Abstract
Purpose
Autosomal Dominant Hyper IgE Recurrent Infection Syndrome (AD-HIES) is caused by mutations in STAT3 and characterized by eczema, recurrent bacterial infections, and skeletal and connective tissue abnormalities. To further understand the minimal trauma fractures of AD-HIES, we examined bone mineral density (BMD) and laboratory markers of bone turnover.
Methods
Patients with AD-HIES enrolled in a prospective natural history study were examined with dual x-ray absorptiometry (DEXA) scans and laboratory studies of bone metabolism. The number of fractures was recorded as well as clinical features of AD-HIES including scoliosis and retained primary teeth. Patients on medications with skeletal effects, including bisphosphonates, were examined separately.
Results
Twenty-three AD-HIES children (6–18 years) and 33 AD-HIES adults (21–50 years) not receiving bone-active drugs were studied. Fourteen of the 23 children (61 %) had histories of minimal trauma fractures, as did 26 of the 33 adults (79 %). Osteopenia or osteoporosis was found in 79% of children and adults. Only radial BMD correlated with the qualitative occurrence of fractures but it did not correlate with the numbers of fractures. Markers of bone metabolism did not correlate with minimal trauma fractures or BMD. Patients on bone-active medications had improved BMD, but still sustained fractures.
Conclusions
Minimal trauma fractures and decreased BMD are common in AD-HIES. Low radial BMD is associated with fractures, but hip and spine BMD are not. Treatment with bisphosphonates increased BMD but its role in fracture prevention remains undefined.
Keywords: Autosomal dominant hyper IgE syndrome (AD-HIES), job’s syndrome, signal transducer and activator of transcription 3 (STAT3), osteoporosis, retained primary teeth
Introduction
Autosomal dominant Hyper IgE syndrome (AD-HIES or Job’s syndrome) is characterized by mutations in the STAT3 gene, resulting in recurrent pneumonias, boils, eczema, elevated serum immunoglobulin E (IgE), and connective tissue, vascular, and skeletal abnormalities [1, 2]. STAT3 is a necessary signaling protein for many cytokines and growth factors, explaining why AD-HIES has such diverse non-immunologic manifestations [3, 4]. To better understand the minimal trauma fractures in AD-HIES, we examined bone mineral density (BMD), markers of bone metabolism, and connective and skeletal features of AD-HIES.
Methods
Study Subjects
We recorded minimal trauma fractures, BMD and bone metabolic markers for 56 individuals with AD-HIES not receiving bisphosphonates or parathyroid hormone therapy (teriparatide). All patients or their parents provided consent on an IRB-approved natural history protocol to study HIES at the National Institutes of Health (NIH), Bethesda, MD. Patients were diagnosed clinically and by STAT3 mutational analysis. Patients were excluded if they received steroid treatment for at least a month or any chemotherapy, or had other conditions associated with osteoporosis. After initial analysis suggested a correlation between radius BMD and fractures, 12 additional individuals were analyzed to further evaluate this association.
Four adult patients with AD- HIES on bisphosphonate or parathyroid hormone therapy were analyzed along with eight age- and gender-matched AD-HIES patients with no history of these medications and two BMD measures over a similar period. A pre-treatment (baseline) and a roughly 4–5 year follow-up measure were used to calculate an annual rate of change in BMD for patients and controls.
Laboratory and Radiologic Investigation
Evaluations included complete history and physical, dental examination, BMD by dual x-ray absorptiometry (DEXA) scans, and scoliosis radiographs. Bone metabolism studies included serum calcium, 25-hydroxyvitamin D, phosphorous, osteocalcin, intact parathyroid hormone, testosterone, and urine N-telopeptides of collagen (NTX).
Statistics
The Wilcoxon rank sum test and Spearman’s rank correlation were used to detect associations with continuous variables; Fisher’s exact test was used for categorical variables. The rate of change in BMD was compared between treated adults and matched controls using the paired t-test. Statistical tests were two-sided and performed at the 0.05 level. Statistical analysis was done in R software (http://www.r-project.org).
Results
Demographics
The 56 AD-HIES patients not on osteoporosis therapy included 33 adults ages 21–50 years and 23 children, aged 6–18 years. DNA genotyping was available for all but one deceased adult with an HIES score of 89 (Table I).
Table I.
Demographics and diagnostic features of patients included in main study analysis (N=56)
| All (N=56) | Adult (age≥20) (N=33) |
Children (age<20) (N=23) |
|
|---|---|---|---|
| Age in years | |||
| Median (IQR) | 22.5 (12,34) | 31 (24,37) | 11 (9,14.5) |
| Male n (%) | 26 (46.4 %) | 13 (39.4 %) | 13 (56.5 %) |
| STAT3 mutation n (%) | |||
| DNA binding domain | 30 (53.6 %) | 17 (51.5 %) | 13 (56.5 %) |
| SH2 binding | 23 (41.1 %) | 14 (42.4 %) | 9 (39.1 %) |
| Transactivation | 2 (3.6 %) | 1 (3 %) | 1 (4.3 %) |
| Not determined | 1 (1.8 %) | 1 (3 %) | 0 (0 %) |
| HIES score | |||
| Median (IQR) | 70 (58,80) | 78 (68,82) | 60 (54,67) |
| BMI | |||
| Median (IQR) | 22.15 (18.9,26.7) | 25.4 (20.5,29.4) | 18.9 (17.3,21.6) |
| Smoker n (%) | 7 (21 %) | ||
IQR inter-quartile range, the 25th and 75th percentiles
Bone Mineral Density (BMD)
For most patients, DEXA scores were obtained for AP spine, total hip and radius. On at least one BMD measure, 79 % of subjects had either osteopenia or osteoporosis.
Spine BMD determination in 23 children found that 12 had normal, 9 had osteopenic and 2 had osteoporotic BMD Z-scores. Of the 32 adults with available studies, 10 had normal, 13 had osteopenic, and 9 had osteoporotic BMD T-scores. Similar distributions were found for hip and radius (not shown). Children with DNA binding region mutations had lower median AP spine z-scores compared to those with SH2 mutations (p-value=0.02); no association was seen in adults. Overall, males had lower hip (p-value=0.047) and radius (p-value=0.013) BMDs than females (Table II). Adult men had significantly lower median AP spine (−2.5) and radius (−2.8) z-scores than women (−1.3 spine and −0.95 radius) (p=0.008 and p=0.0004, respectively).
Table II.
Bone mineral density and bone-related HIES features for all patients and by gender
| N* | All | Male | Female | p-valuea | |
|---|---|---|---|---|---|
| BMD median (IQR) | |||||
| Hip | 55 | −0.8 (−1.3,0) | −0.9 (−1.6,−0.5) | −0.35 (−1.1,0.2) | 0.047 |
| Radius | 56 | −1.2 (−2.1,−0.6) | −1.85 (−2.9,−0.7) | −1 (−1.6,−0.5) | 0.013 |
| Spine | 55 | −1.4 (−2.2,−0.6) | −1.4 (−2.8,−0.7) | −1.3 (−2.2,−0.5) | 0.423 |
| Fractures median (IQR) | 56 | 1.5 (0,4) | 2.5 (0,4) | 1.5 (0,4) | 0.675 |
| Scoliosis >15° (adults) n (%) | 33 | 15 (45.5) | 4 (30.8) | 11 (55) | 0.284 |
| Retained primary teeth >3 (adults) n (%) | 33 | 23 (69.7) | 8 (61.5) | 15 (75) | 0.461 |
IQR inter-quartile range, the 25th and 75th percentiles
p-value comparing the listed variables (BMDs, fractures, scoliosis, retained teeth) for males and females calculated using Wilcoxon rank sum test for BMD and number of fractures, Fisher’s exact test for scoliosis and retained primary teeth
Fractures
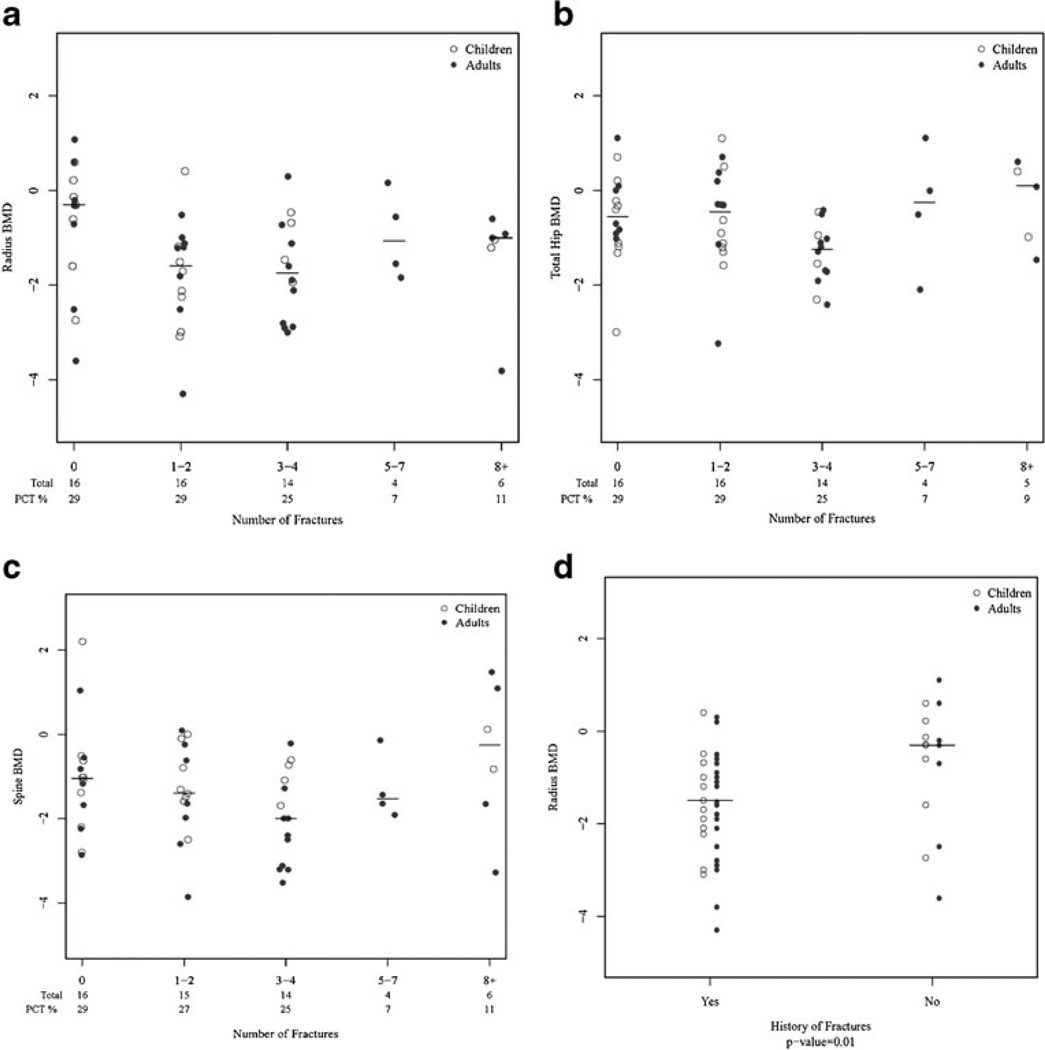
Forty (71 %) of the 56 patients had experienced minimal trauma fractures, ranging from one to 18 or more fractures. There were no significant associations between any of the BMD measures and increasing number of fractures (Fig. 1a, b, c). There was an association between any minimal trauma fractures and low radial BMD (p=0.01), but not with hip or spine BMD (Fig. 1d). A similar trend was seen in 12 confirmatory patients, with median radius BMD of −1.6 for those with fractures compared to −0.5 in the no fracture group (p=0.17). Fracture location was limited to long bones and ribs, with the exception of one individual who had a vertebral fracture. Having at least two abnormally low BMD measures was associated with a 4-fold increase in the odds of a history of fractures (p=0.039) compared to fewer abnormal measures, suggesting that this may be another important risk factor.
Fig. 1.
Bone mineral density (BMD) with increasing number of fractures of (a) radius (b) hip and (c) spine. d Radius Bone mineral density (BMD) determinations in the presence or absence of minimal trauma fractures
Retained Primary Dentition and Scoliosis
Since tooth root resorption involves an interaction of the alveolar bone and the dentin, and scoliosis involves bone deposition and remodeling, we examined the association of BMD and fracture, with retained primary teeth and scoliosis in adults. No associations were found.
Measures of Bone Metabolism
There were no significant associations between any bone metabolism marker and BMD or fractures in either adults or children. There was a weak association for increased urine N-telopeptides, a marker of osteoclast activity, and minimal trauma fractures in adult patients (p=0.10); however, there was a wide range of values in both groups. Serum vitamin D ranged from low to normal and was not correlated with number of fractures. Calcium was insignificantly lower for adults with fractures (p=0.06) and vitamin D was insignificantly lower in children with fractures (p=0.06). Neither calcium nor vitamin D correlated with BMD.
Treatment
Two men and two women ages 32–61 received osteoporosis therapy over 3 to 10 years (average 5.5 years). One man received teriparatide after not improving with alendronate; all others received alendronate. All recipients had improved BMD from baseline. The average change in spine BMD over 5 years was a 0.42 unit increase for treated patients compared to 0.31 unit decrease for matched non-treated controls. Despite improvements in BMD, three of the four patients suffered fractures during treatment; three of the eight without therapy fractured.
Discussion
While fractures and reduced BMD are common in this population, the number of minimal trauma fractures did not strongly correlate with overall BMD. The correlation between fractures and radius BMD, which consists primarily of cortical bone, was interesting in light of the fracture distribution favoring long bones.
Several lines of data suggest that the osteoporosis of AD-HIES results from increased osteoclast activity. Monocytes from patients with AD-HIES resorb bone at an increased rate, similar to that of postmenopausal women [5]. Hematopoietic cell-specific Stat3 deficient mice develop osteoporosis through increased osteoclastogenesis resulting in decreased trabecular and cortical bone [6]. However, the significant number of fractures in some patients despite relatively normal BMD as well as the lack of correlation between urine N-telopeptides, a marker of osteoclasts, and fractures or BMD suggests there are factors beyond bone density that affect bone fragility. The factors affecting fragility likely differ dependent on the bone type since fractures occurred predominantly in long bones, primarily cortical bone, and the radius BMD, as compared to hip or spine BMD, correlated with history of fractures. The etiology of low BMD in AD-HIES is likely multifactorial and may result from a combination of increased osteoclast function, chronic inflammation, nutritional and hormonal factors, as well as other undefined effects of STAT3 on bone metabolism. The relative rarity of this disease as well as the difficulties of measurement and interpretation of BMD in children limited our study. Fracture history was retrospective and therefore affected by recall. The analysis was exploratory and should be interpreted with caution.
Our findings suggest that AD-HIES has a high risk of pathologic fractures that is incompletely explained by BMD or bone resorption and likely not addressed by measures directed at inhibition of osteoclastogenesis. These data suggest that STAT3 mediates here-to-fore unrecognized aspects of bone integrity that may be separate from its role in osteoclast activity.
Acknowledgments
Publication disclaimer This research was supported by the Division of Intramural Research, NIAID, NIH. The views expressed in this article are those of the authors and do not reflect the official policy of the U.S. Government. This project has been funded in part with federal funds from the National Cancer Institute, National Institutes of Health, under Contract No.HHSN261200800001E. The content of this publication does not necessarily reflect the views or policies of the Department of Health and Human Services, nor does mention of trade names, commercial products, or organizations imply endorsement by the U.S. government. This research was supported [in part] by the Division of Intramural Research, National Institute of Allergy and Infectious Diseases, National Institutes of Health.
Contributor Information
Kathryn J. Sowerwine, Laboratories of Clinical Infectious Diseases, NIAID, NIH, Bethesda, MD, USA
Pamela A. Shaw, Biostatistics Research Branch, National Institute of Allergy and Infectious Diseases, Bethesda, MD, USA
Wenjuan Gu, Biostatistics Research Branch, Clinical Research Directorate/CMRP SAIC-Frederick, National Laboratory for Cancer Research, Frederick, MD 21703, USA.
Jennifer C. Ling, Department of Allergy and Infectious Diseases, University of Washington, Seattle, WA, USA
Michael T. Collins, Skeletal Clinical Studies Unit, Craniofacial and Skeletal Diseases Branch, NIDCR, NIH, Bethesda, MD, USA
Dirk N. Darnell, Laboratories of Clinical Infectious Diseases, NIAID, NIH, Bethesda, MD, USA
Victoria L. Anderson, Laboratories of Clinical Infectious Diseases, NIAID, NIH, Bethesda, MD, USA
Joie Davis, Laboratories of Clinical Infectious Diseases, NIAID, NIH, Bethesda, MD, USA.
Amy Hsu, Laboratories of Clinical Infectious Diseases, NIAID, NIH, Bethesda, MD, USA.
Pamela Welch, Biostatistics Research Branch, Clinical Research Directorate/CMRP SAIC-Frederick, National Laboratory for Cancer Research, Frederick, MD 21703, USA.
Jennifer M. Puck, Department of Pediatrics, University of California San Francisco, San Francisco, CA, USA
Steven M. Holland, Laboratories of Clinical Infectious Diseases, NIAID, NIH, Bethesda, MD, USA
Alexandra F. Freeman, Email: freemaal@mail.nih.gov, Laboratories of Clinical Infectious Diseases, NIAID, NIH, Bethesda, MD, USA.
References
- 1.Minegishi Y, Saito M, Tsuchiya S, Tsuge I, Takada H, Hara T, et al. Dominant-negative mutations in the DNA-binding domain of STAT3 cause hyper-IgE syndrome. Nature. 2007;448(7157):1058–1062. doi: 10.1038/nature06096. Research Support, Non-U.S. Gov’t. [DOI] [PubMed] [Google Scholar]
- 2.Buckley RH, Wray BB, Belmaker EZ. Extreme hyperimmunoglobulinemia E and undue susceptibility to infection. Pediatrics. 1972;49(1):59–70. In Vitro. [PubMed] [Google Scholar]
- 3.Holland SM, DeLeo FR, Elloumi HZ, Hsu AP, Uzel G, Brodsky N, et al. STAT3 mutations in the hyper-IgE syndrome. N Engl J Med. 2007;357(16):1608–1619. doi: 10.1056/NEJMoa073687. Research Support, N.I.H., Extramural Research Support, N.I.H., Intramural Research Support, Non-U.S. Gov’t. [DOI] [PubMed] [Google Scholar]
- 4.Grimbacher B, Holland SM, Gallin JI, Greenberg F, Hill SC, Malech HL, et al. Hyper-IgE syndrome with recurrent infections—an autosomal dominant multisystem disorder. N Engl J Med. 1999;340(9):692–702. doi: 10.1056/NEJM199903043400904. Research Support, Non-U.S. Gov’t. [DOI] [PubMed] [Google Scholar]
- 5.Cohen-Solal M, Prieur AM, Prin L, Denne MA, Launay JM, Graulet AM, et al. Cytokine-mediated bone resorption in patients with the hyperimmunoglobulin E syndrome. Clin Immunol Immunopathol. 1995;76(1 Pt 1):75–81. doi: 10.1006/clin.1995.1090. Comparative Study In Vitro. [DOI] [PubMed] [Google Scholar]
- 6.Zhang Z, Welte T, Troiano N, Maher SE, Fu XY, Bothwell AL. Osteoporosis with increased osteoclastogenesis in hematopoietic cell-specific STAT3-deficient mice. Biochem Biophys Res Commun. 2005;328(3):800–807. doi: 10.1016/j.bbrc.2005.01.019. Research Support, Non-U.S. Gov’t Research Support, U.S. Gov’t, P.H.S. [DOI] [PubMed] [Google Scholar]