Abstract
Studying the reproductive strategies of insect species that transmit diseases to humans can identify new exploitable targets for the development of vector control methods. Here we describe shared characteristics and individual features of the reproductive biology of three major disease vectors: Anopheles gambiae, Aedes aegypti and Glossina morsitans. Current studies are identifying i) species-specific molecular cascades that determine female monandrous behavior, ii) core aspects of egg development that could be disrupted for controlling natural populations, and iii) the increasingly apparent role of resident microbiota in shaping reproductive success and disease transmission potential. The recent completion of multiple genome sequencing projects is allowing comparative genomics studies that not only increase our knowledge of reproductive processes but also facilitate the identification of novel targets for vector control.
Introduction
The global burden of diseases spread by the biting of insect vectors is a heavy one: more than 17% of all infectious diseases are transmitted by vectors, and it is estimated over half the world's population is at risk, with more than 1 million deaths every year (World Health Organization; URL: www.who.int). Blood feeding is necessary for insect vectors to obtain nutrients required for energy and reproduction, and various viruses and parasites have evolved to exploit this requirement as a way to move between hosts. Current vector control strategies heavily rely on insecticides, which are nevertheless thwarted by the spread of resistance alleles in insect populations. Studying the basic reproductive biology of insect vectors of human disease can identify broad-ranging or species-specific reproductive targets that can be exploited for the development of novel control methods as alternatives or to complement the use of insecticides.
In this review, we will focus on recent findings concerning the reproductive biology of disease vectors from the genera Anopheles (malaria), Aedes (yellow fever, dengue fever, chikungunya), and Glossina (human African trypanosomiasis), which account for the vast majority of global vector-borne mortality. Crucial aspects of mating, egg development and symbiotic relationships will be discussed with the end goal to highlight possible weak links in these life cycles that can be exploited for disease control.
Mating strategies and post-mating behavior of insect vectors
Insect vectors show various mating strategies depending on species-specific behaviors and ecologies. Mosquitoes (sub-Order Nematocera) are distant relatives of the tsetse flies (sub-Order Brachycera). Nevertheless, their mating behaviors bear some similarities (Table 1). Most Anopheles species mate in crepuscular swarms formed over particular markers on the ground [1-3], where males gather at dusk and attract females by as yet unknown mechanisms, likely based on visual and chemical cues. Aedes mosquitoes, although they may show swarming behavior, prefer instead to mate in proximity to the hosts on which they feed [3]. Similarly, tsetse flies mate in close proximity to their vertebrate hosts and utilize visual cues to identify mating partners. Mating begins once a contact-based pheromone on the female is detected by the male [4] and pairs must remain coupled for 1.5-2 hours for the pairing to be successful [5]. In contrast, mosquito matings are short (10-20 seconds).
Table 1. Comparison of the mating biology of major disease vectors.
| Mating biology | Anopheles | Aedes | Glossina |
|---|---|---|---|
| Mating in swarms | Yes | Possible | No |
| Mating near host | No | Yes | Yes |
| Mating duration | 15-20 sec | 10-15 sec | 90-120 min |
| Coagulated seminal fluids (SF) | Yes | No | Yes |
| Identified SF components | 20E, TG3 | JH | None |
| Post-mating changes | |||
| Female monandry | Yes | Yes | Yes |
| Fecundity increased | Yes | Yes | No |
| Ovulation induced | Yes | Yes | Yes |
| Sperm storage | Yes | Yes | Yes |
Regardless of the mating strategy, in all three genera sperm transferred during mating are stored in a dedicated sperm storage organ: a single spermatheca in Anopheles, two in tsetse, and two spermathecae and a bursa inseminalis in Aedes. Males of most Anopheles species are exceptional in the coagulation of their seminal fluid to form a mating plug, a gelatinous rod rich in proteins, lipids and steroid hormones produced in the male accessory glands (MAGs) which upon sexual transfer is processed in the female reproductive tract [6-11]. In An. gambiae, the major malaria vector, transfer of the mating plug is linked to sperm storage, as females that do not receive a plug do not store sperm in their spermatheca [7]. Aedes seminal fluid is not coagulated but nevertheless contains a complex mix of bioactive peptides [12, 13].
Seminal fluids in Glossina are transferred to the female reproductive tract, where they coagulate into a structure called a spermatophore, which also contains the sperm bundle [14]. After mating, the spermatophore is broken down over 24 hours and sperm migrate to the spermathecae. The constituent proteins and chemical moieties associated with this structure remain however undefined. Despite wide evolutionary distance, Anopheles, Aedes and Glossina share a female monandrous behavior (i.e. the occurrence of a single mating event during the female's lifespan). This mating strategy could potentially be targeted using chemical analogs that mimic monandry-inducing factors, preventing virgin females from mating, thereby decreasing vector populations. The male triggers of monandry have been recently identified in An. gambiae: high titers of the steroid hormone 20-hydroxyecdysone (20E) transferred to the female atrium (uterus) within the mating plug contribute to switching the female to a mated status, rendering her refractory to further copulation (among other physiological changes – see below)[10]. As discussed later, both 20E and its precursor ecdysone (E) are also produced by the female after a blood meal, where they are essential for egg development.
Conversely, the molecular basis of monandry is unknown in Aedes, although a number of early studies suggest a role for peptides synthesized in the MAGs [15]. MAG protein extracts were able to induce mating refractoriness and oviposition when injected into virgin female mosquitoes [16, 17]. Recent work aimed at molecularly characterizing components of Aedes seminal fluid has identified a number of male proteins that are transferred to the female, so that the specific factors required to induce monandry in these mosquitoes may be pinned down in the near future [12, 13].
Female tsetse flies also become refractory to further copulation after sex [18], a behavior that starts 24 hours after mating [19, 20]. Injection of MAG extracts can induce mating refractoriness [21], suggesting that factors produced by the male glands are the trigger of this behavior. Spermatophore digestion over 24 hours correlates with the initiation of refractoriness behavior in females, but the nature of the molecular triggers is not known.
Egg development is a conserved process in different vectors
Much of what we know of the molecular mechanisms of oogenesis comes from studies in Ae. aegypti. Here, egg development is triggered by both the nutritional status of the female and the taking of a blood meal (reviewed in [22]). After emergence, the sesquiterpene juvenile hormone (JH) is secreted by the corpora allata in the brain and coordinates the maturation of multiple tissues. As JH levels increase and peak over the first 2 days of adulthood, the multifunctional fat body, with roles in nutrient storage, detoxification and protein synthesis, undergoes structural remodeling and large JH-dependent changes in gene expression that render this tissue competent to respond to ecdysone produced by the ovary after blood feeding [23]. JH also causes the pre-vitellogenic development and maintenance of ovarian follicles, which accumulate lipids and transcripts for key proteins involved in uptake of yolk protein precursors (YPPs) [24, 25]. Moreover, JH delivered by Ae. aegypti males during mating [26] also increases female fecundity [17, 27] by directing available nutrient resources towards reproduction, enlarging ovarian follicles and preventing follicle resorption [28].
After taking a blood meal Aedes mosquitoes develop eggs over 2-3 days. The mosquito brain stops JH synthesis and releases the ovarian ecdysiotropic hormone (OEH)[29], triggering the ovaries to produce the steroid hormone ecdysone [30]. Ecdysone is hydroxylated in turn to 20E in the fat body. Through the 20E receptor EcR/USP and early-acting genes E74, E75 and Broad [31-33], 20E stimulates the transcription of YPPs, such as vitellogenin and lipophorin, which are released into the hemolymph and taken up by the ovaries by receptor-mediated endocytosis [34-36]. Additionally, levels of extracellular amino acids released by blood meal digestion trigger YPP production via the TOR-signaling pathway [37].
These oogenic processes are considered largely conserved in Anopheles as many of the molecular components are found in the genome [38], however published data confirming a role for JH and OEH is limited [39, 40]. In An. gambiae, fecundity is also augmented by mating, and the mating-induced trigger of egg development occurs via the interaction between sexually transferred 20E received in the mating plug and the atrial protein Mating-Induced Stimulator of Oogenesis (MISO) [9]. The MISO-dependent increase in egg numbers is characterized by an enhanced expression of the YPP lipophorin after a blood meal, possibly suggesting that male 20E primes the fat body for the production of YPPs.
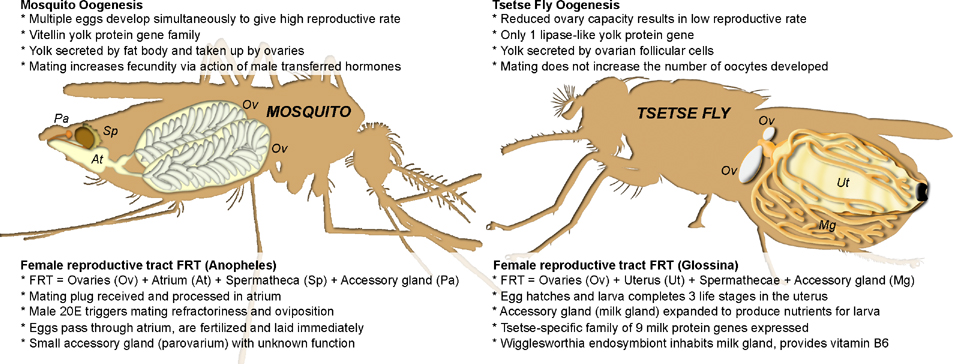
The fundamental biology behind oocyte development in tsetse is similar to that observed in other Diptera, yet is markedly reduced in scale; tsetse ovaries are significantly smaller and contain only two ovarioles per ovary for a total of four ovarioles. During each gonotrophic cycle a single oocyte develops at any given time, independently of mating. The remaining ovarioles are held in a state of arrest that is broken when oocyte development is complete and the oocyte is ovulated into the uterus. Flies utilize lipase-derived YPPs (similar to other Brachyceran flies) that are synthesized and secreted exclusively by the ovarian follicle cells, as opposed to mosquitoes that utilize vitellogenins secreted by the fat body [41]. Egg development processes in mosquitoes and tsetse are summarized in Figure 1.
Figure 1. Oogenesis in mosquitoes and tsetse flies.
An Anopheles mosquito is shown on the left and a tsetse fly on the right. Eggs are shown within the ovaries. The spermathecae of the tsetse fly are not shown for clarity and a 3rd instar larva is shown within the uterus. Aedes mosquitoes have a similar reproductive tract structure to Anopheles, but have three sperm storage organs (two spermathecae and a bursa inseminalis).
After development in the ovary, oocytes are ovulated and fertilized as they move through the female's reproductive tract. In mosquitoes, fertilized embryos are laid seconds later on (Anopheles) or close to (Aedes) water, where they hatch into an aquatic larval life stage (Figure 2). As virgins can develop eggs but do not lay them, mating triggers ovulation and egg-laying behavior. In An. gambiae once again the male-transferred 20E has recently been identified as both a necessary and sufficient trigger of oviposition [10]: 38% of females that were mated to males with experimentally reduced 20E levels were not able to lay their eggs compared to 14% of females mated to control males; consistently, oviposition was stimulated in virgin blood-fed females by the injection of 20E in a dose-dependent manner [10]. 20E also regulates fertility over multiple blood feedings preserving sperm function by up-regulating a spermathecal detoxifying enzyme, the heme peroxidase HPX15, and possible other mechanisms [42]. No information is yet available on the identity of the triggers of oviposition in Aedes, although, again similar to Drosophila, small proteins and peptides produced by the MAGs are likely candidates.
Figure 2. Life cycles of mosquitoes and tsetse flies.
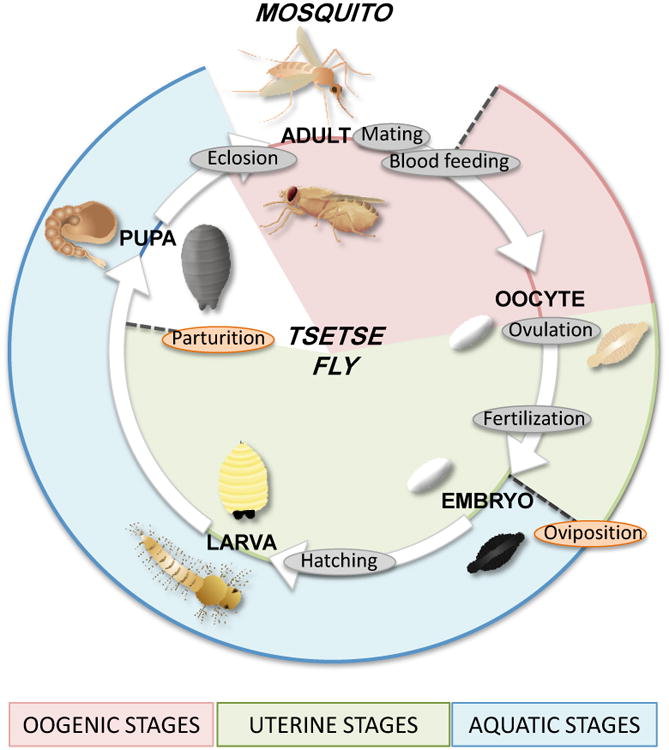
Life cycles are shown on the same circle, with mosquitoes on the outside and tsetse flies on the inside of the circle. Common events are labeled in gray, and species-specific events in orange. After blood feeding, mosquitoes develop multiple oocytes within the ovaries, which are ovulated and fertilized as they pass through the reproductive tract. The embryos are laid immediately on (Anopheles) or close to (Aedes) water and hatch into larvae. The larvae molt thought 4 instars and become pupae, before eclosing into adult mosquitoes. Tsetse flies begin egg development during the pupal stage and continue to develop oocytes after blood meals. A single oocyte is developed at one time and is ovulated into the uterus. The oocyte is fertilized and the embryo remains in the uterus, where it hatches into a larva. The larva is fed by nutrients from the milk gland and completes 3 further molts within the uterus. The female gives birth to a 3rd instar larva, which rapidly pupates, before eclosing into an adult.
In tsetse, the oocyte is ovulated and fertilized as it enters into the uterus. Ovulation in tsetse is dependent upon the mating and pregnancy status of the fly [18]. Like mosquitoes, unmated females develop oocytes but are unable to ovulate until after mating. MAG proteins do not appear to regulate ovulation as mechanical stimulation by intrauterine implantation of glass beads has the same effect as mating [21]. However, ovulation can be induced in virgin females by the injection of hemolymph from mated pregnant females, which indicates an ovulation factor is systemically released in response to mating and oogenic stimuli [5].
At this point tsetse's reproductive cycle veers dramatically from that of oviparous insects (Figure 2). The embryo is retained within the uterus and hatches into a larva, which grows within the mother for its entire 3 instar developmental cycle and is fed by nutrients generated by an adapted female accessory gland termed the milk gland. This is the defining characteristic of obligate adenotrophic viviparity. The milk secretions generated by the mother consist of roughly 50% lipids and 50% protein. During lactation stored lipids are mobilized from the fat body to the milk gland, and this transfer is regulated by JH and insulin signaling [43]. Milk contains at least 12 proteins, 10 of which are specific to tsetse [44] and are partly regulated in a milk gland- and pregnancy-specific manner by a transcription factor called Ladybird late [45]. This factor/regulatory system appears conserved among the Brachyceran dipterans and may also regulate female accessory function in other vector species within the Nematocera.
Microbes influence the reproductive success of insect vector species
It has long been known that tsetse fly reproduction is strongly dependent upon its relationship with an obligate endosymbiont, Wigglesworthia [46]. Wigglesworthia live intracellularly in a specialized organ in the gut named the bacteriome, and extracellularly within the lumen of the milk gland, allowing bacteria to be vertically transmitted to the developing larva [47, 48]. These symbionts supplement tsetse's rich but nutritionally limited blood diet with essential nutrients and cofactors required for energy metabolism. The Wigglesworthia genome encodes the synthesis pathways for multiple B vitamins, one of which (vitamin B6) functions as a co-factor for proline biosynthesis in the fly [49]. Proline functions as tsetse's primary energy source and is required to maintain the energetic process of milk production during pregnancy. In addition to Wigglesworthia, laboratory strains of tsetse have endosymbiotic relationships with Sodalis and Wolbachia bacteria. Sodalis do not affect tsetse's reproductive physiology but are required for longevity and can reduce trypanosome infection intensity [50], while Wolbachia induce strong cytoplasmic incompatibility [51], a phenomenon discussed below. All three symbionts may be exploited in paratransgenic anti-pathogen strategies to create trypanosome-resistant tsetse populations [52].
In mosquitoes, antibiotic treatment does affect reproductive output, indirectly implying roles for microbiota in reproductive fitness [53, 54]. Well studied are the reproductive effects of Wolbachia, an endosymbiont which resides within the germlines of many arthopod species (including tsetse and some mosquitoes), and is vertically transmitted from the female parent to progeny. Initially characterized in Culex mosquitoes [55], Wolbachia can cause cytoplasmic incompatibility (CI - reviewed within [56]) whereby matings between uninfected females and infected males result in embryonic lethality in the progeny, while matings of infected females produce fertile progeny regardless of the infection status of the male. Wolbachia infection can cause additional reproductive phenotypes, including increased fecundity and hatching rates in Aedes albopictus [57, 58]. Because of these reproductive phenotypes, Wolbachia infections can rapidly spread through natural insect populations, and have been detected in Aedes [59], Glossina [51, 60] and more recently in Anopheles species from West Africa [61]. Additionally, Wolbachia infections have also been shown to block human pathogen transmission in Ae. aegypti [62, 63] and in An. stephensi [64, 65], prompting their current and proposed use in disease control programs [61, 66, 67] (Eliminate Dengue Program; URL: www.eliminatedengue.com). A specific effect of Wolbachia on trypanosome infection intensity or on tsetse fecundity has not yet been fully established.
Comparative genomics to identify shared and species-specific reproductive pathways
Recent publication of the genomes of the major tsetse fly vector species G. morsitans morsitans [68] and 16 Anopheles species [69] has provided exceptional opportunity to study key biological questions of vector species. How do tsetse achieve their unusual reproductive biology? What are the individual and common determinants of reproductive success in anophelines? What species are likely to be targeted by particular reproductive control strategies? The anticipated genome release of the invasive mosquito species Ae. albopictus [70] and improvements to the assembly of the Ae. aegypti genome [71] will enable similar comparative analysis for Aedes mosquitoes.
The analysis of the tsetse genome has allowed a first insight into how reproduction diverged so significantly in these vectors [68]. The reduced capacity for oogenesis in tsetse may have resulted in the reduction in YPP genes; while close relatives of tsetse carry three or more YPPs, tsetse has only one, YP1. Conversely, the evolution of lactation has resulted in the expansions of families of milk protein genes, likely via a series of gene duplication events. Nine of these genes are clustered within a single 40 kb region of the genome and have no known orthologs in other Diptera. They are only expressed in the secretory cells of the milk gland and in coordination with the pregnancy status of the mother. Novel insights into the reproductive biology of Anopheles mosquitoes have been provided by the 16 genome sequencing project [69]. Previous work in the African vector An. gambiae had shown that formation of the mating plug depends on the crosslinking activity of a MAG-specific transglutaminase enzyme, AgTG3 [7, 72]. Phylogenetic analysis across 16 anophelines showed that AgTG3 is highly divergent and more rapidly evolving than the other two TGs present in the genome [69], possibly reflecting divergence in mating plug phenotypes. This hypothesis was confirmed by a phenotypic study where semen coagulation and 20E synthesis by the MAGs were determined in eight Anopheles species besides An. gambiae. While three anophelines (An. arabiensis, An. funestus and An. stephensi) had a fully coagulated plug and high 20E levels in the MAGs similar to An. gambiae, others (An. atroparvus, An. dirus, An. farauti, and An. sinensis) showed intermediate coagulation and hormone synthesis, while the New World species An. albimanus was the only species that completely lacked both plug formation and male 20E synthesis [11]. These findings indicate the occurrence of different reproductive strategies across the Anopheles genus. Ancestral state reconstruction analyses determined that plug coagulation and 20E synthesis in the MAGs are derived characters that have co-evolved in anophelines from a plugless and 20E-less ancestor [11]. Given the role of 20E transfer in switching off the female receptivity to further mating and in inducing oviposition [10], these data suggest that strategies targeting this steroid hormone may be successful in preventing successful mating and reproduction in a number of malaria vectors, demonstrating the power of comparative genomics.
Conclusions
The 200 million years of divergence separating mosquitoes and tsetse flies is reflected in the remarkable differences in their reproductive biology. Control methods based on reducing the reproductive output of these insect vectors of disease are likely to be highly specific compared to wide-spectrum insecticides, and less harmful to the native ecology. On the other hand, these species share a male-triggered monandrous behavior that suggests these species could all be vulnerable to control strategies that either mimic or disrupt key factors transferred at mating, such as the steroid hormone 20E in Anopheles. The slow, reduced rate and unusual method of reproduction in tsetse makes this species an especially attractive target for control strategies based on disrupting the reproductive cycle, perhaps by interfering with milk protein production in lactation or exploiting its obligate symbiotic relationships. The next steps are to translate these laboratory findings into effective strategies to control insect populations in disease-endemic areas, producing a significant impact on the global burden of vector-borne disease.
Highlights.
Targeting reproduction may lead to new ways to control vector-borne disease
Monandry is a weak link in the life cycles of disease vectors
Tsetse fly reproduction is unique and includes pregnancy and lactation
The microbiome can influence the reproductive success of disease vectors
Comparative genomics powerfully reveals shared and species-specific mechanisms
Acknowledgments
W. Robert Shaw and Flaminia Catteruccia were supported by grants from the National Institutes of Health R21 AI117313 and R01 AI104956, and the European Research Council FP7 Starting Grant project “Anorep” Grant 260897. Geoffrey M. Attardo and Serap Aksoy were supported by grants from the National Institutes of Health R01 AI081774 and R21 AI109263, and the Ambrose Monell Foundation.
Footnotes
The authors declare no competing financial interests
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Marchand RP. Field observations on swarming and mating in Anopheles gambiae mosquitoes in Tanzania. Netherlands Journal of Zoology. 1984;34:367–387. [Google Scholar]
- 2.Charlwood JD, Pinto J, Sousa CA, Madsen H, Ferreira C, do Rosario VE. The swarming and mating behaviour of Anopheles gambiae s.s. (Diptera: Culicidae) from Sao Tome Island. J Vector Ecol. 2002;27:178–83. [PubMed] [Google Scholar]
- 3.Yuval B. Mating systems of blood-feeding flies. Annu Rev Entomol. 2006;51:413–40. doi: 10.1146/annurev.ento.51.110104.151058. [DOI] [PubMed] [Google Scholar]
- 4.Carlson DA, Langley PA, Huyton P. Sex pheromone of the tsetse fly: isolation, identification, and synthesis of contact aphrodisiacs. Science. 1978;201:750–3. doi: 10.1126/science.675256. [DOI] [PubMed] [Google Scholar]
- 5.Chaudhury MF, Dhadialla TS. Evidence of hormonal control of ovulation in tsetse flies. Nature. 1976;260:243–4. doi: 10.1038/260243a0. [DOI] [PubMed] [Google Scholar]
- 6.Giglioli M, Mason G. The mating plug of anopheline mosquitoes. Proc R Ent Soc Lond. 1966;41:123–129. [Google Scholar]
- 7.Rogers DW, Baldini F, Battaglia F, Panico M, Dell A, Morris HR, Catteruccia F. Transglutaminase-Mediated Semen Coagulation Controls Sperm Storage in the Malaria Mosquito. PLoS Biology. 2009;7:e1000272. doi: 10.1371/journal.pbio.1000272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pondeville E, Maria A, Jacques JC, Bourgouin C, Dauphin-Villemant C. Anopheles gambiae males produce and transfer the vitellogenic steroid hormone 20-hydroxyecdysone to females during mating. Proc Natl Acad Sci U S A. 2008;105:19631–6. doi: 10.1073/pnas.0809264105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Baldini F, Gabrieli P, South A, Valim C, Mancini F, Catteruccia F. The Interaction between a Sexually Transferred Steroid Hormone and a Female Protein Regulates Oogenesis in the Malaria Mosquito Anopheles gambiae. PLoS Biology. 2013;11:e1001695. doi: 10.1371/journal.pbio.1001695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- **10.Gabrieli P, Kakani EG, Mitchell SN, Mameli E, Want EJ, Mariezcurrena Anton A, Serrao A, Baldini F, Catteruccia F. Sexual transfer of the steroid hormone 20E induces the postmating switch inAnopheles gambiae. Proceedings of the National Academy of Sciences. 2014;111:16353–16358. doi: 10.1073/pnas.1410488111. This paper demonstrates a key role of male-transferred steroid hormone 20E in triggering mating refractoriness and egg laying in Anopheles gambiae females. It underlines the pivotal nature of this hormone in Anopheles reproduction. [DOI] [PMC free article] [PubMed] [Google Scholar]
- **11.Mitchell SN, Kakani EG, South A, Howell PI, Waterhouse RM, Catteruccia F. Evolution of sexual traits influencing vectorial capacity in anopheline mosquitoes. Science. 2015;347:985–988. doi: 10.1126/science.1259435. This paper describes the co-evolution of mating plug and male 20E synthesis in anophelines, and determines that co-evolution of these two male reproductive traits has driven adaptive changes in female reproductive processes that may have influenced malaria transmission dynamics. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sirot LK, Poulson RL, McKenna MC, Girnary H, Wolfner MF, Harrington LC. Identity and transfer of male reproductive gland proteins of the dengue vector mosquito, Aedes aegypti: potential tools for control of female feeding and reproduction. Insect Biochem Mol Biol. 2008;38:176–89. doi: 10.1016/j.ibmb.2007.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sirot LK, Hardstone MC, Helinski ME, Ribeiro JM, Kimura M, Deewatthanawong P, Wolfner MF, Harrington LC. Towards a semen proteome of the dengue vector mosquito: protein identification and potential functions. PLoS Negl Trop Dis. 2011;5:e989. doi: 10.1371/journal.pntd.0000989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kokwaro ED, Odhiambo TR. Spermatophore of the tsetse, Glossina mortisans mortisans Westwood: an ultrastructural study. International Journal of Tropical Insect Science. 1981;1:185–190. [Google Scholar]
- 15.Craig GB. Mosquitoes: female monogamy induced by male accessory gland substance. Science. 1967;156:1499–1501. doi: 10.1126/science.156.3781.1499. [DOI] [PubMed] [Google Scholar]
- 16.Hiss EA, Fuchs MS. The effect of matrone on oviposition in the mosquito, Aedes Aegypti. J Insect Physiol. 1972;18:2217–27. doi: 10.1016/0022-1910(72)90250-8. [DOI] [PubMed] [Google Scholar]
- 17.Klowden MJ, Chambers GM. Male accessory gland substances activate egg development in nutritionally stressed Aedes aegypti mosquitoes. J Insect Physiol. 1991;37:721–726. [Google Scholar]
- 18.Saunders DS, Dodd CWH. Mating, insemination, and ovulation in the tsetse fly, Glossina mortisans. J Insect Physiol. 1972;18:187–198. [Google Scholar]
- 19.Curtis CF. Radiation sterilization and the effect of multiple mating of females in Glossina austeni. J Insect Physiol. 1968;14:1365–80. [Google Scholar]
- 20.Pollock JN. The evolution of sperm transfer mechanisms in the Diptera. Journal of Entomology Series A. 1972;47:29–35. [Google Scholar]
- 21.Gillott C, Langley PA. The control of receptivity and ovulation in the tsetse fly, Glossina morsitans. Physiological Entomology. 1981;6:269–281. [Google Scholar]
- 22.Attardo GM, Hansen IA, Raikhel AS. Nutritional regulation of vitellogenesis in mosquitoes: Implications for anautogeny. Insect Biochemistry and Molecular Biology. 2005;35:661–675. doi: 10.1016/j.ibmb.2005.02.013. [DOI] [PubMed] [Google Scholar]
- **23.Zou Z, Saha TT, Roy S, Shin SW, Backman TW, Girke T, White KP, Raikhel AS. Juvenile hormone and its receptor, methoprene-tolerant, control the dynamics of mosquito gene expression. Proc Natl Acad Sci U S A. 2013;110:E2173–81. doi: 10.1073/pnas.1305293110. This paper captures the cascade of developmental changes in the fat body after eclosion, in preparation for YPP production. These changes are mediated largely by JH and the authors identify a consensus binding site for the JH receptor in the promoters of these genes. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Clifton ME, Noriega FG. Nutrient limitation results in juvenile hormone-mediated resorption of previtellogenic ovarian follicles in mosquitoes. Journal of Insect Physiology. 2011;57:1274–1281. doi: 10.1016/j.jinsphys.2011.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Clifton ME, Noriega FG. The fate of follicles after a blood meal is dependent on previtellogenic nutrition and juvenile hormone in Aedes aegypti. Journal of Insect Physiology. 2012;58:1007–1019. doi: 10.1016/j.jinsphys.2012.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Borovsky D, Carlson DA, Hancock RG, Rembold H, van Handel E. De novo biosynthesis of juvenile hormone III and I by the accessory glands of the male mosquito. Insect Biochem Mol Biol. 1994;24:437–44. doi: 10.1016/0965-1748(94)90038-8. [DOI] [PubMed] [Google Scholar]
- 27.Klowden MJ. Mating and nutritional state affect the reproduction of Aedes albopictus mosquitoes. J Am Mosq Control Assoc. 1993;9:169–73. [PubMed] [Google Scholar]
- **28.Clifton ME, Correa S, Rivera-Perez C, Nouzova M, Noriega FG. Male Aedes aegypti mosquitoes use JH III transferred during copulation to influence previtellogenic ovary physiology and affect the reproductive output of female mosquitoes. Journal of Insect Physiology. 2014;64:40–47. doi: 10.1016/j.jinsphys.2014.03.006. This paper shows that in Aedes mosquitoes a male-transferred hormone (JH) increases oogenesis, and also includes a mechanistic explanation by follicle resorption. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Brown MR, Graf R, Swiderek KM, Fendley D, Stracker TH, Champagne DE, Lea AO. Identification of a steroidogenic neurohormone in female mosquitoes. J Biol Chem. 1998;273:3967–71. doi: 10.1074/jbc.273.7.3967. [DOI] [PubMed] [Google Scholar]
- 30.Hagedorn HH, O'Connor JD, Fuchs MS, Sage B, Schlaeger DA, Bohm MK. The ovary as a source of alpha-ecdysone in an adult mosquito. Proc Natl Acad Sci U S A. 1975;72:3255–9. doi: 10.1073/pnas.72.8.3255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sun G, Zhu J, Chen L, Raikhel AS. Synergistic action of E74B and ecdysteroid receptor in activating a 20-hydroxyecdysone effector gene. Proc Natl Acad Sci U S A. 2005;102:15506–11. doi: 10.1073/pnas.0503501102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chen L, Zhu J, Sun G, Raikhel AS. The early gene Broad is involved in the ecdysteroid hierarchy governing vitellogenesis of the mosquito Aedes aegypti. J Mol Endocrinol. 2004;33:743–61. doi: 10.1677/jme.1.01531. [DOI] [PubMed] [Google Scholar]
- 33.Cruz J, Mane-Padros D, Zou Z, Raikhel AS. Distinct roles of isoforms of the heme-liganded nuclear receptor E75, an insect ortholog of the vertebrate Rev-erb, in mosquito reproduction. Mol Cell Endocrinol. 2012;349:262–71. doi: 10.1016/j.mce.2011.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cheon HM, Seo SJ, Sun J, Sappington TW, Raikhel AS. Molecular characterization of the VLDL receptor homolog mediating binding of lipophorin in oocyte of the mosquito Aedes aegypti. Insect Biochem Mol Biol. 2001;31:753–60. doi: 10.1016/s0965-1748(01)00068-6. [DOI] [PubMed] [Google Scholar]
- 35.Sappington TW, Kokoza VA, Cho WL, Raikhel AS. Molecular characterization of the mosquito vitellogenin receptor reveals unexpected high homology to the Drosophila yolk protein receptor. Proc Natl Acad Sci U S A. 1996;93:8934–9. doi: 10.1073/pnas.93.17.8934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Raikhel AS, Dhadialla TS. Accumulation of yolk proteins in insect oocytes. Annu Rev Entomol. 1992;37:217–51. doi: 10.1146/annurev.en.37.010192.001245. [DOI] [PubMed] [Google Scholar]
- 37.Hansen IA, Attardo GM, Park JH, Peng Q, Raikhel AS. Target of rapamycin-mediated amino acid signaling in mosquito anautogeny. Proc Natl Acad Sci U S A. 2004;101:10626–31. doi: 10.1073/pnas.0403460101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Noriega FG, Ribeiro JM, Koener JF, Valenzuela JG, Hernandez-Martinez S, Pham VM, Feyereisen R. Comparative genomics of insect juvenile hormone biosynthesis. Insect Biochem Mol Biol. 2006;36:366–74. doi: 10.1016/j.ibmb.2006.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lu YH, Hagedorn HH. Egg development in the mosquito Anopheles albimanus. International Journal of Invertebrate Reproduction and Development. 1986;9:79–94. [Google Scholar]
- 40.Bai H, Gelman DB, Palli SR. Mode of action of methoprene in affecting female reproduction in the African malaria mosquito, Anopheles gambiae. Pest Manag Sci. 2010;66:936–43. doi: 10.1002/ps.1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Attardo GM, Guz N, Strickler-Dinglasan P, Aksoy S. Molecular aspects of viviparous reproductive biology of the tsetse fly (Glossina morsitans morsitans): Regulation of yolk and milk gland protein synthesis. Journal of Insect Physiology. 2006;52:1128–1136. doi: 10.1016/j.jinsphys.2006.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Shaw WR, Teodori E, Mitchell SN, Baldini F, Gabrieli P, Rogers DW, Catteruccia F. Mating activates the heme peroxidase HPX15 in the sperm storage organ to ensure fertility in Anopheles gambiae. Proc Natl Acad Sci U S A. 2014;111:5854–9. doi: 10.1073/pnas.1401715111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Baumann AA, Benoit JB, Michalkova V, Mireji PO, Attardo GM, Moulton JK, Wilson TG, Aksoy S. Juvenile hormone and insulin suppress lipolysis between periods of lactation during tsetse fly pregnancy. Molecular and Cellular Endocrinology. 2013;372:30–41. doi: 10.1016/j.mce.2013.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *44.Benoit JB, Attardo GM, Michalkova V, Krause TB, Bohova J, Zhang Q, Baumann AA, Mireji PO, Takac P, Denlinger DL, et al. A novel highly divergent protein family identified from a viviparous insect by RNA-seq analysis: a potential target for tsetse fly-specific abortifacients. PLoS Genet. 2014;10:e1003874. doi: 10.1371/journal.pgen.1003874. This paper identifies the components of tsetse milk by transcriptomic and proteomic analyses. These in depth analyses find a novel milk gene family and demonstrate their essential requirement for larva development. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Attardo GM, Benoit JB, Michalkova V, Patrick KR, Krause TB, Aksoy S. The homeodomain protein ladybird late regulates synthesis of milk proteins during pregnancy in the tsetse fly (Glossina morsitans) PLoS Negl Trop Dis. 2014;8:e2645. doi: 10.1371/journal.pntd.0002645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Aksoy S. Wigglesworthia gen. nov. and Wigglesworthia glossinidia sp. nov., taxa consisting of the mycetocyte-associated, primary endosymbionts of tsetse flies. Int J Syst Bacteriol. 1995;45:848–51. doi: 10.1099/00207713-45-4-848. [DOI] [PubMed] [Google Scholar]
- 47.Attardo GM, Lohs C, Heddi A, Alam UH, Yildirim S, Aksoy S. Analysis of milk gland structure and function in Glossina morsitans: milk protein production, symbiont populations and fecundity. J Insect Physiol. 2008;54:1236–42. doi: 10.1016/j.jinsphys.2008.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Balmand S, Lohs C, Aksoy S, Heddi A. Tissue distribution and transmission routes for the tsetse fly endosymbionts. J Invertebr Pathol. 2013;112 Suppl:S116–22. doi: 10.1016/j.jip.2012.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Michalkova V, Benoit JB, Weiss BL, Attardo GM, Aksoy S. Vitamin B6 generated by obligate symbionts is critical for maintaining proline homeostasis and fecundity in tsetse flies. Appl Environ Microbiol. 2014;80:5844–53. doi: 10.1128/AEM.01150-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Dale C, Welburn SC. The endosymbionts of tsetse flies: manipulating host-parasite interactions. Int J Parasitol. 2001;31:628–31. doi: 10.1016/s0020-7519(01)00151-5. [DOI] [PubMed] [Google Scholar]
- 51.Alam U, Medlock J, Brelsfoard C, Pais R, Lohs C, Balmand S, Carnogursky J, Heddi A, Takac P, Galvani A, et al. Wolbachia Symbiont Infections Induce Strong Cytoplasmic Incompatibility in the Tsetse Fly Glossina morsitans. PLoS Pathogens. 2011;7:e1002415. doi: 10.1371/journal.ppat.1002415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Medlock J, Atkins KE, Thomas DN, Aksoy S, Galvani AP. Evaluating Paratransgenesis as a Potential Control Strategy for African Trypanosomiasis. PLoS Neglected Tropical Diseases. 2013;7:e2374. doi: 10.1371/journal.pntd.0002374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sharma A, Dhayal D, Singh OP, Adak T, Bhatnagar RK. Gut microbes influence fitness and malaria transmission potential of Asian malaria vector Anopheles stephensi. Acta Trop. 2013;128:41–7. doi: 10.1016/j.actatropica.2013.06.008. [DOI] [PubMed] [Google Scholar]
- 54.Gendrin M, Rodgers FH, Yerbanga RS, Ouedraogo JB, Basanez MG, Cohuet A, Christophides GK. Antibiotics in ingested human blood affect the mosquito microbiota and capacity to transmit malaria. Nat Commun. 2015;6:5921. doi: 10.1038/ncomms6921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Laven H. Eradication of Culex pipiens fatigans through cytoplasmic incompatibility. Nature. 1967;216:383–4. doi: 10.1038/216383a0. [DOI] [PubMed] [Google Scholar]
- 56.Serbus LR, Casper-Lindley C, Landmann F, Sullivan W. The Genetics and Cell Biology ofWolbachia-Host Interactions. Annual Review of Genetics. 2008;42:683–707. doi: 10.1146/annurev.genet.41.110306.130354. [DOI] [PubMed] [Google Scholar]
- 57.Dobson SL, Marsland EJ, Rattanadechakul W. Mutualistic Wolbachia infection in Aedes albopictus: accelerating cytoplasmic drive. Genetics. 2002;160:1087–94. doi: 10.1093/genetics/160.3.1087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Dobson SL, Rattanadechakul W, Marsland EJ. Fitness advantage and cytoplasmic incompatibility in Wolbachia single- and superinfected Aedes albopictus. Heredity (Edinb) 2004;93:135–42. doi: 10.1038/sj.hdy.6800458. [DOI] [PubMed] [Google Scholar]
- 59.Kittayapong P, Baisley KJ, Baimai V, O'Neill SL. Distribution and diversity of Wolbachia infections in Southeast Asian mosquitoes (Diptera: Culicidae) J Med Entomol. 2000;37:340–5. doi: 10.1093/jmedent/37.3.340. [DOI] [PubMed] [Google Scholar]
- 60.Alam U, Hyseni C, Symula RE, Brelsfoard C, Wu Y, Kruglov O, Wang J, Echodu R, Alioni V, Okedi LM, et al. Implications of microfauna-host interactions for trypanosome transmission dynamics in Glossina fuscipes fuscipes in Uganda. Appl Environ Microbiol. 2012;78:4627–37. doi: 10.1128/AEM.00806-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Baldini F, Segata N, Pompon J, Marcenac P, Shaw WR, Dabire KR, Diabate A, Levashina E, Catteruccia F. Evidence of natural Wolbachia infections in field populations of Anopheles gambiae. Nature Communications. 2014;5:3985. doi: 10.1038/ncomms4985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Moreira LA, Iturbe-Ormaetxe I, Jeffery JA, Lu G, Pyke AT, Hedges LM, Rocha BC, Hall-Mendelin S, Day A, Riegler M, et al. A Wolbachia Symbiont in Aedes aegypti Limits Infection with Dengue, Chikungunya, and Plasmodium. Cell. 2009;139:1268–1278. doi: 10.1016/j.cell.2009.11.042. [DOI] [PubMed] [Google Scholar]
- 63.Kambris Z, Cook PE, Phuc HK, Sinkins SP. Immune activation by life-shortening Wolbachia and reduced filarial competence in mosquitoes. Science. 2009;326:134–6. doi: 10.1126/science.1177531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Hughes GL, Koga R, Xue P, Fukatsu T, Rasgon JL. Wolbachia infections are virulent and inhibit the human malaria parasite Plasmodium falciparum in Anopheles gambiae. PLoS Pathog. 2011;7:e1002043. doi: 10.1371/journal.ppat.1002043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Bian G, Joshi D, Dong Y, Lu P, Zhou G, Pan X, Xu Y, Dimopoulos G, Xi Z. Wolbachia Invades Anopheles stephensi Populations and Induces Refractoriness to Plasmodium Infection. Science. 2013;340:748–751. doi: 10.1126/science.1236192. [DOI] [PubMed] [Google Scholar]
- 66.Hoffmann AA, Montgomery BL, Popovici J, Iturbe-Ormaetxe I, Johnson PH, Muzzi F, Greenfield M, Durkan M, Leong YS, Dong Y, et al. Successful establishment of Wolbachia in Aedes populations to suppress dengue transmission. Nature. 2011;476:454–457. doi: 10.1038/nature10356. [DOI] [PubMed] [Google Scholar]
- 67.Hoffmann AA, Iturbe-Ormaetxe I, Callahan AG, Phillips BL, Billington K, Axford JK, Montgomery B, Turley AP, O'Neill SL. Stability of the wMel Wolbachia Infection following invasion into Aedes aegypti populations. PLoS Negl Trop Dis. 2014;8:e3115. doi: 10.1371/journal.pntd.0003115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- **68.IGGI. Genome Sequence of the Tsetse Fly (Glossina morsitans): Vector of African Trypanosomiasis. Science. 2014;344:380–386. doi: 10.1126/science.1249656. This paper reports the genome of Glossina morsitans morsitans, the first tsetse fly to be sequenced. This identifies how multiple processes in tsetse flies have diverged from other insects, including reproduction, immunity, and relationships with symbiotic bacteria. [DOI] [PMC free article] [PubMed] [Google Scholar]
- **69.Neafsey DE, Waterhouse RM, Abai MR, Aganezov SS, Alekseyev MA, Allen JE, Amon J, Arca B, Arensburger P, Artemov G, et al. Highly evolvable malaria vectors: The genomes of 16 Anopheles mosquitoes. Science. 2015;347:1258522. doi: 10.1126/science.1258522. This paper provides a comparative genomic analysis of 16 anopheline species with a wide range of abilities to transmit malaria. It is a large body of work that identifies differences in genome structure and evolution and provides an essential resource for future vector biology studies. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Bonizzoni M, Gasperi G, Chen X, James AA. The invasive mosquito species Aedes albopictus: current knowledge and future perspectives. Trends in Parasitology. 2013;29:460–468. doi: 10.1016/j.pt.2013.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Nene V, Wortman JR, Lawson D, Haas B, Kodira C, Tu ZJ, Loftus B, Xi Z, Megy K, Grabherr M, et al. Genome sequence of Aedes aegypti, a major arbovirus vector. Science. 2007;316:1718–23. doi: 10.1126/science.1138878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Le BV, Nguyen JB, Logarajah S, Wang B, Marcus J, Williams HP, Catteruccia F, Baxter RHG. Characterization of Anopheles gambiae Transglutaminase 3 (AgTG3) and Its Native Substrate Plugin. Journal of Biological Chemistry. 2013;288:4844–4853. doi: 10.1074/jbc.M112.435347. [DOI] [PMC free article] [PubMed] [Google Scholar]