Abstract
Objective
We previously demonstrated that subjects with functional ATP-binding cassette (ABC) A1 mutations have increased atherosclerosis, which has been attributed to the role of ABCA1 in reverse cholesterol transport. More recently, a pro-inflammatory effect of Abca1 deficiency was shown in mice, potentially contributing to atherogenesis. In the present study, we investigated whether ABCA1 deficiency was associated with pro-inflammatory changes in humans.
Approach and Results
31 heterozygous, 5 homozygous ABCA1 mutation carriers and 21 matched controls were studied. 18fluordeoxyglucose (18F-FDG) positron emission tomography with computed tomography (PET/CT) scanning was performed in a subset of carriers and controls to assess arterial wall inflammation (target-background-ratio, TBR). Heterozygous ABCA1 mutation carriers had a 20% higher TBR compared to controls (TBR; p=0.008). In carriers using statins (n=7), TBR was 21% reduced compared to non-statin users (n=7, p=0.03). We then measured plasma cytokine levels. Tumor Necrosis Factor α (TNFα), Monocyte Chemoattractant Protein-1 (MCP-1) and Interleukin-6 (IL-6) levels were increased in heterozygous and homozygous ABCA1 mutation carriers. We isolated monocytes from carriers and controls and measured inflammatory gene expression. Only TNFα mRNA was increased in monocytes from heterozygous ABCA1 mutation carriers. Further studies in THP-1 macrophages showed that both ABCA1 deficiency and lipoprotein deficient plasma from ABCA1 mutation carriers increased inflammatory gene expression.
Conclusions
Our data suggest a pro-inflammatory state in ABCA1 mutation carriers as reflected by an increased PET-MRI signal in non-statin using subjects, and increased circulating cytokines. The increased inflammation in ABCA1 mutation carriers seems to be attenuated by statins.
Introduction
High-Density Lipoprotein Cholesterol (HDL-C) levels are inversely correlated with cardiovascular risk.1-3 The atheroprotective effects of HDL have traditionally been attributed to its role in reverse cholesterol transport. The ATP-Binding Cassette Transporter A1 (ABCA1) transporter plays a crucial role in mediating cholesterol efflux from peripheral cells, including arterial wall macrophages, to lipid-poor apolipoprotein AI (apoAI) or pre-β HDL particles.4;5 Homozygous ABCA1 mutation carriers display near absent HDL-C levels, whereas heterozygous carriers are characterized by half-normal HDL-C. Single nucleotide polymorphisms in the ABCA1 gene have variously been reported to have no impact on CVD,6;7 or to be associated with an increased CVD risk.8;9 However, studies in ABCA1 mutation carriers, displaying marked defects in cholesterol efflux and profound decreases in HDL levels, showed increased arterial wall thickness,10;11 and CVD risk8;9 in carriers compared to controls.
The paradigm that a macrophage-dominated inflammatory process, initiated by the deposition of cholesterol-rich lipoproteins in the arterial wall, is central to atherosclerosis has been widely accepted.12 The molecular mechanisms linking defective cholesterol homeostasis to increased inflammation are not well understood. Recent studies have implicated defective cellular cholesterol efflux pathways in increased inflammatory gene expression in monocytes and macrophages, as well as the increased production of inflammatory cells such as monocytes and neutrophils.13, 14 Deficiency of ABCA1 and/or ABCG1 is associated with a pro-inflammatory phenotype in mouse peritoneal macrophages as well as in the macrophages of atherosclerotic plaques.13-16 Whether ABCA1 deficiency in humans represents a pro-inflammatory state is presently unknown.
In the present study, we assessed whether ABCA1 mutation carriers exhibit pro-inflammatory changes in the arterial wall as measured by 18fluorodeoxyglucose (18F-FDG) positron emission tomography with computed tomography (PET/CT). We also measured plasma cytokine levels and assessed inflammatory gene expression in ABCA1 deficient monocytes/macrophages in vitro.
Methods
An expanded version of the methods can be found in the online Data Supplement.
Study participants
Subjects with low High-Density Lipoprotein Cholesterol (HDL-C) levels, defined as HDL-C < 5th percentile, were selected from a cohort of hypoalphalipoproteinemia patients17 and screened for ABCA1 (GenBank No. AF275948) mutations. Family members of ATP-Binding Cassette Transporter A1 (ABCA1) mutation carriers were recruited. Carriers of functional ABCA1 gene mutations and controls matched for age and gender were enrolled in this study. Body mass index was calculated from weight and length. Hypertension was defined as systolic blood pressure >140 mmHg, diastolic blood pressure >90 mmHg or use of antihypertensive medication. Blood was obtained after an overnight fast and stored at -80 °C. All participants provided written informed consent. The study protocol was approved by the Institutional Review Board at the AMC, The Netherlands.
Results
Baseline characteristics
Baseline characteristics of study participants are listed in the Table. 36 ABCA1 mutation carriers from 14 separate families were included, comprising 3 homozygous, 2 compound heterozygous, and 31 heterozygous patients. Homozygous and compound heterozygous subjects suffer from Tangier Disease. Subjects were carriers of the following mutations: p.Leu1056Pro, c.3535+1G>C, c.6401+2T>C, p.Asn1800his, p.Ser930Phe, p.Phe1760Valfs*21, p.Ser824Leu, p.Gln1038Ter, p.Thr929Ile, p.Arg587Trp, p.Asn935Ser and p.Arg579Gln. Heterozygosity for these mutations has been shown to impair cholesterol efflux by 40 to 85 %.11;17-20 Fourteen of the 36 ABCA1 mutation carriers were on statin therapy, including 3 homozygous subjects. All statin users had been on statin therapy for at least 2 years. Statin therapy was initiated by the patients' treating physicians based on the current guidelines.
Table.
Values are indicated as mean ± SD unless otherwise indicated. Het indicates heterozygous subjects, hom indicates homozygous subjects. P1 tests ABCA1 mutation carriers versus controls; P2 tests ABCA1 mutation carriers not on statins versus ABCA1 mutation carriers on statins. P-value for student's T-test, compared to control, unless otherwise specified.
| ABCA1 mutation carriers (n=36) | |||||||||
|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||
| Controls (n=21) | No statin tot (n=22) | No statin het (20) | No statin hom (2) | Statin tot (n=14) | Statin het (11) | Statin hom (3) | P2 | P1 | |
| Characteristics | |||||||||
| Age (years) | 51.0±11.3 | 50.2±13.1 | 50.7±12.6 | 41.9±22.9 | 54.6±14.6 | 54.6±16.0 | 54.7±10.5 | 0.37 | 0.93 |
| Male sex, n (%)* | 9 (43) | 11 (48) | 9 (45) | 1 (50) | 6 (43) | 5 (46) | 1 (33) | 0.77 | 0.82 |
| Body Mass Index (kg/m2) | 24.1±3.1 | 25.8±3.8 | 26.1±3.8 | 22.4±1.5 | 25.8±4.2 | 27.9±4.5 | 24.5±7.1 | 1.00 | 0.10 |
| Smokers, n (%)* | 2 (10) | 5 (25) | 5 (25) | 0 (0) | 1 (8) | 1 (9) | 0 (0) | 0.21 | 0.52 |
| Diabetes, n (%)* | 1 (5) | 1 (5) | 1 (5) | 0 (0) | 1 (8) | 1 (9) | 0 (0) | 0.74 | 0.80 |
| Statin use (%)* | 0 | 0 (0) | 0 (0) | 0 (0) | 14 (100) | 11 (100) | 3 (100) | n.a. | n.a. |
| Blood pressure | |||||||||
| Systolic (mmHg)# | 129 (122-138) | 133 (132-143) | 133 (132-144) | 118 (-) | 147 (132-165) | 147 (130-169) | 147 (145-147) | 0.11 | 0.75 |
| Diastolic (mmHg)# | 80 (74-85) | 83 (74-88) | 83 (79-89) | 62 (-) | 82 (73-87) | 84 (72-88) | 80 (79-80) | 0.86 | 0.74 |
| Hypertension, n (%)* | 3 (14) | 2 (11) | 3 (15) | 0 (0) | 4 (36) | 5 (46) | 0 | 0.09 | 0.60 |
| Lipid metabolism | |||||||||
| Total cholesterol (mmol/L) | 5.39±0.92 | 4.86±1.30 | 4.89±1.16 | 4.02±2.81 | 3.54±1.29 | 3.68±0.84 | 3.07±2.52 | 0.007 | 0.005 |
| LDL-cholesterol (mmol/L) | 3.49±0.79 | 3.53±1.07 | 3.50±1.00 | 3.40±2.26 | 2.59±0.85 | 2.63±0.57 | 2.43±1.75 | 0.009 | 0.23 |
| HDL-cholesterol (mmol/L) | 1.53±0.40 | 0.89±0.36 | 0.97±0.28 | 0.14±0.19 | 0.60±0.42 | 0.74±0.35 | 0.07±0.11 | 0.04 | <0.001 |
| Triglycerides (mmol/L)# | 1.01 (0.64-1.42) | 1.09 (0.88-1.46) | 1.08 (0.80-1.32) | 1.24 (-) | 1.16 (0.73-1.59) | 1.05 (0.64-1.42) | 1.55 (1.10-1.55) | 0.96 | 0.17 |
| Apolipoprotein B (mg/dL) | 110.65±21.44 | 125.82±28.70 | 124.41±29.46 | 122.00 ± 21.23 | 114.15±44.12 | 101.53±17.41 | 152.00±81.43 | 0.37 | 0.25 |
| Apolipoprotein A-I (mg/dL) | 161.99±19.87 | 109.64±32.34 | 124.41±19.00 | 122.00±12.76 | 78.87±54.59 | 104.02±35.23 | 3.40±5.72 | 0.10 | <0.001 |
| Vessel wall thickness | |||||||||
| NWI | 0.32±0.03 | 0.37±0.06 | 0.38±0.06 | 0.32±0.04 | 0.37±0.06 | 0.39±0.06 | 0.32±0.06 | 0.94 | <0.001 |
| MWT (mm) | 0.66±0.11 | 0.82±0.21 | 0.84±0.21 | 0.61±0.16 | 0.88±0.21 | 0.89±0.21 | 0.82±0.22 | 0.43 | <0.001 |
indicates parameters tested by means of χ2 test.
indicates parameters for which median and interquartile range are given and testing was performed by Mann Whitney U test. NWI is normalized wall index; MWT is mean wall thickness. N.a. is not applicable.
21 controls from the general population were matched for age and sex to carriers. Statin users were excluded from the control cohort (Table). Total cholesterol levels were significantly lower in ABCA1 mutation carriers (p=0.005), largely due to a 50% reduction in HDL-C (p<0.001). ApoAI was decreased by 40% (p<0.001). Carriers on statins displayed a significantly lower Low-density lipoprotein-cholesterol (LDL-C) level (p=0.009). Other parameters were not significantly different (Table). Normalized wall index and mean wall thickness of the carotid arteries as determined by 3.0 Tesla magnetic resonance imaging were increased in both statin using heterozygous ABCA1 mutation carriers (p<0.001 and p=0.006 respectively), as well as non-statin using heterozygous ABCA1 mutation carriers (p<0.001 and p=0.002 respectively, Supplementary Figure I), consistent with earlier reports.11
Vascular 18fluordeoxyglucose positron emission tomography with computed tomography
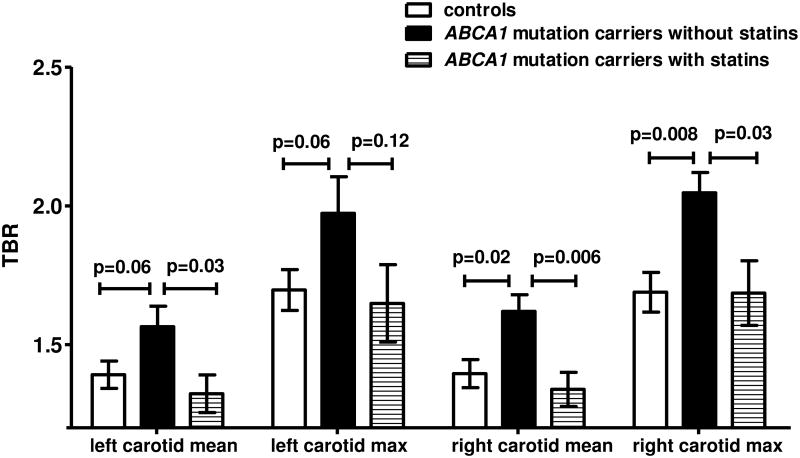
18Fluordeoxyglucose positron emission tomography with computed tomography (18F-FDG PET/CT) scanning was performed in a random subset of heterozygous ABCA1 mutation carriers (n=14) and controls (n=15). In the whole group, the Target to Background Ratio (TBR) was not significantly different in heterozygous ABCA1 mutation carriers compared to controls (data not shown). However, the average mean TBR of the left and right carotid was 20% higher in non-statin using heterozygous ABCA1 mutation carriers compared to statin using heterozygous ABCA1 mutation carriers (p=0.03 for left mean TBR; p=0.006 for right mean TBR, Figure 1). After excluding the heterozygous ABCA1 mutation carriers using statins, the mean TBR in the left and right carotid was higher in heterozygous ABCA1 mutation carriers compared to controls (p=0.06 and 0.02, respectively, Figure 1). Max TBR was significantly higher for the right carotid (p=0.008, Figure 1), and showed a trend to an increase in the left carotid (p=0.06). In Supplementary Figure II, representative images of CT and 18F-FDG PET/CT right carotid arteries of heterozygous ABCA1 mutation carriers, controls, and heterozygous ABCA1 mutation carriers using statins are displayed.
Figure 1.
Increased vessel wall inflammation in heterozygous ABCA1 mutation carriers. Vessel wall inflammation was assessed by PET-CT in controls (n=15), and heterozygous ABCA1 mutation carriers without (n=7) and with statin (n=7) treatment. TBR denotes target to background ratio. Data are presented as mean ± SEM. P-values are indicated.
Since the TBR signal depends on glucose uptake in macrophages in the arterial wall21 and ABCA1 has been reported to have a role in glucose uptake,22 we evaluated a potential direct effect of ABCA1 expression on macrophage glucose uptake. Macrophage-glucose uptake did not differ between heterozygous ABCA1 mutation carriers and controls (Supplementary Figure III), indicating that the differences in TBR signal cannot be explained by a direct effect of ABCA1 on glucose uptake.
Systemic inflammatory phenotype in ABCA1 mutation carriers
To assess whether the apparent inflammatory phenotype in the arterial wall of heterozygous ABCA1 mutation carriers also manifested itself systemically, plasma cytokines were measured in both heterozygous and homozygous ABCA1 mutation carriers. Plasma levels of tumor necrosis factor α (TNFα) were significantly higher in homozygous ABCA1 mutation carriers versus controls (Figure 2A). In line with the PET/CT data, TNFα levels were significantly higher in non-statin using ABCA1 heterozygous mutation carriers compared to statin using heterozygous carriers who had levels similar to controls (Figure 2A). TNFα levels also appeared to be higher in the non-statin using homozygous carriers compared to statin using homozygous carriers. Plasma levels of monocyte chemoattractant protein-1 (MCP-1) and interleukin-6 (IL-6) were increased in heterozygous ABCA1 mutation carriers and homozygous ABCA1 mutation carriers compared to controls (Figure 2B). In contrast to TNFα, no effect of statin use was observed (Figure 2B and 2C).
Figure 2.
Pro-inflammatory cytokines in plasma of controls, heterozygous and homozygous ABCA1 mutation carriers. Plasma was isolated from controls, heterozygous ABCA1 mutation carriers not using (ABCA1 het) or using a statin (ABCA1 het statin), and homozygous ABCA1 mutation carriers not using (ABCA1 hom) or using a statin (ABCA1 hom statin). Levels of pro-inflammatory cytokines were assessed using ELISA. A. Tumor Necrosis Factor α (TNFα). B. Monocyte Chemoattractant Protein-1 (MCP-1). C. Interleukin-6 (IL-6). In all graphs, each data point represents one patient or control. The mean is indicated. *P<0.05, **P<0.01, ***P<0.001.
Inflammatory gene expression in monocytes from heterozygous ABCA1 carriers
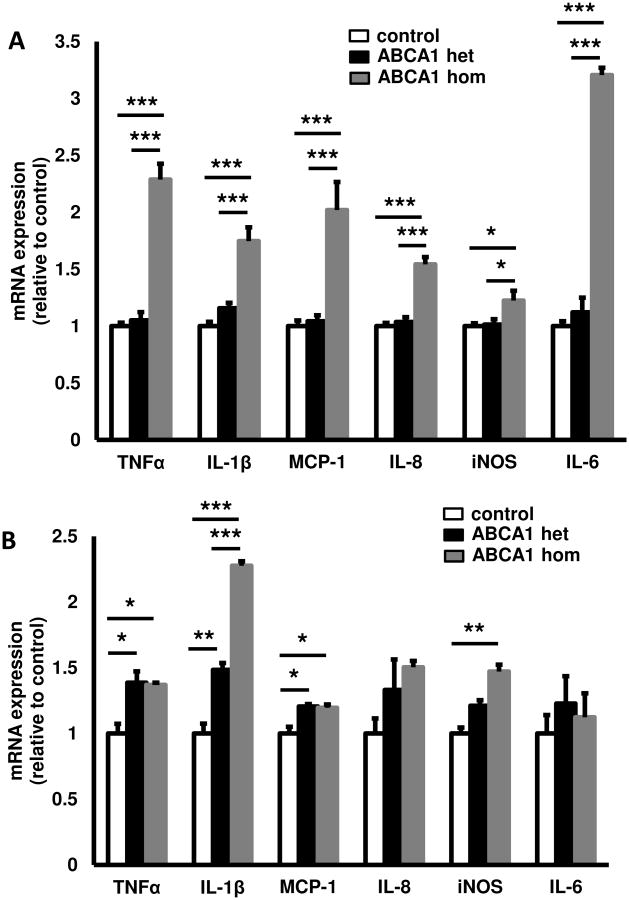
To assess whether monocytes showed increased inflammatory gene expression, we isolated CD14+ monocytes from heterozygous ABCA1 mutation carriers and controls and measured TNFα, IL-1β, MCP-1, and IL-6 mRNA expression. TNFα mRNA was increased in heterozygous ABCA1 mutation carriers compared to controls (2.5-fold; P<0.01) (Figure 3). Interestingly, statin use did not affect the increase in TNFα mRNA. Expression of IL-1β, MCP1 and IL-6 was not different from controls (Figure 3).
Figure 3.

Inflammatory cytokine expression in monocytes from controls and heterozygous ABCA1 mutation carriers. Peripheral Blood Mononuclear Cells (PBMCs) were isolated from heterozygous ABCA1 mutation carriers or controls (n=6 per group). CD14+ monocytes were isolated from PBMCs using magnetic beads and mRNA expression for TNFα, IL-1β, MCP-1, and IL-6 was assessed, and corrected for the housekeeping gene cyclophilin. Data are presented as mean ± SEM. **P<0.01.
ABCA1 deficiency and macrophage inflammation
To examine whether knock-down of ABCA1 in human macrophages resulted in increased expression of inflammatory genes, we used siRNA to knock-down ABCA1 in human THP-1 macrophages. This resulted in a 64% decrease in ABCA1 mRNA expression and a 23% increase in ABCG1 mRNA expression (Supplementary Figure IVA and IVB). ABCA1 protein was also decreased by ∼50% (Supplementary Figure IVC and IVD). There were marked increases in expression of IL-1β and inducible nitric oxide synthase (iNOS) mRNA, a small increase in IL-6 mRNA and no change in TNFα mRNA (Figure 4). Addition of a cholesterol-poor reconstituted HDL (rHDL) preparation resulted in decreased IL-1β, iNOS and TNFα mRNA expression, while IL-6 expression was relatively unchanged (Figure 4), likely reflecting cholesterol efflux via non-ABCA1 dependent pathways such as ABCG1.
Figure 4.

Effect of cellular ABCA1 deficiency on inflammatory cytokine expression. THP-1 macrophages were incubated with ABCA1 or scrambled siRNA. After 48 h, cells were treated o/n with or without rHDL. Macrophage RNA was isolated and the mRNA expression of TNFα (A), IL-1β (B), IL6 (C), and inducible nitric oxide synthase (iNOS) (D) was measured and corrected for the housekeeping gene cyclophilin. Data are presented as mean ± SEM. *P<0.05, ***P<0.001.
Pro-inflammatory effects of apoB depleted plasma and lipoprotein deficient plasma from ABCA1 deficient subjects
To determine if the increased plasma inflammatory cytokines and decreased HDL from ABCA1 mutation carriers could contribute to enhanced macrophage inflammation, we added polyethylene glycol (PEG) supernatant (apoB-depleted plasma still containing HDL) from controls, heterozygous and homozygous ABCA1 mutation carriers to THP-1 macrophages. Only the apoB depleted plasma from homozygous ABCA1 mutation carriers increased mRNA expression of inflammatory cytokines (Figure 5A). This could reflect the virtual absence of HDL in the plasma from homozygous subjects, while the half normal levels of HDL in the heterozygous subjects may have been sufficient to suppress inflammatory cytokine production. To test this idea further, we added pooled lipoprotein deficient plasma (LPDS) from controls, heterozygous and homozygous ABCA1 mutation carriers to THP-1 macrophages. LPDS from heterozygous ABCA1 mutation carriers increased TNFα, IL-1β, and MCP-1 mRNA expression, whereas LPDS from homozygous ABCA1 mutation carriers caused a more marked an widespread increase in mRNA expression of inflammatory cytokines (TNFα, IL-1β, MCP-1, IL-8, and iNOS) (Figure 5B). These experiments suggest that in ABCA1 mutation carriers half normal HDL levels may be sufficient to counteract the effect of pro-inflammatory cytokines in plasma, while in homozygous subjects the near absence of HDL is permissive for the pro-inflammatory effect. Thus in heterozygotes, partial ABCA1 deficiency (comparable to the partial knockdown in Figure 4) in monocytes and macrophages may make a key contribution to inflammatory cytokine expression.
Figure 5.
Effect of apoB-depleted plasma and lipoprotein deficient plasma from ABCA1 mutation carriers on macrophage inflammation. (A) ApoB was peg-precipitated from plasma and apoB-depleted plasma was incubated overnight with THP-1 macrophages (n=13 per group; n=4 for homozygous ABCA1 mutation carriers). (B) Plasma samples (n=10 per group; n=4 for homozygous ABCA1 mutation carriers) were pooled and lipoprotein deficient plasma (LPDS) was obtained after ultracentrifugation and incubated with THP-1 macrophages (n=4 per pool). mRNA expression was corrected for the housekeeping gene cyclophilin. Data are presented as mean ± SEM representing individual samples in panel A, and replicates of pooled samples in panel B. *P<0.05, **P<0.01, ***P<0.001.
Discussion
ABCA1 mutation carriers displayed both increased vessel wall inflammation as assessed by 18F-FDG PET/CT as well as increased systemic inflammation, as reflected by a pro-inflammatory plasma cytokine profile and increased inflammatory gene expression in circulating monocytes. In vitro experiments with human THP-1 macrophages revealed a pro-inflammatory effect of LPDS from heterozygous and homozygous ABCA1 mutation carriers, most likely secondary to increased levels of plasma cytokines, as well as a cell intrinsic effect of ABCA1 deficiency. Both cellular ABCA1 deficiency and reduced levels of plasma HDL may contribute to increased monocyte and macrophage inflammatory responses; however, our findings suggest that in heterozygotes cellular ABCA1 deficiency may have the predominant role, while in homozygous subjects both cellular ABCA1 deficiency and the absence of plasma HDL may also contribute. Our findings are consistent with studies in mouse macrophages, in which genetic deficiency of Abca1 leads to enhanced inflammatory gene expression.13-16 This has been attributed to increased plasma membrane lipid raft formation promoting signalling via Toll like receptors 2, 3 and 4.14-16;23 Our data show an association between ABCA1 deficiency and increased systemic and plaque inflammation in humans, probably contributing to the increased atherosclerotic plaque volume that has been observed in ABCA1 mutation carriers.10;11
18F-FDG PET/CT has emerged as a reliable non-invasive technique for visualization of metabolic activity in the arterial wall in humans.24 Metabolic activity likely reflects the inflammatory state of the arterial wall, since the arterial uptake of 18F-FDG has been shown to correlate with circulating inflammatory biomarkers,25 inflammatory gene expression,26 CVD risk factors,27 as well as the number of plaque macrophages.28 We showed that the 18F-FDG uptake in the arterial wall of non-statin using heterozygous ABCA1 mutation carriers was increased compared to matched controls, paralleling an increase in vessel wall thickness. Although suspected based on studies in macrophages from Abca1-/- mice,13-16 this is the first confirmation of an in vivo role for ABCA1 in the suppression of inflammation in humans. The finding of increased vessel wall inflammation in heterozygous ABCA1 mutation carriers is likely to contribute to their increased cardiovascular risk,8;9 since carotid arterial wall 18F-FDG uptake has been associated with increased cardiovascular risk, independent of the degree of stenosis.29;30
Interestingly, the increased inflammatory status in the vessel wall of heterozygous ABCA1 mutation carriers was manifested systemically, as plasma levels of TNFα, MCP-1, and IL-6 were also increased. This is consistent with previous reports, showing that ABCA1 suppresses secretion of IL-1β, IL-6 and TNFα.15;16;31;32 Furthermore, TNFα mRNA expression was increased in circulating monocytes, consistent with a systemic pro-inflammatory state. These findings are also consistent with reports that plasma C-reactive protein levels are negatively associated with ABCA1 mRNA levels in human peripheral monocytes.33 The cross-sectional design of this study precludes us from answering whether plaque inflammation is causal or secondary to atherosclerosis. However, since the increases in inflammatory mediators in our study are secondary to genetic changes in ABCA1, and knockdown of ABCA1 increases inflammatory gene expression, it is reasonable to conclude that excessive plaque inflammation contributes to increased atherosclerotic burden. 23, 14 Our findings suggest an anti-inflammatory effect of statin treatment in humans as determined both by reduced 18F-FDG uptake in the arterial wall and decreased circulating levels of cytokines. Although the effect of statins on 18F-FDG PET/CT signal in atherosclerotic subjects is in line with previous publications,34-36 the ∼20% decrease in TBR in statin users in our study is larger than the ∼10% 34 and ∼9%36 decreases in other reports. This may be explained by the short treatment period of 3-6 months in these intervention trials versus long-term use in our patients or the increased inflammatory status of ABCA1 mutation carriers. The finding of a statin effect on 18F-FDG PET/CT, but not on MRI underlines the capacity of 18F-FDG PET/CT to visualize inflammation. Folco et al29 have suggested a specific effect of statins to decrease the uptake of 18F-FDG-glucose by macrophages in a hypoxic plaque environment;21 a contribution of such an effect to our results cannot be excluded.
Interestingly, amongst the inflammatory cytokines measured, TNFα mRNA levels were clearly increased in monocytes of ABCA1 heterozygotes and TNFα protein levels were increased in plasma; however, statins lowered TNFα plasma levels while appearing not to affect monocyte TNFα mRNA levels. TNFα is an important inflammatory cytokine that has pro-atherogenic effects especially on the endothelium and smooth muscle cells. TNFα is synthesized as a Type 2 membrane protein and released from cells as result of the activity of Adam17 (TACE).37 This raises the intriguing possibility of an independent effect of statins on TNF processing.
In conclusion, our data demonstrate a pro-inflammatory state in heterozygous and homozygous ABCA1 mutation carriers as reflected by increased circulating cytokines. This is attributed to a cellular effect of ABCA1 deficiency with an additional contribution of lower HDL levels especially in homozygous subjects. Our findings suggest that the increased inflammation documented in ABCA1 deficient cells and animal models14-16;23 is also present in humans. The increased inflammation in ABCA1 mutation carriers, documented by an increased carotid 18F-FDG PET/CT signal, seems to be attenuated by statins, as shown by normalization of 18F-FDG PET/CT and plasma cytokine levels.
Supplementary Material
Significance.
Plasma high-density lipoprotein (HDL)-cholesterol levels inversely correlate with CVD in humans. Recent studies have suggested that this is mainly due to the ability of HDL to induce cholesterol efflux from macrophage foam cells in the arterial wall, mediated by the cholesterol transporters ATP Binding Cassette A1 and G1 (ABCA1 and ABCG1). In mouse models, deficiency of ABCA1 and ABCG1 in macrophages accelerates atherosclerosis, not only due to enhanced foam cell formation but also to an increased level of pro-inflammatory cytokines in the atherosclerotic plaque as well as in plasma. Our current data reveal a similar increase in inflammation in humans carrying loss of function mutations for ABCA1, who have an increased atherosclerotic burden, thus suggesting that our previous findings in animal models may also play a role in humans. Importantly, statins reduce inflammation in these patients. These data reveal an association between vascular cholesterol accumulation, inflammation, and atherosclerosis in humans.
Acknowledgments
The research was supported by a grant from Fondation LeDucq and NIH grant HL107653. Part of the research was supported by a grant from the Netherlands Heart Foundation (2011-B019: generating the best evidenced based pharmaceutical targets for atherosclerosis (GENIUS)). A.E. Bochem is supported by fellowship WdL/HE/12-029 from the Saal van Zwanenbergstichting, the Netherlands. M. Westerterp has received funding from The Netherlands Organization of Scientific Research (NWO VENI-grant 916.11.072).
Abbreviations
- ABCA1/G1
ATP Binding Cassette Transporter A1/G1
- ApoAI/B
Apolipoprotein AI/B
- 18F-FDG
18Fluordeoxyglucose
- HDL/LDL-C
High-Density Lipoprotein/Low-Density Lipoprotein-Cholesterol
- IL-1β/6
Interleukin-1β/6
- iNOS
inducible nitric oxide synthase
- LPDS
Lipoprotein deficient serum
- MCP-1
Monocyte Chemoattractant Protein-1
- MWT
Mean Wall Thickness
- NWI
Normalized Wall Index
- PEG
Polyethylene Glycol
- PET/CT
Positron Emission Tomography with Computed Tomography
- TBR
Target to Background Ratio
- TNFα
Tumor Necrosis Factor α
Footnotes
Disclosures: A.R. Tall is a consultant to Amgen, Arisaph, and CSL. The other authors report no conflicts.
References
- 1.Murray CJ, Lopez AD. Global mortality, disability, and the contribution of risk factors: Global Burden of Disease Study. Lancet. 1997;349:1436–42. doi: 10.1016/S0140-6736(96)07495-8. [DOI] [PubMed] [Google Scholar]
- 2.Gordon DJ, Probstfield JL, Garrison RJ, Neaton JD, Castelli WP, Knoke JD, Jacobs DR, Jr, Bangdiwala S, Tyroler HA. High-density lipoprotein cholesterol and cardiovascular disease. Four prospective American studies. Circulation. 1989;79:8–15. doi: 10.1161/01.cir.79.1.8. [DOI] [PubMed] [Google Scholar]
- 3.Assmann G, Schulte H. Relation of high-density lipoprotein cholesterol and triglycerides to incidence of atherosclerotic coronary artery disease (the PROCAM experience). Prospective Cardiovascular Munster study. Am J Cardiol. 1992;70:733–7. doi: 10.1016/0002-9149(92)90550-i. [DOI] [PubMed] [Google Scholar]
- 4.McNeish J, Aiello RJ, Guyot D, Turi T, Gabel C, Aldinger C, Hoppe KL, Roach ML, Royer LJ, de Wet J, Broccardo C, Chimini G, Francone OL. High density lipoprotein deficiency and foam cell accumulation in mice with targeted disruption of ATP-binding cassette transporter-1. Proc Natl Acad Sci U S A. 2000;97:4245–50. doi: 10.1073/pnas.97.8.4245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Timmins JM, Lee JY, Boudyguina E, Kluckman KD, Brunham LR, Mulya A, Gebre AK, Coutinho JM, Colvin PL, Smith TL, Hayden MR, Maeda N, Parks JS. Targeted inactivation of hepatic Abca1 causes profound hypoalphalipoproteinemia and kidney hypercatabolism of apoA-I. J Clin Invest. 2005;115:1333–42. doi: 10.1172/JCI23915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Teslovich TM, Musunuru K, Smith AV, et al. Biological, clinical and population relevance of 95 loci for blood lipids. Nature. 2010;466:707–13. doi: 10.1038/nature09270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Frikke-Schmidt R, Nordestgaard BG, Stene MC, Sethi AA, Remaley AT, Schnohr P, Grande P, Tybjaerg-Hansen A. Association of loss-of-function mutations in the ABCA1 gene with high-density lipoprotein cholesterol levels and risk of ischemic heart disease. JAMA. 2008;299:2524–32. doi: 10.1001/jama.299.21.2524. [DOI] [PubMed] [Google Scholar]
- 8.Frikke-Schmidt R, Nordestgaard BG, Jensen GB, Steffensen R, Tybjaerg-Hansen A. Genetic variation in ABCA1 predicts ischemic heart disease in the general population. Arterioscler Thromb Vasc Biol. 2008;28:180–6. doi: 10.1161/ATVBAHA.107.153858. [DOI] [PubMed] [Google Scholar]
- 9.Frikke-Schmidt R, Nordestgaard BG, Schnohr P, Steffensen R, Tybjaerg-Hansen A. Mutation in ABCA1 predicted risk of ischemic heart disease in the Copenhagen City Heart Study Population. J Am Coll Cardiol. 2005;46:1516–20. doi: 10.1016/j.jacc.2005.06.066. [DOI] [PubMed] [Google Scholar]
- 10.van Dam MJ, de Groot E, Clee SM, Hovingh GK, Roelants R, Brooks-Wilson A, Zwinderman AH, Smit AJ, Smelt AH, Groen AK, Hayden MR, Kastelein JJ. Association between increased arterial-wall thickness and impairment in ABCA1-driven cholesterol efflux: an observational study. Lancet. 2002;359:37–42. doi: 10.1016/S0140-6736(02)07277-X. [DOI] [PubMed] [Google Scholar]
- 11.Bochem AE, van Wijk DF, Holleboom AG, Duivenvoorden R, Motazacker MM, Dallinga-Thie GM, de Groot E, Kastelein JJ, Nederveen AJ, Hovingh GK, Stroes ES. ABCA1 mutation carriers with low high-density lipoprotein cholesterol are characterized by a larger atherosclerotic burden. Eur Heart J. 2013;34:286–91. doi: 10.1093/eurheartj/ehs376. [DOI] [PubMed] [Google Scholar]
- 12.Ross R. Atherosclerosis--an inflammatory disease. N Engl J Med. 1999;340:115–26. doi: 10.1056/NEJM199901143400207. [DOI] [PubMed] [Google Scholar]
- 13.Yvan-Charvet L, Welch C, Pagler TA, et al. Deficiency of ATP-Binding Cassette Transporters A1 and G1 in Macrophages Increases Inflammation and Accelerates Atherosclerosis in Mice. Circ Res. 2013;112:1456–65. doi: 10.1161/CIRCRESAHA.113.301086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Koseki M, Hirano K, Masuda D, Ikegami C, Tanaka M, Ota A, Sandoval JC, Nakagawa-Toyama Y, Sato SB, Kobayashi T, Shimada Y, Ohno-Iwashita Y, Matsuura F, Shimomura I, Yamashita S. Increased lipid rafts and accelerated lipopolysaccharide-induced tumor necrosis factor-alpha secretion in Abca1-deficient macrophages. J Lipid Res. 2007;48:299–306. doi: 10.1194/jlr.M600428-JLR200. [DOI] [PubMed] [Google Scholar]
- 15.Yvan-Charvet L, Welch C, Pagler TA, Ranalletta M, Lamkanfi M, Han S, Ishibashi M, Li R, Wang N, Tall AR. Increased inflammatory gene expression in ABC transporter-deficient macrophages: free cholesterol accumulation, increased signaling via toll-like receptors, and neutrophil infiltration of atherosclerotic lesions. Circulation. 2008;118:1837–47. doi: 10.1161/CIRCULATIONAHA.108.793869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhu X, Lee JY, Timmins JM, Brown JM, Boudyguina E, Mulya A, Gebre AK, Willingham MC, Hiltbold EM, Mishra N, Maeda N, Parks JS. Increased cellular free cholesterol in macrophage-specific Abca1 knock-out mice enhances pro-inflammatory response of macrophages. J Biol Chem. 2008;283:22930–41. doi: 10.1074/jbc.M801408200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Candini C, Schimmel AW, Peter J, Bochem AE, Holleboom AG, Vergeer M, Dullaart RP, Dallinga-Thie GM, Hovingh GK, Khoo KL, Fasano T, Bocchi L, Calandra S, Kuivenhoven JA, Motazacker MM. Identification and characterization of novel loss of function mutations in ATP-binding cassette transporter A1 in patients with low plasma high-density lipoprotein cholesterol. Atherosclerosis. 2010;213:492–8. doi: 10.1016/j.atherosclerosis.2010.08.062. [DOI] [PubMed] [Google Scholar]
- 18.Singaraja RR, Visscher H, James ER, Chroni A, Coutinho JM, Brunham LR, Kang MH, Zannis VI, Chimini G, Hayden MR. Specific mutations in ABCA1 have discrete effects on ABCA1 function and lipid phenotypes both in vivo and in vitro. Circ Res. 2006;99:389–97. doi: 10.1161/01.RES.0000237920.70451.ad. [DOI] [PubMed] [Google Scholar]
- 19.Havel RJ, Eder HA, Bragdon JH. The distribution and chemical composition of ultracentrifugally separated lipoproteins in human serum. J Clin Invest. 1955;34:1345–53. doi: 10.1172/JCI103182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pisciotta L, Bocchi L, Candini C, Sallo R, Zanotti I, Fasano T, Chakrapani A, Bates T, Bonardi R, Cantafora A, Ball S, Watts G, Bernini F, Calandra S, Bertolini S. Severe HDL deficiency due to novel defects in the ABCA1 transporter. J Intern Med. 2009;265:359–72. doi: 10.1111/j.1365-2796.2008.02019.x. [DOI] [PubMed] [Google Scholar]
- 21.Folco EJ, Sheikine Y, Rocha VZ, Christen T, Shvartz E, Sukhova GK, Di Carli MF, Libby P. Hypoxia but not inflammation augments glucose uptake in human macrophages: Implications for imaging atherosclerosis with 18fluorine-labeled 2-deoxy-D-glucose positron emission tomography. J Am Coll Cardiol. 2011;58:603–14. doi: 10.1016/j.jacc.2011.03.044. [DOI] [PubMed] [Google Scholar]
- 22.Gautier EL, Westerterp M, Bhagwat N, Cremers S, Shih A, Abdel-Wahab O, Lütjohann D, Randolph GJ, Levine RL, Tall AR, Yvan-Charvet L. HDL and Glut1 inhibition reverse a hypermetabolic state in mouse models of myeloproliferative disorders. J Exp Med. 2013;210:339–53. doi: 10.1084/jem.20121357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhu X, Owen JS, Wilson MD, Li H, Griffiths GL, Thomas MJ, Hiltbold EM, Fessler MB, Parks JS. Macrophage ABCA1 reduces MyD88-dependent Toll-like receptor trafficking to lipid rafts by reduction of lipid raft cholesterol. J Lipid Res. 2010;51:3196–206. doi: 10.1194/jlr.M006486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rudd JH, Myers KS, Bansilal S, Machac J, Rafique A, Farkouh M, Fuster V, Fayad ZA. (18)Fluorodeoxyglucose positron emission tomography imaging of atherosclerotic plaque inflammation is highly reproducible: implications for atherosclerosis therapy trials. J Am Coll Cardiol. 2007;50:892–6. doi: 10.1016/j.jacc.2007.05.024. [DOI] [PubMed] [Google Scholar]
- 25.Rudd JH, Myers KS, Bansilal S, Machac J, Woodward M, Fuster V, Farkouh ME, Fayad ZA. Relationships among regional arterial inflammation, calcification, risk factors, and biomarkers: a prospective fluorodeoxyglucose positron-emission tomography/computed tomography imaging study. Circ Cardiovasc Imaging. 2009;2:107–15. doi: 10.1161/CIRCIMAGING.108.811752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pedersen SF, Graebe M, Fisker Hag AM, Hojgaard L, Sillesen H, Kjaer A. Gene expression and 18FDG uptake in atherosclerotic carotid plaques. Nucl Med Commun. 2010;31:423–9. doi: 10.1097/MNM.0b013e32833767e0. [DOI] [PubMed] [Google Scholar]
- 27.Bucerius J, Duivenvoorden R, Mani V, Moncrieff C, Rudd JH, Calcagno C, Machac J, Fuster V, Farkouh ME, Fayad ZA. Prevalence and risk factors of carotid vessel wall inflammation in coronary artery disease patients: FDG-PET and CT imaging study. JACC Cardiovasc Imaging. 2011;4:1195–205. doi: 10.1016/j.jcmg.2011.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tawakol A, Migrino RQ, Hoffmann U, Abbara S, Houser S, Gewirtz H, Muller JE, Brady TJ, Fischman AJ. Noninvasive in vivo measurement of vascular inflammation with F-18 fluorodeoxyglucose positron emission tomography. J Nucl Cardiol. 2005;12:294–301. doi: 10.1016/j.nuclcard.2005.03.002. [DOI] [PubMed] [Google Scholar]
- 29.Paulmier B, Duet M, Khayat R, Pierquet-Ghazzar N, Laissy JP, Maunoury C, Hugonnet F, Sauvaget E, Trinquart L, Faraggi M. Arterial wall uptake of fluorodeoxyglucose on PET imaging in stable cancer disease patients indicates higher risk for cardiovascular events. J Nucl Cardiol. 2008;15:209–17. doi: 10.1016/j.nuclcard.2007.10.009. [DOI] [PubMed] [Google Scholar]
- 30.Marnane M, Merwick A, Sheehan OC, et al. Carotid plaque inflammation on 18F-fluorodeoxyglucose positron emission tomography predicts early stroke recurrence. Ann Neurol. 2012;71:709–18. doi: 10.1002/ana.23553. [DOI] [PubMed] [Google Scholar]
- 31.Tang C, Liu Y, Kessler PS, Vaughan AM, Oram JF. The macrophage cholesterol exporter ABCA1 functions as an anti-inflammatory receptor. J Biol Chem. 2009;284:32336–43. doi: 10.1074/jbc.M109.047472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Francone OL, Royer L, Boucher G, Haghpassand M, Freeman A, Brees D, Aiello RJ. Increased cholesterol deposition, expression of scavenger receptors, and response to chemotactic factors in Abca1-deficient macrophages. Arterioscler Thromb Vasc Biol. 2005;25:1198–205. doi: 10.1161/01.ATV.0000166522.69552.99. [DOI] [PubMed] [Google Scholar]
- 33.Li C, Guo R, Lou J, Zhou H. The Transcription Levels of ABCA1, ABCG1 and SR-BI are Negatively Associated with Plasma CRP in Chinese Populations with Various Risk Factors for Atherosclerosis. Inflammation. 2012;35:1641–8. doi: 10.1007/s10753-012-9479-9. [DOI] [PubMed] [Google Scholar]
- 34.Tahara N, Kai H, Ishibashi M, Nakaura H, Kaida H, Baba K, Hayabuchi N, Imaizumi T. Simvastatin attenuates plaque inflammation: evaluation by fluorodeoxyglucose positron emission tomography. J Am Coll Cardiol. 2006;48:1825–31. doi: 10.1016/j.jacc.2006.03.069. [DOI] [PubMed] [Google Scholar]
- 35.Wu YW, Kao HL, Huang CL, Chen MF, Lin LY, Wang YC, Lin YH, Lin HJ, Tzen KY, Yen RF, Chi YC, Huang PJ, Yang WS. The effects of 3-month atorvastatin therapy on arterial inflammation, calcification, abdominal adipose tissue and circulating biomarkers. Eur J Nucl Med Mol Imaging. 2012;39:399–407. doi: 10.1007/s00259-011-1994-7. [DOI] [PubMed] [Google Scholar]
- 36.Ishii H, Nishio M, Takahashi H, Aoyama T, Tanaka M, Toriyama T, Tamaki T, Yoshikawa D, Hayashi M, Amano T, Matsubara T, Murohara T. Comparison of atorvastatin 5 and 20 mg/d for reducing F-18 fluorodeoxyglucose uptake in atherosclerotic plaques on positron emission tomography/computed tomography: a randomized, investigator-blinded, open-label, 6-month study in Japanese adults scheduled for percutaneous coronary intervention. Clin Ther. 2010;32:2337–47. doi: 10.1016/j.clinthera.2010.12.001. [DOI] [PubMed] [Google Scholar]
- 37.Pietri M, Dakowski C, Hannaoui S, Alleaume-Butaux A, Hernandez-Rapp J, Ragagnin A, Mouillet-Richard S, Haik S, Bailly Y, Peyrin JM, Launay JM, Kellermann O, Schneider B. PDK1 decreases TACE-mediated α-secretase activity and promotes disease progression in prion and Alzheimer's diseases. Nat Med. 2013;19:1124–31. doi: 10.1038/nm.3302. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.