Abstract
Aim
In recent years, several studies with large sample sizes and recent follow-up data have been published comparing outcomes between laparoscopic Nissen fundoplication and laparoscopic Toupet fundoplication. It is now timely to be re-evaluated and synthesized long-term efficacy and adverse events of both total and partial posterior fundoplication.
Materials and Methods
Electronic searches for RCTs comparing the outcome after laparoscopic Nissen fundoplication and laparoscopic Toupet fundoplication were performed in the databases of MEDLINE, EMBASE, and the Cochrane Center Register of Controlled Trials. The data of evaluation in positive and adverse results of laparoscopic Nissen fundoplication and laparoscopic Nissen fundoplication were extracted and compared using meta-analysis.
Results
13 RCTs were ultimately identified involving 814 (52.05%) and 750 (47.95%) patients who underwent laparoscopic Nissen fundoplication and laparoscopic Toupet fundoplication, respectively. The operative time, perioperative complications, postoperative satisfaction, recurrence, and the rates of medication adoption or re-operation due to recurrence were not significantly different between two groups. The two types of fundoplication both reinforced the anti-reflux barrier and elevated the lower esophageal sphincter pressure. However, rates of adverse results involving dysphasia, gas-bloat syndrome, inability to belch and re-operation due to severe dysphasia were significantly higher after LNF. In the subgroup analysis of wrap length≤2cm, laparoscopic Nissen fundoplication was associated with a significantly higher incidence of postoperative dysphagia. However, in the subgroup wrap length>2cm, the difference was not statistically significant.
Conclusion
Laparoscopic Toupet fundoplication might be the better surgery approach for gastroesophageal reflux disease with a lower rate of postoperative adverse results and equal effectiveness as Laparoscopic Nissen fundoplication.
Introduction
The lower esophageal sphincter (LES) plays an important role in the pathogenesis of gastroesophageal reflux disease (GERD) [1]. In order to inhibit LES relaxation, fundoplication is considered to be an essential and important part of antireflux surgery. Laparoscopic Nissen fundoplication (LNF), a total wrap that surrounds the esophagus 360°, is the most commonly used, gold standard technique worldwide for antireflux surgery [2]. However, LNF is associated with a high incidence of postoperative dysphagia and gas-bloat syndrome [3,4]. Laparoscopic Toupet fundoplication (LTF), a 270° partial wrap, was introduced to counteract these side effects. An ongoing discussion has focused on the ideal approach to antireflux surgery, including durable reflux control, as well as minimal postoperative dysphagia and gas-related symptoms.
Many surgeons advocate that the incidence of regurgitation and heartburn are similar in both LNF and LTF, while postoperative dysphagia may have a higher incidence following LNF [5]. Besides fundoplication type, other variables such as the length of the wrap and impaired esophageal peristalsis may also be associated with postoperative dysphagia [6,7]. The original LNF procedure, which purports a 6-cm wrap length, is associated with a higher dysphagia rate [8]. Two studies on the length of the Nissen fundoplication showed that a loose wrap of 1–2 cm was sufficient to suppress reflux and reduce the incidence of postoperative bloating and dysphagia [9,10]. An early study showed that LTF was more effective when esophageal motility (EM) was abnormal (less than 50% peristaltic waveforms) [11]. However, a previous study reported a similar incidence of dysphagia between LTF and LNF 1 year postoperatively [12]. Whether LTF has a benefit on abnormal esophageal peristalsis remains controversial.
Several meta-analyses have been performed comparing outcomes between LNF and LTF until 2011 [13,14]. However, a comprehensive study collecting randomized clinical trials (RCTs) has not been conducted to date. In recent years, several studies with large sample sizes and recent follow-up data have been published comparing long-term efficacy and adverse events of both total and partial fundoplication [7, 15–17]. Therefore, in order to better weigh the potential benefits against the potential side effects, data from these recent trials is now timely to be re-evaluated and synthesized with the existing trials. To address this need, we performed a meta-analysis of RCTs to determine the optimal surgical approach for GERD, providing better reflux control with minimal postoperative complications.
Methods
Search strategy
All RCTs in the English language comparing outcomes of LNF with LTF were eligible for the meta-analysis, regardless of publication status (published, unpublished, in press, or in progress).
Electronic searches were performed for relevant reports in the MEDLINE, EMBASE, and the Cochrane Center Register of Controlled Trials databases, until June 2014. We adopted our search strategy using disease-specific terms (e.g. gastroesophageal reflux disease), management-specific terms (e.g. laparoscopic fundoplication), and terms related to surgical procedures (e.g. Nissen, Toupet, partial, and total). The abstracts of all potential articles, references, and related articles were reviewed according to their titles.
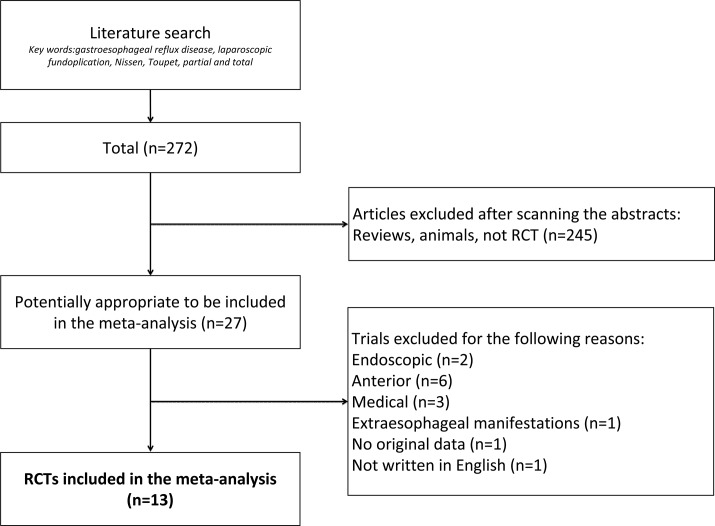
Each article was independently assessed for eligibility using inclusion and exclusion criteria. Inclusion criteria were as follows: (1) randomized controlled trials (RCTs) comparing efficacy and negative outcomes of laparoscopic fundoplication (LF), including LNF and LTF; and (2) exact and intact dichotomous-type or continuous-type data with standard deviations. Exclusion criteria were as follows: (1) non-randomized controlled trials; (2) trials comparing total and non-posterior partial fundoplication (e.g. total vs. anterior partial fundoplication); and (3) patients younger than 16 years. The literature search was performed independently by two authors (Bin Wang and Zhi-chao Tian). The third author (Ming Qiu) determined whether or not to include the study when controversy occurred. Our procedure for screening and selection is shown in Fig 1.
Fig 1. A flow chart showing the details of search strategy and the trials selection process according to the including and excluding criteria.
The criteria for grading methodologic quality of included studies was determined by group discussion. The determination of whether methodologic bias existed was made according to Cochrane criteria guidelines, including selection, performance, detection, attrition, reporting, and other biases [18]. All items assessed were shown in Forest-plot figures.
Selection criteria
Two investigators (Bin Wang and Zhi-chao Tian) independently extracted and collected data using a standardized data-extraction protocol. Data of baseline patient characteristics were collected, including first author, publication year, sample size, age, sex, BMI, wrap length (cm), and duration of mean follow-up. For repeated publications, we only extracted the most recent data of interesting outcomes. For example, Mickevicius et al. reported their results twice at different periods of the same trial [6, 7]. Therefore, we extracted different outcomes from the corresponding article (e.g. perioperative complications from Mickevicius et al. 2008 [6], and dysphagia from Mickevicius et al. 2013 [7] for long-term outcome evaluation. Otherwise, Mickevicius et al. [6, 7] further reported effectiveness and adverse results according to the length of wrap (1.5 cm vs. 3.0 cm). Therefore, we extracted and showed data from the 1.5 cm group and 3.0 cm group, respectively, in some fields.
Statistical analysis
Data extracted from included trials were integrated with Review Manager Software version 5.2 (Cochrane Collaborative, Oxford, England). The risk ratio was used for dichotomous-type data and the standard mean difference for continuous-type data. The fixed-effects model was used only if no significant heterogeneity was detected among trials, or if the random-effects model was adopted to ignore heterogeneity. Subgroup analyses were performed to assess the impact of esophageal motility and wrap length on dysphagia, according to esophageal function (subgroup 1: EM both normal; subgroup 2: EM both abnormal; subgroup 3: EM normal before LNF vs. abnormal before LTF) and wrap length (subgroup 1: WL ≤2 cm; subgroup 2: WL >2 cm).
Results
Baseline characteristics of included trials
Finally, the literature search identified 13 RCTs [6, 7, 12, 15–17, 19, 20–25] published between June 1997 and July 2013. A total of 814 (52.05%) patients underwent LNF and 750 (47.95%) patients underwent LTF. The LNF group was comprised of 212 (57.92%) males and 207 (58.97%) females in the LTF, respectively. Patient ages ranged from 19 to 82 years. Five trials [7, 16, 20, 24–25] calculated the body mass index (BMI) of patients, which were similar within a range from 26 to 29 kg/m2. More preoperative details are shown in Table 1.
Table 1. The baseline characteristics of included trials.
| Included references | Sample Capcity* | Follow-up (mo) | Sex** | Age(yr) * | Weight(Kg) or BMI(Kg/m2) * | Wrap length (cm) * | PPI use (%)* | Duration of symptoms* |
|---|---|---|---|---|---|---|---|---|
| Laws 1997[19] | 23/16 | Mean 27.2 | 19/20 | 45.5 / 55.5 | No detail | Average 2.2 [1.6–3] / - | No detail | No detail |
| Chrysos 2003[12] | 14/19 | 12 | 18/15 | 61.7±8.7 / 59.2±11.5 | No detail | 3–4 / 3–4 | No detail | No detail |
| Guérin 2007[20] | 77/63 | 36 | 86/54 | No detail | Median BMI 27/26.6 | 3 / - | No detail | No detail |
| Booth 2008[21] | 64/63 | 12 | 84/43 | 45.3[21–86]/44.2[19–69] | Mean Weight 81.6/80.2 | 2 / - | 92% / 90% | 94.5 (7–516) / 95.6 (6–248) mo |
| Fibbe 2001[22] | 100/100 | 24 | 121/79 | 56[20–80] | Mean BMI 26.4[18.9–40.4] | 2 / - | Average 24 (0.2–180) mo | Median 7 (0.2–50) yr |
| Zornig 2002[23] | ||||||||
| Strate 2008[24] | ||||||||
| Shaw 2010[25] | 50/50 | 60 | 60/40 | 45.2[28–72]/45.6[25–67] | Mean BMI 29.3±5.2/29.2±5.2 | 1 / 2 | No detail | 2.36±0.5 / 2.58±0.6 |
| Mucio 2012[15] | 133/131 | 180 | No detail | No detail | No detail | 2.54 (1 inch) / - | No detail | No detail |
| Koch 2013[16] | 62/63 | 12 | 78/47 | 50.32[20–76]/51.87[25–81] | Mean BMI 28.18[19.47–41.80]/27.32[19.66–3.86] | - / - | No detail | No detail |
| Qin 2013[17] | 215/168 | 12 | 194/189 | 56.3[34–82] | No detail | - / - | No detail | No detail |
| Mickevicius 2008[6] | 76/77 | 60 | 74/79 | 49.2±14.4 / 54.8±12.6 | Mean BMI 27.7±4.6/29.3±3.2 | 1.5 vs. 3 / 1.5 vs. 3 | No detail | 7.2±7.7 / 7.1±7.6 yr |
| Mickevicius 2013[7] |
LNF: Laparoscopic Nissen fundoplication; LTF: Laparoscopic Toupet fundoplication; Mo: Month; yr: year; BMI: Body mass index; PPI: Proton pump inhibitors.
*Data in these columns are showed as data in LNF group/data in LTF group
**Data are showed as number of males/number of female
Perioperative characteristics
Operative time and hospital stay
Seven trials [6, 12, 15, 17, 19, 21, 25] reported mean operative time (S2 Fig), which ranged from 35.5 [15] to 155 [19] min in the LNF group and 32.5 [15] to 162 [19] min in the LTF group. Operative duration was shorter in the LNF group, but no significant difference was found between groups (LNF vs. LTF, standard mean difference -0.45, 95% CI [-1.00, 0.11], p = 0.11). Four trials reported mean hospital stay in both groups, which ranged from 2.6 [12] to 3.8 [17] days in the LNF group and from 2.5 [19] to 4.2 [17] days in the LTF group.
Perioperative complications
Five trials [7, 12, 15, 19, 21] reported perioperative complications (S3 Fig), which occurred in 19 patients with LNF and 29 patients with LTF, and included lacerations of the gastric fundus and spleen, bleeding from the spleen or short gastric blood vessels, and pneumothorax. Although the incidence of perioperative complications was slightly lower in the LNF compared with LTF group, this trend was not found to be statistically significant (6.13% vs. 9.48%, RR 0.67, 95% CI [0.39, 1.14], p = 0.14).
Postoperative satisfaction
Four trials [12, 20, 24, 25] evaluated patient satisfaction during the follow-up period (S4 Fig), which were categorized as excellent, good, unchanged, or worse. Overall satisfaction rates were remarkably high after both LNF and LTF, without a significant difference between groups (89.30% LNF vs. 85.38% LTF, RR 1.05, 95% CI [0.97, 1.13], p = 0.22). The mean general gastrointestinal quality of life index score in the LNF and LTF group was 96.3 ± 16.6/93.7 ± 21.2 preoperatively and 119.8 ± 15.71/115.2 ± 15.96 postoperatively [16]. Healthy individuals scored, on average, 122.6 ± 8.5 points.
Postoperative symptoms
Dysphagia
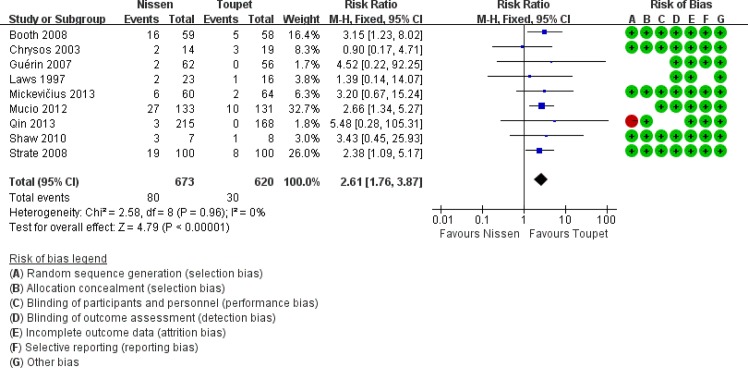
Nine trials [7, 12, 15, 17, 19, 20–21, 24–25] reported postoperative dysphagia (Fig 2), which occurred in 80/637 (12.56%) and 30/620 (4.84%) of patients in the LNF and LTF groups, respectively, and was significantly higher after LNF compared with LTF (RR 2.61, 95% CI [1.76, 3.87], p <0.01). Two trials [7,12] evaluated dysphagia severity. Even though the incidence of moderate-to-severe dysphagia failed to show a significant difference between groups (S5 Fig), it was clear that LNF was associated with moderate-to-severe dysphagia (8.86% vs. 3.85%, RR 2.28, 95% CI [0.63, 8.30], p = 0.21). Additionally, 14/100 (14.00%) patients after LNF and 5/100 (5.00%) after LTF had severe dysphagia, requiring endoscopic bougie dilatation [24].
Fig 2. Overall rates of dysphagia after LNF and LTF.
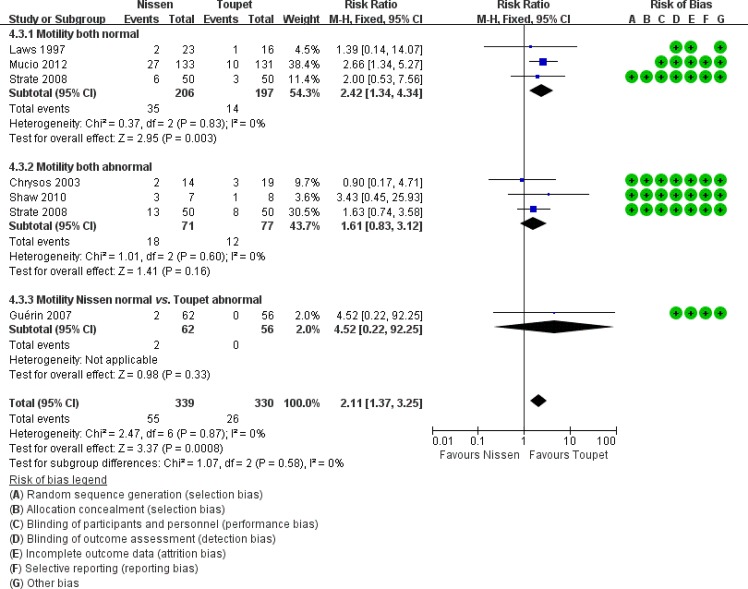
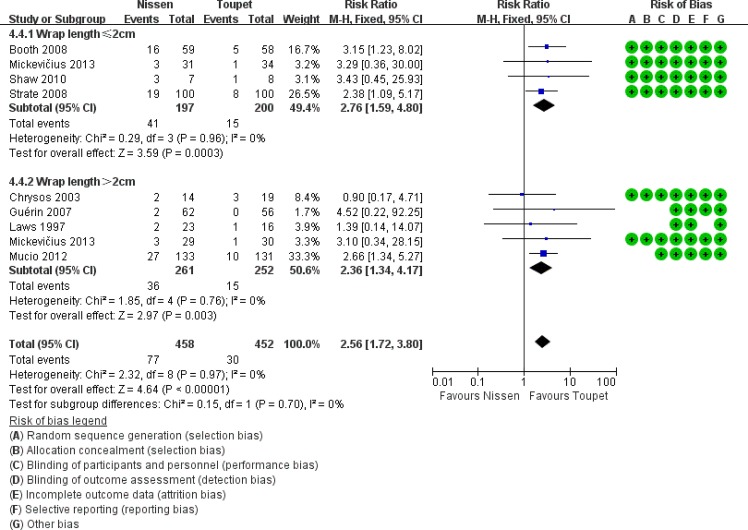
Apart from fundoplication type, EM and wrap length are two important factors for postoperative dysphagia; therefore, a subgroup analysis was performed in this area (Figs 3 and 4). In preoperative normal EM subgroups, postoperative dysphagia occurred more commonly following LTF compared with LNF (LNF 17% vs. LTF 7.11%, RR 2.42, 95% CI [1.34, 4.34], p = 0.003). In the subgroups of LTF with normal EM or abnormal EM, the prevalence of postoperative dysphagia were both showed lower than that in the subgroup of LNF with normal EM, even if there were no significant differences. In the subgroup analysis of patients with a wrap length ≤2 cm, LNF was associated with a significantly higher incidence of postoperative dysphagia. However, in the subgroup analysis of patients with a wrap length >2 cm, this difference was not found to be statistically significant (subgroup 1: LNF 20.81% vs. LTF 7.50%, RR 2.76, 95% CI [1.59, 4.80], p = 0.0003; subgroup 2: LNF 7.03% vs. LTF 4.13%, RR 1.79, 95% CI [0.64, 5.03], p = 0.27).
Fig 3. Subgroup analysis of dysphagia according to esophageal motility.
Fig 4. Subgroup analysis of dysphagia according to wrap length.
Gas-bloat syndrome or gas-related symptoms
The symptoms of gas-bloat syndrome include bloating, inability to belch, postprandial fullness, flatulence, and epigastric pain. Five trials [7, 12, 20–21, 24] assessed the outcome of gas-related symptoms (S6 Fig). The overall prevalence of gas-related symptoms was significantly higher after LNF vs. LTF (31.19% vs. 23.91%, RR 1.31, 95% CI [1.05, 1.65], p = 0.02). Inability to belch occurred in 33 of 221 (14.93%) patients following LNF and 18 of 214 (8.41%) patients following LTF, respectively. Therefore, there was a higher incidence of inability to belch for patients following LNF (RR 1.79, 95% CI [1.06, 3.03], p = 0.03) (S7 Fig). In addition, Booth et al. [21] reported that 18.64%/10.34% suffered from gas-bloat symptoms, 62.71%/63.79% had postprandial fullness, 74.58%/67.24% complained of flatulence, and 25.42%/31.03% experienced epigastric pain after both LNF and LTF.
Reappearance of GERD symptoms
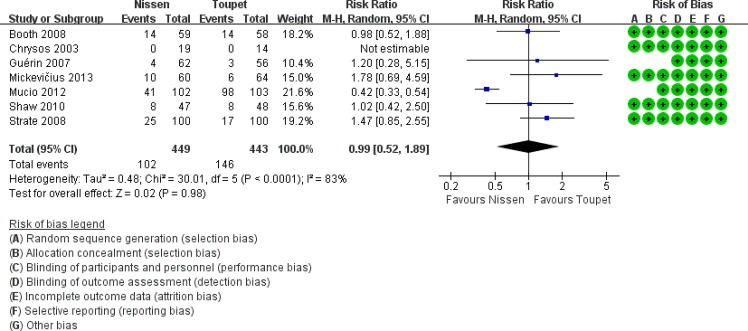
The postoperative reappearance of typical GERD symptomsincludes heartburn, regurgitation, chest pain. Heartburn is the most typical symptom of GERD and this symptom was considered to indicate recurrence. Reappearance of GERD symptoms was reported in seven trials [7, 12, 15, 20–21, 24–25], including 449 patients following LNF and 443 patients following LTF (Fig 5). The overall recurrence rate was 22.72% (102/449) after LNF and 32.96% (146/443) after LTF (RR 0.99, 95% CI [0.52, 1.89], p = 0.59). Subjective reflux control was similar for complete and partial fundoplication. Of the included patients, 13.01% suffered from heartburn after LF (LNF 14.00% vs. LTF 11.98%, p = 0.45), 6.57% had regurgitation (LNF vs. LTF: 3.41% vs. 10.42%, p = 0.54), and 6.72% complained of chest pain (LNF vs. LTF: 10.71% vs. 2.34%, p = 0.01).
Fig 5. Rates of recurrence of GERD after LNF and LTF.
Requirement for antireflux medication and reoperation
The need for antireflux medication was described in two trials [7, 25] and reoperation was described in four trials [15–16, 24–25] (S8 Fig and S9 Fig). A total of 14 of 209 patients after LNF and 13 of 112 patients after LTF required medication due to severe reflux after LF, but this difference was not found to be statistically significant (LNF vs. LTF: 13.08% vs. 11.61%, RR = 1.13, p = 0.74). Redo fundoplications for GERD recurrence were similar for complete and partial fundoplication (LNF vs. LTF: 4.74% vs. 6.54%, p = 0.77) [15–16, 25]. However, 11 patients [16, 24–25] in the LNF group suffered from severe postoperative dysphagia and underwent reoperation, whereas there were no patients with severe dysphagia in patients undergoing reoperation in the LTF group (S10 Fig).
Objective evaluations
LES resting pressure
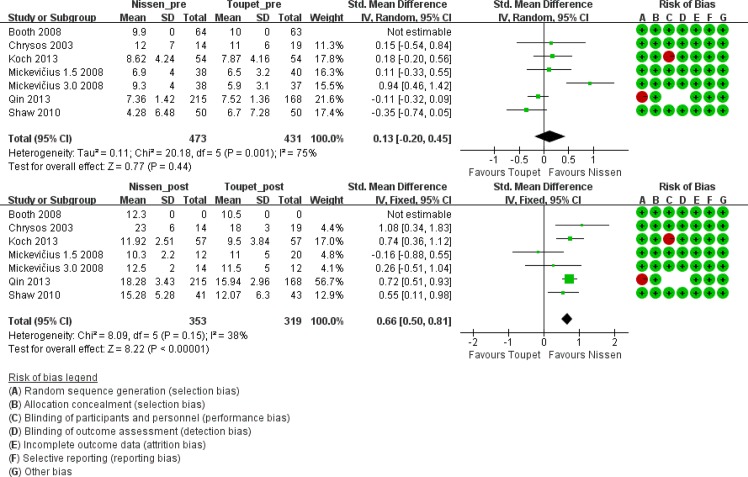
Six trials [6, 12, 16, 17, 21, 25] measured LES resting pressure before and after LF. Preoperative average LES pressure ranged from 4.28 to 12 mm Hg in the LNF group and from 5.9 to 11 mm Hg in the LTF group. A significant improvement in LES pressure was achieved in both groups (10.3 to 23 mm Hg after LNF and 9.5 to 18 mm Hg after LTF). Preoperative LES pressure was approximate between groups. However, postoperative LES pressure increased significantly in the LNF group compared with the LTF group (Fig 6).
Fig 6. LES resting pressure before and after LNF and LTF.
DeMeester score based on gastric monitoring of 24-pH
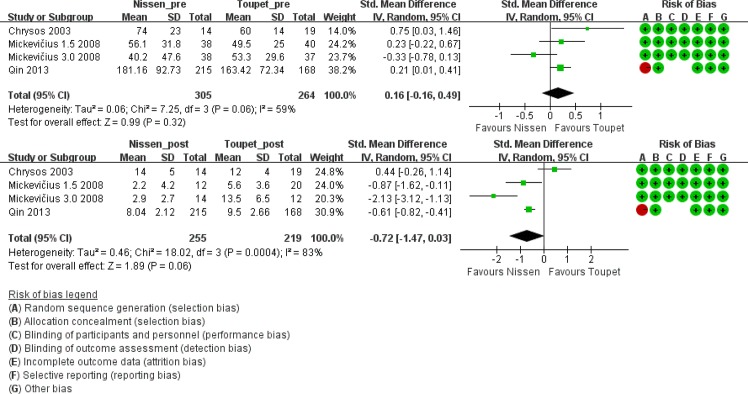
Three trials [6, 12, 17] performed 24-hour pH monitoring and provided both pre- and postoperative DeMeester scores. Although the preoperative score was not significantly different between groups, the postoperative DeMeester score was lower after LNF (pre-LNF vs. pre-LTF, standard mean difference 0.16, 95% CI [-0.16, 0.49], p = 0.32; post-LNF vs. post-LTF, standard mean difference -0.72, 95% CI [-1.47, 0.03], p = 0.06). However, the mean scores after both LNF and LTF were normal (i.e. <14.7) (Fig 7).
Fig 7. DeMeester score before and after LNF and LTF.
Discussion
In our meta-analysis, we included 13 RCTs that reported outcome and postoperative complications of 1564 patients undergoing LNF or LTF. We found that LTF was equally effective in improving quality of life and controlling reflux compared to LNF, but had a lower incidence of postoperative dysphagia and other gas-related symptoms.
Subjective outcomes are accurate and important to evaluate the efficacy of LF for GERD. We demonstrated that both LNF and LTF significantly improve patients’ quality of life while providing optimal control of GERD symptoms. While the efficacy of laparoscopic antireflux surgery has been confirmed by our analysis, adverse effects such as dysphagia, gas-bloat syndrome, and others remain major postoperative complications. The prevalence of dysphagia after LTF was found to be significantly lower than after LNF. Anatomic factors including a tight wrap, distal migration of the wrap over the stomach, migration of the wrap into the mediastinum due to a shortened esophagus, or tight approximation of the crura had been initially implicated in the pathogenesis of postoperative dysphagia [12]. Modifications of total fundoplication, such as partial fundoplication or performing a loose fundoplication are now in common practice. Postoperative dysphagia was reported to occur in 20% to 40% of patients in previous studies [3, 4, 9, 26]. However, in the current analysis, the prevalence was only 12.56% and 4.84% after LNF and LTF, respectively. This phenomenon may be due to the fact that more trials with long-term follow-up were included in our analysis, with improving results as the follow-up period continued.
In the patients with preoperative esophageal dysmotility, dysphagia due to the restriction of LES opening during swallowing, caused by distortion of the distal esophagus at the wrap segment, is generally worse. Inadequate esophageal peristalsis cannot overcome this obstacle, and the emptying of the bolus into the stomach will be blocked [27, 28]. Based on this concept, it has been advocated that there is no need to conduct a Toupet procedure over a full wrap in these patients [29, 30]. Our subgroup analysis, which demonstrated a higher prevalence in both the abnormal EM subgroup compared with the normal subgroup, is consistent with this concept. However, the results in the three subgroups altogether favored LTF, even though it is not significantly different from subgroups 2 and 3. Thus, we still recommend partial fundoplication. It is the surgical approach that affects the prevalence of postoperative dysphagia, instead of preoperative esophageal motility.
It should be noted that dysphagia and preoperative esophageal dysmotility was not accurately defined in most included studies. To evaluate postoperative dysphagia, a subjective questionnaire was used to determine whether they had dysphagia or not during follow-up by Laws et al. [19]. Grading of dysphagia was done by applying Likert scale in the postoperative clinical assessment conducted by Mickevičius et al. [7]. To identify preoperative esophageal dysmotility, dynamic esophageal peristalsis amplitude at the distal esophagus or percentage of effective primary peristalsis during several swallows was most widely used. However, we found that the evaluation criterions were of great variation among studies. For example, Mucio et al. and Strate et al. determined esophageal dysmotility as less than 70% and 60% effective peristalsis, respectively [15][24]. This factor might affect the ability to draw meaningful conclusions about choosing the adequate surgery approach, in spite of the negligible heterogeneity of analysis concerning the postoperative dysphagia with or without preoperative impaired EM. Chicago classification is the newest and the most comprehensive criteria of diagnosing EM disorders to date, which was defined by utilizing high resolution esophageal pressure topography incorporating the combination of high resolution manometry instead of traditional esophageal manometry [31]. Unfortunately, none of included studies in our meta-analysis used Chicago classification to evaluate esophageal dysmotility. For accurate definitions of EM disorders, Chicago classification is required to be used in further studies.
The cause of the gas-bloat syndrome has been attributed to several complex anatomic and functional factors, including vagus nerve injury, slippage, dislocation or disruption of the wrap, defective LES relaxation, preexisting gastric motility disorders, or even to a completely different mechanism of belching in postfundoplication patients [12]. A recent study demonstrated that belching pattern is altered by LNF, by reducing gastric belching (air venting from the stomach) and increasing supragastric belching (no air venting from the stomach) [32]. In our study, LTF was superior in terms of gas-bloat syndrome. A tendency for fewer gas bloat symptoms after LTF in the short- and long-term follow up period has also been reported in prior studies [33, 34]. Taking into consideration the technical details of constructing a partial posterior fundic wrap, LTF appears to be more physiologic than the LNF [35].
In this meta-analysis, reappearance of GERD symptoms was not found to correlate with GERD recurrence. GERD symptoms may result from acid reflux, esophageal hypersensitivity, sustained esophageal contractions or abnormal tissue resistance [36]. Esophageal hypersensitivity may be an independent phenomenon or may overlap with GERD. It describes a condition in which an esophageal stimulus induces GERD symptoms but without any esophageal injury. In other words, patients with esophageal hypersensitivity have a lower threshold for the perception of physiologically nonpainful stimuli [37]. In our study, reappearance of GERD symptoms occurred in 55.2% (449/814) patients following LNF and 59.1% (443/750) patients following LTF. However, the mean scores of DeMeester after both LNF and LTF were normal (i.e. <14.7), which was not accordance with occurrence of postoperative GERD symptoms. Therefore, several recent publications held the view that the recurrence of GERD should be identified by pH studies instead of simply subjective symptoms [38, 39]. Actually, only 26% of symptomatic patients in these studies had abnormal pH studies results. There was no need for these patients to return to PPI treatment. The indication for postoperative medication or re-operations should rely on objective results. As the indication for therapy in GERD depends mainly on clinical symptoms [24], LTF has the same success as LNF in the terms of recurrence. Previous studies reported possible higher recurrence rates of GERD after partial posterior fundoplication [40, 41]. It has been shown that the technique of LTF, consisting of a more than 300° wrap without dissection of the greater curvature, creates an antireflux barrier which is as effective as in LNF [40, 42–43]. In addition, the requirement for postoperative antireflux medications or reoperation due to severe symptoms can also help to determine which approach has a more satisfactory result. Because LNF is a total wrap, postoperative LES pressure was higher compared with LTF, whereas this difference was not apparent in the preoperative period. Although the DeMeester score was lower after LNF, the mean postoperative score was normal after both LNF and LTF, which suggested that reflux can be controlled by LTF to normal level. Meanwhile, we observed a higher prevalence of postoperative adverse results after LNF, corresponding with higher LES pressure. LTF tends to have less outflow resistance as suggested by a lower LES pressure and greater degree of LES relaxation, resulting in postoperative improvement of esophageal peristalsis and esophageal emptying by constructing a physiologic antireflux barrier, while LNF may result in a decreased ability to clear the esophagus [40, 44]. In our current analysis, however, those included studies didn’t provide objective long-term data beyond 5 years. The data of postoperative asymptomatic patients who refused the physiological invasive examinations or lost to follow-up was unavailable [25]. The LTF has always been criticized as having decreased durability in the long-term as several large prospective non-randomized and retrospective studies with long-term follow-up suggested poorer long-term reflux control after LTF [45, 46]. In the study conducted by Kamolz et al., LES pressure decreased over time following LTF but increased over time following LNF. But DeMeester scores were both decreased after 5 years following LNF and LTF [47]. Uncertainty exists over what level of LES pressure can prevent reflux while still avoiding dysphagia. Based on our experience, a postoperative increase in LES pressure of 2 to 5 mmHg below the normal value (10–12 mm Hg) is sufficient to control reflux. Moreover, even though the increase in LES pressure was significantly less after LTF than after LNF, the increase seemed sufficient to prevent the reflux of gastric contents. Results of the present study also support the view that LTF can control reflux symptoms for GERD.
Finally, we concluded that LTF may be the superior surgical approach for GERD with a lower morbidity rate and equal effectiveness compared with LNF.
Supporting Information
(PDF)
(TIF)
(PNG)
(PNG)
(PNG)
(PNG)
(PNG)
(PNG)
(PNG)
(PNG)
(PNG)
Acknowledgments
We gratefully thank Xin Song and Nian-cun Qiu for literature research and data collection.
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
The authors have no support or funding to report.
References
- 1. Kahrilas PJ. (2003) GERD pathogenesis, pathophysiology, and clinical manifestations. Cleveland Clinic journal of medicine. 70 Suppl 5: S4–19. [DOI] [PubMed] [Google Scholar]
- 2. DeMeester SR, DeMeester TR. (2000) Columnar mucosa and intestinal metaplasia of the esophagus: fifty years of controversy. Annals of surgery 231: 303–321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Hunter JG, Swanstrom L, Waring JP. (1996) Dysphagia after laparoscopic antireflux surgery. The impact of operative technique. Annals of surgery 224: 51–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Rantanen TK, Salo JA, Salminen JT, Kellokumpu IH. (1999) Functional outcome after laparoscopic or open Nissen fundoplication: a follow-up study. Archives of surgery. 134: 240–244. [DOI] [PubMed] [Google Scholar]
- 5. Owers C, Ackroyd R. (2013) Management of gastroesophageal reflux disease and hiatus hernia: Overview and authors' perspective. Journal of Surgery 1: 51–58. [Google Scholar]
- 6. Mickevicius A, Endzinas Z, Kiudelis M, Jonaitis L, Kupcinskas L, Maleckas A, et al. (2008) Influence of wrap length on the effectiveness of Nissen and Toupet fundoplication: a prospective randomized study. Surgical endoscopy 22: 2269–2276. 10.1007/s00464-008-9852-9 [DOI] [PubMed] [Google Scholar]
- 7. Mickevičius A, Endzinas Ž, Kiudelis M, Jonaitis L, Kupčinskas L, Pundzius J, et al. (2013) Influence of wrap length on the effectiveness of Nissen and Toupet fundoplications: 5-year results of prospective, randomized study[J]. Surgical endoscopy 27: 986–991. 10.1007/s00464-012-2550-7 [DOI] [PubMed] [Google Scholar]
- 8. Nissen R. (1956) Gastropexy as the lone procedure in the surgical repair of hiatus hernia. The American Journal of Surgery 92: 389–392. [DOI] [PubMed] [Google Scholar]
- 9. DeMeester TR, Bonavina L, Albertucci M. (1986) Nissen fundoplication for gastroesophageal reflux disease. Evaluation of primary repair in 100 consecutive patients. Annals of surgery 204: 9–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. del Pino Porres FJ, Sancho Fornos S, Benages Martinez A, Mora F. (2000) Manometric comprobation of esophagogastric junction competence after Nissen fundoplication and its relation to the length of fundic wrap. World journal of surgery 24: 870–873. [DOI] [PubMed] [Google Scholar]
- 11. Worrell SG, Greene CL, DeMeester TR. (2014) The state of surgical treatment of gastroesophageal reflux disease after five decades. Journal of the American College of Surgeons 219: 819–830. 10.1016/j.jamcollsurg.2014.05.018 [DOI] [PubMed] [Google Scholar]
- 12. Chrysos E, Tsiaoussis J, Zoras OJ, Athanasakis E, Mantides A, Katsamouris A, et al. (2003) Laparoscopic surgery for gastroesophageal reflux disease patients with impaired esophageal peristalsis: total or partial fundoplication? Journal of the American College of Surgeons 197: 8–15. [DOI] [PubMed] [Google Scholar]
- 13. Shan CX, Zhang W, Zheng XM, Jiang DZ, Liu S, Qiu M. (2010) Evidence-based appraisal in laparoscopic Nissen and Toupet fundoplications for gastroesophageal reflux disease. World journal of gastroenterology: WJG, 16: 3063–3071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Tan G, Yang Z, Wang Z. (2011) Meta-analysis of laparoscopic total (Nissen) versus posterior (Toupet) fundoplication for gastro-oesophageal reflux disease based on randomized clinical trials. ANZ journal of surgery 81: 246–252. 10.1111/j.1445-2197.2010.05481.x [DOI] [PubMed] [Google Scholar]
- 15. Mucio M, Rojano M, Herrera JJ, Valdovinos MA, Córdova JÁ, Bravo C, et al. (2012) Novel surgical concept in antireflux surgery: long-term outcomes comparing 3 different laparoscopic approaches[J]. Surgery 151: 84–93. 10.1016/j.surg.2011.06.024 [DOI] [PubMed] [Google Scholar]
- 16. Koch OO, Kaindlstorfer A, Antoniou SA, Luketina RR, Emmanuel K, Pointner R. (2013) Comparison of results from a randomized trial 1 year after laparoscopic Nissen and Toupet fundoplications[J]. Surgical endoscopy 27: 2383–2390. 10.1007/s00464-013-2803-0 [DOI] [PubMed] [Google Scholar]
- 17. Qin M, Ding G, Yang H. (2013) A clinical comparison of laparoscopic nissen and toupet fundoplication for gastroesophageal reflux disease. Journal of Laparoendoscopic & Advanced Surgical Techniques 23: 601–604. [DOI] [PubMed] [Google Scholar]
- 18. Higgins JP, Altman DG, Gøtzsche PC, Jüni P, Moher D, Oxman AD, et al. (2011) The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ, 343:d5928 10.1136/bmj.d5928 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Laws HL, Clements RH, Swillie CM. (1997) A randomized, prospective comparison of the Nissen fundoplication versus the Toupet fundoplication for gastroesophageal reflux disease. Annals of surgery 225: 647–653; discussion 654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Guérin E, Bétroune K, Closset J, Mehdi A, Lefèbvre JC, Houben JJ, et al. (2007) Nissen versus Toupet fundoplication: results of a randomized and multicenter trial. Surgical endoscopy 21: 1985–1990. [DOI] [PubMed] [Google Scholar]
- 21. Booth MI, Stratford J, Jones L, Dehn TC. (2008) Randomized clinical trial of laparoscopic total (Nissen) versus posterior partial (Toupet) fundoplication for gastro-oesophageal reflux disease based on preoperative oesophageal manometry. British Journal of Surgery 95: 57–63. [DOI] [PubMed] [Google Scholar]
- 22. Fibbe C, Layer P, Keller J, Strate U, Emmermann A, Zornig C. (2001) Esophageal motility in reflux disease before and after fundoplication: a prospective, randomized, clinical, and manometric study[J]. Gastroenterology 121: 5–14. [DOI] [PubMed] [Google Scholar]
- 23. Zornig C, Strate U, Fibbe C, Emmermann A, Layer P. (2002) Nissen vs Toupet laparoscopic fundoplication. Surgical Endoscopy and Other Interventional Techniques 16: 758–766. [DOI] [PubMed] [Google Scholar]
- 24. Strate U, Emmermann A, Fibbe C, Layer P, Zornig C. (2008) Laparoscopic fundoplication: Nissen versus Toupet two-year outcome of a prospective randomized study of 200 patients regarding preoperative esophageal motility. Surgical endoscopy 22: 21–30. [DOI] [PubMed] [Google Scholar]
- 25. Shaw JM, Bornman PC, Callanan MD, Beckingham IJ, Metz DC. (2010) Long-term outcome of laparoscopic Nissen and laparoscopic Toupet fundoplication for gastroesophageal reflux disease: a prospective, randomized trial. Surgical endoscopy 24: 924–932. 10.1007/s00464-009-0700-3 [DOI] [PubMed] [Google Scholar]
- 26. Watson DI, Jamieson GG, Devitt PG, Matthew G, Britten-Jones RE, Game PA, et al. (1995) Changing strategies in the performance of laparoscopic Nissen fundoplication as a result of experience with 230 operations. Surgical endoscopy 9: 961–966. [DOI] [PubMed] [Google Scholar]
- 27. Beckingham IJ, Cariem AK, Bornman PC, Callanan MD, Louw JA. (1998) Oesophageal dysmotility is not associated with poor outcome after laparoscopic Nissen fundoplication. Br J Surg 85:1290–1293. [DOI] [PubMed] [Google Scholar]
- 28. Anderson JA, Myers JC, Watson DI, Gabb M, Mathew G, Jamieson GG. (1998) Concurrent fluoroscopy and manometry reveal differences in laparoscopic Nissen and anterior fundoplication. Dig Dis Sci 43: 847–853. [DOI] [PubMed] [Google Scholar]
- 29. McKernan JB, Champion JK. (1995) Minimally invasive surgery for gastroesophageal reflux disease. AmJ Surg 170: 614–618. [DOI] [PubMed] [Google Scholar]
- 30. Stein HJ, DeMeester TR. (1992) Who benefits from antireflux surgery? World J Surg 16:313–319. [DOI] [PubMed] [Google Scholar]
- 31. Bredenoord AJ, Fox M, Kahrilas PJ, Pandolfino JE, Schwizer W, Smout AJ, et al. (2012) Chicago classification criteria of esophageal motility disorders defined in high resolution esophageal pressure topography. Neurogastroenterology and motility: the official journal of the European Gastrointestinal Motility Society 24 Suppl 1:57–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Broeders JA, Bredenoord AJ, Hazebroek EJ, Broeders IA, Gooszen HG, Smout AJ. (2011) Effects of anti-reflux surgery on weakly acidic reflux and belching. Gut 60: 435–441. 10.1136/gut.2010.224824 [DOI] [PubMed] [Google Scholar]
- 33. Lundell L, Abrahamsson H, Ruth M, Sandberg N, Olbe LC. (1991) Lower esophageal sphincter characteristics and esophageal acid exposure following partial or 360 degrees fundoplication: Results of a prospective, randomized, clinical study. World journal of surgery 15: 115–120. [DOI] [PubMed] [Google Scholar]
- 34. Trus TL, Laycock WS, Branum G, Waring JP, Mauren S, Hunter JG. (1996) Intermediate follow-up of laparoscopic antireflux surgery[J]. The American journal of surgery 171: 32–35. [DOI] [PubMed] [Google Scholar]
- 35. Wykypiel H, Hugl B, Gadenstaetter M, Bonatti H, Bodner J, Wetscher GJ. (2008) Laparoscopic partial posterior (Toupet) fundoplication improves esophageal bolus propagation on scintigraphy. Surgical endoscopy 22: 1845–1851. [DOI] [PubMed] [Google Scholar]
- 36. Barlow WJ, Orlando RC. (2005) The pathogenesis of heartburn in nonerosive reflux disease: a unifying hypothesis. Gastroenterology 128(3):771–8. [DOI] [PubMed] [Google Scholar]
- 37. Prakash GC. (2010) Esophageal hypersensitivity. Gastroenterology & hepatology 6(8):497–500. [PMC free article] [PubMed] [Google Scholar]
- 38. Thompson SK, Jamieson GG, Myers JC, Chin KF, Watson DI, Devitt PG. (2007) Recurrent heartburn after laparoscopic fundoplication is not always true reflux. Journal of gastrointestinal surgery: official journal of the Society for Surgery of the Alimentary Tract 11(5): 642–7. [DOI] [PubMed] [Google Scholar]
- 39. Thompson SK, Cai W, Jamieson GG, Zhang AY, Myers JC, Parr ZE. (2009) Recurrent symptoms after fundoplication with a negative pH study—recurrent reflux or functional heartburn. Journal of gastrointestinal surgery: official journal of the Society for Surgery of the Alimentary Tract 13:54–60. [DOI] [PubMed] [Google Scholar]
- 40. Patti MG, De Bellis M, De Pinto M, Bhoyrul S, Tong J, Arcerito M. (1997) Partial fundoplication for gastroesophageal reflux. Surgical endoscopy 11: 445–448. [DOI] [PubMed] [Google Scholar]
- 41. Farrell TM, Archer SB, Galloway KD, Branum GD, Smith CD, Hunter JG. (2000) Heartburn is more likely to recur after Toupet fundoplication than Nissen fundoplication. The American surgeon 66: 229–36; discussion 236–7. [PubMed] [Google Scholar]
- 42. Wykypiel H, Gadenstaetter M, Klaus A, Klingler P, Wetscher GJ. (2005) Nissen or partial posterior fundoplication: which antireflux procedure has a lower rate of side effects?. Langenbeck's Archives of Surgery 390: 141–147. [DOI] [PubMed] [Google Scholar]
- 43. Wykypiel H, Wetscher GJ, Klaus A, Schmid T, Gadenstaetter M, Bodner J. (2003) Robot-assisted laparoscopic partial posterior fundoplication with the DaVinci system: initial experiences and technical aspects. Langenbeck's Archives of Surgery 387: 411–416. [DOI] [PubMed] [Google Scholar]
- 44. Lund RJ, Wetcher GJ, Raiser F, Glaser K, Perdikis G, Gadenstätter M, et al. (1997) Laparoscopic Toupet fundoplication for gastroesophageal reflux disease with poor esophageal body motility. Journal of gastrointestinal surgery 1: 301–308. [DOI] [PubMed] [Google Scholar]
- 45. Jobe BA, Wallace J, Hansen PD, Swanstrom LL. (1997) Evaluation of laparoscopic Toupet fundoplication as a primary repair for all patients with medically resistant gastroesophageal reflux. Surgical endoscopy 11(11):1080–3. [DOI] [PubMed] [Google Scholar]
- 46. Patti MG, Robinson T, Galvani C, Gorodner MV, Fisichella PM, Way LW. (2004) Total fundoplication is superior to partial fundoplication even when esophageal peristalsis is weak. Journal of the American College of Surgeons. 198(6):863–9; discussion 869–70. [DOI] [PubMed] [Google Scholar]
- 47. Kamolz T, Granderath FA, Bammer T, Wykypiel H Jr, Pointner R. (2002) "Floppy" Nissen vs. Toupet laparoscopic fundoplication: quality of life assessment in a 5-year follow-up (part 2). Endoscopy 34(11):917–22. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(PDF)
(TIF)
(PNG)
(PNG)
(PNG)
(PNG)
(PNG)
(PNG)
(PNG)
(PNG)
(PNG)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.