:1072–1087. doi: 10.1523/JNEUROSCI.19-03-01072.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jhuang H, Garrote E, Mutch J, Yu X, Khilnani V, Poggio T, Steele AD, Serre T. Automated home-cage behavioural phenotyping of mice. Nat. Commun. 2010;1:68. doi: 10.1038/ncomms1064. [DOI] [PubMed] [Google Scholar]
- Kanatani A, Mashiko S, Murai N, Sugimoto N, Ito J, Fukuroda T, Fukami T, Morin N, MacNeil DJ, Van der Ploeg LH, et al. Role of the Y1 receptor in the regulation of neuropeptide Y-mediated feeding: comparison of wild-type, Y1 receptor-deficient, and Y5 receptor-deficient mice. Endocrinology. 2000;141:1011–1016. doi: 10.1210/endo.141.3.7387. [DOI] [PubMed] [Google Scholar]
- Karvat G, Kimchi T. Systematic autistic-like behavioral pheno-typing of 4 mouse strains using a novel wheel-running assay. Behav. Brain Res. 2012;233:405–414. doi: 10.1016/j.bbr.2012.05.028. [DOI] [PubMed] [Google Scholar]
- Kheifets A, Gallistel CR. Mice take calculated risks. Proc. Natl. Acad. Sci. USA. 2012;109:8776–8779. doi: 10.1073/pnas.1205131109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krashes MJ, DasGupta S, Vreede A, White B, Armstrong JD, Wad-dell S. A neural circuit mechanism integrating motivational state with memory expression in Drosophila. Cell. 2009;139:416–427. doi: 10.1016/j.cell.2009.08.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krashes MJ, Koda S, Ye C, Rogan SC, Adams AC, Cusher DS, Maratos-Flier E, Roth BL, Lowell BB. Rapid, reversible activation of AgRP neurons drives feeding behavior in mice. J. Clin. Invest. 2011;121:1424–1428. doi: 10.1172/JCI46229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krashes MJ, Shah BP, Koda S, Lowell BB. Rapid versus delayed stimulation of feeding by the endogenously released AgRP neuron mediators GABA, NPY, and AgRP. Cell Metab. 2013;18:588–595. doi: 10.1016/j.cmet.2013.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kyzar EJ, Pham M, Roth A, Cachat J, Green J, Gaikwad S, Kalueff AV. Alterations in grooming activity and syntax in heterozygous SERT and BDNF knockout mice: the utility of behavior-recognition tools to characterize mutant mouse phenotypes. Brain Res. Bull. 2012;89:168–176. doi: 10.1016/j.brainresbull.2012.08.004. [DOI] [PubMed] [Google Scholar]
- Lam TKT, Schwartz GJ, Rossetti L. Hypothalamic sensing of fatty acids. Nat. Neurosci. 2005;8:579–584. doi: 10.1038/nn1456. [DOI] [PubMed] [Google Scholar]
- Liu T, Kong D, Shah BP, Ye C, Koda S, Saunders A, Ding JB, Yang Z, Sabatini BL, Lowell BB. Fasting activation of AgRP neurons requires NMDA receptors and involves spinogenesis and increased excitatory tone. Neuron. 2012;73:511–522. doi: 10.1016/j.neuron.2011.11.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Londei T, Valentini AM, Leone VG. Investigative burying by laboratory mice may involve non-functional, compulsive, behaviour. Behav. Brain Res. 1998;94:249–254. doi: 10.1016/s0166-4328(97)00162-9. [DOI] [PubMed] [Google Scholar]
- Luquet S, Perez FA, Hnasko TS, Palmiter RD. NPY/AgRP neurons are essential for feeding in adult mice but can be ablated in neonates. Science. 2005;310:683–685. doi: 10.1126/science.1115524. [DOI] [PubMed] [Google Scholar]
- Matsunaga H, Kiriike N, Iwasaki Y, Miyata A, Yamagami S, Kaye WH. Clinical characteristics in patients with anorexia nervosa and obsessive-compulsive disorder. Psychol. Med. 1999;29:407–414. doi: 10.1017/s003329179800796x. [DOI] [PubMed] [Google Scholar]
- Merle JV, Haas V, Burghardt R, Döhler N, Schneider N, Lehmkuhl U, Ehrlich S. Agouti-related protein in patients with acute and weight-restored anorexia nervosa. Psychol. Med. 2011;41:2183–2192. doi: 10.1017/S0033291711000365. [DOI] [PubMed] [Google Scholar]
- Moriya J, Takimoto Y, Yoshiuchi K, Shimosawa T, Akabayashi A. Plasma agouti-related protein levels in women with anorexia nervosa. Psychoneuroendocrinology. 2006;31:1057–1061. doi: 10.1016/j.psyneuen.2006.06.006. [DOI] [PubMed] [Google Scholar]
- Ollmann MM, Wilson BD, Yang YK, Kerns JA, Chen Y, Gantz I, Barsh GS. Antagonism of central melanocortin receptors in vitro and in vivo by agouti-related protein. Science. 1997;278:135–138. doi: 10.1126/science.278.5335.135. [DOI] [PubMed] [Google Scholar]
- Pedrazzini T, Seydoux J, Künstner P, Aubert JF, Grouzmann E, Beer-mann F, Brunner HR. Cardiovascular response, feeding behavior and locomotor activity in mice lacking the NPY Y1 receptor. Nat. Med. 1998;4:722–726. doi: 10.1038/nm0698-722. [DOI] [PubMed] [Google Scholar]
- Rogan SC, Roth BL. Remote control of neuronal signaling. Pharmacol. Rev. 2011;63:291–315. doi: 10.1124/pr.110.003020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossi M, Kim MS, Morgan DG, Small CJ, Edwards CM, Sunter D, Abusnana S, Goldstone AP, Russell SH, Stanley SA, et al. A C-terminal fragment of Agouti-related protein increases feeding and antagonizes the effect of alpha-melanocyte stimulating hormone in vivo. Endocrinology. 1998;139:4428–4431. doi: 10.1210/endo.139.10.6332. [DOI] [PubMed] [Google Scholar]
- Sarrar L, Ehrlich S, Merle JV, Pfeiffer E, Lehmkuhl U, Schneider N. Cognitive flexibility and Agouti-related protein in adolescent patients with anorexia nervosa. Psychoneuroendocrinology. 2011;36:1396–1406. doi: 10.1016/j.psyneuen.2011.03.014. [DOI] [PubMed] [Google Scholar]
- Stanley BG, Kyrkouli SE, Lampert S, Leibowitz SF. Neuro-peptide Y chronically injected into the hypothalamus: a powerful neurochemical inducer of hyperphagia and obesity. Peptides. 1986;7:1189–1192. doi: 10.1016/0196-9781(86)90149-x. [DOI] [PubMed] [Google Scholar]
- Takahashi KA, Cone RD. Fasting induces a large, leptin-dependent increase in the intrinsic action potential frequency of orexigenic arcuate nucleus neuropeptide Y/Agouti-related protein neurons. Endocrinology. 2005;146:1043–1047. doi: 10.1210/en.2004-1397. [DOI] [PubMed] [Google Scholar]
- Thiel A, Broocks A, Ohlmeier M, Jacoby GE, Schüssler G. Obsessive-compulsive disorder among patients with anorexia nervosa and bulimia nervosa. Am. J. Psychiatry. 1995;152:72–75. doi: 10.1176/ajp.152.1.72. [DOI] [PubMed] [Google Scholar]
- Thomas A, Burant A, Bui N, Graham D, Yuva-Paylor LA, Paylor R. Marble burying reflects a repetitive and perseverative behavior more than novelty-induced anxiety. Psychopharmacology (Berl.) 2009;204:361–373. doi: 10.1007/s00213-009-1466-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witkin JM. Animal models of obsessive-compulsive disorder. Curr. Protoc. in Neurosci., Unit. 2008;9:30. doi: 10.1002/0471142301.ns0930s45. [DOI] [PubMed] [Google Scholar]
- Wolak ML, DeJoseph MR, Cator AD, Mokashi AS, Brownfield MS, Urban JH. Comparative distribution of neuropeptide Y Y1 and Y5 receptors in the rat brain by using immunohistochemistry. J. Comp. Neurol. 2003;464:285–311. doi: 10.1002/cne.10823. [DOI] [PubMed] [Google Scholar]
- Wu Q, Clark MS, Palmiter RD. Deciphering a neuronal circuit that mediates appetite. Nature. 2012;483:594–597. doi: 10.1038/nature10899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu AW, Kaelin CB, Morton GJ, Ogimoto K, Stanhope K, Graham J, Baskin DG, Havel P, Schwartz MW, Barsh GS. Effects of hypothalamic neurodegeneration on energy balance. PLoS Biol. 2005;3:e415. doi: 10.1371/journal.pbio.0030415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y, Atasoy D, Su HH, Sternson SM. Hunger states switch a flip-flop memory circuit via a synaptic AMPK-dependent positive feedback loop. Cell. 2011;146:992–1003. doi: 10.1016/j.cell.2011.07.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.